Abstract
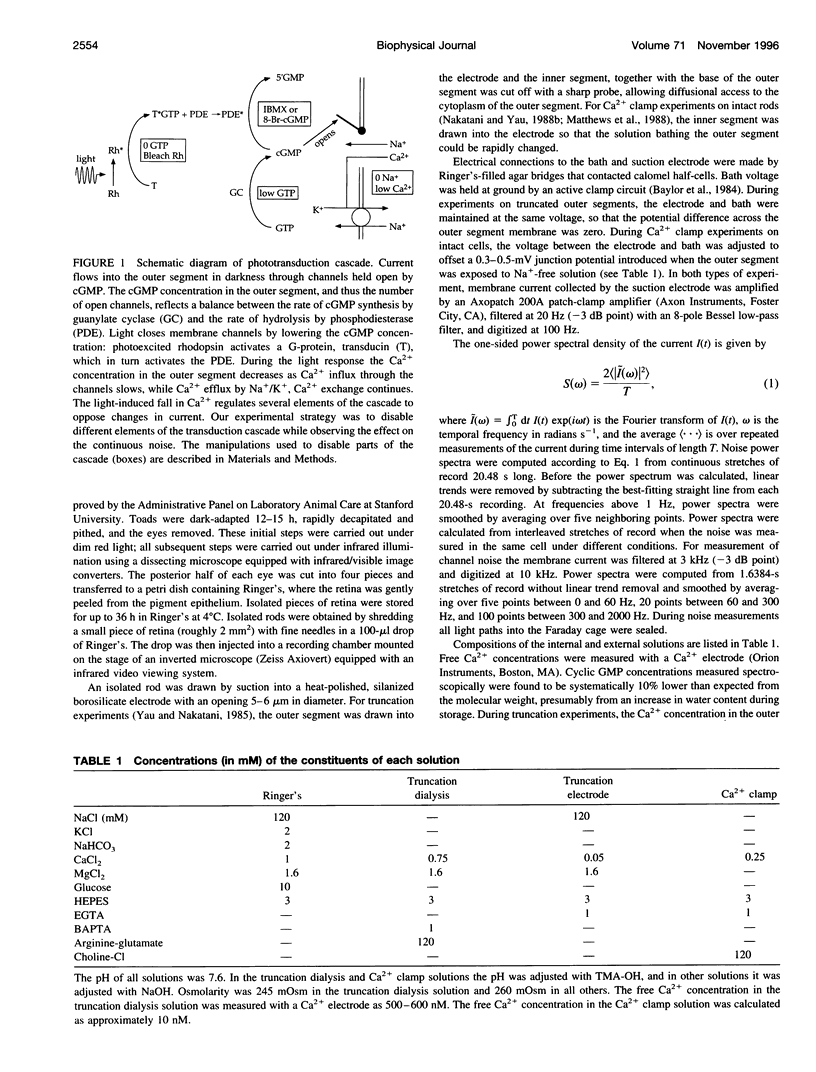
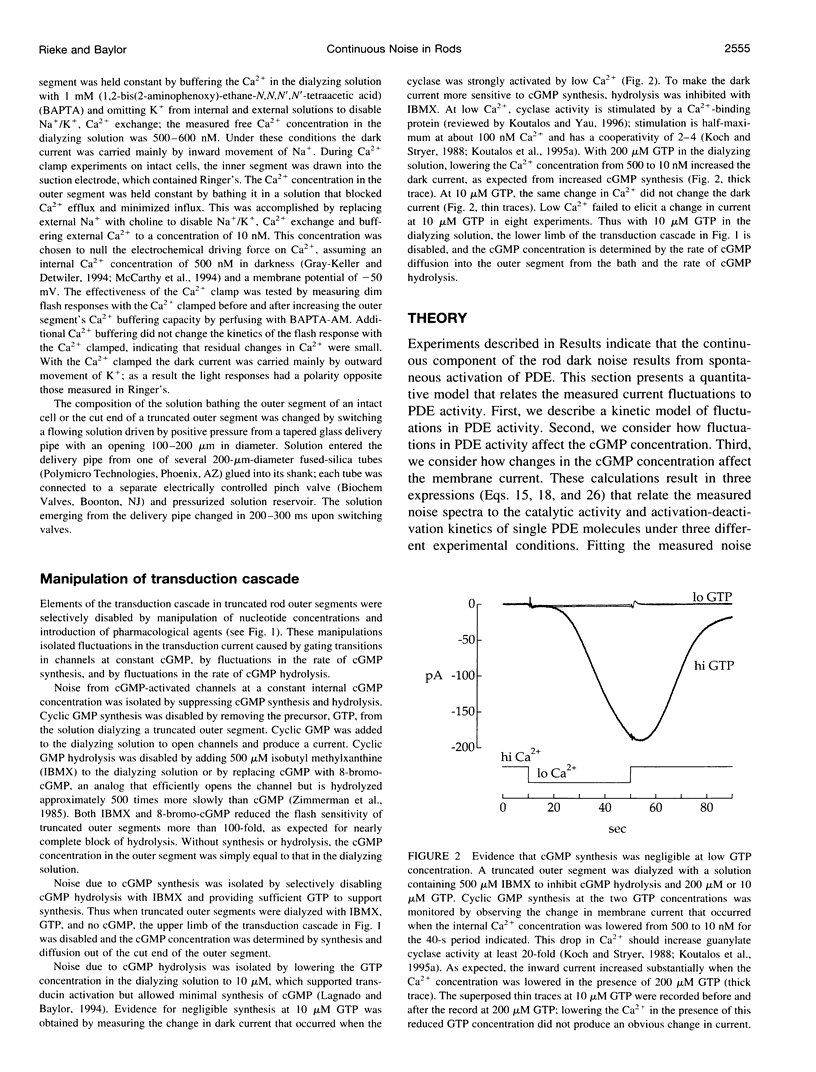
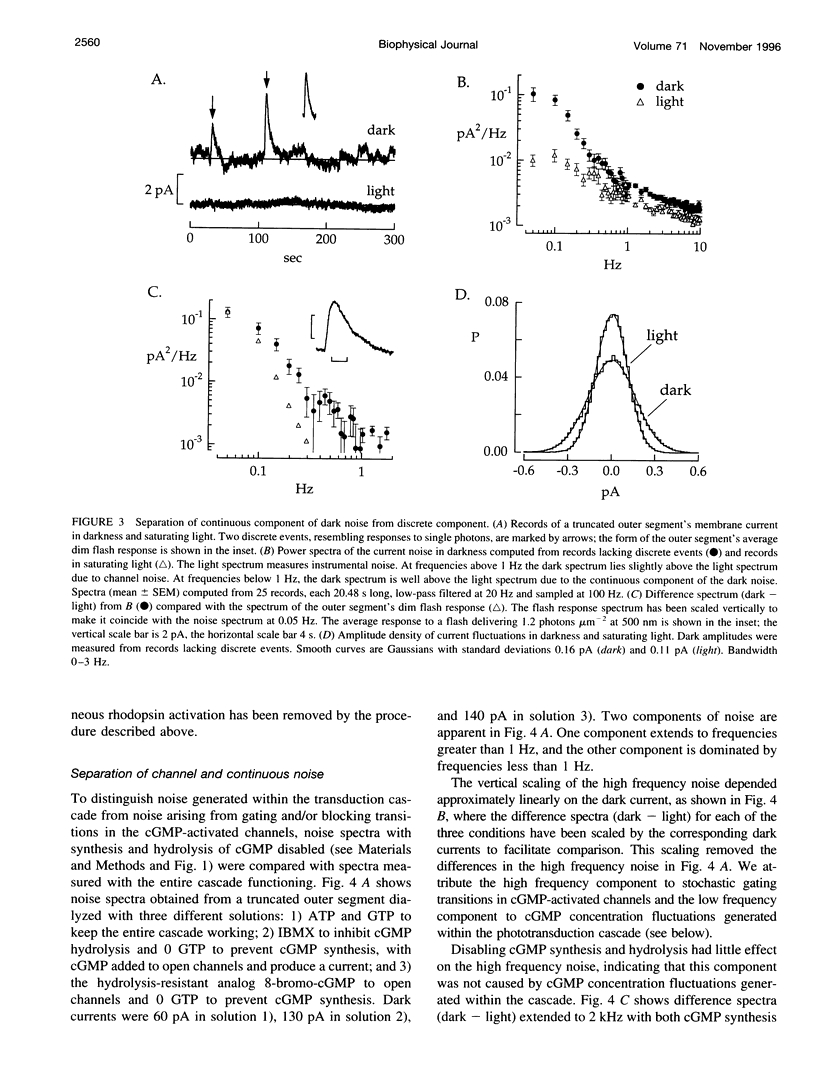
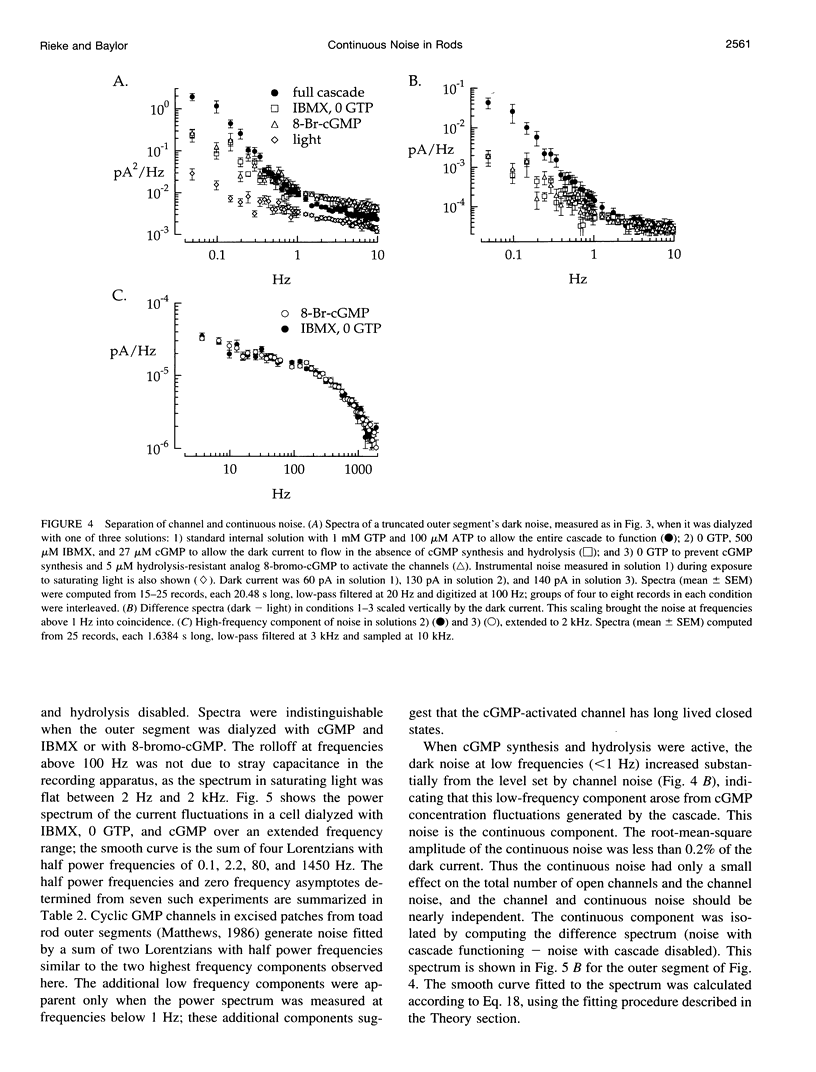
Noise in the rod photoreceptors limits the ability of the dark-adapted visual system to detect dim lights. We investigated the molecular mechanism of the continuous component of the electrical dark noise in toad rods. Membrane current was recorded from intact, isolated rods or truncated, internally dialyzed rod outer segments. The continuous noise was separated from noise due to thermal activation of rhodopsin and to transitions in the cGMP-activated channels. Selectively disabling different elements of the phototransduction cascade allowed examination of their contributions to the continuous noise. These experiments indicate that the noise is generated by spontaneous activation of cGMP phosphodiesterase (PDE) through a process that does not involve transducin. The addition of recombinant gamma, the inhibitory subunit of PDE, did not suppress the noise, indicating that endogenous gamma does not completely dissociate from the catalytic subunit of PDE during spontaneous activation. Quantitative analysis of the noise provided estimates of the rate constants for spontaneous PDE activation and deactivation and the catalytic activity of a single PDE molecule in situ.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho A. C., Donner K., Hydén C., Larsen L. O., Reuter T. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 1988 Jul 28;334(6180):348–350. doi: 10.1038/334348a0. [DOI] [PubMed] [Google Scholar]
- Aho A. C., Donner K., Reuter T. Retinal origins of the temperature effect on absolute visual sensitivity in frogs. J Physiol. 1993 Apr;463:501–521. doi: 10.1113/jphysiol.1993.sp019608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Retinal noise and absolute threshold. J Opt Soc Am. 1956 Aug;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L. Functional regions of the inhibitory subunit of retinal rod cGMP phosphodiesterase identified by site-specific mutagenesis and fluorescence spectroscopy. Biochemistry. 1992 Jun 30;31(25):5918–5925. doi: 10.1021/bi00140a031. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Stryer L. Expression in bacteria of functional inhibitory subunit of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4922–4926. doi: 10.1073/pnas.86.13.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Donner K., Reuter T. Ganglion cell performance at absolute threshold in toad retina: effects of dark events in rods. J Physiol. 1987 Dec;393:667–680. doi: 10.1113/jphysiol.1987.sp016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke C. L., Arshavsky V. Y., Calvert P. D., Bownds M. D., Pugh E. N., Jr Rod outer segment structure influences the apparent kinetic parameters of cyclic GMP phosphodiesterase. J Gen Physiol. 1994 Jun;103(6):1071–1098. doi: 10.1085/jgp.103.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Tamura T., Yau K. W. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995 Nov;106(5):863–890. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995 Jan;68(1):373–382. doi: 10.1016/S0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol. 1995 Nov;106(5):891–921. doi: 10.1085/jgp.106.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Yau K. W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996 Feb;19(2):73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Kutuzov M., Pfister C. Activation of the retinal cGMP-specific phosphodiesterase by the GDP-loaded alpha-subunit of transducin. Eur J Biochem. 1994 Mar 15;220(3):963–971. doi: 10.1111/j.1432-1033.1994.tb18700.x. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Sources of noise in photoreceptor transduction. J Opt Soc Am A. 1987 Dec;4(12):2295–2300. doi: 10.1364/josaa.4.002295. [DOI] [PubMed] [Google Scholar]
- Leibrock C. S., Reuter T., Lamb T. D. Dark adaptation of toad rod photoreceptors following small bleaches. Vision Res. 1994 Nov;34(21):2787–2800. doi: 10.1016/0042-6989(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McCarthy S. T., Younger J. P., Owen W. G. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994 Nov;67(5):2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Ramdas L., Disher R. M., Wensel T. G. Nucleotide exchange and cGMP phosphodiesterase activation by pertussis toxin inactivated transducin. Biochemistry. 1991 Dec 17;30(50):11637–11645. doi: 10.1021/bi00114a005. [DOI] [PubMed] [Google Scholar]
- Rispoli G., Sather W. A., Detwiler P. B. Visual transduction in dialysed detached rod outer segments from lizard retina. J Physiol. 1993 Jun;465:513–537. doi: 10.1113/jphysiol.1993.sp019691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitt B. Counting every quantum. J Physiol. 1972 May;223(1):131–150. doi: 10.1113/jphysiol.1972.sp009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Yarfitz S., Hurley J. B. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994 May 20;269(20):14329–14332. [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]



