Abstract
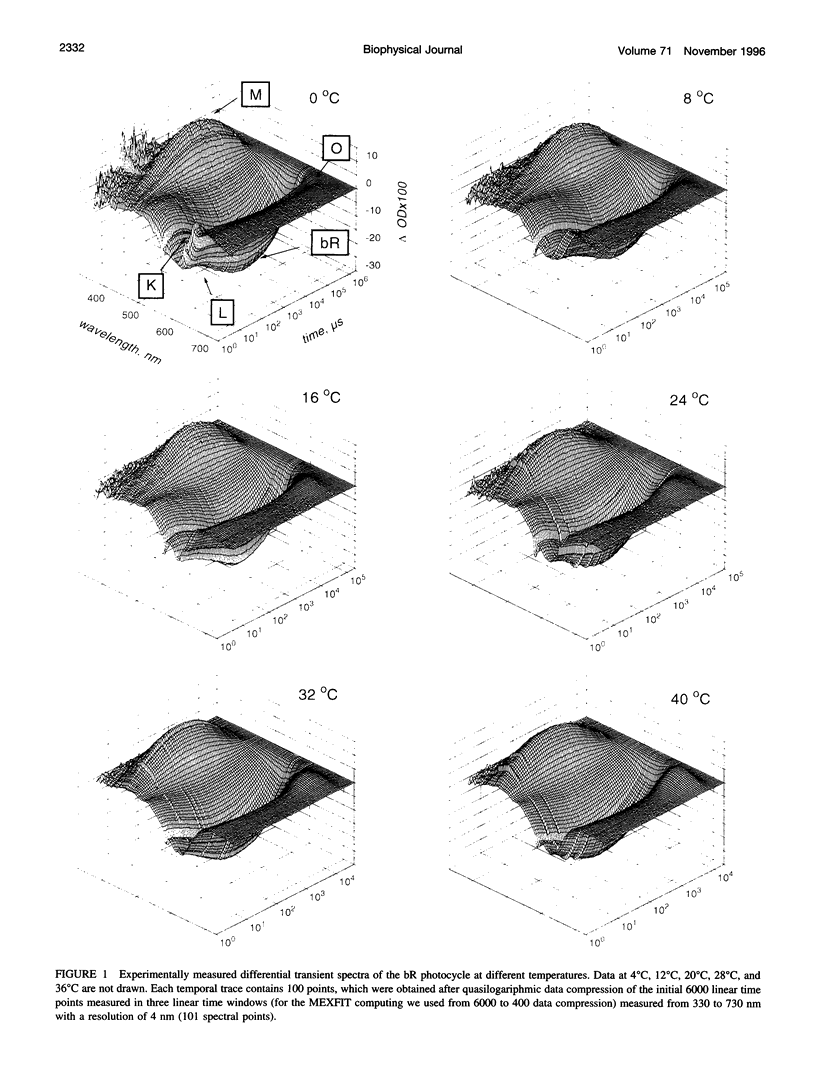
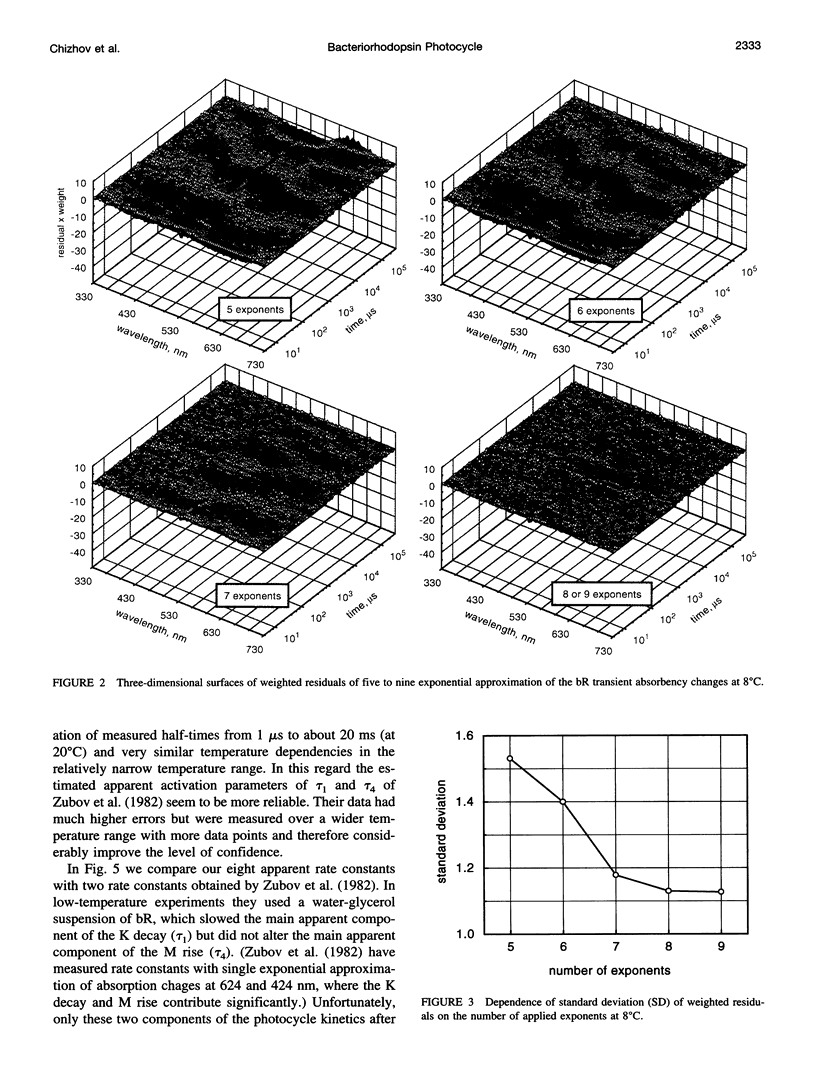
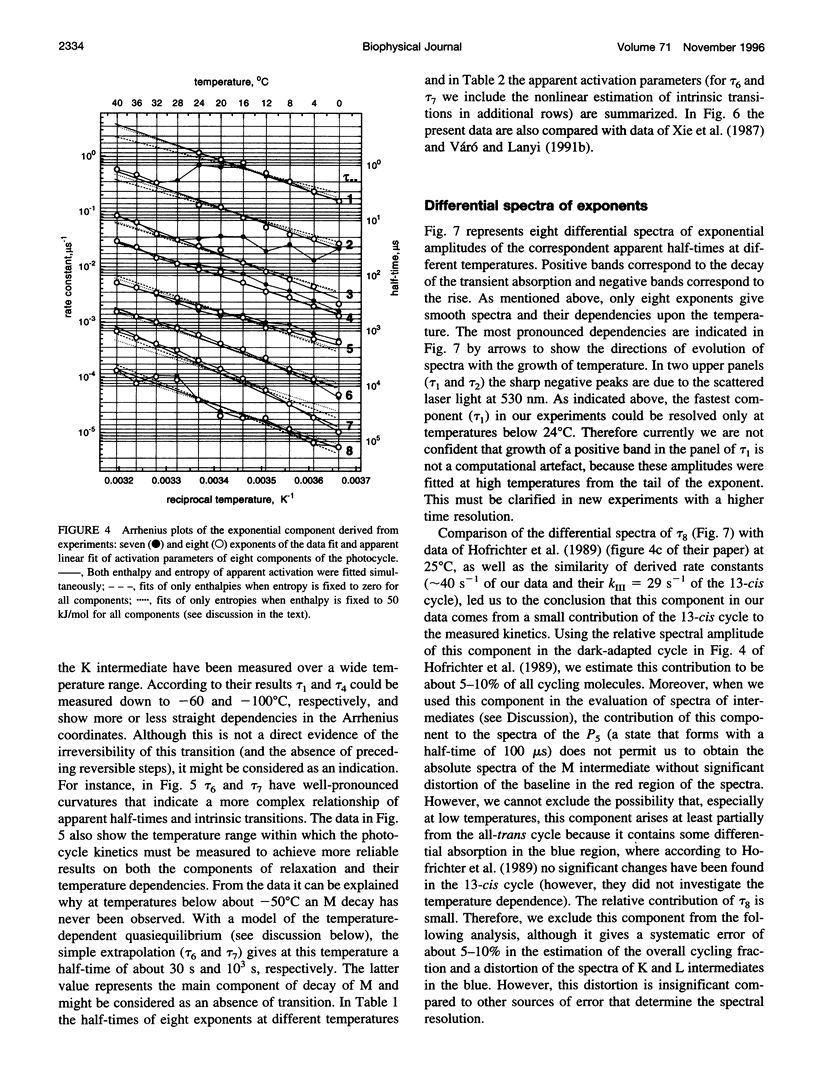
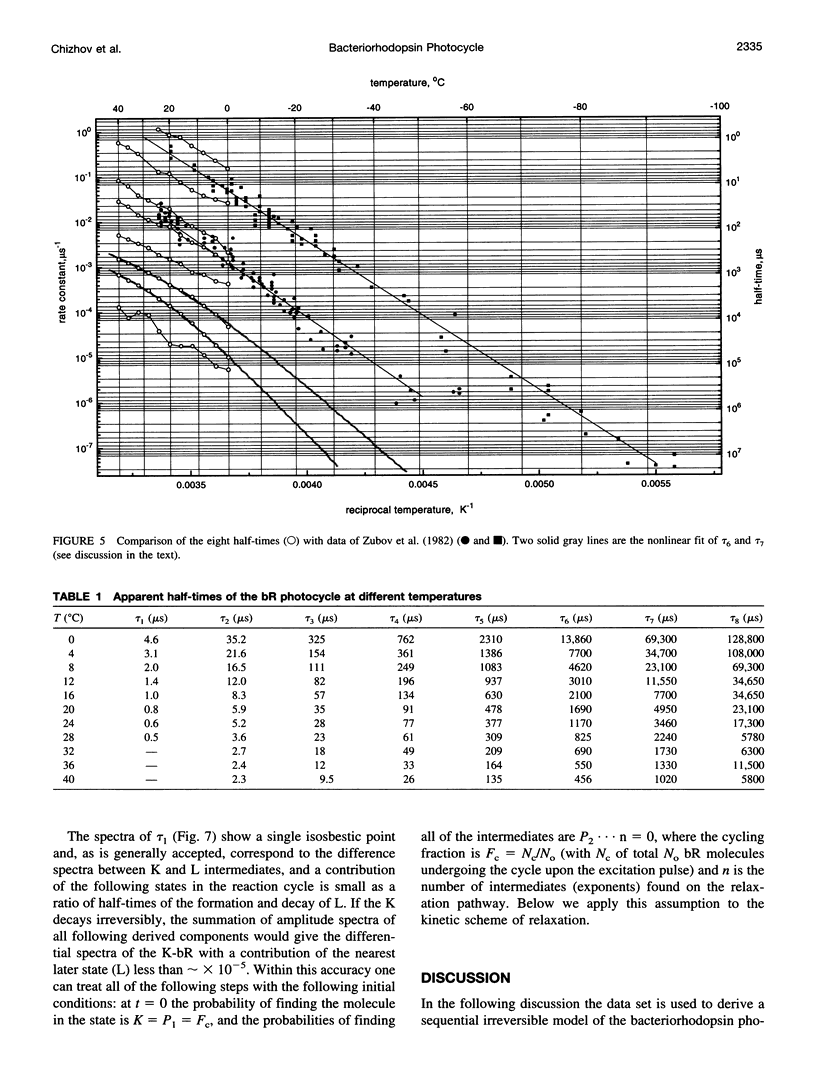
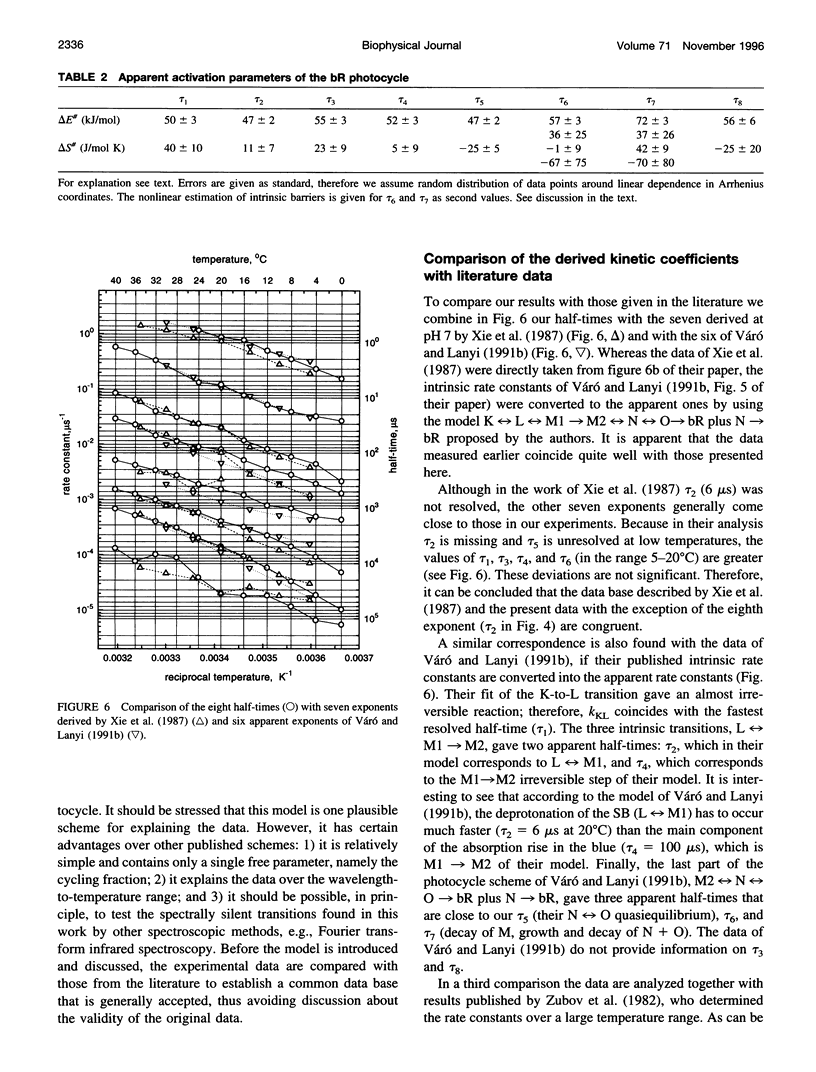
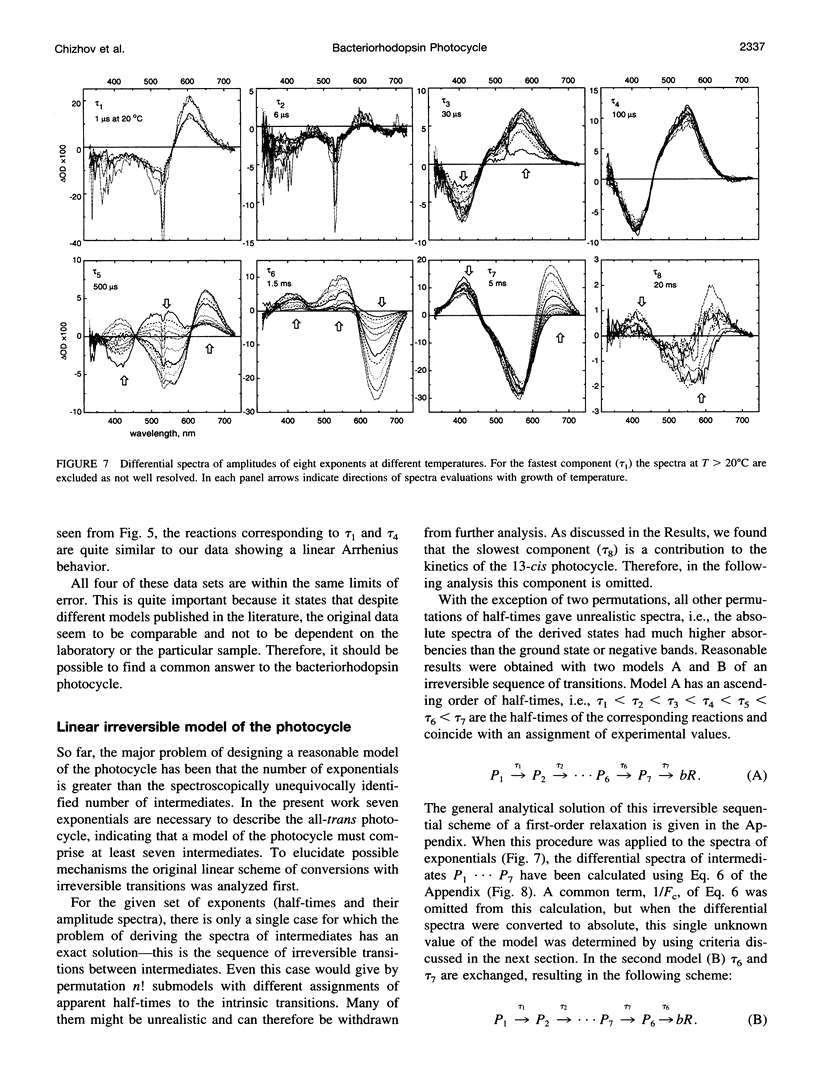
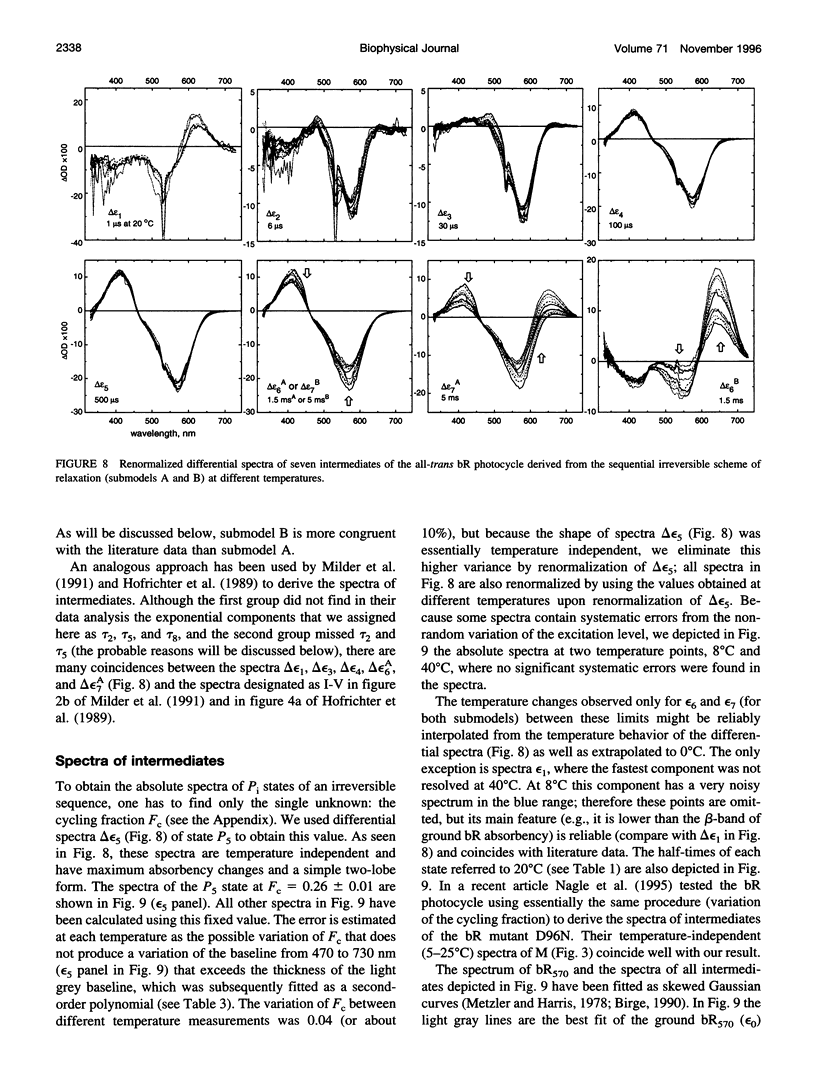
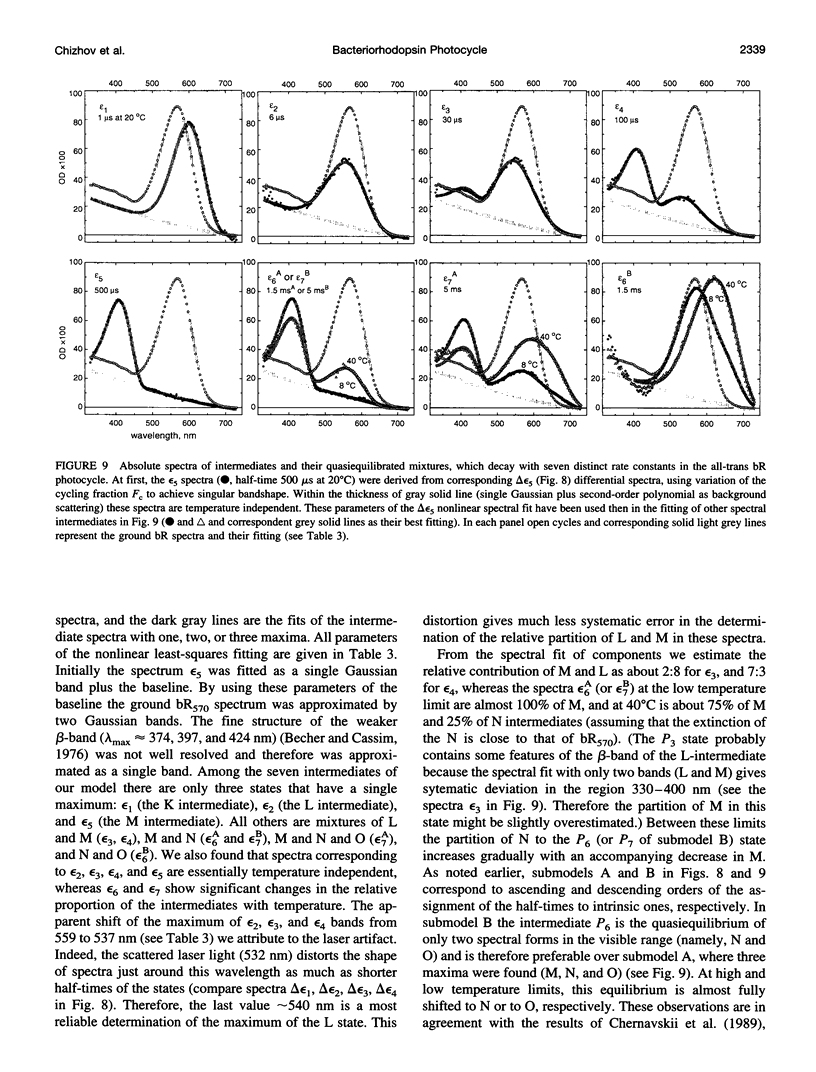
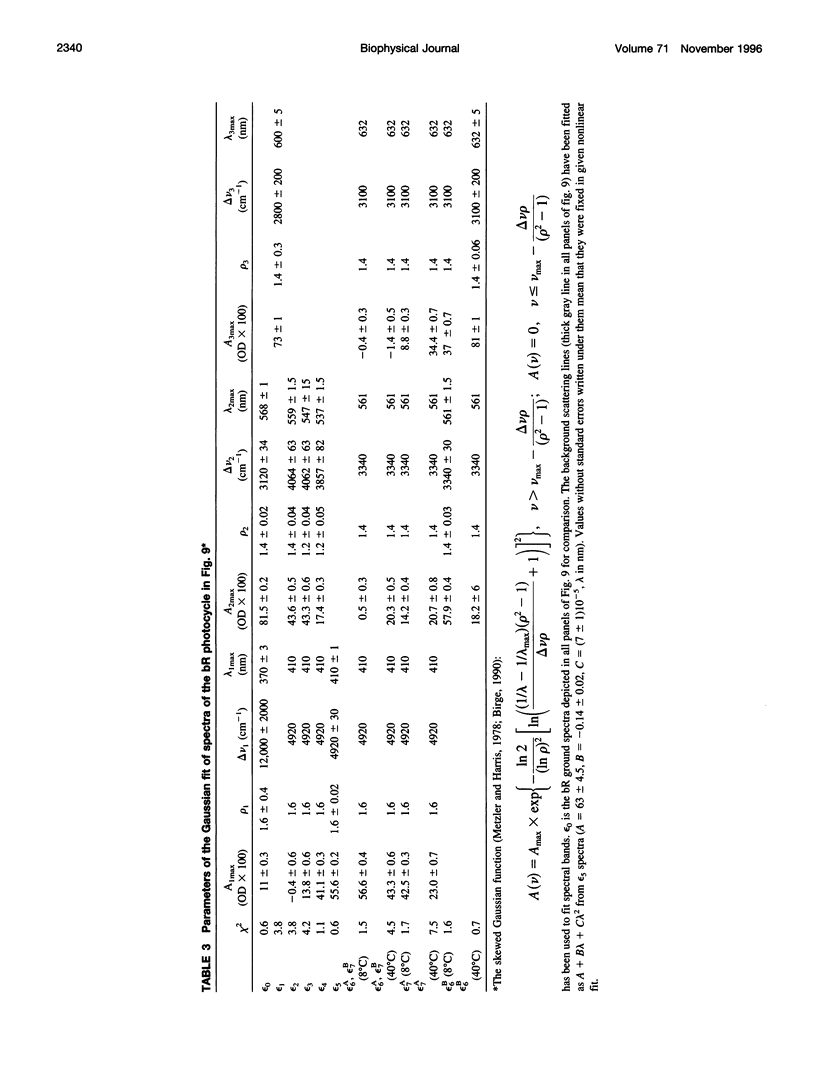
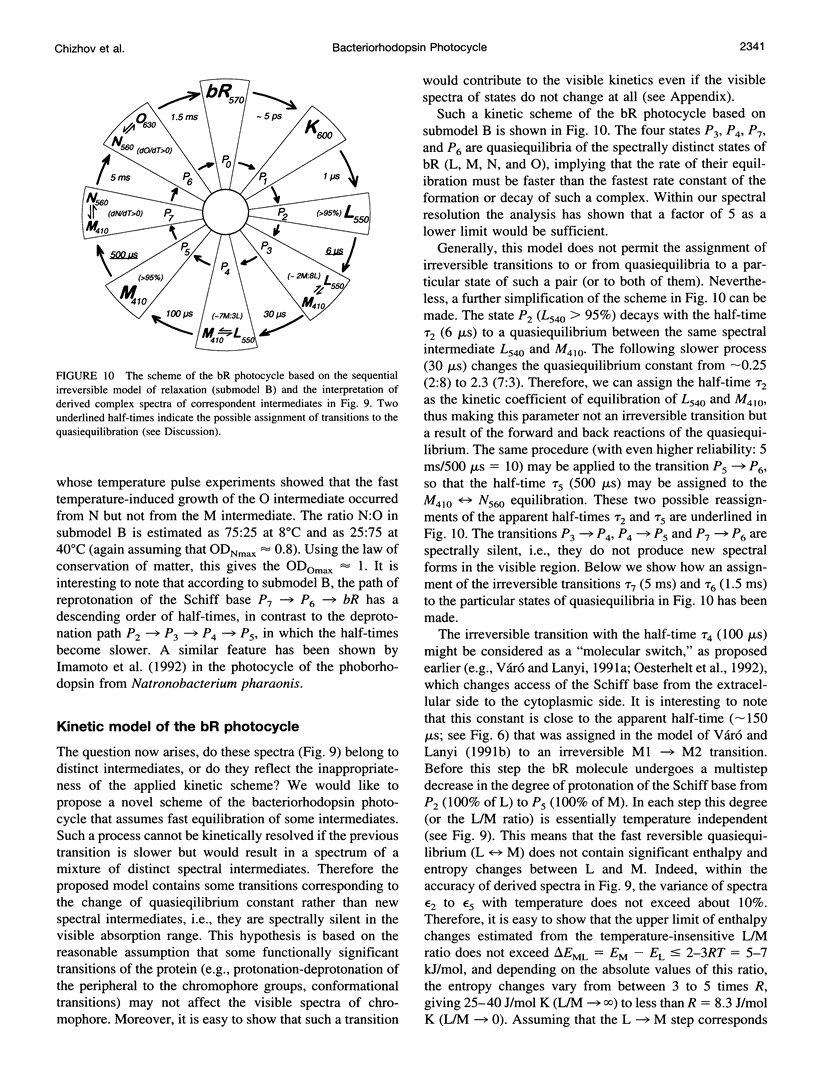
The photocycle kinetics of bacteriorhodopsin were analyzed from 0 to 40 degrees C at 101 wavelengths (330-730 nm). The data can be satisfactorily approximated by eight exponents. The slowest component (half-time 20 ms at 20 degrees C) belongs to the 13-cis cycle. The residual seven exponentials that are sufficient to describe the all-trans photocycle indicate that at least seven intermediates of the all-trans cycle must exist, although only five spectrally distinct species (K, L, M, N, and O) have been identified. These seven exponentials and their spectra at different temperatures provide the basis for the discussion of various kinetic schemes of the relaxation. The simplest model of irreversible sequential transitions includes after the first K--> L step the quasiequilibria of L<-->M, M<-->N, and N<-->O intermediates. These quasiequilibria are controlled by rate-limiting dynamics of the protein and/or proton transfer steps outside the chromophore region. Thus there exists an apparent kinetic paradox (i.e., why is the number of exponents of relaxation (at least seven) higher than the number of distinct spectral intermediates (only five)), which can be explained by assuming that some of the transitions correspond to changes in the quasiequilibria between spectrally distinct intermediates (i.e., are spectrally silent).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexiev U., Mollaaghababa R., Scherrer P., Khorana H. G., Heyn M. P. Rapid long-range proton diffusion along the surface of the purple membrane and delayed proton transfer into the bulk. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):372–376. doi: 10.1073/pnas.92.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Cao Y., Brown L. S., Sasaki J., Maeda A., Needleman R., Lanyi J. K. Relationship of proton release at the extracellular surface to deprotonation of the schiff base in the bacteriorhodopsin photocycle. Biophys J. 1995 Apr;68(4):1518–1530. doi: 10.1016/S0006-3495(95)80324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Porte M., Hess B., Oesterhelt D. Low temperature kinetics of H+ changes of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):913–917. doi: 10.1016/S0006-3495(75)85865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernavskii D. S., Chizhov I. V., Lozier R. H., Murina T. M., Prokhorov A. M., Zubov B. V. Kinetic model of bacteriorhodopsin photocycle: pathway from M state to bR. Photochem Photobiol. 1989 May;49(5):649–653. doi: 10.1111/j.1751-1097.1989.tb08437.x. [DOI] [PubMed] [Google Scholar]
- Chizhov I., Engelhard M., Chernavskii D. S., Zubov B., Hess B. Temperature and pH sensitivity of the O(640) intermediate of the bacteriorhodopsin photocycle. Biophys J. 1992 Apr;61(4):1001–1006. doi: 10.1016/s0006-3495(92)81907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Kung M., DeVault D., Hess B., Oesterhelt D. Photolysis of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):907–911. doi: 10.1016/S0006-3495(75)85864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Heyn M. P., Lanyi J. K., Ottolenghi M., Zimanyi L. Thermal equilibration between the M and N intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1993 Sep;65(3):1231–1234. doi: 10.1016/S0006-3495(93)81166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillbro T. Flash kinetic study of the last steps in the photoinduced reaction cycle of bacteriorhodopsin. Biochim Biophys Acta. 1978 Oct 11;504(1):175–186. doi: 10.1016/0005-2728(78)90016-6. [DOI] [PubMed] [Google Scholar]
- Heberle J., Oesterhelt D., Dencher N. A. Decoupling of photo- and proton cycle in the Asp85-->Glu mutant of bacteriorhodopsin. EMBO J. 1993 Oct;12(10):3721–3727. doi: 10.1002/j.1460-2075.1993.tb06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Hess B., Kuschmitz D. Branching reactions in the photocycle of bacteriorhodopsin. FEBS Lett. 1978 Sep 15;93(2):266–270. doi: 10.1016/0014-5793(78)81118-1. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler D. E., Harris C. M. Shapes of spectral bands of visual pigments. Vision Res. 1978;18(10):1417–1420. doi: 10.1016/0042-6989(78)90235-3. [DOI] [PubMed] [Google Scholar]
- Milder S. J., Thorgeirsson T. E., Miercke L. J., Stroud R. M., Kliger D. S. Effects of detergent environments on the photocycle of purified monomeric bacteriorhodopsin. Biochemistry. 1991 Feb 19;30(7):1751–1761. doi: 10.1021/bi00221a004. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Solving complex photocycle kinetics. Theory and direct method. Biophys J. 1991 Feb;59(2):476–487. doi: 10.1016/S0006-3495(91)82241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Zimanyi L., Lanyi J. K. Testing BR photocycle kinetics. Biophys J. 1995 Apr;68(4):1490–1499. doi: 10.1016/S0006-3495(95)80321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Parodi L. A., Lozier R. H., Bhattacharjee S. M., Nagle J. F. Testing kinetic models for the bacteriorhodopsin photocycle--II. Inclusion of an O to M backreaction. Photochem Photobiol. 1984 Oct;40(4):501–506. doi: 10.1111/j.1751-1097.1984.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Korenstein R., Caplan S. R. Energetics and chronology of phototransients in the light response of the purple membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Jun 8;430(3):454–458. doi: 10.1016/0005-2728(76)90021-9. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Váró G., Chang M., Ni B., Needleman R., Lanyi J. K. Pathways of proton release in the bacteriorhodopsin photocycle. Biochemistry. 1992 Sep 15;31(36):8535–8543. doi: 10.1021/bi00151a022. [DOI] [PubMed] [Google Scholar]