Abstract
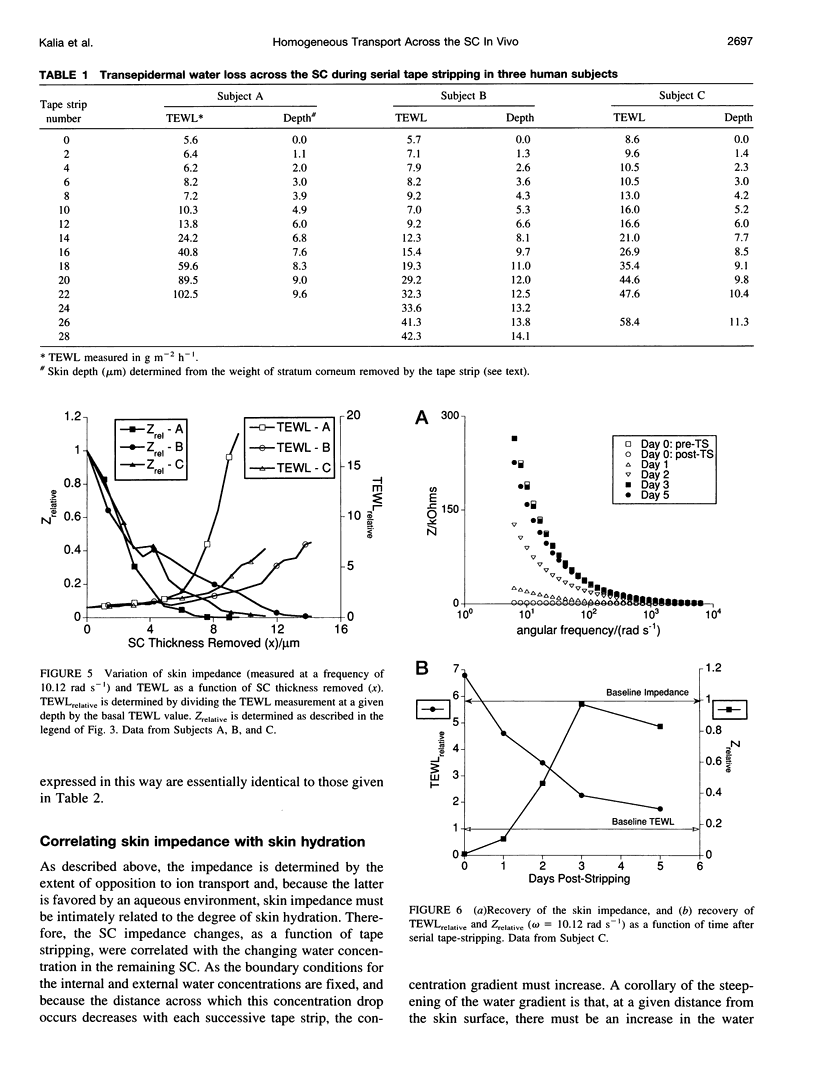
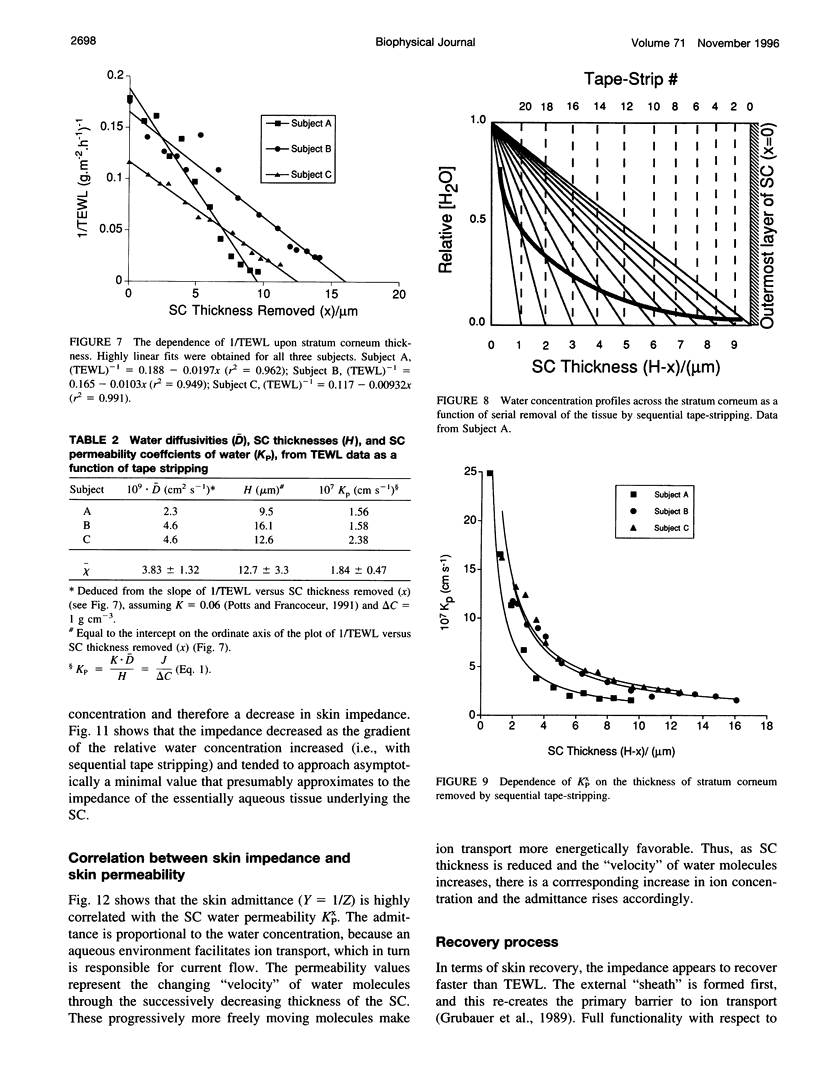
The objective of this study was to determine whether a structurally heterogeneous biomembrane, human stratum corneum (SC), behaved as a homogeneous barrier to water transport. The question is relevant because the principal function of the SC in vivo is to provide a barrier to the insensible loss of tissue water across the skin. Impedance spectra (IS) of the skin and measurements of the rate of transepidermal water loss (TEWL) were recorded sequentially in vivo in human subjects as layers of the SC were progressively removed by the serial application of adhesive tape strips. The low-frequency (< or = 100 rad s-1) impedance of skin was much more significantly affected by tape stripping than the higher frequency values; removal of the outermost SC layer had the largest effect. In contrast, TEWL changed little as the outer SC layers were stripped off, but increased dramatically when 6-8 microns of the tissue had been removed. It follows that the two noninvasive techniques probe SC barrier integrity in somewhat different ways. After SC removal, recovery of barrier function, as assessed by increasing values of the low-frequency impedance, apparently proceeded faster than TEWL decreased to the prestripping control. The variation of TEWL as a function of SC removal behaved in a manner entirely consistent with a homogeneous barrier, thereby permitting the apparent SC diffusivity of water to be found. Skin impedance (low frequency) was correlated with the relative concentration of water within the SC, thus providing an in vivo probe for skin hydration. Finally, the SC permeability coefficient to water, as a function of SC thickness, was calculated and correlated with the corresponding values of skin admittance derived from IS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Cassidy J. M. Variation in physical dimensions and chemical composition of human stratum corneum. J Invest Dermatol. 1973 Jul;61(1):30–32. doi: 10.1111/1523-1747.ep12674117. [DOI] [PubMed] [Google Scholar]
- Astley J. P., Levine M. Effect of dimethyl sulfoxide on permeability of human skin in vitro. J Pharm Sci. 1976 Feb;65(2):210–215. doi: 10.1002/jps.2600650210. [DOI] [PubMed] [Google Scholar]
- Blank I. H., Moloney J., 3rd, Emslie A. G., Simon I., Apt C. The diffusion of water across the stratum corneum as a function of its water content. J Invest Dermatol. 1984 Feb;82(2):188–194. doi: 10.1111/1523-1747.ep12259835. [DOI] [PubMed] [Google Scholar]
- Bond J. R., Barry B. W. Limitations of hairless mouse skin as a model for in vitro permeation studies through human skin: hydration damage. J Invest Dermatol. 1988 Apr;90(4):486–489. doi: 10.1111/1523-1747.ep12460958. [DOI] [PubMed] [Google Scholar]
- Bouwstra J. A., Gooris G. S., van der Spek J. A., Bras W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol. 1991 Dec;97(6):1005–1012. doi: 10.1111/1523-1747.ep12492217. [DOI] [PubMed] [Google Scholar]
- Chapman S. J., Walsh A. Desmosomes, corneosomes and desquamation. An ultrastructural study of adult pig epidermis. Arch Dermatol Res. 1990;282(5):304–310. doi: 10.1007/BF00375724. [DOI] [PubMed] [Google Scholar]
- Chapman S. J., Walsh A., Jackson S. M., Friedmann P. S. Lipids, proteins and corneocyte adhesion. Arch Dermatol Res. 1991;283(3):167–173. doi: 10.1007/BF00372057. [DOI] [PubMed] [Google Scholar]
- Downing D. T. Lipid and protein structures in the permeability barrier of mammalian epidermis. J Lipid Res. 1992 Mar;33(3):301–313. [PubMed] [Google Scholar]
- Frödin T., Skogh M. Measurement of transepidermal water loss using an evaporimeter to follow the restitution of the barrier layer of human epidermis after stripping the stratum corneum. Acta Derm Venereol. 1984;64(6):537–540. [PubMed] [Google Scholar]
- Gay C. L., Guy R. H., Golden G. M., Mak V. H., Francoeur M. L. Characterization of low-temperature (i.e., < 65 degrees C) lipid transitions in human stratum corneum. J Invest Dermatol. 1994 Aug;103(2):233–239. doi: 10.1111/1523-1747.ep12393214. [DOI] [PubMed] [Google Scholar]
- Golden G. M., Guzek D. B., Kennedy A. H., McKie J. E., Potts R. O. Stratum corneum lipid phase transitions and water barrier properties. Biochemistry. 1987 Apr 21;26(8):2382–2388. doi: 10.1021/bi00382a045. [DOI] [PubMed] [Google Scholar]
- Grubauer G., Elias P. M., Feingold K. R. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- Harrison S. M., Barry B. W., Dugard P. H. Effects of freezing on human skin permeability. J Pharm Pharmacol. 1984 Apr;36(4):261–262. doi: 10.1111/j.2042-7158.1984.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Kalia Y. N., Guy R. H. The electrical characteristics of human skin in vivo. Pharm Res. 1995 Nov;12(11):1605–1613. doi: 10.1023/a:1016228730522. [DOI] [PubMed] [Google Scholar]
- King C. S., Barton S. P., Nicholls S., Marks R. The change in properties of the stratum corneum as a function of depth. Br J Dermatol. 1979 Feb;100(2):165–172. doi: 10.1111/j.1365-2133.1979.tb05556.x. [DOI] [PubMed] [Google Scholar]
- Kontturi K., Murtomäki L. Impedance spectroscopy in human skin. A refined model. Pharm Res. 1994 Sep;11(9):1355–1357. doi: 10.1023/a:1018915100150. [DOI] [PubMed] [Google Scholar]
- Liron Z., Clewell H. J., McDougal J. N. Kinetics of water vapor sorption in porcine stratum corneum. J Pharm Sci. 1994 May;83(5):692–698. doi: 10.1002/jps.2600830520. [DOI] [PubMed] [Google Scholar]
- Obata M., Tagami H. Electrical determination of water content and concentration profile in a simulation model of in vivo stratum corneum. J Invest Dermatol. 1989 Jun;92(6):854–859. doi: 10.1111/1523-1747.ep12696875. [DOI] [PubMed] [Google Scholar]
- Ongpipattanakul B., Burnette R. R., Potts R. O., Francoeur M. L. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm Res. 1991 Mar;8(3):350–354. doi: 10.1023/a:1015845632280. [DOI] [PubMed] [Google Scholar]
- PINKUS H. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J Invest Dermatol. 1951 Jun;16(6):383–386. doi: 10.1038/jid.1951.45. [DOI] [PubMed] [Google Scholar]
- Potts R. O., Francoeur M. L. Lipid biophysics of water loss through the skin. Proc Natl Acad Sci U S A. 1990 May;87(10):3871–3873. doi: 10.1073/pnas.87.10.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R. O., Francoeur M. L. The influence of stratum corneum morphology on water permeability. J Invest Dermatol. 1991 Apr;96(4):495–499. doi: 10.1111/1523-1747.ep12470197. [DOI] [PubMed] [Google Scholar]
- Scheuplein R. J., Blank I. H. Permeability of the skin. Physiol Rev. 1971 Oct;51(4):702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- Scheuplein R. J. Mechanism of percutaneous adsorption. I. Routes of penetration and the influence of solubility. J Invest Dermatol. 1965 Nov;45(5):334–346. doi: 10.1038/jid.1965.140. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Swartzendruber D. C., Abraham W., Madison K. C., Downing D. T. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987 Oct;123(10):1381–1384. [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. The extracellular matrix of stratum corneum: role of lipids in normal and pathological function. Crit Rev Ther Drug Carrier Syst. 1987;3(2):95–122. [PubMed] [Google Scholar]
- Yamamoto T., Yamamoto Y. Electrical properties of the epidermal stratum corneum. Med Biol Eng. 1976 Mar;14(2):151–158. doi: 10.1007/BF02478741. [DOI] [PubMed] [Google Scholar]
- van der Valk P. G., Maibach H. I. A functional study of the skin barrier to evaporative water loss by means of repeated cellophane-tape stripping. Clin Exp Dermatol. 1990 May;15(3):180–182. doi: 10.1111/j.1365-2230.1990.tb02068.x. [DOI] [PubMed] [Google Scholar]


