Abstract
Membrane properties that vary as a result of isotropic and transmembrane osmolality variations (osmotic stress) are of considerable relevance to mechanisms such as osmoregulation, in which a biological system "senses" and responds to changes in the osmotic environment. In this paper the light-scattering behavior of a model system consisting of large unilamellar vesicles of dioleoyl phosphatidyl glycerol (DOPG) is examined as a function of their osmotic environment. Osmotic downshifts lead to marked reductions in the scattered intensity, whereas osmotic upshifts lead to strong intensity increases. It is shown that these changes in the scattering intensity involve changes in the refractive index of the membrane bilayer that result from an alteration in the extent of hydration and/or the phospholipid packing density. By considering the energetics of osmotically stressed vesicles, and from explicit analysis of the Rayleigh-Gans-Debye scattering factors for spherical and ellipsoidal shells, we quantitatively demonstrate that although changes in vesicle volume and shape can arise in response to the imposition of osmotic stress, these factors alone cannot account for the observed changes in scattered intensity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972 Dec 5;11(25):4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
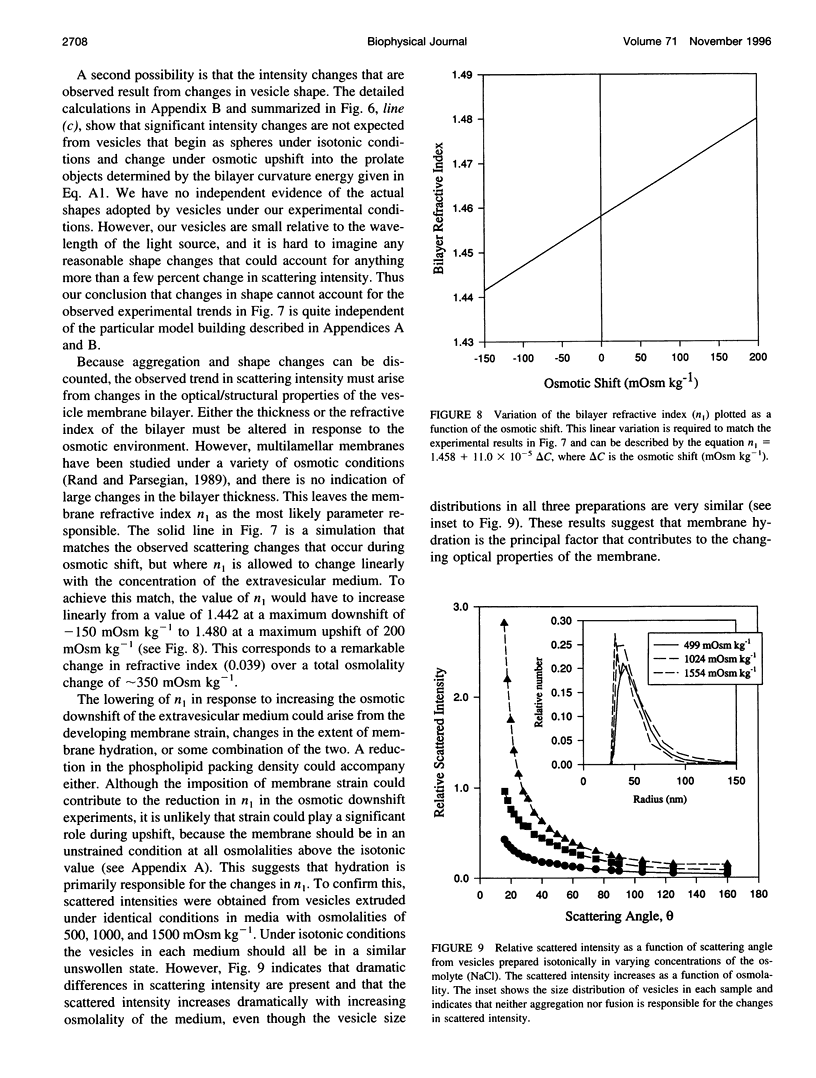
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):167–188. doi: 10.1016/0009-3084(86)90069-1. [DOI] [PubMed] [Google Scholar]
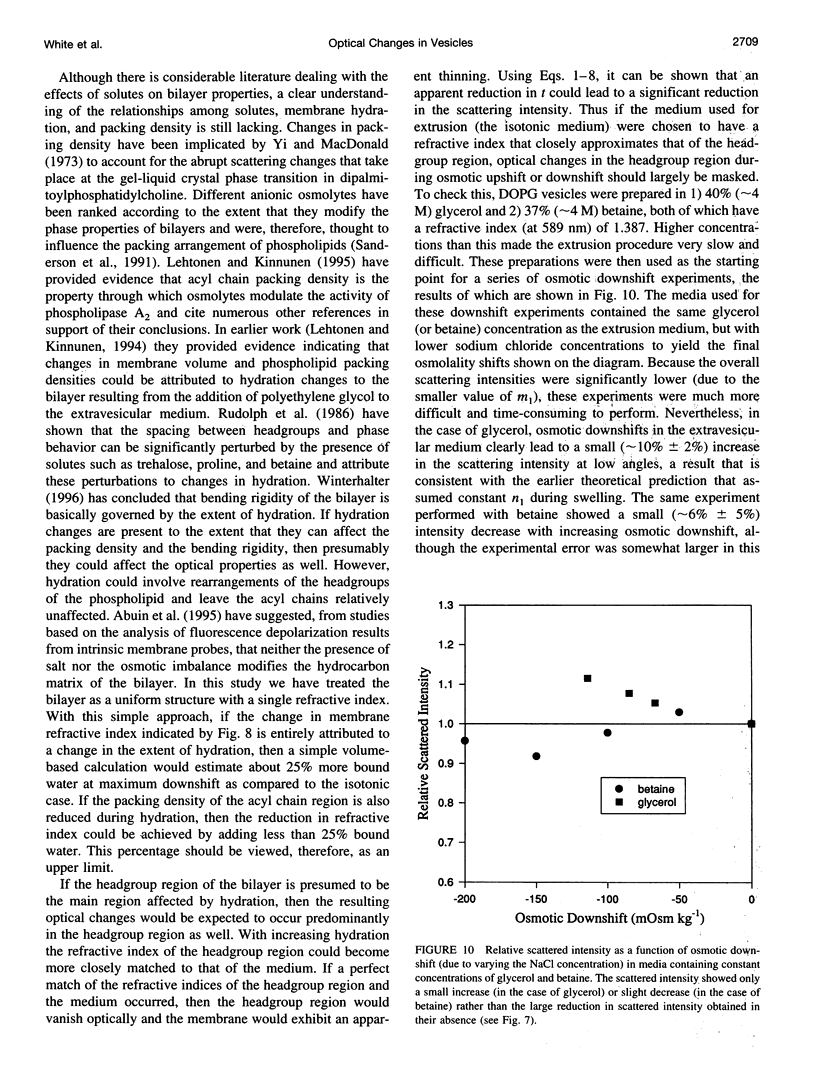
- Engelbert H. P., Lawaczeck R. Isotopic light scattering of lipid vesicles. Water permeation and effect of alpha-tocopherol. Chem Phys Lipids. 1985 Nov-Dec;38(4):365–379. doi: 10.1016/0009-3084(85)90030-1. [DOI] [PubMed] [Google Scholar]
- Ertel A., Marangoni A. G., Marsh J., Hallett F. R., Wood J. M. Mechanical properties of vesicles. I. Coordinated analysis of osmotic swelling and lysis. Biophys J. 1993 Feb;64(2):426–434. doi: 10.1016/S0006-3495(93)81383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995 Dec;13(12):527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- Grothe S., Krogsrud R. L., McClellan D. J., Milner J. L., Wood J. M. Proline transport and osmotic stress response in Escherichia coli K-12. J Bacteriol. 1986 Apr;166(1):253–259. doi: 10.1128/jb.166.1.253-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett F. R., Marsh J., Nickel B. G., Wood J. M. Mechanical properties of vesicles. II. A model for osmotic swelling and lysis. Biophys J. 1993 Feb;64(2):435–442. doi: 10.1016/S0006-3495(93)81384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett F. R., Watton J., Krygsman P. Vesicle sizing: Number distributions by dynamic light scattering. Biophys J. 1991 Feb;59(2):357–362. doi: 10.1016/S0006-3495(91)82229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Ayalon P. Precipitation membranes: III. Reversible changes of membrane properties induced by alterations in ionic concentrations. J Membr Biol. 1979 Dec 12;51(1):1–6. doi: 10.1007/BF01869339. [DOI] [PubMed] [Google Scholar]
- Jansen M., Blume A. A comparative study of diffusive and osmotic water permeation across bilayers composed of phospholipids with different head groups and fatty acyl chains. Biophys J. 1995 Mar;68(3):997–1008. doi: 10.1016/S0006-3495(95)80275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Active transport in Escherichia coli: passage to permease. Annu Rev Biophys Biophys Chem. 1986;15:279–319. doi: 10.1146/annurev.bb.15.060186.001431. [DOI] [PubMed] [Google Scholar]
- Lawaczeck R. Water permeability through biological membranes by isotopic effects of fluorescence and light scattering. Biophys J. 1984 Mar;45(3):491–494. doi: 10.1016/S0006-3495(84)84184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogs. Biophys J. 1994 Jun;66(6):1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Phospholipase A2 as a mechanosensor. Biophys J. 1995 May;68(5):1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- Milner J. L., Grothe S., Wood J. M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14900–14905. [PubMed] [Google Scholar]
- Mui B. L., Cullis P. R., Evans E. A., Madden T. D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys J. 1993 Feb;64(2):443–453. doi: 10.1016/S0006-3495(93)81385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski C. A., Williams L. M., Haines T. H., Cummins H. Z. The elasticity of synthetic phospholipid vesicles obtained by photon correlation spectroscopy. Biochemistry. 1991 Jun 11;30(23):5688–5696. doi: 10.1021/bi00237a008. [DOI] [PubMed] [Google Scholar]
- Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A. 1991 Jul 15;44(2):1182–1202. doi: 10.1103/physreva.44.1182. [DOI] [PubMed] [Google Scholar]


