Abstract
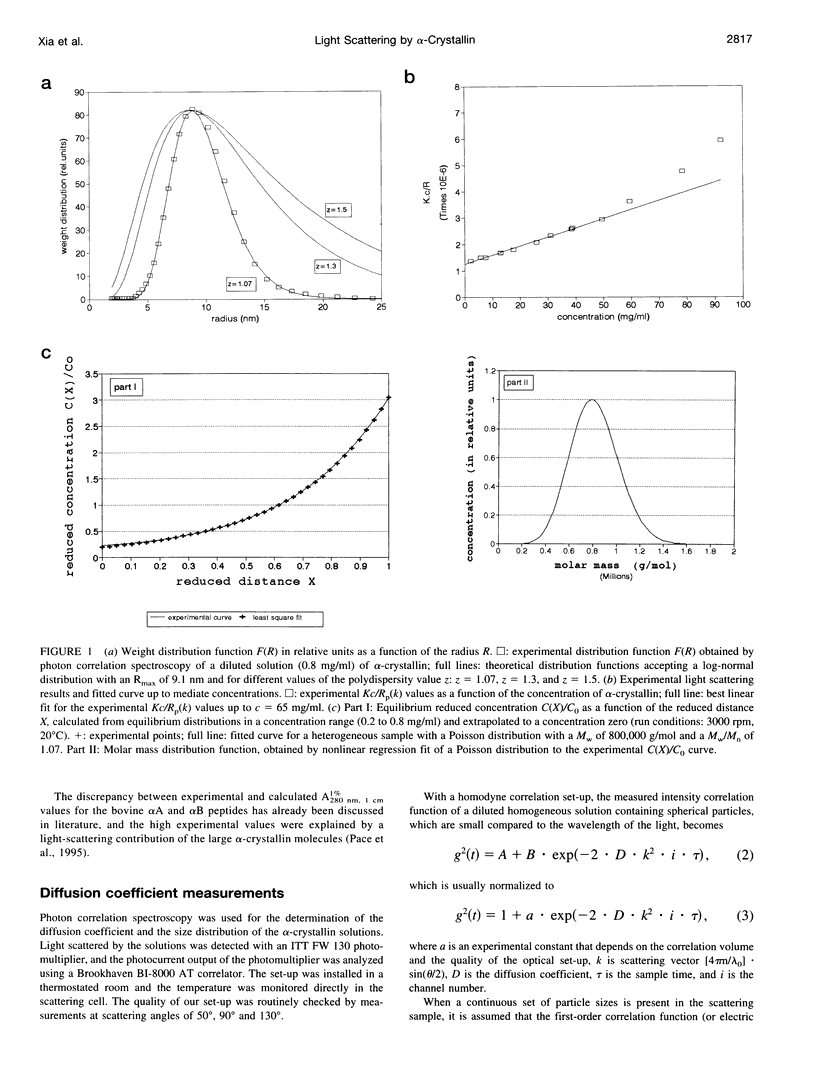
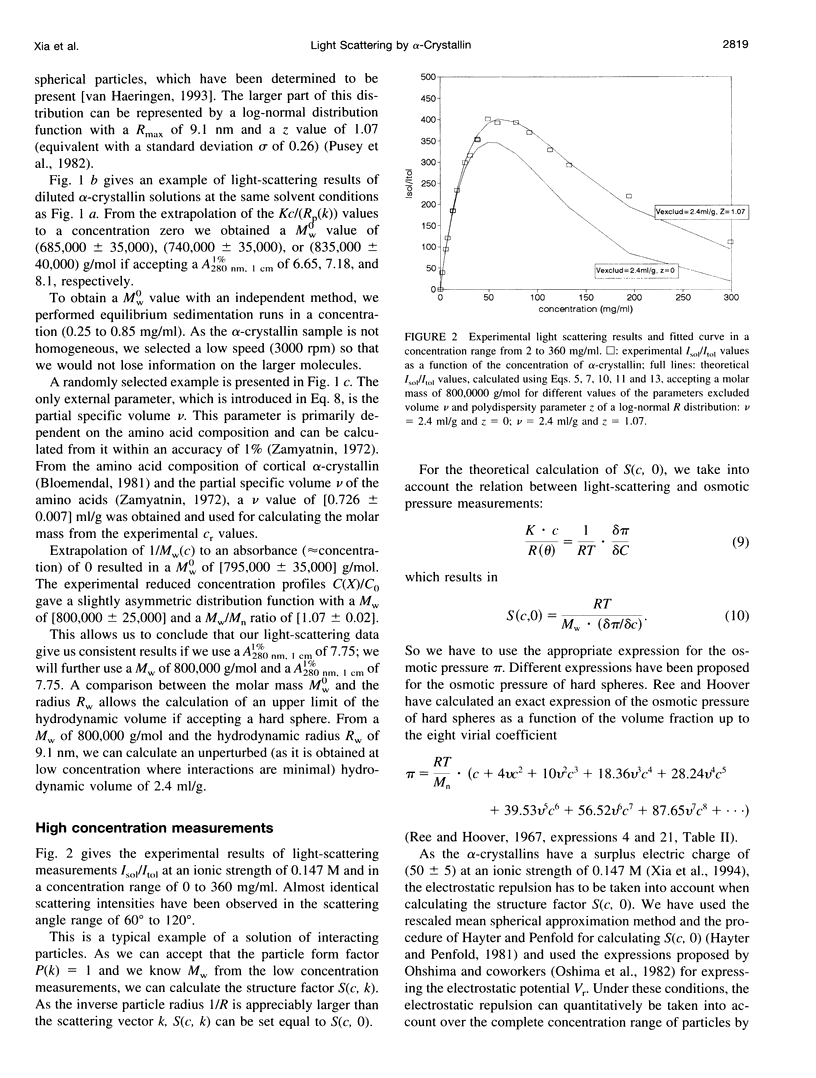
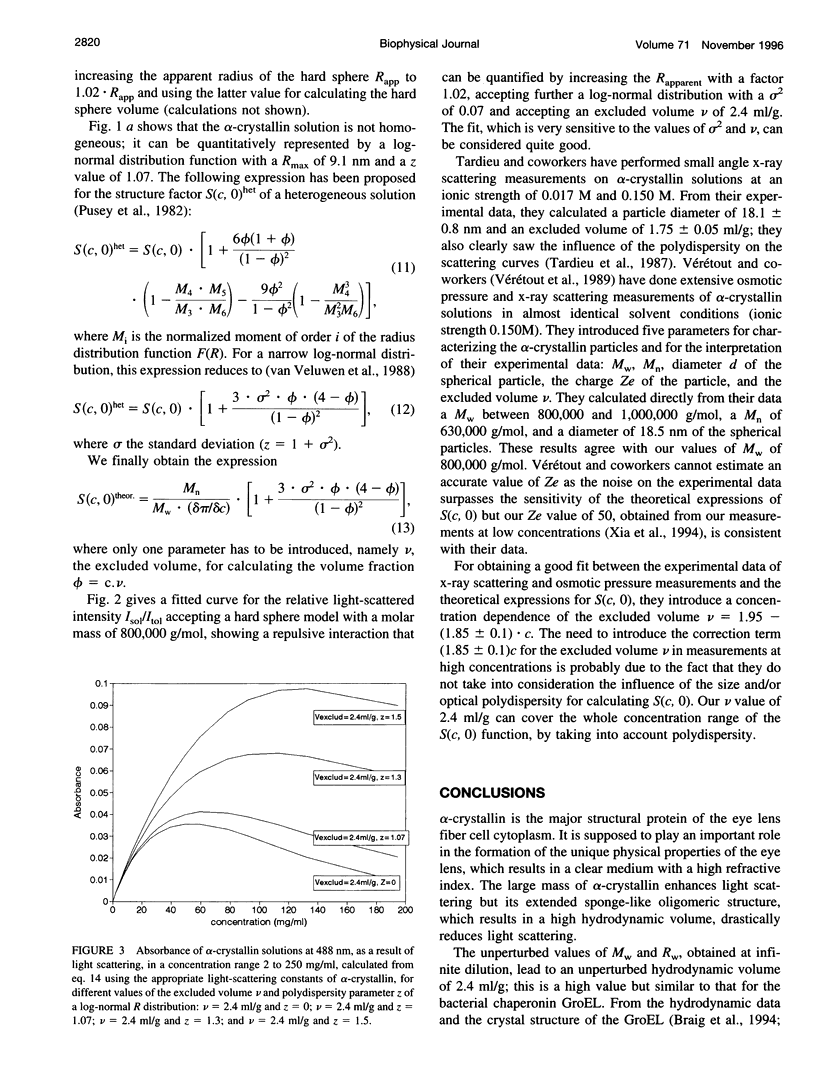
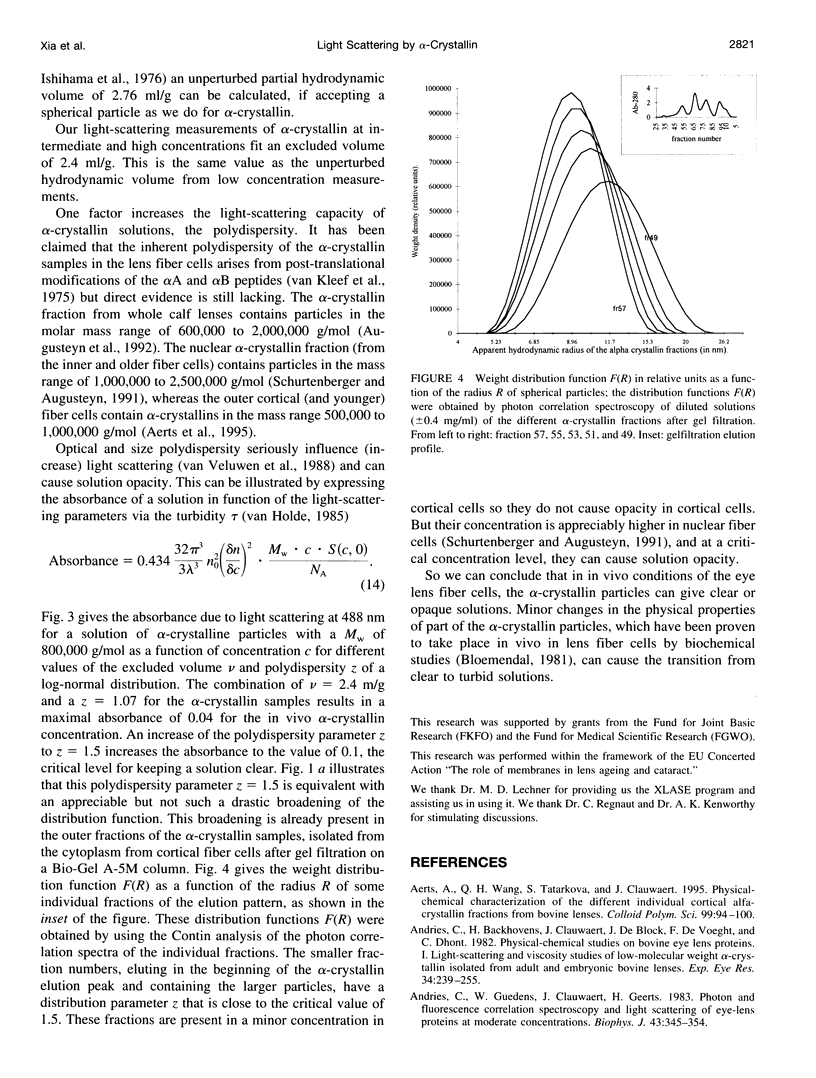
Short range order of the crystallins does account for the transparency of the eye lens. To explain the solution structure of this highly concentrated protein solution on a quantitative basis, the hydrodynamic structure and the interparticle interactions of the proteins have to be known. For that purpose, the light scattering of concentrated solutions of alpha-crystallin has been studied. Starting from the detailed knowledge of the solution parameters of alpha-crystallin in diluted solutions, the structure of concentrated solutions up to 360 mg/ml has been studied using light scattering. Our results indicate that subtle changes in the macromolecular structure such as optical anisotropy or structural asymmetry for part of the alpha-crystallins, which results in solute light-scattering heterogeneity, can dramatically increase the light scattering by the alpha-crystallins and cause solution opacity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andries C., Backhovens H., Clauwaert J., De Block J., De Voeght F., Dhont C. Physical-chemical studies on bovine eye lens proteins. I. Light-scattering and viscosity studies of low-molecular weight alpha-crystallin isolated from adult and embryonic bovine lenses. Exp Eye Res. 1982 Feb;34(2):239–255. doi: 10.1016/0014-4835(82)90058-6. [DOI] [PubMed] [Google Scholar]
- Andries C., Guedens W., Clauwaert J., Geerts H. Photon and fluorescence correlation spectroscopy and light scattering of eye-lens proteins at moderate concentrations. Biophys J. 1983 Sep;43(3):345–354. doi: 10.1016/S0006-3495(83)84358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison D. A., Smith G. Continuous gradient index and shell models of the human lens. Vision Res. 1995 Sep;35(18):2529–2538. doi: 10.1016/0042-6989(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Augusteyn R. C., Parkhill E. M., Stevens A. The effects of isolation buffers on the properties of alpha-crystallin. Exp Eye Res. 1992 Feb;54(2):219–228. doi: 10.1016/s0014-4835(05)80211-8. [DOI] [PubMed] [Google Scholar]
- Braig K., Otwinowski Z., Hegde R., Boisvert D. C., Joachimiak A., Horwich A. L., Sigler P. B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994 Oct 13;371(6498):578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Darragh A. J., Garrick D. J., Moughan P. J., Hendriks W. H. Correction for amino acid loss during acid hydrolysis of a purified protein. Anal Biochem. 1996 May 1;236(2):199–207. doi: 10.1006/abio.1996.0157. [DOI] [PubMed] [Google Scholar]
- Delaye M., Gromiec A. Mutual diffusion of crystallin proteins at finite concentrations: a light-scattering study. Biopolymers. 1983 Apr;22(4):1203–1221. doi: 10.1002/bip.360220413. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen P. J., Merck K. B., de Jong W. W., Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem. 1994 Oct 1;225(1):1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Ikeuchi T., Matsumoto A., Yamamoto S. A novel adenosine triphosphatase isolated from RNA polymerase preparations of Escherichia coli. II. Enzymatic properties and molecular structure. J Biochem. 1976 May;79(5):927–937. doi: 10.1093/oxfordjournals.jbchem.a131160. [DOI] [PubMed] [Google Scholar]
- Jagger W. S. The optics of the spherical fish lens. Vision Res. 1992 Jul;32(7):1271–1284. doi: 10.1016/0042-6989(92)90222-5. [DOI] [PubMed] [Google Scholar]
- Kröger R. H., Campbell M. C., Munger R., Fernald R. D. Refractive index distribution and spherical aberration in the crystalline lens of the African cichlid fish Haplochromis burtoni. Vision Res. 1994 Jul;34(14):1815–1822. doi: 10.1016/0042-6989(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995 Nov;4(11):2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurtenberger P., Augusteyn R. C. Structural properties of polydisperse biopolymer solutions: a light scattering study of bovine alpha-crystallin. Biopolymers. 1991 Sep;31(10):1229–1240. doi: 10.1002/bip.360311011. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Berger H. The quaternary structure of bovine alpha-crystallin. Size and shape studies by sedimentation, small-angle X-ray scattering and quasi-elastic light scattering. Eur J Biochem. 1978 Nov 15;91(2):397–405. doi: 10.1111/j.1432-1033.1978.tb12692.x. [DOI] [PubMed] [Google Scholar]
- Spector A., Chiesa R., Sredy J., Garner W. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4712–4716. doi: 10.1073/pnas.82.14.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Laporte D., Licinio P., Krop B., Delaye M. Calf lens alpha-crystallin quaternary structure. A three-layer tetrahedral model. J Mol Biol. 1986 Dec 20;192(4):711–724. doi: 10.1016/0022-2836(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Van Kleef F. S., De Jong W. W., Hoenders H. J. Stepwise degradations and deamidation of the eye lens protein alpha-crystallin in ageing. Nature. 1975 Nov 20;258(5532):264–266. doi: 10.1038/258264a0. [DOI] [PubMed] [Google Scholar]
- Vérétout F., Delaye M., Tardieu A. Molecular basis of eye lens transparency. Osmotic pressure and X-ray analysis of alpha-crystallin solutions. J Mol Biol. 1989 Feb 20;205(4):713–728. doi: 10.1016/0022-2836(89)90316-1. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Bettelheim F. A. Second virial coefficient of alpha-crystallin. Proteins. 1989;5(2):166–169. doi: 10.1002/prot.340050211. [DOI] [PubMed] [Google Scholar]
- Xia J. Z., Aerts T., Donceel K., Clauwaert J. Light scattering by bovine alpha-crystallin proteins in solution: hydrodynamic structure and interparticle interaction. Biophys J. 1994 Mar;66(3 Pt 1):861–872. doi: 10.1016/s0006-3495(94)80862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]
- van Haeringen B., van den Bogaerde M. R., Eden D., van Grondelle R., Bloemendal M. Further characterization of structural and electric properties of non-spherical alpha-crystallin. Eur J Biochem. 1993 Oct 1;217(1):143–150. doi: 10.1111/j.1432-1033.1993.tb18229.x. [DOI] [PubMed] [Google Scholar]
- van Iersel J., Jzn J. F., Duine J. A. Determination of absorption coefficients of purified proteins by conventional ultraviolet spectrophotometry and chromatography combined with multiwavelength detection. Anal Biochem. 1985 Nov 15;151(1):196–204. doi: 10.1016/0003-2697(85)90072-7. [DOI] [PubMed] [Google Scholar]
- van der Ouderaa F. J., de Jong W. W., Bloemendal H. The amino-acid sequence of the alphaA2 chain of bovine alpha-crystallin. Eur J Biochem. 1973 Nov 1;39(1):207–222. doi: 10.1111/j.1432-1033.1973.tb03119.x. [DOI] [PubMed] [Google Scholar]


