Abstract
An image-based technique of fluorescence recovery after photobleaching (video-FRAP) was used to measure the lateral diffusion coefficients of a series of nine fluorescent probes in two model lipid bilayer systems, dimyristoylphosphatidylcholine (DMPC) and DMPC/cholesterol (40 mol%), as well as in human stratum corneum-extracted lipids. The probes were all lipophilic, varied in molecular weight from 223 to 854 Da, and were chosen to characterize the lateral diffusion of small compounds in these bilayer systems. A clear molecular weight dependence of the lateral diffusion coefficients in DMPC bilayers was observed. Values ranged from 6.72 x 10(-8) to 16.2 x 10(-8) cm2/s, with the smaller probes diffusing faster than the larger ones. Measurements in DMPC/cholesterol bilayers, which represent the most thorough characterization of small-solute diffusion in this system, exhibited a similar molecular weight dependence, although the diffusion coefficients were lower, ranging from 1.62 x 10(-8) to 5.60 x 10(-8) cm2/s. Lateral diffusion measurements in stratum corneum-extracted lipids, which represent a novel examination of diffusion in this unique lipid system, also exhibited a molecular weight dependence, with values ranging from 0.306 x 10(-8) to 2.34 x 10(-8) cm2/s. Literature data showed that these strong molecular weight dependencies extend to even smaller compounds than those examined in this study. A two-parameter empirical expression is presented that describes the lateral diffusion coefficient in terms of the solute's molecular weight and captures the size dependence over the range examined. This study illustrates the degree to which small-molecule lateral diffusion in stratum corneum-extracted lipids can be represented by diffusion in DMPC and DMPC/cholesterol bilayer systems, and may lead to a better understanding of small-solute transport across human stratum corneum.
Full text
PDF

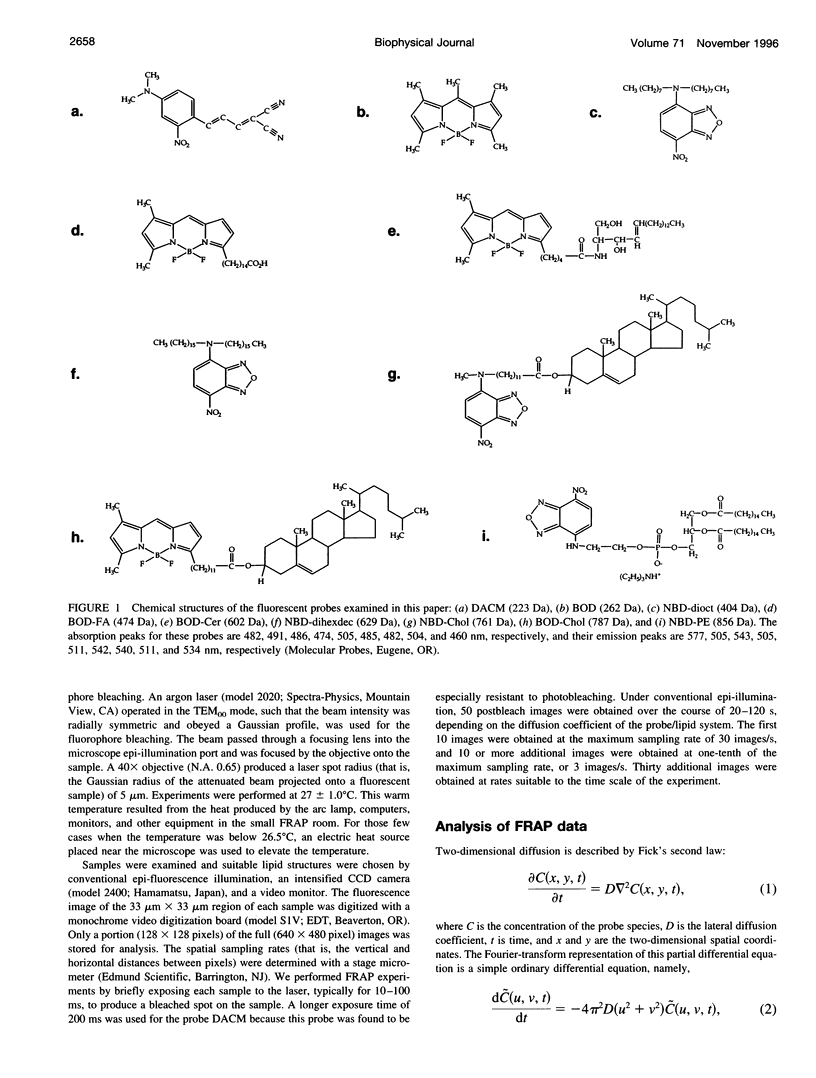


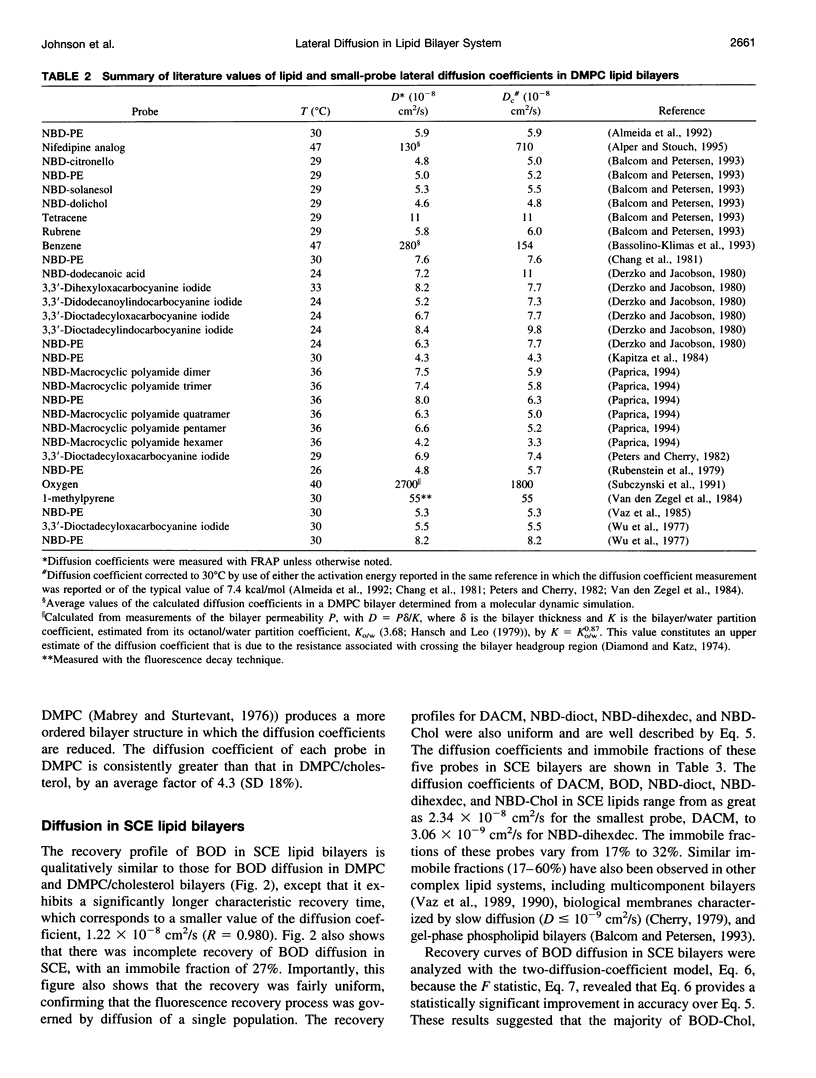

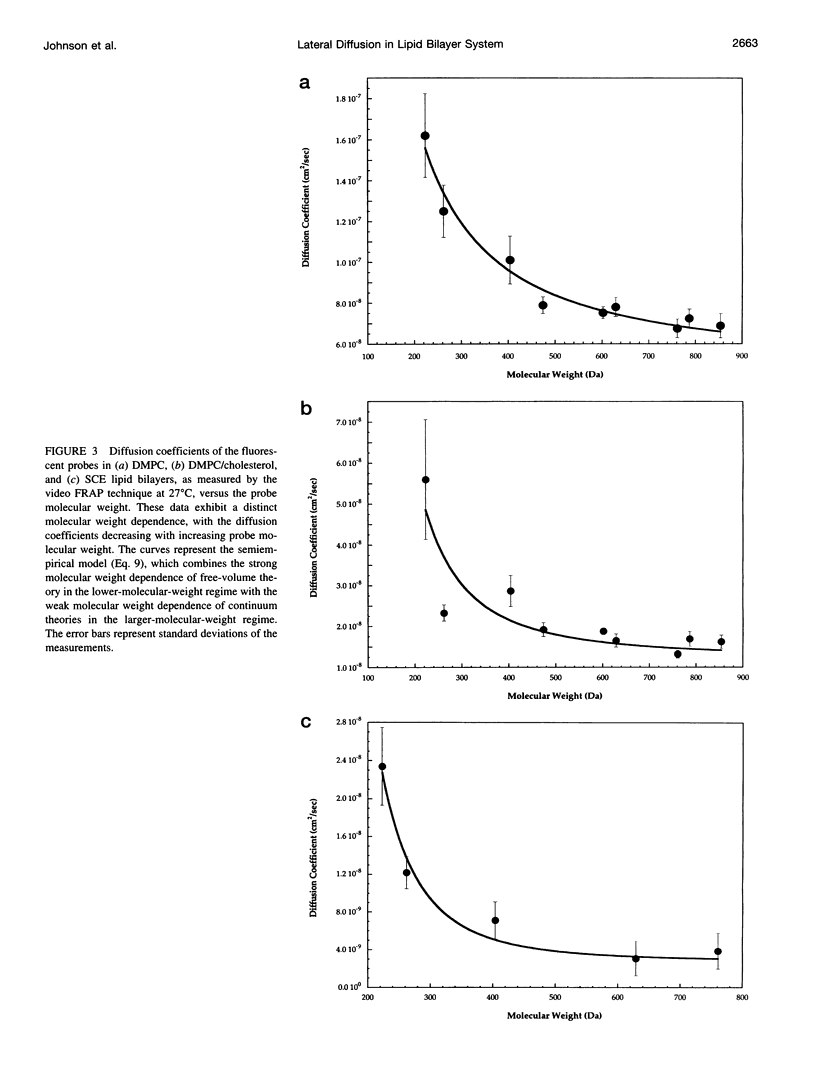


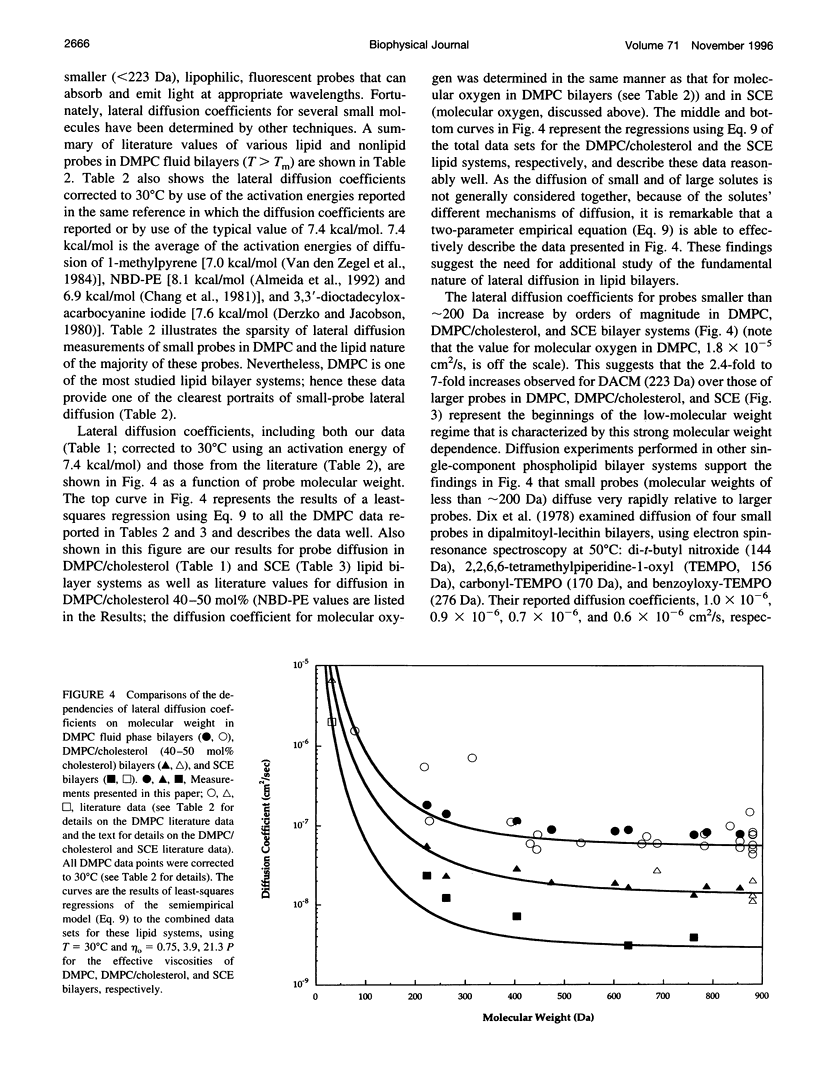


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992 Jul 28;31(29):6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- Anderson B. D., Higuchi W. I., Raykar P. V. Heterogeneity effects on permeability-partition coefficient relationships in human stratum corneum. Pharm Res. 1988 Sep;5(9):566–573. doi: 10.1023/a:1015989929342. [DOI] [PubMed] [Google Scholar]
- Anderson B. D., Raykar P. V. Solute structure-permeability relationships in human stratum corneum. J Invest Dermatol. 1989 Aug;93(2):280–286. doi: 10.1111/1523-1747.ep12277592. [DOI] [PubMed] [Google Scholar]
- Balcom B. J., Petersen N. O. Lateral diffusion in model membranes is independent of the size of the hydrophobic region of molecules. Biophys J. 1993 Aug;65(2):630–637. doi: 10.1016/S0006-3495(93)81106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk D. A., Yuan F., Leunig M., Jain R. K. Fluorescence photobleaching with spatial Fourier analysis: measurement of diffusion in light-scattering media. Biophys J. 1993 Dec;65(6):2428–2436. doi: 10.1016/S0006-3495(93)81326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwstra J. A., Gooris G. S., van der Spek J. A., Bras W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol. 1991 Dec;97(6):1005–1012. doi: 10.1111/1523-1747.ep12492217. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Takeuchi H., Ito T., Machida K., Ohnishi S. Lateral mobility of erythrocyte membrane proteins studied by the fluorescence photobleaching recovery technique. J Biochem. 1981 Oct;90(4):997–1004. doi: 10.1093/oxfordjournals.jbchem.a133586. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Müller U., Holenstein C., Heyn M. P. Lateral segregation of proteins induced by cholesterol in bacteriorhodopsin-phospholipid vesicles. Biochim Biophys Acta. 1980 Feb 15;596(1):145–151. doi: 10.1016/0005-2736(80)90179-0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Daems D., Van den Zegel M., Boens N., De Schryver F. C. Fluorescence decay of pyrene in small and large unilamellar L, alpha-dipalmitoylphosphatidylcholine vesicles above and below the phase transition temperature. Eur Biophys J. 1985;12(2):97–105. doi: 10.1007/BF00260432. [DOI] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Katz Y. Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. J Membr Biol. 1974;17(2):121–154. doi: 10.1007/BF01870176. [DOI] [PubMed] [Google Scholar]
- Dix J. A., Kivelson D., Diamond J. M. Molecular motion of small nonelectrolyte molecules in lecithin bilayers. J Membr Biol. 1978 Jun 9;40(4):315–342. doi: 10.1007/BF01874162. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Hatcher M. E., Plachy W. Z. Dioxygen diffusion in the stratum corneum: an EPR spin label study. Biochim Biophys Acta. 1993 Jun 18;1149(1):73–78. doi: 10.1016/0005-2736(93)90026-v. [DOI] [PubMed] [Google Scholar]
- Homan R., Pownall H. J. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 1988 Feb 18;938(2):155–166. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- Hou S. Y., Mitra A. K., White S. H., Menon G. K., Ghadially R., Elias P. M. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991 Feb;96(2):215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Huang Z., Pearce K. H., Thompson N. L. Translational diffusion of bovine prothrombin fragment 1 weakly bound to supported planar membranes: measurement by total internal reflection with fluorescence pattern photobleaching recovery. Biophys J. 1994 Oct;67(4):1754–1766. doi: 10.1016/S0006-3495(94)80650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Berk D. A., Jain R. K., Deen W. M. Diffusion and partitioning of proteins in charged agarose gels. Biophys J. 1995 Apr;68(4):1561–1568. doi: 10.1016/S0006-3495(95)80328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Blankschtein D., Langer R. Permeation of steroids through human skin. J Pharm Sci. 1995 Sep;84(9):1144–1146. doi: 10.1002/jps.2600840922. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., Rüppel D. A., Galla H. J., Sackmann E. Lateral diffusion of lipids and glycophorin in solid phosphatidylcholine bilayers. The role of structural defects. Biophys J. 1984 Mar;45(3):577–587. doi: 10.1016/S0006-3495(84)84195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson N., Thewalt J., Lafleur M., Bloom M. A model membrane approach to the epidermal permeability barrier. Biochemistry. 1994 May 31;33(21):6707–6715. doi: 10.1021/bi00187a042. [DOI] [PubMed] [Google Scholar]
- Lampe M. A., Burlingame A. L., Whitney J., Williams M. L., Brown B. E., Roitman E., Elias P. M. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983 Feb;24(2):120–130. [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Biological membranes behave as non-porous polymeric sheets with respect to the diffusion of non-electrolytes. Nature. 1969 Oct 18;224(5216):240–243. doi: 10.1038/224240a0. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Non-Stokesian nature of transverse diffusion within human red cell membranes. J Membr Biol. 1986;92(2):111–119. doi: 10.1007/BF01870701. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison K. C., Swartzendruber D. C., Wertz P. W., Downing D. T. Presence of intact intercellular lipid lamellae in the upper layers of the stratum corneum. J Invest Dermatol. 1987 Jun;88(6):714–718. doi: 10.1111/1523-1747.ep12470386. [DOI] [PubMed] [Google Scholar]
- Martel P., Makriyannis A., Mavromoustakos T., Kelly K., Jeffrey K. R. Topography of tetrahydrocannabinol in model membranes using neutron diffraction. Biochim Biophys Acta. 1993 Sep 5;1151(1):51–58. doi: 10.1016/0005-2736(93)90070-g. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Ongpipattanakul B., Francoeur M. L., Potts R. O. Polymorphism in stratum corneum lipids. Biochim Biophys Acta. 1994 Feb 23;1190(1):115–122. doi: 10.1016/0005-2736(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R. O., Guy R. H. Predicting skin permeability. Pharm Res. 1992 May;9(5):663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuplein R. J., Blank I. H. Permeability of the skin. Physiol Rev. 1971 Oct;51(4):702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Nemecz G., Wood W. G., Joiner C., Morrot G., Ayraut-Jarrier M., Devaux P. F. Transmembrane distribution of sterol in the human erythrocyte. Biochim Biophys Acta. 1991 Jul 22;1066(2):183–192. doi: 10.1016/0005-2736(91)90185-b. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S., Kusumi A. Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study. Biochemistry. 1991 Sep 3;30(35):8578–8590. doi: 10.1021/bi00099a013. [DOI] [PubMed] [Google Scholar]
- Tocanne J. F., Dupou-Cézanne L., Lopez A., Tournier J. F. Lipid lateral diffusion and membrane organization. FEBS Lett. 1989 Oct 23;257(1):10–16. doi: 10.1016/0014-5793(89)81774-0. [DOI] [PubMed] [Google Scholar]
- Tsay T. T., Jacobson K. A. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys J. 1991 Aug;60(2):360–368. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Fluid phase connectivity in an isomorphous, two-component, two-phase phosphatidylcholine bilayer. Biophys J. 1990 Jul;58(1):273–275. doi: 10.1016/S0006-3495(90)82373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. L., Palmer A. G., 3rd, Thompson N. L. Inhomogeneous translational diffusion of monoclonal antibodies on phospholipid Langmuir-Blodgett films. Biophys J. 1988 Sep;54(3):463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Zegel M., Boens N., de Schryver F. C. Fluorescence decay of 1-methylpyrene in small unilamellar l-alpha-dimyristoylphosphatidylcholine vesicles. A temperature and concentration dependence study. Biophys Chem. 1984 Nov;20(4):333–345. doi: 10.1016/0301-4622(84)80023-x. [DOI] [PubMed] [Google Scholar]



