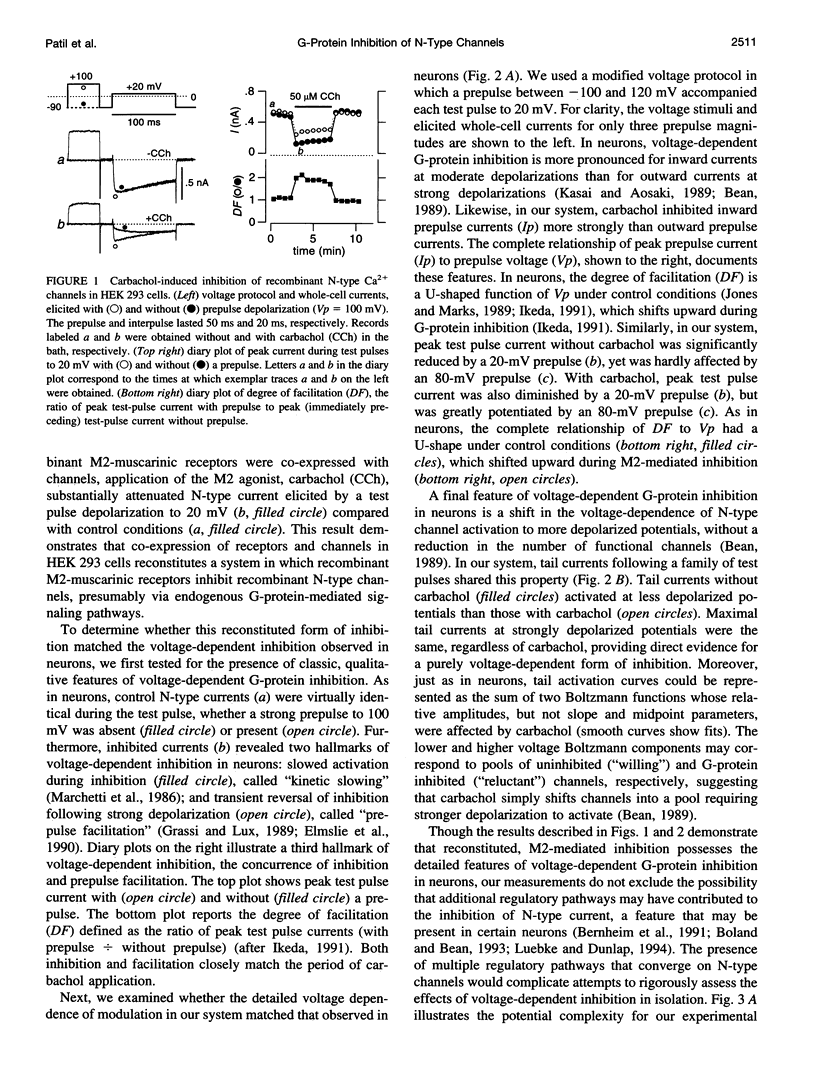
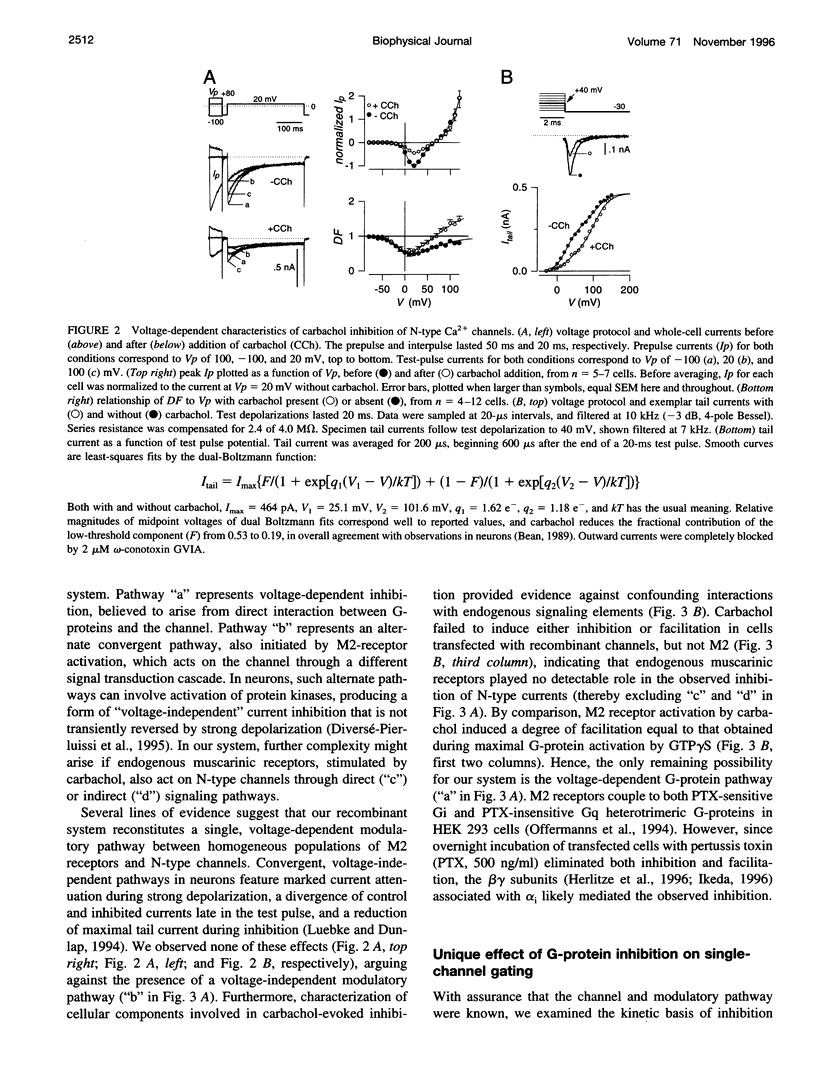
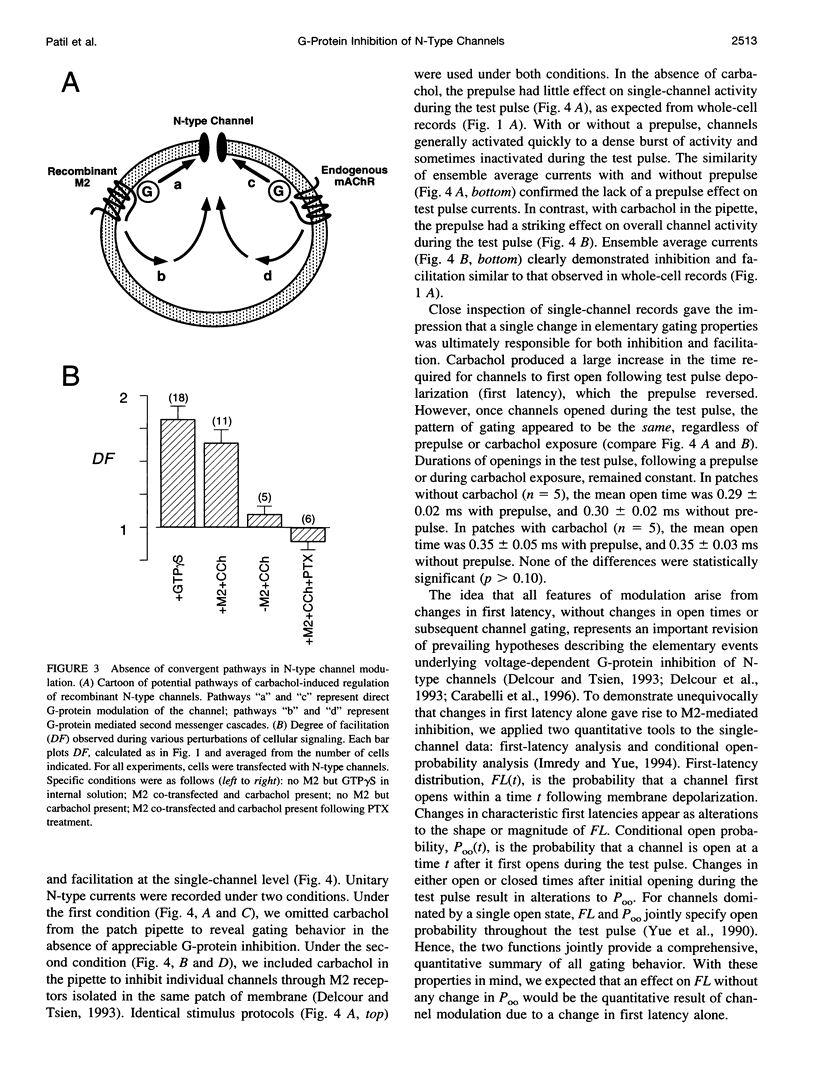
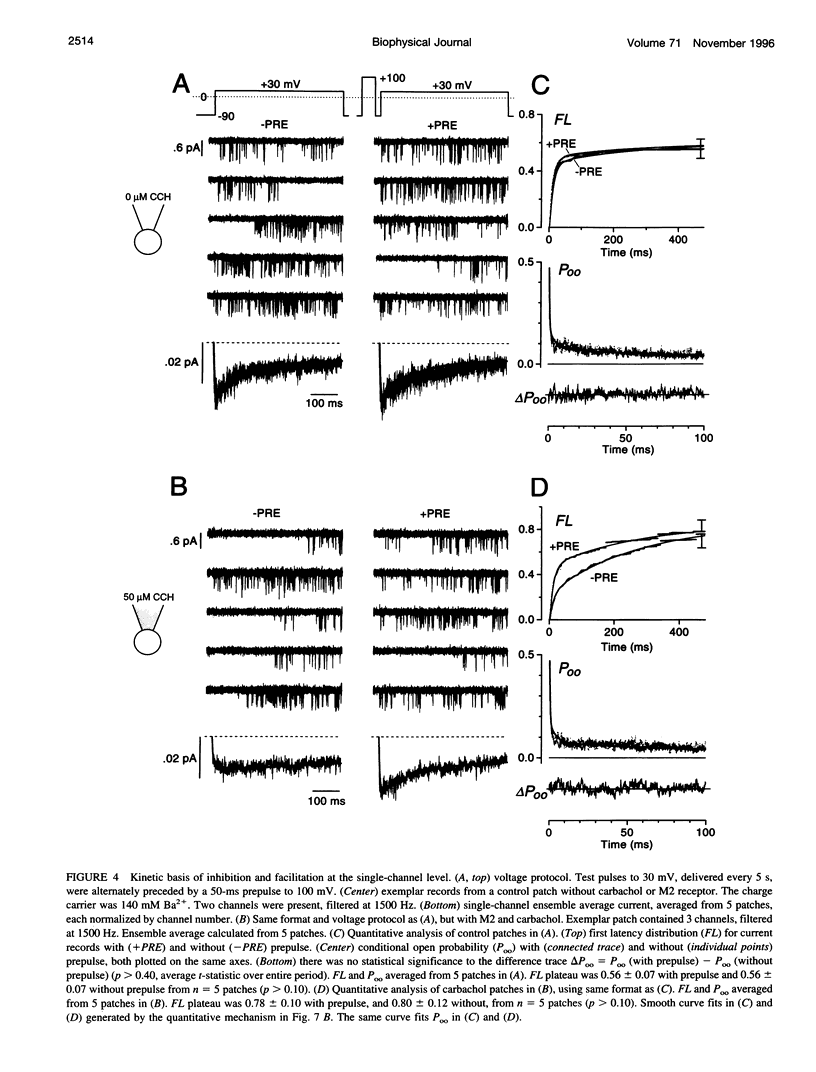
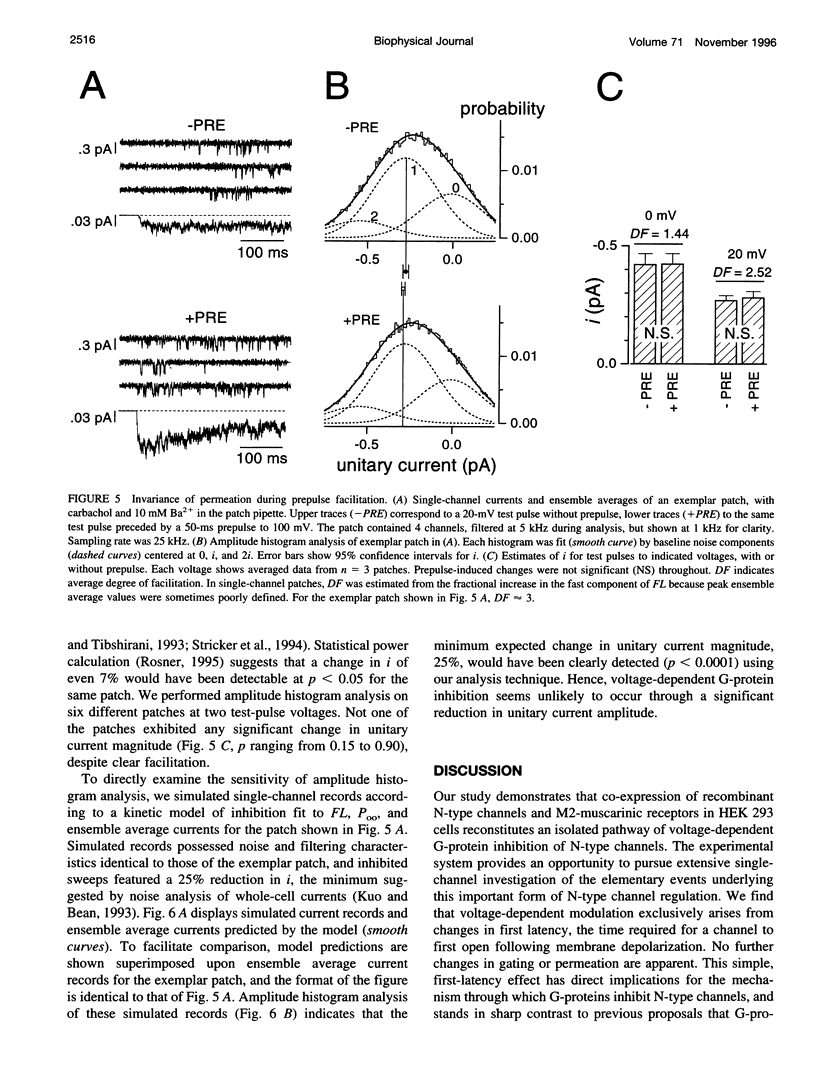
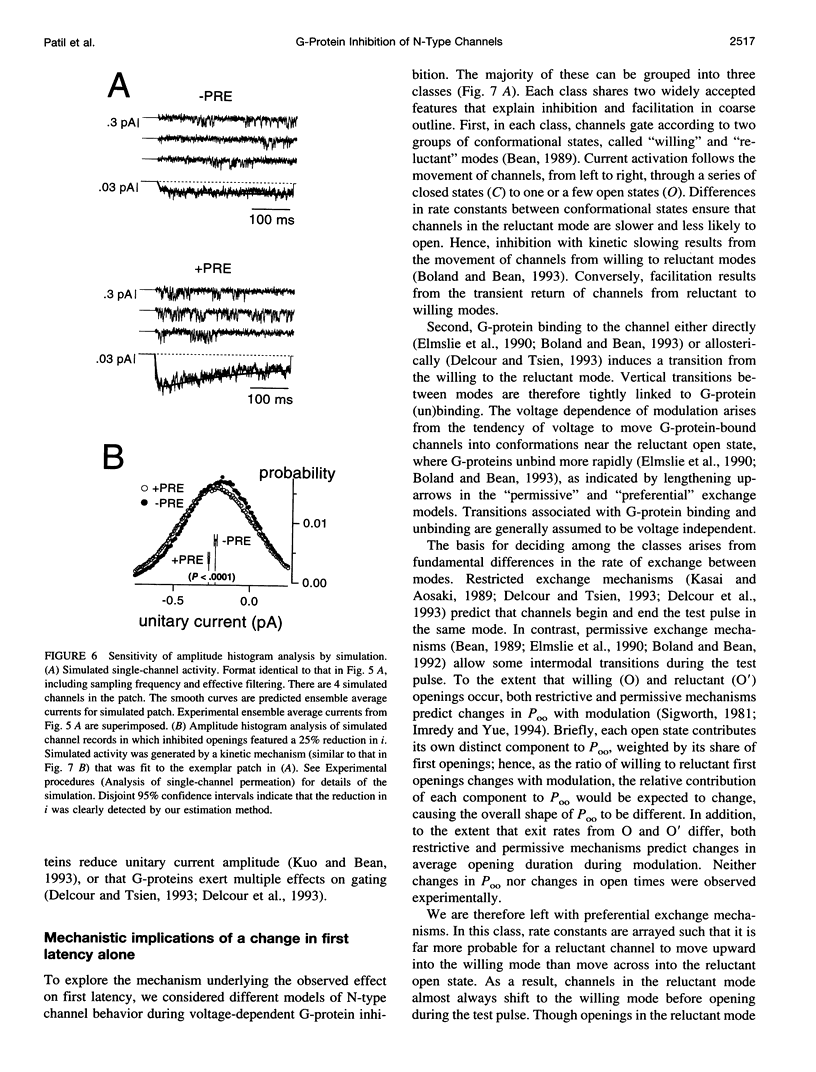
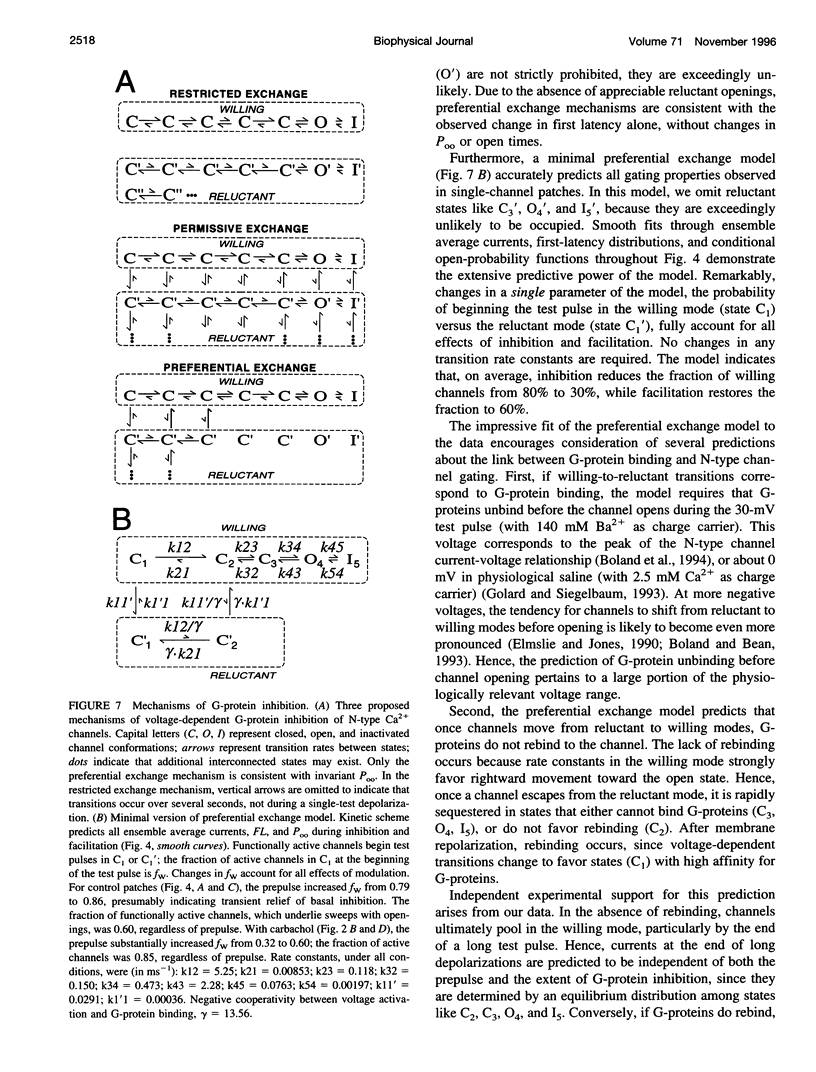
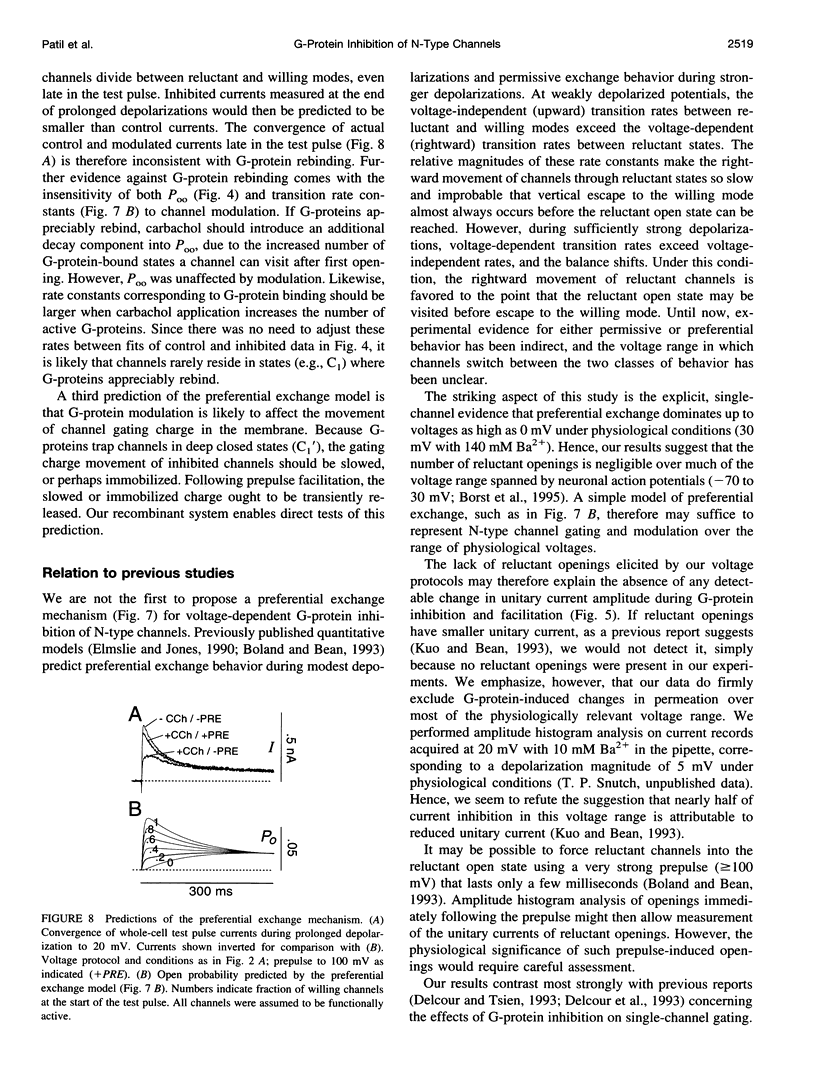
Abstract
Voltage-dependent G-protein inhibition of N-type calcium channels reduces presynaptic calcium entry, sharply attenuating neurotransmitter release. Studies in neurons demonstrate that G-proteins have multiple modulatory effects on N-type channels. The observed changes may reflect genuine complexity in G-protein action and/or the intricate interactions of multiple channels and receptors in neurons. Expression of recombinant M2-muscarinic receptors and N-type channels in HEK 293 cells allowed voltage-dependent inhibition to be studied in isolation. In this system, receptor-activated G-proteins had only one effect: a 10-fold increase in the time required for channels to first open following membrane depolarization. There were no changes in gating after the channel first opened, and unitary currents were not detectably altered by modulation. Despite its simplicity, this single change successfully accounts for the complex alterations in whole-cell current observed during G-protein inhibition in neurons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Beech D. J., Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991 Jun;6(6):859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- Boland L. M., Bean B. P. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993 Feb;13(2):516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland L. M., Morrill J. A., Bean B. P. omega-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J Neurosci. 1994 Aug;14(8):5011–5027. doi: 10.1523/JNEUROSCI.14-08-05011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E., Soong T. W., Stea A., Snutch T. P. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V., Lovallo M., Magnelli V., Zucker H., Carbone E. Voltage-dependent modulation of single N-Type Ca2+ channel kinetics by receptor agonists in IMR32 cells. Biophys J. 1996 May;70(5):2144–2154. doi: 10.1016/S0006-3495(96)79780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Delcour A. H., Lipscombe D., Tsien R. W. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J Neurosci. 1993 Jan;13(1):181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour A. H., Tsien R. W. Altered prevalence of gating modes in neurotransmitter inhibition of N-type calcium channels. Science. 1993 Feb 12;259(5097):980–984. doi: 10.1126/science.8094902. [DOI] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Diversé-Pierluissi M., Goldsmith P. K., Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995 Jan;14(1):191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- Dubel S. J., Starr T. V., Hell J., Ahlijanian M. K., Enyeart J. J., Catterall W. A., Snutch T. P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Luebke J. I., Turner T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995 Feb;18(2):89–98. [PubMed] [Google Scholar]
- Elmslie K. S., Kammermeier P. J., Jones S. W. Reevaluation of Ca2+ channel types and their modulation in bullfrog sympathetic neurons. Neuron. 1994 Jul;13(1):217–228. doi: 10.1016/0896-6273(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Golard A., Siegelbaum S. A. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993 Sep;13(9):3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Herlitze S., Garcia D. E., Mackie K., Hille B., Scheuer T., Catterall W. A. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996 Mar 21;380(6571):258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996 Mar 21;380(6571):255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992 Aug;9(2):197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 1989 Jun;414(2):145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Bean B. P. G-protein modulation of ion permeation through N-type calcium channels. Nature. 1993 Sep 16;365(6443):258–262. doi: 10.1038/365258a0. [DOI] [PubMed] [Google Scholar]
- Luebke J. I., Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and -independent mechanisms. Pflugers Arch. 1994 Oct;428(5-6):499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Receptor-mediated regulation of calcium channels and neurotransmitter release. FASEB J. 1990 Dec;4(15):3291–3299. [PubMed] [Google Scholar]
- Offermanns S., Wieland T., Homann D., Sandmann J., Bombien E., Spicher K., Schultz G., Jakobs K. H. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994 May;45(5):890–898. [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Pollo A., Lovallo M., Sher E., Carbone E. Voltage-dependent noradrenergic modulation of omega-conotoxin-sensitive Ca2+ channels in human neuroblastoma IMR32 cells. Pflugers Arch. 1992 Oct;422(1):75–83. doi: 10.1007/BF00381516. [DOI] [PubMed] [Google Scholar]
- Pragnell M., Sakamoto J., Jay S. D., Campbell K. P. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 1991 Oct 21;291(2):253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Covariance of nonstationary sodium current fluctuations at the node of Ranvier. Biophys J. 1981 Apr;34(1):111–133. doi: 10.1016/S0006-3495(81)84840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993 Dec;11(6):1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Stricker C., Redman S., Daley D. Statistical analysis of synaptic transmission: model discrimination and confidence limits. Biophys J. 1994 Aug;67(2):532–547. doi: 10.1016/S0006-3495(94)80513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker C., Redman S. Statistical models of synaptic transmission evaluated using the expectation-maximization algorithm. Biophys J. 1994 Aug;67(2):656–670. doi: 10.1016/S0006-3495(94)80514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson W. J., Stea A., Bourinet E., Charnet P., Nargeot J., Snutch T. P. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993 Nov;32(11):1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- Wickman K., Clapham D. E. Ion channel regulation by G proteins. Physiol Rev. 1995 Oct;75(4):865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Wilding T. J., Womack M. D., McCleskey E. W. Fast, local signal transduction between the mu opioid receptor and Ca2+ channels. J Neurosci. 1995 May;15(5 Pt 2):4124–4132. doi: 10.1523/JNEUROSCI.15-05-04124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E. Permeation in the dihydropyridine-sensitive calcium channel. Multi-ion occupancy but no anomalous mole-fraction effect between Ba2+ and Ca2+. J Gen Physiol. 1990 May;95(5):911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]



