Abstract
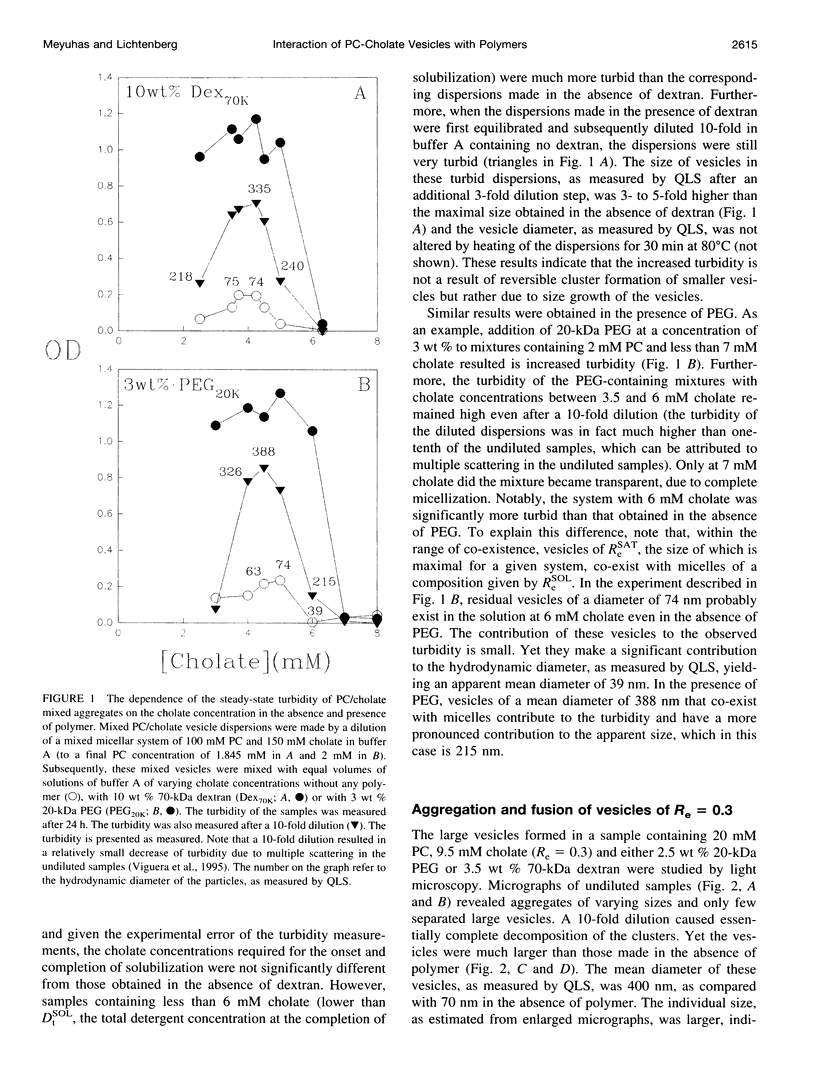
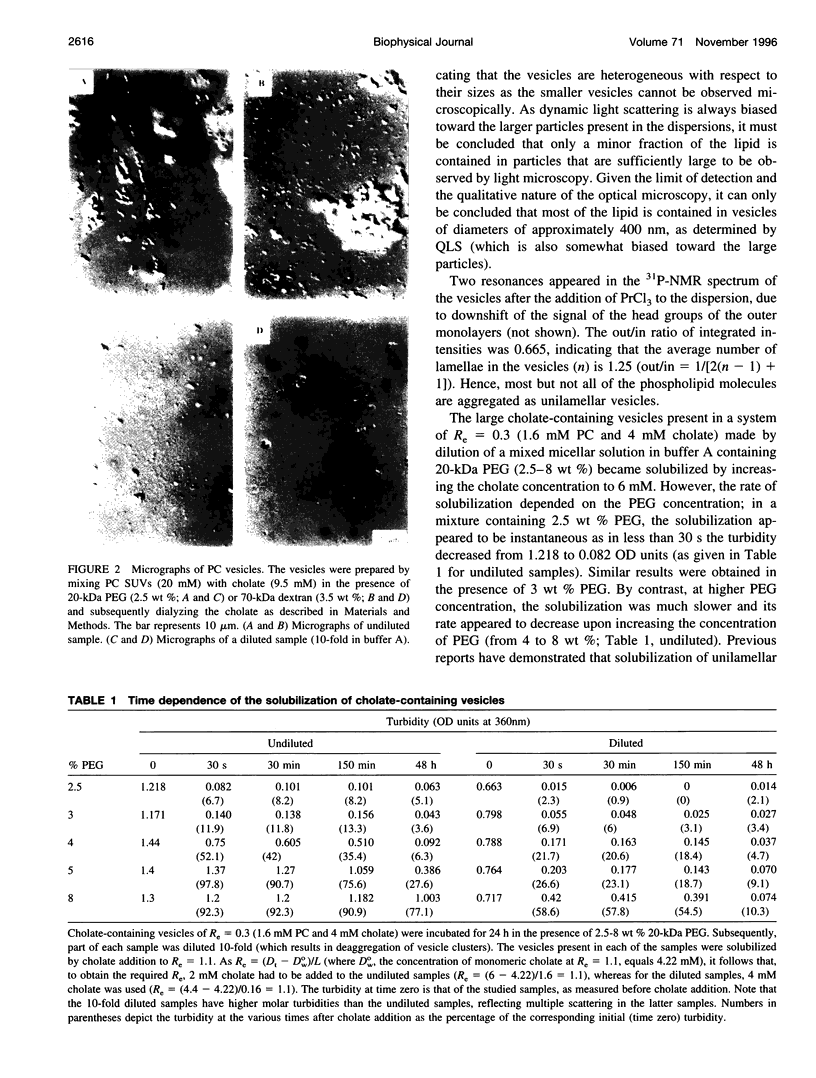
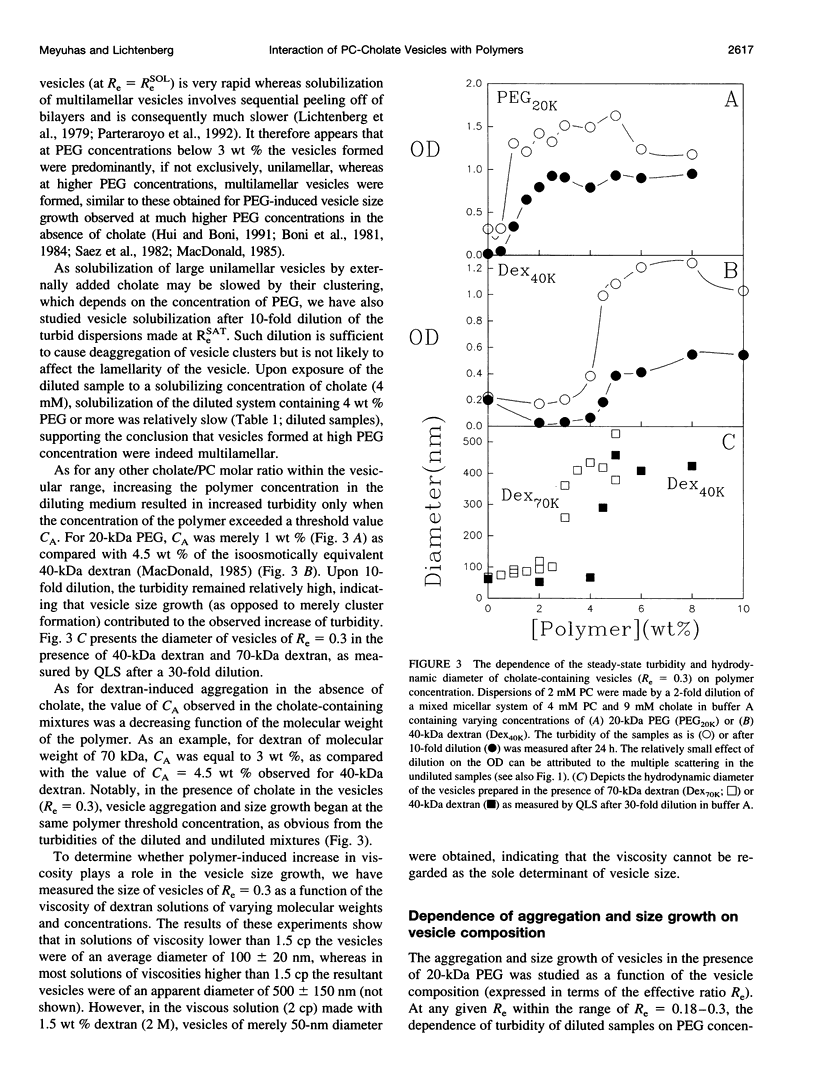
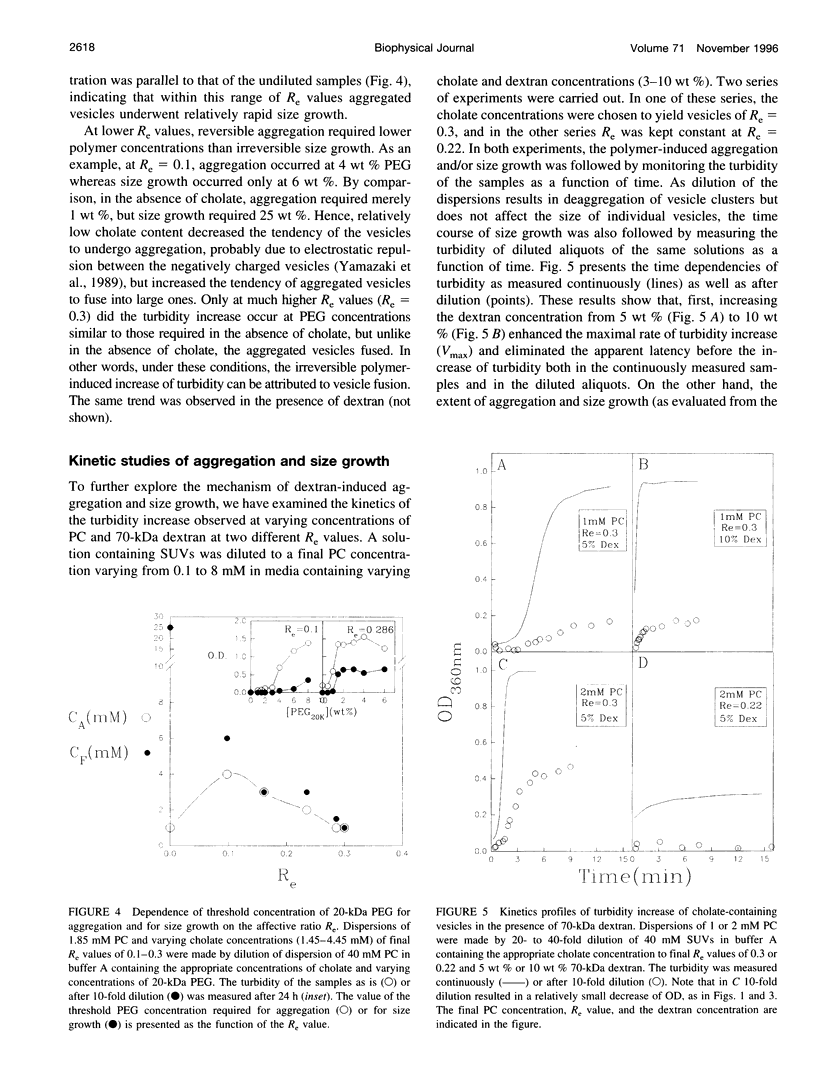
The state of aggregation and the steady-state size of mixed aggregates made of phospholipids and surfactants are both determined by the surfactant/lipid ratio in the mixed aggregates (Re). Water-soluble polymers, such as dextrans and polyethylene glycols (PEGs) of different molecular weights, induce reversible aggregation of phospholipid vesicles, mostly due to dehydration of the vesicle surface and depletion forces, and only at much higher concentrations, PEGs (but not dextran) also induce irreversible size growth of the vesicles. Here we show that the water-soluble polymers dextrans and PEGs do not affect the vesicle-micelle phase boundaries in mixtures of phosphatidylcholine and the anionic surfactant sodium cholate. By contrast, these polymers affect markedly the steady-state size of cholate-containing vesicles. As compared with pure phosphatidylcholine vesicles, the cholate-containing vesicles have a lower tendency to undergo polymer-induced aggregation, probably due to the electrostatic repulsion between the negatively charged vesicles, but a higher tendency to undergo irreversible size growth at relatively low polymer concentrations. Such irreversible size growth was observed not only for PEG but also for dextran, which in the absence of cholate is incapable of inducing vesicle size growth. These findings are consistent with the prevailing concept that the polymer-induced size growth is due to the effect of large structural fluctuations in the bilayers of deformed aggregated vesicles, the surface of which is dehydrated by the polymer. The presence of cholate in the bilayers at sufficiently high concentrations induces such fluctuations, yielding irreversible size growth within the clusters of dehydrated vesicles formed upon mixing with polymers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Kushnir T., Nir S., Lichtenberg D. Kinetic and structural aspects of reconstitution of phosphatidylcholine vesicles by dilution of phosphatidylcholine-sodium cholate mixed micelles. Biochemistry. 1986 May 6;25(9):2597–2605. doi: 10.1021/bi00357a048. [DOI] [PubMed] [Google Scholar]
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Hah J. S., Hui S. W., Mukherjee P., Ho J. T., Jung C. Y. Aggregation and fusion of unilamellar vesicles by poly(ethylene glycol). Biochim Biophys Acta. 1984 Sep 5;775(3):409–418. doi: 10.1016/0005-2736(84)90198-6. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Stewart T. P., Alderfer J. L., Hui S. W. Lipid-polyethylene glycol interactions: I. Induction of fusion between liposomes. J Membr Biol. 1981;62(1-2):65–70. doi: 10.1007/BF01870200. [DOI] [PubMed] [Google Scholar]
- Gheriani-Gruszka N., Almog S., Biltonen R. L., Lichtenberg D. Hydrolysis of phosphatidylcholine in phosphatidylcholine-cholate mixtures by porcine pancreatic phospholipase A2. J Biol Chem. 1988 Aug 25;263(24):11808–11813. [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogs. Biophys J. 1994 Jun;66(6):1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Carpenter T. J., Alford D. R. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987 Aug 25;26(17):5389–5397. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985 Dec 19;821(3):470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Zilberman Y., Greenzaid P., Zamir S. Structural and kinetic studies on the solubilization of lecithin by sodium deoxycholate. Biochemistry. 1979 Aug 7;18(16):3517–3525. doi: 10.1021/bi00583a013. [DOI] [PubMed] [Google Scholar]
- MacDonald R. I. Membrane fusion due to dehydration by polyethylene glycol, dextran, or sucrose. Biochemistry. 1985 Jul 16;24(15):4058–4066. doi: 10.1021/bi00336a039. [DOI] [PubMed] [Google Scholar]
- Massenburg D., Lentz B. R. Poly(ethylene glycol)-induced fusion and rupture of dipalmitoylphosphatidylcholine large, unilamellar extruded vesicles. Biochemistry. 1993 Sep 7;32(35):9172–9180. doi: 10.1021/bi00086a024. [DOI] [PubMed] [Google Scholar]
- Meyuhas D., Nir S., Lichtenberg D. Aggregation of phospholipid vesicles by water-soluble polymers. Biophys J. 1996 Nov;71(5):2602–2612. doi: 10.1016/S0006-3495(96)79452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti M., Aducci P., Viti V. Interaction of neutral polysaccharides with phosphatidylcholine multilamellar liposomes. Phase transitions studied by the binding of fluorescein-conjugated dextrans. Biochemistry. 1979 Jun 12;18(12):2541–2548. doi: 10.1021/bi00579a017. [DOI] [PubMed] [Google Scholar]
- Otten D., Löbbecke L., Beyer K. Stages of the bilayer-micelle transition in the system phosphatidylcholine-C12E8 as studied by deuterium- and phosphorous-NMR, light scattering, and calorimetry. Biophys J. 1995 Feb;68(2):584–597. doi: 10.1016/S0006-3495(95)80220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partearroyo M. A., Urbaneja M. A., Goñi F. M. Effective detergent/lipid ratios in the solubilization of phosphatidylcholine vesicles by Triton X-100. FEBS Lett. 1992 May 11;302(2):138–140. doi: 10.1016/0014-5793(92)80424-f. [DOI] [PubMed] [Google Scholar]
- Sáez R., Alonso A., Villena A., Goñi F. M. Detergent-like properties of polyethyleneglycols in relation to model membranes. FEBS Lett. 1982 Jan 25;137(2):323–326. doi: 10.1016/0014-5793(82)80376-1. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Fisher D. The interaction of phospholipid membranes with poly(ethylene glycol). Vesicle aggregation and lipid exchange. Biochim Biophys Acta. 1982 Jun 14;688(2):645–652. doi: 10.1016/0005-2736(82)90375-3. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Lentz B. R. Mechanism of poly(ethylene glycol)-induced lipid transfer between phosphatidylcholine large unilamellar vesicles: a fluorescent probe study. Biochemistry. 1991 Jul 9;30(27):6780–6787. doi: 10.1021/bi00241a022. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Ohnishi S., Ito T. Osmoelastic coupling in biological structures: decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry. 1989 May 2;28(9):3710–3715. doi: 10.1021/bi00435a013. [DOI] [PubMed] [Google Scholar]




