Abstract
Spinal fusion is considered today as the last treatment option for different spinal conditions, such as degenerative and infectious illnesses. It consists of fusing two or more vertebrae to obtain reinforcement/fixation based on several methods used to sustain osteosynthesis and grafting, such as cage insertion in the intervertebral space, which provides an important level of mechanical stability, impacting only a low amount of the natural biomechanics of the spine and facilitating the implant bony ingrowth. This review paper first explores the background of intervertebral fusion, emphasizing medical applications and material properties of interbody fusion cages. It then provides a brief historical overview and discusses antibacterial efficacy-related issues. Additionally, some of the most met-in-clinical practice lumbar interbody cages with a detailed description of their geometry and examples of clinical trials performed worldwide are provided. The biomaterials used in lumbar cage manufacture are comprehensively described. In the last part of this review paper, special attention is devoted to prospective biomaterials and coatings for spine fusion cages. Firstly, the rationale for using Mg-based alloys or high osteogenic polycaprolactone as biodegradable and bioresorbable alternatives in the spinal cage industry, addressing the clinical limitations of traditional Ti alloys and polyether ether ketone, is provided. Then, a more conservative approach, focusing on the use of bioactive or antibacterial coatings on the already certified biomaterials, is presented as a second alternative to the existing products on the market. Relevant literature studies are reviewed, and the osteointegrative, bioactive, or antibacterial character of the coatings is explained. Finally, our review identifies current clinical limitations and offers future perspectives that will provide better bioactive solutions, improving the existing biomaterials.
Keywords: Spinal fusion, Clinical trials, Biodegradable cages, Bioactive solutions, Mg-based alloys, High osteogenic polycaprolactone
Graphical abstract
Highlights
-
•
Medical applications and materials used for interbody fusion cages.
-
•
Surgical approach for lumbar fusion cage insertion.
-
•
Overview of lumbar fusion cages in clinical practice.
-
•
Biomaterials used, mechanical and biological matching, bioactive solutions.
-
•
Prospective biomaterials and coatings for enhanced spine fusion cages.
1. Introduction
The main scope of this review paper is to present innovative bioactive and high osteogenic solutions for interbody spinal cage manufacture that could be further developed, adopted, or improved. Nowadays, an increased awareness regarding spinal conditions in the framework of a continuously aging population has been identified, so spinal fusion remains one of the principal research topics worldwide. The paper will present some medical applications of interbody spinal cages, a historical background, and the most used types of material employed in commercial products. Then, the potential of prospective bioactive materials will be detailed with examples of in vitro or in vivo studies extracted from the literature, and solutions such as complex coatings will be offered to underline their efficiency in improving the osteointegrative, bioactive, or antibacterial behavior of the spinal cages. In the end, clinical limitations of the products available on the market and future perspectives will be presented. The review can be divided into two essential parts as follows: the first one consists of a clinical investigation that includes the most used in practice commercial cages with detailed analyses regarding their geometry, materials, and medical outcomes, while the second part is dedicated to biomaterials. Initially, the traditional materials are presented with devoted attention to their mechanical and biological properties. The innovative and bioactive solutions, which can be applied shortly, are sustained with a detailed literature investigation. Last but not least, the conservative approach of developing coatings for traditional materials is presented by explaining the importance of bioactive or antibacterial properties.
1.1. Medical applications and materials used for interbody fusion cages
Nowadays, spinal degenerative condition is considered a main priority, exhibiting an incidence worldwide higher than 50 % [[1], [2], [3]]. Spine degeneration usually deteriorates the anatomical structure and biomechanics of the vertebrae, surrounding soft tissue, nerves, and, last but not least, the intervertebral discs. These modifications are considered of utmost importance because they have an influence on loading patterns, range of movements, and tolerance to traumatic situations, which usually implies a higher mechanical load application due to an impaired mechanical activity of the spine. Some of the most important degenerative pathologies that lead to spine damage are disc degeneration and rupture, spondylosis – degenerative disease, spondylitis – inflammatory or infections in the bone or soft tissue, scoliosis and other congenital spine conditions with progressive deformity, spinal stenosis, and spondylolisthesis – degenerative or traumatic vertebrae slippage [4]. Intervertebral Disc (IVD) degeneration is one of the most common illnesses and can include the following conditions: a decrease in disc height, disc bulges, disc desiccation, disc herniations, and bone spurs formation [5,6]. A study developed by Brinjikji et al. [5] investigated the prevalence of disk degeneration in pain-free individuals and patients with back pain, based on a systematic literature review of Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) images published in English. They observed that in young asymptomatic patients, IVD degeneration occurred in 37 % of cases, while in individuals aged 80, this rate was approximately 96 %. In 20-year-old individuals, the prevalence of disc bulge was 30 %, disc protrusion 29 %, and annular fissure 19 %. In contrast, older patients show higher prevalence rates: disk bulge 84 %, disk protrusion 43 %, and annular fissure 29 %. The authors concluded that spine degeneration could be present even if a patient exhibits asymptomatic features. It is well known that, due to current lifestyle factors, diseases once considered associated with advanced age and part of the normal aging process are occurring much more frequently in the younger population. As previously mentioned, spinal fusion surgery is also performed to correct the spine shape. Weiss et al. [7] found that spinal fusion surgery is recommended when the spine curvature exceeds 40–45°. The authors noticed in the literature that the old biomaterials used for implants generated important side effects during patient life, such as loss of normal spinal function, neurological damage, higher strain on un-fused vertebrae, inflammatory effects, infections due to the implant contamination, curvature progression, and increased torso deformity. Spinal fusion is an effective surgical technique for complex clinical situations to resolve issues ranging from deformity correction and biomechanical stability to microbial resistance. A recent study developed by Zhao et al. [8] proved that in the case of a 10-year-old boy exhibiting an advanced kyphosis with a thoracic angle of 113° associated with Gaucher disease, a posterior spinal fusion was obtained based on pedicle screw fixation and Ponte osteotomy. By analyzing the medical images of the patient, the authors concluded after a 2-year follow-up period that posterior spinal fusion without an anterior spinal release was a good choice to treat the boy's advanced deformity. Another reason to use spinal fusion consists of spinal instability and weakness as a result of severe arthritis. Crawford et al. [9] investigated the lumbar fusion outcomes in the case of patients with rheumatoid arthritis. Their study was based on two groups of patients with an average age of about 65 years old, including arthritis and non-arthritis patients. They noticed an increased level of surgery complications, wound infections, and non-union in the case of the arthritis group. The main conclusion of this study was that about 74 % of the patients exhibited good results, but the lumbar fusion remains still a challenge because, in some cases, it can be accompanied by serious side effects related to immunosuppression treatment of arthritis.
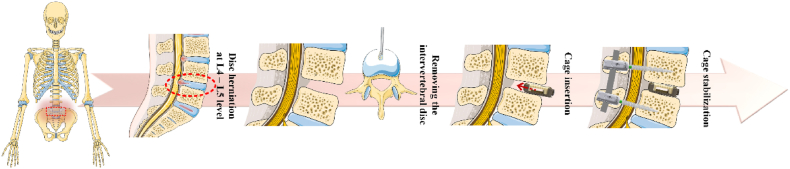
As an overall finding, an important purpose of spinal fusion surgery is nerve root decompression, which is sustained by the IVD anatomical height in a direct correlation with a complete bone fusion achievement [10]. Nowadays, the gold standard is represented by autogenous iliac graft [11,12], but due to the limited quantity of tissue and complications related to donor-site morbidity, this method cannot be applied as a general rule. Another solution consists of allogenic bone graft use, but unfortunately, it is often accompanied by side effects such as disease transmission and graft rejection due to the patient's immune response [13,14]. The use of interbody fusion cages seems to be a viable choice, but in some cases, vertebrae fusion cannot be achieved due to cage loosening and other problems, which occur in 5–35 % of the patients [10]. Consequently, choosing an adequate cage does not guarantee an increased rate of success [15]. Zhang et al. [10] established 3 modalities of bone cage enhancement dedicated to better bone fusion. First and foremost, the cage must be designed to support natural bone ingrowth. Next, the biomaterials should be selected based on their close alignment with the physical and mechanical properties of the human bone. Lastly, secondary post-processing steps, such as applying coatings to the cage, can enhance the biocompatibility and bioactivity of the implant. Fig. 1 presents an example of an anterior lumbar interbody fusion procedure to treat disc herniation based on intervertebral cage insertion between the L4-L5 spinal level. Other authors such as Warburton et al. [16] and Verma et al. [17] considered that an adequate bone substituent must have a certain value of mechanical strength almost equal to that of the human bone, a high biocompatibility correlated with a good bone induction, and a suitable ratio between its performance and final price. The most used materials for interbody fusion cages can be classified as metallic or non-metallic, permanent or temporary, and inert or bioactive. Commercial devices with European Certification (CE) or Food and Drug Administration (FDA) approval are typically made from medical-grade titanium (Ti) alloys and polyether ether ketone (PEEK). Unfortunately, these types of biomaterials are characterized by important drawbacks such as impaired radiographical opacity and stress shielding that occur in the case of Ti alloy cages due to an important mismatch between mechanical properties compared to those of the human bone, and in both cases, it can be mentioned the inertia of the materials, which hinder their active participation to the bone remodeling process. In addition, inflammatory responses can also occur in both cases [18]. To overcome the limitations of the aforementioned commercial devices, ongoing research and development of bioabsorbable materials, including biocompatible polymers and bioactive metals like magnesium and zinc, are essential. Also, three-dimensional (3D) porous implants can be considered a good alternative because even if they are made of inert materials, the pore presence will enhance bone ingrowth, blood and nutrient circulation, and the insertion of biological materials directly connected to bone regeneration. Biocompatible coatings can be applied alongside drugs such as strontium ranelate (SRR) or simvastatin (SIM) to support implant osteointegration in osteoporotic patients. Additionally, the combination of Epidermal Growth Factor and Bone Morphogenetic Protein (BMP), which regulates the expression of osteogenic genes and promotes interbody fusion, should also be considered [19].
Fig. 1.
Exemplification of anterior lumbar interbody fusion surgical procedure consisting of an intervertebral cage insertion between spinal levels. This Figure was generated using images assembled from Servier Medical Art, which are licensed under CC BY 4.0 (https://smart.servier.com, accessed on January 23, 2025).
It can be concluded that interbody cages are an important alternative to the full-biological solutions in intervertebral fusion surgery. However, today, many models of fusion cages exhibit a hollow center that is filled with bone grafts to improve bone fusion. As stated before, the grafts could be autogenic, allogenic, or synthetic. The surgeons could use a Demineralized Bone Matrix (DBM), which consists of an allograft collected from the patient iliac crest and combined with calcium phosphates. DBM is obtained in powder form with a given carrier as a graft extender. Another bioactive solution could be achieved by replacing DBM with ceramics and Bone Morphogenetic Protein-2 (BMP-2). In clinical practice, inert fusion cages are combined with orthobiologics to increase the natural osteogenesis that occurs after cage implantation. However, it is important to emphasize the need for ongoing research into innovative materials to mitigate the common risks associated with inert implants.
1.2. History of lumbar interbody fusion surgery
To fully understand the current needs and challenges in developing new and innovative interbody fusion cages, it is essential to examine the history of lumbar interbody fusion surgery and the prevalence of spinal infections. The new biomaterials considered for spinal cage manufacturing must facilitate effective osteointegration and protect against various infections.
The first surgeon who performed a spinal fusion to treat a dislocated fracture between the C6 and C7 vertebra chose a silver wire that was wrapped around the spine and used a posterior approach in 1891 [20]. After that, the posterior approach was applied by Hibbs [21] and Albee [22], who used a bridge created from patients’ bones to fix the negative effects of posterolateral spondylosis. Classical Posterior Lumbar Interbody Fusion (PLIF) was introduced in 1994, and later, it was modified by Cloward [23,24] and applied to more than 300 patients. He noticed a higher fusion rate when he used a partial bilateral laminectomy combined with a full facetectomy. The surgeon entirely removed the damaged IVD and both endplates by a posterior surgical procedure and used to fill the obtained void with bone grafts collected from the patient iliac crest. During the time, many studies [[25], [26], [27], [28]] described case reports in which autologous grafts from the fibula or iliac crest and allografts were involved to fix spinal problems, but as described in Section 1.1, the risks mentioned above were noticed. Couture and Branch [29] found that pseudarthrosis developed in cases where bone grafts were used alone, without association with other implants, and this was directly related to graft collapse. Between 1970 and 1980, a supplementary stabilization procedure was proposed based on a plate interconnected with pedicular screws or in combination with the so-called Harrington rod [30]. Steffee and Sitkowski [31] proved that better fusion rates and higher stability were achieved when the interbody fusion procedure was combined with pedicle screw fixation. Blume [32] applied the posterior unilateral transforaminal approach, Transforaminal Lumbar Interbody Fusion (TLIF) to solve lumbar interbody spondylosis. The TLIF procedure was adapted and improved later by Harms [33]. He used one midline posterior incision to perform a posterior unilateral TLIF based on an anterior interbody fusion procedure using classical pedicle implants. In Ref. [34], morselized autologous bone grafts were inserted around a screw-rod ensemble to improve the spinal fusion. Today, in order to obtain a proper osteointegration process for the spinal cages, morselized autologous bone grafts are used to fill the voids between the intervertebral space and cages. Through osteointegration, the cages will offer good nerve decompression and stability to the affected spine segment, but it is usually conditioned to a certain quality and physical properties of the implant surface.
In addition, systematic reviews and important studies were conducted to provide information regarding the best treatment choice for different spine diseases. As an overall finding, spinal fusion procedure is commonly accepted to induce patient relief and have good outcomes. The so-called Spine Patient Outcomes Research Trial (SPORT) developed by Weinstein et al. [35] is considered today one of the most comprehensive and interesting studies. It was conducted as a random clinical trial, including patients who supported spine surgery between March 2000 and November 2004, coming from 13 multidisciplinary spine clinics. There were chosen 501 candidates, who were 42 years old on average, of which 42 % were women. Based on those results, other studies were conducted and proved that even after 8 years of follow-up, the spinal fusion intervention was beneficial and suitable to be applied to patients with spinal stenosis and degenerative spondylolisthesis [36,37]. In the last decades, an increasing number of spinal fusion procedures have been reported in Europe and the USA. Provaggi et al. [38] found an increased number of spinal fusions of about 63 % starting from 2005 and ending in 2015 in the United Kingdom, while Grotle et al. [39] noticed an increased number of spinal fusions in Norway between 1999 and 2013. Beyer et al. [40] conducted a study in Köln, Germany, in which they considered patients treated for vertebral osteomyelitis (52 patients) and degenerative spondylolisthesis (48 patients) based on one- or two-stage fusion of the ill spine segments. They found similar outcomes related to patients and concluded that spinal fusion is recommended in many cases to alleviate the patient's pain. Regarding other continents, a very interesting analysis was performed by Kim et al. [41], who noticed after a detailed comparison between Asia (South Korea and Japan) and the USA that many more spine surgical interventions were performed in the last case. This fact was attributed to the lifestyle of the investigated population, and it was concluded that Asian people exhibit their particularities and have healthier behavior. A comprehensive study was conducted by Deyo et al. [42], who observed an increased number of spinal fusions in the USA by 77 % between 1996 and 2004. After that, Rajaee et al. [43] noticed an increase in spinal fusion application by about 137 %, starting in 1998 and ending in 2008. Seven million cases were studied by Sheikh et al. [44]. The authors observed a steady increase in spine fusion intervention, with a rise of 118 %, compared to decompression, a non-spinal procedure, across the North American continent from 1998 to 2014.
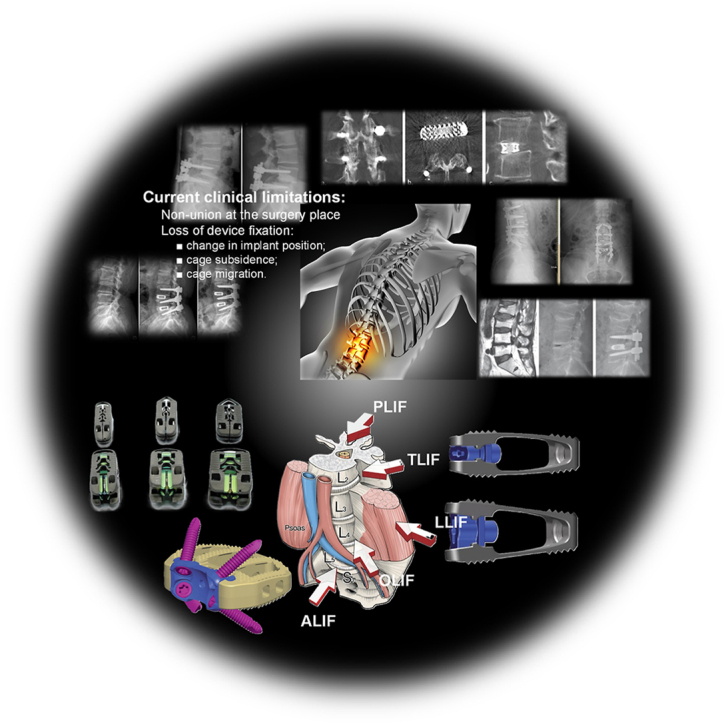
Today, minimally invasive surgical techniques are applied in lumbar interbody fusion surgery. Traditionally, this procedure was conducted as a PLIF based on a posterior approach combined with an extended facet joint resection. Then, a cage made of different biocompatible materials was inserted after the nerve roots and dura mater were correctly repositioned. The dura mater is the outermost and toughest layer of the three membranes (meninges) that surround and protect the spinal cord. Another method that was applied in practice is TLIF, which allows the surgeon to use the space between the dura mater and nerve roots through a posterior approach. The Anterior Lumbar Interbody Fusion (ALIF) was considered a safe and less traumatic surgical intervention in comparison with PLIF and TLIF because it allows direct visualization of the IVDs, permitting also to perform complete discectomy and introduction of a large interbody cage that improves the patient's position with a better sagittal alignment. In addition, ALIF was usually linked to a higher fusion rate and posterior structure preservation [20]. Another procedure met in practice is the Lateral Lumbar Interbody Fusion (LLIF) that is associated with minimum blood loss, soft tissue damage, and shorter hospitalization time. It is also practiced in revision cases and usually restores foraminal height and central canal surface [45,46]. The minimally invasive approaches performed through the lateral anterior corridors include Direct Lateral Interbody Fusion (DLIF) and Oblique Lumbar Interbody Fusion (OLIF) [47] and, in most cases, minimize the invasiveness grade of the spine and the deterioration of the healthy tissue [48]. The expandable lumbar interbody cages can increase the lordotic angle and the space between IVDs compared to the classical static cages, which makes them widely used [49]. Also, supplementary, the controlled expansion of the medical implant keeps the iatrogenic endplates undamaged, and these devices show a larger footprint. However, despite the advantages described above, the literature has shown that both static and expandable cages yield similar fusion outcomes and maintain favorable sagittal parameters [[50], [51], [52], [53], [54]]. Table 1 presents a comparative analysis of the main surgical access' impact on fusion effects underlying the advantages and drawbacks associated with each surgical technique [[55], [56], [57], [58]], [[59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]].
Table 1.
Indications, contraindications, possible risks, and surgery steps for main lumbar spinal surgical accesses.
| Surgical procedure | Indications | Possible risks and complications | Contraindications | Surgery steps | Average fusion rates reported in the literature |
|---|---|---|---|---|---|
| Posterior Lumbar Interbody Fusion (PLIF) | Permits access to the anterior and posterior columns in a single step and complete visualization of the nerves with circumferential decompression. It is indicated for pseudoarthrosis, spinal stenosis, disc hernia, spondylolisthesis, and disc instability [55,56] | Deterioration of paravertebral muscle, long time of muscular contraction, retraction of nerves to permit the insertion into intervertebral space, epidural bleeding, fibrosis. By retracting the dura mater, durotomy could occur [57,58] | Arachnoiditis, infections [55] | Laminectomy, medial facetectomy, annulotomy, discectomy, cage insertion [61] | 71 % ÷ 96 % [[67], [68], [69]] |
| Transforaminal Lumbar Interbody Fusion (TLIF) | Provides access to the intervertebral disc space through a considerably more lateral approach than PLIF. It reduces nerve retraction and is associated with a low risk of nerve damage and dural tear. Maintains biomechanical stability and the interlaminar surface and facet joints. It can be used as a Minimally Invasive Surgery (MIS) [59,60]. Indications: the same as PLIF | Infection, nerve injury, blood loss, paraspinal iatrogenic injury [61] | Epidural scarring, infection, osteoporosis, arachnoiditis, conjoined nerves [61] | Discectomy, endplate preparation, cage insertion [61] | 82 % ÷ 98 % [[70], [71], [72]] |
| Lateral Lumbar Interbody Fusion (LLIF) | It can reduce the soft tissue deterioration’ area and blood loss, as well as minimize the hospitalization time for each patient. Usually, a lateral incision is performed by dissecting the blunt and abdominal muscles. Maximum attention has to be given after the retroperitoneum is reached so as not to damage the lateral femoral cutaneous nerve, iliac inguinal nerve, subiliac nerve, and intercostal nerve [62]. Indications: degenerative disc disease, spondylolisthesis, scoliosis, revisions, correction of coronal and sagittal spine deformity, restoration of foraminal height and central canal height [45] | Damage of the lumbar plexus, psoas, and organs. Tight pain, operative groin, paresthesia, and hip flexion weakness are listed as possible side effects or risks [63] | Adhesive retroperitoneal cases associated with a history of infection, radiation, or another surgery. A main contraindication consists of surgeries made at L5-S1 level [61] | Discectomy and cage insertion made under fluoroscopic guidance [61] | 80 % ÷ 90 % [68,73] |
| Anterior Lumbar Interbody Fusion (ALIF) | Less traumatic surgery compared to PLIF. Improved biomechanics due to larger cage insertion. Preserves the back muscles and ligaments and is characterized by higher fusion rates. ALIF is suitable for the L5-S1 level. Indications: degenerative disc disease, sagittal plane deformities, postoperative spondylodiscitis, and pseudoarthrosis [61] | Superior hypogastric plexus deterioration could lead to retrograde ejaculation [64]. Also, postoperative hernia could occur. ALIF is associated with risks such as ureters' and abdominal organs' damage, and post-surgical hernias. In some cases, large vessel deterioration could be present [65] | Other abdominal surgery, obesity, previous radiation therapy, or important aortic disease [61] | Discectomy and large cage insertion [61] | 94 % ÷ 98 % [67,74] |
| Oblique Lumbar Interbody Fusion (OLIF) | This surgical procedure was developed as a response to overcome the LLIF drawbacks. It provides access to the discs placed between the psoas muscle and abdominal blood vessels with a low risk of lumbar plexus and muscle damage. The follow of the nerves is unnecessary, and thigh pain or postoperative groin seldom occurs. Indications: spondylolisthesis, pseudoarthrosis from L2-L3 to L5-S1, discitis, degenerative disc disease [61] | Large vessel damage [66], sympathetic deterioration | Infection, radiation therapy, and retroperitoneal surgery [61] | Classic discectomy and cage insertion [61] | 92 % ÷ 98 % [75,76] |
An important problem that is met in medical practice consists of infections associated with orthopedic surgical interventions [77]. A study performed by Darouiche [78] provides a risk of infection between 2 % and 5 % in this domain, while others [79,80] stated that many spinal infections are associated with Staphylococcus epidermis and Staphylococcus aureus. Unfortunately, the number of patients exhibiting negative symptoms related to spondylitis, which can be defined as vertebra osteomyelitis, and spondylodiscitis, an infection of the IVDs, has increased lately [81]. In addition, a mortality rate of about 23 % was registered in the case of spondylodiscitis [[82], [83], [84]], making the spine surgery domain very challenging from a social and economic point of view. Some studies [[85], [86], [87]] established that infections occurred in 3.4–8.5 % of spinal surgeries when implants are used and only in 1 % of cases when no external hardware is involved. It should be noted that infections are not the only drawback associated with spinal cages, as they are also directly linked to the development of pseudoarthrosis, with a risk of 30 % [88]. Sometimes, when surgery is performed, even if everything is clean in the operating room, pathogens on the patient's mucous membranes or skin could populate the spinal cage or other implants and lead to multispecies biofilm formation. This condition is considered in the medical sciences as a life-threatening situation because stronger antibiotics and drugs are required to effectively target and eliminate biofilm-associated infection [89]. Even so, vascularization and implant dislocation or loosening are common side effects of infections in the spinal region [[90], [91], [92]]. Unfortunately, the current strategies to address spinal infections associated with implants are limited [93], with fusion rates of approximately 70 % reported for patients with infections [94,95].
Rapid technological advancements must be considered in the development of advanced and multifunctional spinal cage materials, driven by the complications associated with permanent and inert implants. In the authors' opinion, an ideal interbody fusion cage should be biodegradable or bioresorbable to mitigate the risk of biofilm formation, which increases proportionally with the duration the implant remains in the human body. Additionally, the material should possess intrinsic antibacterial properties to prevent infections and exhibit bioactivity to promote new bone formation, ensuring effective healing and stability of the affected segment. Furthermore, the material should be designed to closely match the mechanical properties of bone, enhancing integration and long-term outcomes.
2. Overview of different lumbar fusion cages in clinical applications
This section will focus on the various lumbar fusion cages currently used in clinical applications worldwide. Some studies [43,44] evidenced that in 2008, one of the most met-in-practice diagnoses was that of lumbar degenerative IVD disease, accounting for about 14 % of the total number of surgeries in the United States of America.
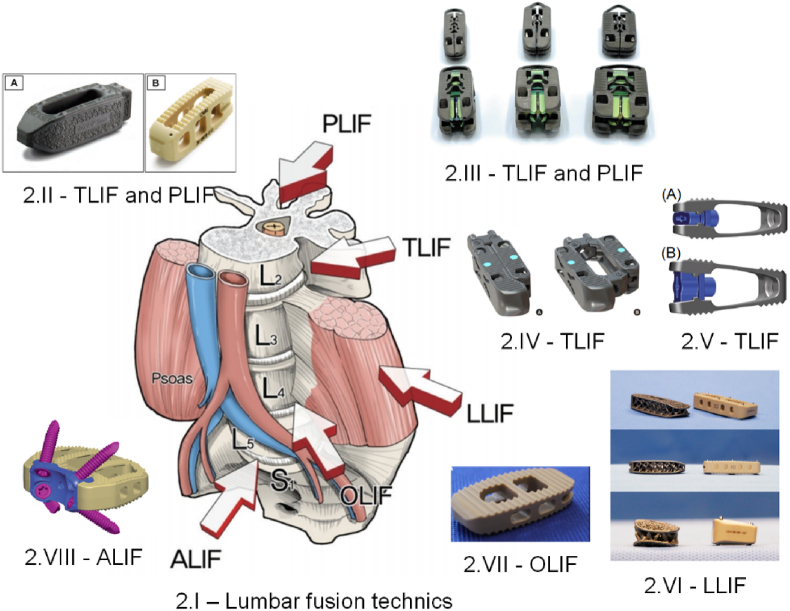
Fig. 2 shows the main types of surgical interventions in the lumbar zone associated with examples of commercial interbody fusion cages made by different producers, as reported in the literature.
Fig. 2.
Examples of commercial fusion cages associated with different surgical interventions. (2.I) Main lumbar interbody fusion surgical approaches [48] (Figure is licensed under CC BY-NC 4.0); (2.II) CTL Amedica, Dallas, TX, USA fusion cages: (A) TLIF Si3N4 Valeo™ OL, (B) PLIF PEEK Phantom™ [52] (Figure is licensed under CC BY-NC-ND 4.0); (2.III) Acellus, Palm Beach Gardens, FL, USA TiHawk7, TiHawk9, and TiHawk11 (from left to right) [96] (Figure is licensed under CC BY-NC-ND 4.0); (2.IV) Amplify Surgical, Inc., Irvine, CA, USA Dual-X TLIF cage [97] (Figure is licensed under CC BY-NC 4.0); (2.V) Signus Medizintechnik GmbH, Alzenau, Germany TLIF Vertaconnect [98] (Figure is licensed under CC BY 4.0); (2.VI) NuVasive, Globus Medical, San Diego, CA, USA 3D Ti Modulus and CoRoent XL PEEK [99] (Figure is licensed under CC BY 4.0); (2.VII) Sanatmetal, Eger, Hungary OLIF PEEK EMERALD™ cage [53] (Figure is licensed under CC BY 4.0); (VIII) DePuy Synthes Spine, PA, USA PEEK SynFix® stand-alone cage [54] (Figure is licensed under CC BY 4.0).
2.1. Permanent lumbar interbody fusion cages
Attention will be focused on permanent lumbar fusion cages currently used in clinical studies and hospitals worldwide, emphasizing implant geometry and biomaterials, the number of treated patients, and key observations related to the investigated models and surgical techniques. Finally, the most current and advanced bioactive solutions will be highlighted at the end of the section.
One of the most used worldwide spinal interbody cages is the FlareHawk® produced by a private company called Accelus. They adopted a minimally invasive surgery based on the FlareHawk® system that was used in Europe (Italy and Portugal) as well as in Taiwan and Colombia. FlareHawk® 7 was reported in Greece, Cyprus, Portugal, Spain, and Switzerland, while FlareHawk® 9 was used in Argentina, Qatar, Australia, and Chile. It must be underlined that FlareHawk® Expandable Lumbar Interbody Fusion Devices received FDA 510(k) clearance and CE mark certification in 2021 [96]. The literature revealed that about 15,000 devices have been involved in US patient treatments, while worldwide, 17,000 implants for spinal fusion were reported. The FlareHawk® Interbody Fusion System was indicated in most medical approaches for spinal fusion surgeries with autogenous or allogeneic bone grafts on skeletally mature patients suffering from Degenerative Disc Diseases (DDD) [100]. Cheng et al. [101] investigated the feasibility assessment of expandable interbody cages by performing a retrospective analysis on 7 patients based on CT images to evaluate the interbody fusion and the modalities of cage accommodation in a direct relationship with the endplates within the intervertebral space. They used FlareHawk® Integrity Implants (Palm Beach Gardens, FL), which consist of 2 parts made of PEEK that expand in two given directions, having an insert manufactured from Ti, to restore the patient lordosis. The authors noticed that their small case series analysis revealed a successful fusion process due to the good endplate-conforming interbody fusion cages. The adaptable geometry of the expandable cage was suitable for each patient's anatomy and proved compliance and conformity to the dimension of the intervertebral space. An anterior average implant deformation of 1.82 mm and posterior cage deformation of 1.41 mm were reported in combination with a suitable endplate concavity between 1.37 mm and 1.90 mm, which agrees with the literature. It was concluded that the analyzed expandable interbody fusion cage exhibited an adequate value of stiffness, which provides the necessary anterior column support by enhancing the arthrodesis. Coric et al. [102] retrospectively analyzed performance and safety regarding FlareHawk® expandable implants. There were selected 58 patients for the investigation related to the 1-year intervertebral fusion process and 45 patients with Oswestry Disability Index (ODI), Visual Analog Scale (VAS) for back pain, or VAS leg pain with an average follow-up of 4.5 months. The surgeons reported that after 12 months, fusion rates ranging from 92 % to 96 % were achieved. An improvement of about 15 ± 19 points in ODI (100-point scale), 4 ± 3.4 points in VAS leg pain (100-point scale), and 3.4 ± 2.6 points in VAS back pain were noticed. The conclusion was that clinically important improvement considering the ODI and VAS scores was obtained in the case of 58 % (ODI), 76 % (VAS back pain), and 71 % (VAS leg pain). The authors considered that their study gave preliminary clinical evidence regarding the safety of FlareHawk® expandable devices and observed that both surgical procedures, PLIF and TLIF, were adequate. Tan et al. [103] analyzed the positive outcomes obtained after a Minimally Invasive (MIS-TLIF) procedure used to treat spondylolisthesis. A retrospective review was performed in the case of 13 patients. All these patients had a FlareHawk® cage for over 1 year. The mean average age was 61, of which about 62 % were female. After the surgical procedure was applied, the VAS back pain score improved from 7.0 ± 2.9 to 3.1 ± 3, the VAS leg pain decreased from 5.1 ± 3.0 to 1.1 ± 1.7, and the Five Dimensions (EQ5D) score improved from 0.37 ± 1.7 to 0.66 ± 0.23 postsurgical. The main conclusion of this study was that the FlareHawk® cage was a good choice to treat spine illness via the MIS-TLIF surgical approach because no side effects, such as cage migration or endplate damage, were reported. In addition, none of the patients required a second surgical intervention. In a very recent study, Zygogiannis et al. [104] investigated the lumbar spinal rahisynthesis based on PLIF by studying the medical documents retrospectively in the case of 58 patients in KAT General Hospital, Athens, Greece. The mean age of the patients was about 60 years old, and 22 patients were female. The follow-up time was set for 1 year. The surgeons used the FlareHawk® 9 implant. The patients' ODI index improved by a high amount in the case of 45 patients exhibiting a decrease from 48.6 preoperative to 23.1 postoperative (p value of 0.05), remains almost unchanged for 8 patients (mean preoperative value of 49.7; mean postoperative value of 48.1 (p value of 0.05)), and in the case of 5 patients, it worsened with an increased value from 50 to 54.2 (p value of 0.05). It was concluded that an improvement of lordosis, with an increase of 2 ± 0.4°, and a good fusion level were achieved, but a longer follow-up time is mandatory for a detailed analysis of the benefits and side effects. By analyzing the literature, it can be concluded that the FlareHawk® class of lumbar interbody fusion devices exhibit the possibility to enlarge in height and lordosis and in width, generating an improvement of sagittal parameters and disc height. Their larger footprint is usually linked to a reduced risk of cage subsidence, providing an adequate medium for bone fusion. In addition, it must also be mentioned that its unique design allows the conformity between the implant and the endplates, and its open architectural geometry permits the use of bone grafts that can enhance new bone apparition.
Another important interbody fusion cages used in clinical research are those made by NuVasive company. One can mention the 3D-printed porous or conventional threaded titanium Modulus cages, as well as the CoRoent type, which are manufactured from sPEEK or Ti alloys. The Modulus Interbody Fusion device is designed in a hollow cylindrical or similar geometrical shape with porous, fenestrated, or threaded features. It is made of Ti alloy, and it is advisable to be filled with bone graft to ensure a successful spinal bone fusion. The CoRoent thoracolumbar non-interfixated fusion cage is usually manufactured of either Ti alloy or PEEK-Optima and includes radiographic markers made of tantalum (Ta) or titanium (Ti). Regarding the interfixated implants, they are fabricated only from PEEK-Optima and are equipped with a canted coil lock mechanism (NiCoCrMo) and screws (medical Ti alloy grade). Segi et al. [99] investigated the effect of native endplate injury that occurred in the case of Extreme Lateral Interbody Fusion (XLIF) procedures. The authors have chosen to perform a retrospective study in Nagoya, Japan, including 32 patients with a mean age of about 74.1 ± 6.7, of which 22 were female. These patients have undergone posterior or anterior surgery combined with XLIF. Two types of implants were used as follows: 3D porous titanium (3DTi, Modulus, NuVasive) (11 patients) and sPEEK (CoRoent XL PEEK, NuVasive) (21 patients). The 3DTi cages were combined with a hydroxyapatite mass, which was inserted in their pores, while in the case of polymeric interbody fusion implants, grafting bone was involved. The 3DTi implant was used in the case of 25 levels since the sPEEK cage was applied for 38 levels. The local lordotic angles were preoperatively equal to 4.3° (3DTi) and 4.7° (sPEEK) (p = 0.90), and then after the implant insertion, these values were corrected to 12.3° (3DTi) and 9.1 (sPEEK) (p = 0.013). The authors [99] noted that the risk of side effects is associated with vertebral endplate concavity (VEC) or other endplate injuries. They found that, after three months postoperatively, none of the patients treated with 3DTi cages exhibited Vertebral Endplate Concavity (VEC) progression (VEC was present in 2 levels, or 0.8 %). In contrast, for the sPEEK cages, VEC progression occurred in 8 levels (21 %), with VEC present in 17 levels (45 %), overall (p = 0.002). The main conclusion of this study was that the 3DTi cages were superior to the polymeric implants and gave the patient an improved local correction, as well as less intraoperative VEC. Although a good prognosis was foreseen, the surgeons noticed that the long-term outcomes must be further investigated to estimate the bony fusion process and spinal alignment. Amini et al. [105] performed a retrospective study based on fusion assessment in standalone lateral lumbar interbody fusion by including two PEEK cage systems (XLIF – NuVasive, Inc., San Diego, CA, USA and COUGAR – DePuy Spine Inc., Raynham, MA, USA) and two 3D-printed Ti cages ((Modulus XLIF (NuVasive, Inc.) and Lateral Spine Truss System (4WEB Medical, Inc., Frisco, TX, USA)). A total number of 91 patients (136 levels) were studied, from which an early group consisting of 49 patients (72 levels) and a late group of 42 patients (64 levels) were admitted. The authors [105] underlined in their study that a main disadvantage of the PEEK consists of the so-called “PEEK-Halo effect” represented by a reduced osseointegration and a biofilm layer formation around the implant surface. In addition, new bone material usually occurs around the implant, facilitating the connection to the patient's vertebrae. Supplementary, they stated that the porous Ti cages are the most advanced, exhibiting an increased biocompatibility due to the fact that they are similar to the trabecular bone. To evaluate the outcomes of the surgical procedure, CT scans were performed at 8 months postoperative for the early group and at 19 months postoperative in the case of the late group. The early group bone fusion was higher in the case of the Ti group (95.8 %) in comparison with the PEEK group (62.5 %) (p = 0.002). For the patients included in the late group, no important differences (94.7 % - 3DTi cages; 80.0 % - PEEK cages (p = 0.258)) were reported. It was concluded that the 3D-printed Ti cages with porous geometry are the most adequate for spine bone fusion surgery due to their enhanced osteoconductivity. Velluto et al. [106] evaluated the fusion patterns obtained after lateral lumbar interbody fusion in the case of conventional and 3D-printed porous titanium cages. The authors used titanium threaded or 3D-printed porous titanium (XLIF Modulus NuVasive, San Diego, CA, USA). The porous implants were filled with synthetic bone from NuVasive AttraX Putty type. A total of 135 patients were included, from which 51 patients (Group A) were treated for degenerative spondylolisthesis with porous cages and 84 patients (Group B) with conventional threaded Ti cages. At 2 years post-surgery, the intervertebral bone bridges (BB) were noticed in 83 segments (94.31 %) in Group A, while for Group B they were present in 87 segments (88.7 %). If one considers also the zygapophyseal joints ankylotic degeneration criterium (ZJ Pathria) [107] grade I (Group A: 2 segments (2.27 %); Group B: 4 segments (4.08 %)), grade II (Group A: 5 segments (5.68 %); Group B: 6 segments (6.12 %)), and grade III denoted as posterior fusion (Group A: 81 segments (92.04 %); Group B: 88 segments (89.79 %)) were noticed (Fig. 3.VI). After analyzing these findings, the authors [107] concluded that the porous Ti cages are adequate for spine diseases by facilitating bone ingrowth and bridging space and offering increased stability and successful bone fusion. In the case of spinal interbody fusion devices produced by NuVasive company, it seems that better fusion rates and improved implant osteointegration were obtained for Ti cages. However, one must also mention that Ti, due to an important difference between its elasticity modulus and native vertebrae Young's modulus, can induce an unwanted effect, called stress shielding, which can generate secondary fractures at the implantation site and hinder bone fusion. Regarding the PEEK, as was underlined above, the Halo effect could lead to reduced new bone formation and infection apparition. Therefore, none of these solutions are ideal, but the literature has already demonstrated their promising and effective application in treating spinal disorders.
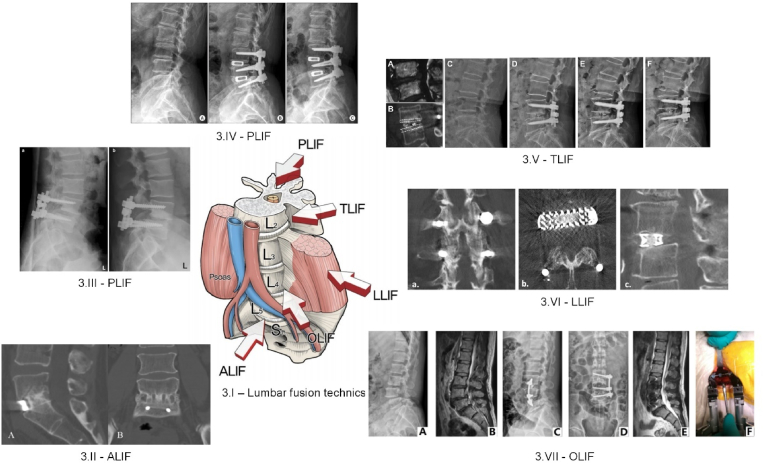
Fig. 3.
Medical images associated with different surgical interventions.(3.I) Main lumbar interbody fusion surgical approaches [48] (Figure is licensed under CC BY-NC 4.0); (3.II) CT scan image confirming a successful grade I fusion: (A) sagittal image, (B) coronal image based on ALIF insertion of DePuy Synthes Spine, PA, USA PEEK SynFix® stand-alone cage [122] (Reprinted from Ref. [122] Copyright (2025), with permission from Elsevier); (3.III) X-ray images of a young patient after a PLIF surgery using a Medtronic Sofamor Danek, Memphis, TN, USA PLDLLA Hydrosob™ taken (a) at 1 day post-surgery, and (b) after 1 year post-surgery [145] (Figure is licensed under CC BY-NC 2.0); (3.IV) Medical images of a patient with spinal stenosis taken before and after PLIF surgery based on Stryker, NJ, USA Titanium O.I.C® cage: (A) preoperative X-ray image evidencing a reduced disc height, (B) lateral view immediately after surgical procedure, (C) lateral view after 3 year post-surgery (Figure is licensed under CC BY 2.0) [140]; (3.V) Medical images of a patient with L4-L5 disc herniation that underwent TLIF surgery with Medtronic, Memphis, Tennessee, USA PEEK Capstone cage: (A) MRI image before surgery, (B) 1 year image evidenced solid fusion and cage subsidence, (C) preoperative upright medical image, (D) reconstruction after TLIF surgery, (E) 1 year image showing loss of disc height and segmental lordosis due to cage subsidence, (F) X-ray image at 33 months post-surgery [119] (Reprinted from Ref. [119] Copyright (2025), with permission from Elsevier); (3.VI) CT scan images presenting different views of a strong fusion (a) coronal view, (b) axial view, (c) sagittal view based on Nuvasive, San Diego, CA, USA Titanium XLIF Modulus cage [106] (Figure is licensed under CC BY 4.0); (3.VII) Medical images of a patient exhibiting grade I spondylolisthesis at L4-L5 level and disc herniation at L3-L4 level treated with Medtronic, Mineapolis, MN, USA Clydesdale Spinal System: (A) and (B) preoperative X-ray and MRI images, (C) anterior-posterior, (D) lateral X-ray and (E) sagittal MRI postoperative images, (F) intraoperative image [124] (Reprinted from Ref. [124] Copyright (2025), with permission from Elsevier).
CTL Amedica is a well-known company that produces Valeo® (Valeo® TL VBR Lumbar Spacer), a special type of implant made of a micro-composite ceramic based on silicon nitride (Si3N4) and endowed with a surface that has a similar structure to that of the cancellous bone. The spinal fusion cage includes a dense load-bearing component and a porous one in combination with an original surface texture. This is highly hydrophilic and is beneficial for protein and cell adhesion, being considered suitable for good implant osteointegration. The cage has a central cavity dedicated to autograft packing, bottom and top ridges to resist the implant migration process, threaded insertion characteristics, a tapered nose dedicated to the facile introduction, and windows, which offer good bone attachment, as well as good visibility of the implant during the surgical procedure [108]. Some literature studies reported successful clinical applications related to these silicon nitride implants. Youssef et al. [109] evaluated the outcomes of Amedica-TL fusion cages in the case of two patients. The first patient was a woman of 47 years old with lumbar spinal stenosis at L4-L5, while the second patient was a 77-year-old female with lumbar spinal stenosis L4-L5 and disc space collapse L4-L5. Both patients did not have osteoporosis. A TLIF procedure was applied. The 1-year follow-up was based on CT scans and dynamic X-rays and demonstrated solid bony fusion. The authors [109] concluded that this outcome was attributed to the material properties that promoted bone growth both on and through the cage. Calvert et al. [110] performed an original study based on 450 patients and 519 silicon nitride implants from four USA centers and compared their outcomes with those obtained in the case of other biomaterials for 1025 patients grouped in 26 cohorts worldwide. The analyzed surgical approaches were ALIF, TLIF, and PLIF, and the authors included two Si3N4 fusion cage generations from the Valeo® types. Different follow-up times between a minimum value of 11.4 ± 9.8 months and a maximum of 6.4 years were reported. In the case of ceramic implants, there was a higher improvement in the VAS pain scores with ΔVAS of 36.8 ± 35.4 points and good bony fusion. It was concluded that due to the fact that Si3N4 is a special material with increased osteoconductivity, good radiolucency, and bacteriostatic effect, it could be used successfully in lumbar fusion approaches, although they were associated with an increased number of complications that were linked to the fact that in this interesting retrospective study, there were also used old implant designs. McEntire et al. [111] made a study that considered the 2-year clinical and radiographic outcomes of a prospective analysis that compared the results obtained in the case of PEEK and Si3N4 fusion cages for treating lumbar degenerative disc illness. The authors hypothesized that Si3N4 cages are not inferior to PEEK implants. Two commercial models, Valeo® OL (CTL Amedica, Dallas, TX, USA) and Phantom™ PLIF, DePuy Synthes, were chosen for comparison. The study included patients aged between 18 and 75 years with chronic low back pain and disc degeneration or Pfirmann grade II or above, or degenerative spondylolisthesis grade I and II. The surgical procedure consisted of a TLIF approach performed at single- or double-level and involved, besides one of the above-mentioned fusion cages, also pedicle screws for implant fixation. Both cages were filled with autografts harvested from the lamina and facet joints. Of the 92 patients, 44 were treated with Si3N4 cages and 48 with PEEK implants. As a primary clinical outcome, the average improvement in Roland-Morris Disability Questionnaire (RMDQ) scores was chosen. It was noticed that the PEEK cohort exhibited lower scores, but with the help of a trendline analysis, no differences regarding the improvement from the pre-operative state at 24 months were reported. The secondary clinical outcomes were related to differences between ODI, back and leg VAS pain scores, and SF-36 Physical or Mental Function. The authors [111] observed that the non-inferiority of ceramic implants could not be established based on a priori protocol margin of 2.6. CT scans taken at the 12-month follow-up revealed that 57 % of Si3N4 cages and 42 % of PEEK implants had formed bone bridges between the patient endplates (p = 0.13). If revision criterium was considered, the study found that in the case of the ceramic cage cohort, 10 patients needed a revision intervention at about 20 months post-surgery due to a neurological necessity, pseudoarthrosis, cage non-union, or screw failure, while in the PEEK group, only 4 patients required a revision due to adjacent level and decompression complications. The main conclusion of this study was that no statistically significant differences regarding the ceramic implant outcomes and PEEK cages could be established. By adding a literature investigation and increasing the non-inferiority margin from 2.6 to 4, the authors confirmed that the Si3N4 cages are non-inferior to their polymeric counterparts. Based on existing literature, it can be concluded that ceramic implants demonstrate good fusion rates and can be confidently used to treat various spinal diseases. Additionally, the properties of Si3N4, compared to other biomaterials used in spinal surgery, have resulted in superior outcomes in some cases, which supports its recommendation. While many studies have reported clinical cases related to cervical fusion surgery, there remains a limited amount of information regarding the use of silicon nitride in lumbar applications. The authors of the present review believe that further studies are needed to establish a higher level of confidence in the use of ceramic implants over traditional PEEK or titanium alloys.
The German company SIGNUS Medizintechnik GmbH produces several fusion cages that have already received the FDA approval and the CE certification. One of the most used models is MOBIS®/MOBIS® II, with a classical geometry, a bullet nose, controlled insertion at different angles, and smooth lateral surfaces [112,113]. This implant is manufactured from Ti6Al4V according to the ASTM F136/ISO 5832-3 and from PEEK-OPTIMA® according to the ASTM F2026. Haeckel et al. [114] made a study in which they analyzed 82 patients that were treated for spinal disorders based on the TLIF approach using only MOBIS® II ST spinal implants filled with synthetic bone grafts in the case of 42 patients and for the rest of the cohort they applied a hybrid fixation with Dynamic Stabilization System (DSS), involving polyaxially pedicle screws at DSS at the adjacent level. The fusion rates were analyzed using CT scans at 12 months postoperatively. In the case of the hybrid group, based on medical images of 37 participants, interbody fusion was achieved for 31 patients (90 %), partial fusion for 14 % (5 patients), and non-union was noticed in 1 case (3 %). Regarding the other cohort, the CT scans were available for 35 patients. A satisfactory interbody fusion was achieved for 28 cases (80 %), partial union was present in the case of 3 participants (9 %), and non-union was observed for 4 people (11 %). The main conclusion of this study was that the minimally invasive TLIF procedure led to important improvements in patient outcomes regardless of whether or not the DSS could be included in the surgical approach. In another study by Duncan and Bailey [115], the fusion cage migration effect was analyzed for the PEEK MOBIS® model. The authors conducted a prospective randomized study on 102 patients who underwent the TLIF procedure. Taking into consideration the fixation technique, there were established two groups: the unilateral one (20 males and 26 females, average age of 53.5 years, DDD, degenerative spondylolisthesis) and the bilateral one (20 males and 36 females, average age of 55.7 years, DDD, spinal stenosis and/or foraminal stenosis associated with instability). The authors noticed the cage migration in the case of 17 patients (11 from the first group and 7 from the second one). Since the implant migration rate was higher in the case of the first analyzed group, it was established that unilateral fixation is not ideal for preventing entirely cage migration in the case of patients who underwent TLIF procedure. The same PEEK implant model was discussed by Brase et al. [116], showing that purulent spondylodiscitis was treated through dorsal decompression and debridement. After that, posterior stabilization was achieved using PEEK lumbar cages and trans-pedicular screws. The authors [116] concluded that PEEK cages are much safer than Ti alloy implants for use in patients undergoing lumbar revision surgery due to an existing infection. Other fusion cage designs produced by SIGNUS Medizintechnik used in clinical research are the VERTACONNECT [98], TETRIS [117], and POSIDON® [118]. Kitsopoulos et al. [98] presented some preliminary results regarding the VERTACONNECT expandable lordotic interbody cages made of Ti alloy. They analyzed 32 patients, exhibiting 40 surgical addressed segments. It was noticed that in the case of 2 patients, a revision surgery was applied to solve the screw loosening and resulting pseudoarthrosis. Another reported side effect consisted of patient endplate damage due to the cage placement observed in the case of 4 patients and cage subsidence was established for 4 patients. As an overall finding, it was concluded that important improvements regarding segmental lordosis and mean degree of spondylolisthesis were present at 2 years postoperative. Regarding the TETRIS cage, Sorge et al. [117] reported the case of a 66-year-old female with lumbar pain. Firstly, she suffered a PLIF surgical intervention at the L4-L5 level, which was solved by using the above-mentioned interbody cage. Unfortunately, this procedure was unsuccessful, and secondary fractures occurred. It was concluded that the treatment route must be carefully chosen in accordance with the patient's health state and bone quality. In some cases, the authors recommended the combination of vertebroplasty and internal fixation lengthening and said that this procedure should be applied as a last alternative. It can be concluded that the interbody fusion cages produced by SIGNUS Medizintechnik are safe enough to ensure a good interbody spinal fusion, and regarding the newest models, they should be carefully treated and analyzed to establish if some improvements are necessary to be performed to avoid revision surgeries.
Another important player in the interbody fusion cage market is Medtronic, based in Minneapolis, MN, USA. One of the most commonly used models in clinical cases is the PEEK bullet-tip design Capstone cage. It features an open volume geometry that allows for the introduction of graft material or synthetic bone, surface teeth to prevent implant expulsion, tantalum markers for X-ray imaging, a convex shape that conforms well to the anatomical structure, and it is available in various sizes to accommodate patient characteristics. Zhou et al. [119] analyzed the influence of vertebral endplate morphology on Capstone cage subsidence in patients who underwent a TLIF surgical approach at the L4-L5 segment. Three groups of patients were analyzed: concave group, flat group, and irregular group, based on their endplate shapes. The authors noticed that the unwanted side effects of cage subsidence occurred in 23 cases (15.9 %) from 145 patients after 1-year follow-up. It was concluded that a higher number of implant failures occurred in patients with irregular endplate geometry. Consequently, anatomical geometry plays a crucial role in ensuring the success of surgical intervention (Fig. 3.V). Mun et al. [120] compared the outcomes of the Perimeter PEEK cage with the ones of the PEEK Capstone cage, both manufactured by the Medtronic company, based on two different surgical approaches: OLIF and TLIF. 74 patients were selected for each type of implant and surgical approach. At 6 months, higher fusion rates of 81.1 % and 87.8 % were observed after OLIF and TLIF, respectively. A lower subsidence rate of 16.2 % was attributed to the OLIF intervention. In conclusion, the authors stated that the OLIF procedure was much more effective than the TLIF due to the better stability of the implant. Other literature studies compared this type of cage with those developed by other producers, and some of them are detailed in Table 1 [[121], [122], [123]] (Fig. 3.II). Clydesdale cage is another implant employed in many clinical treatments for spine diseases. It is made entirely of PEEK with a thin titanium layer with a thickness of 0.1 mm. This coating imparts the implant with an increase in friction coefficient and a larger surface area, which benefits new bone growth. Moreover, because the titanium coating is very thin, it does not alter the mechanical properties or radiolucency of the PEEK substrate. Some advantages of the Clydesdale design include the bullet-nosed tip and the convex geometry placed in the contact zone with the endplates. Liu and Feng [124] performed a preliminary clinical study with devoted attention to the advantages and disadvantages of the OLIF in addition to the use of an anterolateral screw-rod system. 14 patients included in this study suffering from spondylolisthesis, disk hernia, and degenerative lumbar stenosis were treated based on a Clydesdale Spinal System, Medtronic, MN, USA, which was filled with synthetic bone graft from Novabone, FL, USA. After a follow-up of about 45 months, a radiological fusion rate of 95 % was achieved (Fig. 3.VII). No important complications, such as infection or ureteral and vascular injuries, were reported. Unfortunately, a cage subsidence was found in the case of one patient. The authors concluded that the OLIF procedure, in combination with the anterolateral screw-rod fixation, led to good treatment results by reducing, at minimum, the blood loss, hospitalization time, and radiological exposure of the patient. Woods et al. [125] made a retrospective cohort study of 137 patients. For the treatment of different spine diseases, in all cases, a PEEK Clydesdale cage packed with rhBMP-2 (Medtronic, INFUSE) and demineralized bone matrix (MASTERGRAFT Medtronic) mixed with autologous bone marrow was used. At 6-month follow-up, complications such as cage subsidence (4.4 %), vascular injury (2.9 %), and postoperative ileus (2.9 %) were reported. A fusion rate of approximately 97.9 %, coupled with the absence of neurological injury, led to the study's main conclusion: the OLIF procedure and Medtronic cages were well-suited for treating complex spinal diseases and improved the patient's quality of life after 6 months. The Crescent cage is an interbody fusion implant with a distinctive banana-shaped design, bullet-nosed tip, 6° lordosis, and teeth for implant fixation. It is manufactured from Ti alloy medical grade or PEEK [126]. Choi et al. [127] investigated the outcomes and technical feasibility of the MIS-TLIF surgical approach based on a banana-shaped cage (Medtronic) or straight cage (Opal, DePuy Synthes Spine, Massachusetts, USA). They included 21 patients in their study, with an average age of 62 years and a mean follow-up period of 12–36 months. The straight cage was used in the case of 13 patients (61.9 %) and the banana-shaped one for 8 patients (38.1 %). A fusion rate of 86.7 % was reported, together with the observation that in many cases, the cage position was established to be anteromedial. In addition, the VAS scores for leg and back pain and ODI scores improved by a high amount after the surgery, although cage subsidence was noticed for 7 patients (33.3 %). It was concluded that the cages used are adequate for treating conditions such as spondylolisthesis or spinal stenosis based on the MIS-TLIF surgical approach. From the studies mentioned above, it can be concluded that Medtronic fusion cages can be introduced into the human body based on modern and minimally invasive techniques and are very suitable for treating different spine conditions. They are usually used in Asia and the USA and seldom in Europe.
The Cougar® LS lateral cage system was developed by the DePuy Synthes company. It is made of carbon fiber-reinforced PEEK Optima and is available on the market in two configurations: parallel and lordotic. This model can be manufactured in various sizes to match the different anatomical particularities of the patient. The radiopacity of the cage is ensured by tantalum wire markers. Rentenberger et al. [128] analyzed the perioperative risk factors associated with early revisions in the case of stand-alone lateral lumbar interbody fusion. A single- or multi-level LLIF procedure was applied to insert the XLIF system (NuVasive, Inc., San Diego, CA, USA) or COUGAR cage (DePuy Synthes, Raynham, MA, USA). The authors [128] selected 133 patients: 21 needed revision surgery, and 4 were recommended for it after only 1-year post-surgery. All these patients exhibited neurological symptoms, and no important difference was noticed between the revision and nonrevision groups. However, it was assumed that patients with foraminal stenosis may require revision surgery following the LLIF procedure, as this type of intervention could be associated with neurological pain or other complications. Another device commonly encountered in clinical practice is the Synfix-LR, a PEEK spacer implant attached to a Ti locking plate. Its height could be between 12 and 19 mm with a surface area of 26 × 32 mm2 or 30 × 38 mm2 and a lordosis angle equal to 8° or 12°. Stosch-Wiechert et al. [129] studied the incidence of revision surgery and adjacent segment degeneration in the case of 37 patients suffering from spondylolysis. The minimum follow-up was established at 60 months. 8 patients needed secondary surgery due to some complications that occurred at the cranially adjacent level. Regarding facet joint- or disc-degeneration occurred at the adjacent levels, a percentage of 35.1 % and 24.3 % were established. The main conclusion of this study was that preserving the health of the adjacent levels is of utmost importance in clinical practice, and the surgical technique and implant fusion cage must be carefully chosen to ensure optimal conditions for good and efficient bone fusion. Other studies [130,131] that investigated the Synfix-LR device are presented in Table 2. The fusion cages developed by DePuy Synthes showed good confidence in obtaining a satisfactory fusion rate, but some case reports analyzed their side effects and established a certain occurrence of cage subsidence appearance. It can be also concluded that they present an advantage over their counterparts on the market because they are available in different sizes and with various lordosis angles.
Table 2.
Permanent and bioresorbable interbody fusion cages used in current clinical practice.
| Interbody spinal cage commercial name, producers | Locations where the study was made and/or the patients were selected | Geometry/Material | Ref. | Number of patients/Follow-up time | Study design type | Surgical approach | Remarks |
|---|---|---|---|---|---|---|---|
| Permanent implants | |||||||
| FlareHawk®, Integrity Implants Inc./Accelus, Palm Beach Gardens, FL | USA | PEEK shell that expands bidirectionally/Ti shim | [102] | 58 patients for the investigation related to a 1-year intervertebral fusion process and 45 patients with Oswestry Disability Index (ODI), VAS for back pain, or VAS leg pain with an average follow-up of 4.5 months | Retrospective cohort (retrospective analysis) | PLIF or TLIF/1 or 2-levels between L2 and S1 based on autogenous or allogeneic grafts; patients had a supplementary posterior pedicle screw fixation | This investigation gives preliminary clinical evidence regarding the safety of FlareHawk® expandable devices. Both surgical procedures were adequate and led to significant improvements related to patient life quality, as well as good fusion rates |
| FlareHawk® 9, Integrity Implants Inc./Accelus, Palm Beach Gardens, FL | Greece | [104] | 58 patients/36 males and 22 females with diagnoses such as spinal canal stenosis (40), spondylolisthesis with slip percentage 25 % (5), failed back surgery syndrome revision surgery (5), adjacent segment disease (2), and recurrent herniated disc (7) | Retrospective cohort (retrospective analysis) | Open PLIF and decompression | The patients' ODI index improved in the case of 45 patients exhibiting a decrease from 48.6 preoperative to 23.1 postoperative remains almost unchanged for 8 patients (mean preoperative value of 49.7; mean postoperative value of 48.1, and in the case of 5 patients it worsened with an increased value from 50 to 54.2 (p value of 0.05) | |
| FlareHawk®, Integrity Implants Inc./Accelus, Palm Beach Gardens, FL | USA | [101] | 7 patients; 1-year follow-up | Case series (retrospective analysis) | PLIF/1 or 2-level PLIF constructs with 2 cages with fixation consisting of pedicle screw and rods | The cage highly conformed to the interbody space in the case of each patient. The interbody device deformation was not harmful to bone growth and new bone formation | |
| FlareHawk®, Integrity Implants Inc./Accelus, Palm Beach Gardens, FL | USA | [103] | 13 patients/symptomatic spondylolisthesis/7 cases at L5-S1, 1 case at L3-4, acasend 5 cases at L4-5/Grade 1–86 % cases, Grade 2–14 cases | Case series (retrospective review of patient records) | MIS TLIF | The FlareHawk® biplanar expandable cage is adequate for the spondylolisthesis based on MIS TLIF surgical approach. Important improvements in all radiographic parameters were postoperatively obtained | |
| Modulus ALIF – 3D printed porous titanium implant for anterior spine surgery/NuVasive CoRoent XL PEEK; NuVasive | Japan | 3DTi cages exhibited a bullet shape, while the sPEEK implants had spikes, serrations, and angular corners | [99] | 32 patients, 22 females and 10 males | Retrospective cohort | Anterior and posterior combined surgery with XLIF and posterior fusion (pedicle screw-rod system) | The local lordotic angles were preoperatively equal to 4.3° (3DTi) and 4.7° (sPEEK) and postoperatively these values were corrected to 12.3° (3DTi) and 9.1 (sPEEK) (p = 0.013). None of the patients treated with 3DTi cages exhibited VEC progression, but for the sPEEK cages, eight levels (21 %) were characterized by VEC progression. The 3DTi cage generated low endplate injuries compared to the other model |
| PEEK cage systems (XLIF – NuVasive, Inc., San Diego, CA, USA and COUGAR – DePuy Spine Inc., Raynham, MA, USA); 3D-printed Ti cages (Modulus XLIF (NuVasive, Inc.) and Lateral Spine Truss System (4WEB Medical, Inc., Frisco, TX, USA)) | Germany, USA | PEEK cages, 3DTi -printed cages with porous architecture | [105] | 91 patients, 49 patients included in the early group and 42 patients from the late analysis group | Retrospective cohort (retrospective study) | SA-LLIF from L1-L2 to L4-L5 | The porous 3D-printed Ti cages were characterized by an increased fusion rate compared to their PEEK counterparts due to a very good osteoconduction. PEEK was considered an inert material, which usually did not enhance the new bone formation |
| Titanium threaded or 3D-printed porous titanium (XLIF Modulus NuVasive, San Diego, CA, USA) | Italy, USA | Ti conventional and porous cages | [106] | 135 patients, 51 patients (Group A – porous Ti cage), 84 patients (Group B – conventional threaded Ti cage)/degenerative spondylolisthesis | Retrospective cohort (retrospective, single-center radiological study) | LLIF and posterior percutaneous screw fixation | The porous Ti cages are more adequate for spine diseases by facilitating the bone ingrowth and bridging space and offering an increased stability and a successful bone fusion if one considers the criteria of intervertebral BB and ZJ Pathria |
| Valeo I AL, Valeo II AL, Valeo I TL, Valeo II TL, Valeo I PL/OL, Valeo II PL/OL | USA/worldwide analysis | I and II generation of classical Si3N4 cages | [110] | 450 patients USA with Si3N4 cages compared with 1025 patients with other types of implants localized worldwide/variable follow-up time between 12 months and 6.4 years/all patients treated for spondylolisthesis, degenerative disc disease, disc herniation, spinal stenosis, spondylosis, spinal instability, radiculopathy, post-traumatic deformity, and infectious discitis | Retrospective cohort (retrospective review) | ALIF, TLIF, PLIF | The Si3N4 cages exhibited good outcomes compared with other biomaterial cages leading to a decrease in VAS score |
| Valeo® OL (CTL-Amedica, Dallas, TX, USA) and PEEK Phantom™ PLIF, DePuy Synthes | USA, worldwide | Si3N4 cage with 0° lordosis and PEEK implant with 6° lordosis | [111] | 92 patients, 44 were treated with Si3N4 cages and 48 with PEEK implants/follow-up 2 years/all patients treated for degenerative disc disease, isthmic spondylolisthesis, grade 1, isthmic spondylolisthesis, grade 2, degenerative spondylolisthesis, grade 1, degenerative spondylolisthesis, grade 2 | Randomized controlled trial | TLIF | It was confirmed that the Si3N4 cages are non-inferior to their polymeric counterparts. |
| Valeo®TL VBR Lumbar Spacer/Amedica Corporation, Salt Lake City, UT | USA | Silicon nitride (Si3N4) cage with a dense load-bearing component and a porous one in combination with an original surface texture | [109] | 2 patients (47- and 77-year-old women)/1year follow-up/47-year-old patient: TLIF for lumbar spinal stenosis at L4-L5 combined with axial low back pain and neurogenic claudication; 77-year-old patient: TLIF for lumbar spinal stenosis L4-L5 and disc space collapse L4-L5, with no reported neurological problems before surgery | Case report | TLIF | 47-year-old patient: anterior I grade fusion, posterior grade II fusion. 77-year-old patient: grade I fusion both posteriorly and anteriorly with bone bridging the disc space |
| MOBIS®II ST/SIGNUS Medizintechnik GmbH | Australia | A classical geometry that exhibits a bullet nose, controlled insertion at different angles, and smooth lateral surfaces/Ti6Al4V | [114] | 82 patients, average age of 62.1 years, 57 % female | Retrospective cohort (retrospective study) | 41 patients (50 %) underwent TLIF, and 41 people received a hybrid procedure consisting of TLIF and DSS | The minimally invasive TLIF procedure led to important improvement in patient outcomes regardless of the possibility to include in the surgical approach the DSS or not |
| MOBIS®/SIGNUS Medizintechnik GmbH | USA | PEEK | [115] | 102 patients, unilateral fixation technique (20 male and 26 female, average age of 53.5 years, degenerative disc disease, degenerative spondylolisthesis); bilateral fixation technique (20 male and 36 female, average age of 55.7 years, DDD, spinal stenosis and/or foraminal stenosis associated with instability) | Prospective randomized study | TLIF with unilateral and bilateral fixation technique | Due to the fact that the implant migration rate was higher in the case of the first analyzed group it was established that unilateral fixation is not ideal in preventing entirely the cage migration |
| MOBIS® I/SIGNUS Medizintechnik GmbH | Germany | PEEK | [113] | 39 patients (Group I – 8 patients – with fusion in every segment; Group II – 9 patients – with no fusion in one or both segments; Group III – 22 patients – with no fusion in one or both segments, exhibiting a stable pseudarthrosis) | Retrospective cohort (retrospective study) | TLIF | The study did not underline a notable difference between the fusion rates in L3-L4 or L4-L5. It was concluded that the fibrocartilaginous tissue formed in and out of the cage was enough to reduce the patient's pain |
| VERTACONNECT/SIGNUS Medizintechnik GmbH | Germany | Biconvex design with toothed surface and diagonal implant placement/Ti alloy [112] | [98] | 32 patients, 25 patients with monosegmental treatment, 6 were treated bisegmentally, 1 was treated trisegmentally | Retrospective cohort (prospective study) | TLIF, two-year follow-up | Important improvement regarding segmental lordosis and mean degree of spondylolisthesis were present at 2 years postoperative |
| MOBIS®/SIGNUS Medizintechnik GmbH | Germany | PEEK | [116] | 6 patients, average age 65.8 years, treated for lumbar purulent spondylodiscitis | Case series (retrospective study) | TLIF | PEEK cages are much safer than Ti alloy implants to be used in the case of patients with lumbar revision surgeries that occurred due to an existent infection |
| TETRIS/SIGNUS Medizintechnik GmbH | Germany | Toothed surface, adequate for MRI imaging, smoothed lateral surfaces/PEEK - OPTIMA | [117] | 1 patient | Case report | PLIF | The treatment route must be carefully chosen in accordance with the patient's state of health and bone quality. In some cases, it is recommended the combination of vertebroplasty and intern fixation lengthening, but this procedure should be applied as a last alternative |
| Capstone/Medtronic, Memphis, Tennessee, USA | China | Bullet-tip design with an open volume geometry, teeth on its surface to hinder the implant expulsion, tantalum markers, a convex shape that suits well to the place anatomy/PEEK | [119] | 145 patients (41 males and 104 females)/mean follow-up of 33.8 ± 12.3 months | Retrospective cohort (retrospective study) | TLIF and pedicle screw instrumentation via an open posterior approach | The vertebral endplate morphology has an important influence on cage subsidence |
| PEEK Capstone (Medtronic, Minneapolis, MN, USA) and PEEK Perimeter (Medtronic, Memphis, TN, USA) | South Korea | PEEK Capstone cage; PEEK Perimeter cage with a large and round shape, teeth placed on the superior and inferior implant surface, and a hollow geometry for autogenous bone graft | [120] | 74 patients underwent OLIF surgery (Perimeter cages) and 74 were treated with Capstone cages at L5-S1 level based on a TLIF approach | Retrospective cohort (retrospective single-center study) | TLIF and OLIF | The OLIF was considered a much more suitable procedure than TLIF because it produced a successful decompression of foraminal stenosis and generated a higher lordotic angle value. It was concluded that all these positive effects were due to the fact that the Perimeter cage exhibited important mechanical support due to its larger geometry |
| PEEK Capstone (Medtronic Sofamor Danek, Memphis, TN, USA) and PEEK PLIVIOS (Depuy Synthes, Raynham, MA, USA) | South Korea | PEEK bullet-shaped Capstone cage, rotation type PEEK PLIVOS cage | [121] | 784 patients exhibiting 881 lumbar levels/prospective observational longitudinal study/5-year analysis | Retrospective cohort (prospective observational longitudinal study) | TLIF | Cage migration without subsidence was noticed in 20 cases, while cage migration with subsidence was present for 37 patients. Cage retropulsion was observed in 17 patients. The main risk factors for the cage migration side effect were associated with osteoporosis, unilateral cage use, pear-shaped disc, and endplate injury |
| PEEK Synfix cage (DePuy Synthes, Welwyn Garden City, UK)- ALIF; PEEK Capstone Cage (Medtronic, Memphis, TN, USA) – TLIF; two dedicated PEEK cages from Medtronic, Memphis, TN, USA - PLIF |
South Korea | Synfix, standalone implant with a geometrical shape ensuring an increased stability/PEEK, PEEK classical Capstone cage | [122] | 77 patients were divided into 3 groups: 26 patients in the ALIF group, 21 patients in the TLIF group, and 30 patients in the PLIF group/1–2 years of follow-up | Retrospective cohort (comparative retrospective study) | ALIF, TLIF, PLIF | A better segmental lordosis restauration was achieved for the ALIF procedure. The fusion rate was not statistically different (69.2 % -ALIF, 72.7 %-TLIF, 64.3 % -PLIF). PLIF approach exhibited the lowest cage subsidence of 10 % compared to 15.4 % - ALIF and 38.1 %-TLIF (all the reported data are determined at 2 years postsurgical) |
| Capstone (Medtronic Sofamor Danek, Memphis, Tennessee, USA) and Opal (DePuy Synthes Spine, Raynham Massachusetts, USA) – minimally invasive MIS-TLIF; Clydesdale (Medtronic Sofamor Danek, Memphis, Tennessee, USA) OLIF | South Korea | For MIS-TLIF surgical approach there were used 0° cages, and in the case of OLIF group, for 20 patients there were used 6° cages and for 5 patients 12° cages/PEEK | [123] | 25 patients in each MIS-TLIF and OLIF groups | Retrospective cohort (comparative retrospective study) | MIS-TLIF, OLIF | OLIF surgical procedure was linked to a much more reduced hospitalization time, a lower blood quantity, as well as an excellent disc height restauration and earlier time to fusion |
| Clydesdale Spinal System, Medtronic, MN, USA | USA | PEEK Clydesdale cage packed with rhBMP-2 (Medtronic, INFUSE), demineralized bone matrix (MASTERGRAFT Medtronic), which was mixed with autologous bone marrow | [125] | 137 patients/6 months | Retrospective cohort (retrospective cohort study) | OLIF | Complications such as cage subsidence (4.4 %), vascular injury (2.9 %), and postoperative ileus (2.9 %) were reported. A good fusion rate of about 97.9 %, in combination with the absence of neurological injury, led to the main conclusion, which stated that the OLIF procedure and Medtronic cages were properly chosen to treat complicated spine disease |
| Crescent (Medtronic, MN, USA) and Opal, (DePuy Synthes Spine, Massachusetts, USA) | South Korea | The Crescent cage is an interbody fusion implant that has a distinctive banana shape design with a bullet nose, 6° lordosis and teeth for implant fixation. The Opal cage is made of PEEK | [127] | 21 patients/follow-up between 12 and 36 months. The straight cage was used in the case of 13 patients (61.9 %) and the banana-shaped one for 8 patients (38.1 %). | Retrospective cohort (retrospective study) | MIS-TLIF | A fusion rate of 86.7 % was reported together with the observation that, in many cases, the case position was established to be anteromedial. In addition, the VAS scores for leg and back pain and ODI scores improved by a high amount after the surgery, although cage subsidence was noticed for 7 patients (33.3 %) |
| Crescent (Medtronic, MN, USA) and Opal, (DePuy Synthes Spine, Massachusetts, USA) | South Korea | PEEK banana-shaped Crescent cage (length 25–36 mm, height 7–15 mm, 6° lordosis, maximum surface area of 180 mm2, PEEK straight cage Opal (length 28–32 mm, height 7–17 mm, no lordosis, maximum surface area of 175 mm2) | [126] | 84 patients/2 groups (44 patients – Crescent, 40 patients – Opal)/12 months follow-up | Prospective randomized clinical trial | MIS-TLIF | Crescent – solid fusion achieved in 95.2 %, Opal – solid fusion achieved in 96.6 %. Cage subsidence occurred more frequently in the case of the Crescent cage (p < 0.04). VAS and ODI scores decreased for both groups of patients |
| Clydesdale Spinal System, Medtronic, MN, USA | China | The PEEK cage has a thin Ti layer. It exhibits a bullet-nosed tip and convex geometry placed in the contact zone with the endplates | [124] | 14 patients with spondylolisthesis, disk hernia, and degenerative lumbar stenosis/12–45 months | Case series (preliminary clinical study) | OLIF | The OLIF procedure, in combination with the anterolateral screw-rod fixation, led to good results of treatment by reducing at minimum the blood loss, hospitalization time, and radiological exposure of the patient |
| XLIF system (NuVasive, Inc., San Diego, CA, USA) and COUGAR cage (DePuy Synthes, Raynham, MA, USA). | USA, Spain, Switzerland | Classical PEEK XLIF cages; COUGAR cages made of carbon fiber reinforced PEEK Optima and available on the market in two configurations: parallel and lordotic | [128] | 133 patients, of them 21 needed revision surgery, and 4 were recommended for it after only 1-year post-surgery | Retrospective cohort (retrospective observational study) | LLIF | In the case of patients with foraminal stenosis, it is probably possible to occur the necessity of the revision surgery in the case of LLIF procedure because this type of surgical intervention could be linked to neurological pain or other manifestations |
| SynFix-LR (DePuy Synthes, West Chester, PA, USA) | USA, Australia | PEEK SynFix-LR model | [130] | 137 patients: Group 1 (<49 years), Group 2 (50–63 years old), Group 3 (64 years old) | Retrospective cohort (retrospective analysis of patients who underwent surgery by a single primary surgeon) | ALIF | Cage subsidence occurred much more frequently in the case of patients older than 64 years |
| SynFix-LR (DePuy Synthes, West Chester, PA, USA) | Australia | PEEK SynFix-LR model | [131] | 147 patients | Retrospective cohort (prospective study) | ALIF | 15 patients (7 male) had cage subsidence, but bony fusion and clinical outcomes were not influenced in a high amount by subsidence |
| PEEK O.I.C. cages, Stryker, Kalamazoo, MI | South Korea | PEEK cage | [138] | 102 patients (139 segments)/average age of the patients (37–86 years) of 65.2 years/follow-up time of 4.1 years | Retrospective cohort (retrospective review of prospectively collected radiographic and clinical data) | PLIF with pedicle screw fixation | Cage subsidence is influenced by the bone mineral density of the patients. PLIF surgical approach was a good option for the treatment of osteoporotic patients suffering of lumbar degenerative diseases |
| PEEK cages from Stryker | China | Cuboid shape PEEK cage with arched geometry, with height × length × angle-width were 10mm × 25mm × 4°-11 mm, 11mm × 25mm × 4°-11 mm, 12mm × 25mm × 4°-11 mm |
[139] | 76 patients: contoured rod group (CR group, 35 persons), straight rod group (SR group, 41 persons)/more than 5 years follow-up | Retrospective cohort (retrospective review) | MIS-TLIF | Both patient groups exhibited good fusion rates. It was concluded that contoured rods exhibited a better fused segment angle |
| Dual-X LLIF, Amplify Surgical, Inc., Irvine, CA | USA | Ti6Al4V – ELI dual expanding implant in width and height introduced through a surgical corridor with reduced effects on nerve retraction and health tissue | [141] | 5 patients | Case series | MIS-LLIF and pedicle screw instrumentation | The MIS-LLIF method is unique, and it was adequate as a treatment of spinal problems in association with the new fusion cage expandable in two directions |
| Dual-X TLIF, Amplify Surgical, Inc., Irvine, CA | South Korea | [97] | 10 patients/6 months follow-up | Case series (retrospective analysis of prospectively collected data with description of surgical technique) | Biportal endoscopic TLIF surgery | Early radiographic and clinical results were favorable for this new innovative type of spinal cage | |
| Latis® Globus Medical, Audubon, PA | USA | Infuse® (Medtronic Inc., Minneapolis, MN) and Actifuse (Baxter International Inc., Deerfield, IL) was introduced into a lattice 10 × 32 × 10 mm 8° lordotic cage. The cage expands laterally and reduces the cage subsidence, permitting the existence of a large fusion bed and exhibiting a migration resistance due to implant geometry based on a slotted tooth pattern |
[142] | 1 patient (52 years old)/3 months follow-up | Case report | TLIF | It was demonstrated that an oblique insertion of a TLIF-designed cage was safe and led to a good fusion outcome |
| ROI-A® Oblique, Zimmer Biomet (former LDR Médical, Troyes, France) and PEEK cage Libeier; Orthopedic, Beijing, China | China | PEEK ROI-A® Oblique stand-alone fusion cage can be inserted from an anterolateral angle equal to 25° that ensures a better and reduced retraction of nerves and great vessels and bullet-shape PEEK Libeier cage | [144] | 82 patients/42 patients underwent MO-ALIF with self-anchored standalone cages/40 patients underwent TLIF | Retrospective cohort (retrospective comparative study) | Mini Open Anterior Lumbar interbody Fusion (MO-ALIF) with PEEK cage ROI-A® Oblique/TLIF with Libeier PEEK cage | MO-ALIF in combination with self-anchored stand-alone cages was considered an adequate treatment for lumbar disc herniation. Similar radiological and clinical outcomes compared to TLIF were reported for MO-ALIF. In addition, MO-ALIF is associated with reduced surgical trauma |
| China | [143] | 13 patients (ROI-A® Oblique)/27 patients (Libeier cage)/2-year follow-up | Case series (comparative study) | ALIF (ROI-A® Oblique)/PLIF (Libeier cage) | ALIF was linked to a reduced hospitalization time, a low blood loss quantity and mean operative time. No significant variations in the baseline data for PLIF and ALIF were found | ||
| Bioabsorbable implants | |||||||
| HYDROSORB (Medtronic Sofamor Danek, Memphis, TN) | USA | Cylindrical interbody fusion spacer made of PLDLLA (70:30 ratio of PLA to PDLA). Elastic modulus of 3.15 GPa, ultimate compressive strength of 100 MPa, ultimate tensile strength of 58 MPa, and ductility of 5 % elongation to failure [155] | [29] | 27 patients/mean follow-up of 26 months | Retrospective cohort (prospective clinical and radiographic review) | PLIF | PLIF, in combination with the Hydrosorb® implant, was a good choice for the patient's treatment, although some side effects, such as pseudoarthrosis apparition, were foreseen |
| USA | [146] | 31 patients/mean follow-up of 18.4 months | Retrospective cohort (retrospective study performed by one surgeon) | TLIF | The follow-up time was higher than the expected implant life period. It was concluded that the TLIF procedure was adequate in the analyzed cases, but mechanical failure of the implant was foreseen as a drawback | ||
| USA | [155] | 22 patients (17 men, 5 women) with 39 fusion level, average age 41.6 (23–70 years)/mean follow-up duration of about 12.4 months | Retrospective cohort | TLIF | Solid arthrodesis was observed in 38 levels from a total of 39 levels, with a disc space height that remained stable over time. In one case, it was reported a delayed union at L5-S1 due to a screw breakage | ||
| HYDROSORB Telamon (Medtronic Sofamor Danek, Memphis, TN), Infuse bone graft (Medtronic Sofamor Danek), rhBMP-2 | USA | [147] | 43 patients (19 female, 24 male), average age of 48.6 (17–68 years)/1-year follow-up | Retrospective cohort (prospective study) | TLIF | The bioresorbable implant exhibited no side effects, and at 1-year post-surgery, a complete bone bridging was established | |
| HYDROSORB Telamon (Medtronic Sofamor Danek, Memphis, TN) and Leopard CFRP (DePuy spine) | USA | Classical Hydrosorb design/Banana-shaped carbon fiber Leopard cage | [149] | 81 patients: 37 patients (Leopard CFRP) and 44 patients (Hydrosorb PLDLA) | Retrospective cohort (prospective study) | TLIF | The authors noticed an important high incidence of nonunion and cage migration for the PLDLLA cage (8/8 patients). It can be concluded that serious side effects were associated with the bioresorbable cages with a clear inferiority grade on their carbon fiber alternative |
| HYDROSORB Telamon and PEEK Telamon (Medtronic Sofamor Danek, Memphis, TN) | The Netherlands | Classical and similar PDLLA and PEEK designs | [145,150] | 14 patients (PEEK cage), 12 patients (PLDLLA cage)/2-year follow-up | Prospective randomized clinical trial | PLIF | PLDLLA bioresorbable cages were associated with reduced bone fusion and osteolysis. It is much safer to use permanent implants, such as those made of PEEK, due to better outcomes |
Modern and innovative solutions that have recently received FDA approval or CE certification were identified in the literature. These include bioactive coatings in their geometry or are based on highly biocompatible composite materials. A clear example comes from HAPPE Spine company [132], which developed the INTEGRATE®-C fusion cage that is made from a special biomaterial with rod-like hydroxyapatite (HA) particles strongly incorporated in the PEEK matrix. Some studies [[133], [134], [135], [136]] evidenced that the rod-like HA ceramic particles increase the implant capacity to promote new bone development, as well as exhibit stronger mechanical properties compared to the cases of polymers with embedded spherical HA particles. In addition, the area fraction of exposed ceramic particles on the machined surface is eight times higher than other similar implants, as stated in Ref. [136]. One can conclude that the special composite used in the INTEGRATE®-C fusion cage could significantly improve the quality of life for patients with spinal disorders. However, to assess the long-term outcomes of this implant, extensive research based on clinical cases must be conducted in the coming years. Some hope also comes from VySpine, which received FDA clearance for an innovative product, UniVy OsteoVy-Ti NanoVy-HA Cervical fusion device, in partnership with Promimic AB. Firstly, a classical Ti cage is 3D printed and then coated with a very thin HA layer of about 20 nm. This extremely thin layer is much more beneficial to osteoblast adhesion and proliferation than the classical 50 nm HA-coated implants. Since this achievement looks successful, it can be estimated that it will soon extend this solution to lumbar cage models. In addition, the lumbar cage LumiVy™ NanoVy™ made entirely of PEEK coated with a nano-layer of Ti offers the patient a better osteointegration process, hindering the so-called Halo-effect associated with uncoated PEEK use. The producers stated that NanoVy™ can also be manufactured from HA applied onto titanium or PEEK cages. Another promising product is the OSTEOVY™ material, consisting of a porous Ti, HA, or PEEK structure. In the case of HA ceramic, a compression strength (1–5 MPa) very close to that of the human bone was achieved with promising osteointegration and angiogenesis effects [137]. It is expected that this company will develop bioactive coatings or even biodegradable solutions for spinal surgery.
Table 2 includes additional lumbar fusion cages used in clinical studies, provided by manufacturers such as Stryker [[138], [139], [140]] (Fig. 3.IV), Amplify Surgical [97,141], Globus Medical [142], Zimmer Biomet (former LDR Medical) [143,144], or Libeier [143,144].
Fig. 3 presents examples of medical images obtained after different lumbar surgical interventions that include an interbody spinal fusion cage insertion step.
2.2. Bioabsorbable and biodegradable interbody fusion cages
Currently, there are only a few bioabsorbable interbody fusion cage models in clinical practice. For example, in 2000, a poly (l-lactide-co-D, l-lactide) (PLDLLA) cage, commercially known as Hydrosorb® (Medtronic Sofamor Danek, Memphis, TN), received FDA clearance and was used to treat approximately 100 patients in the USA. Later, in 2003, it was included in clinical studies in Europe following its CE certification and was marketed as Hydrosorb™ Telamon. Couture et al. [29] made a prospective study based on a cohort of 27 patients who underwent a PLIF surgical approach with a follow-up time of about 26 months. The authors also provided information regarding the cage material and stated that PLDLLA is a copolymer with a 70:30 ratio of PLA to PDLA, characterized by a degradation time between 18 and 36 months. In addition, no allergic or other inflammatory or systemic reactions were reported at the time of this study. This material was visible on CT scans and MRI, and its degradation time was considered suitable for obtaining an adequate bone fusion. Also, its elastic modulus had a much lower value than the Ti alloys and was estimated to have a reduced stress shielding effect. Based on medical images of the patients, it was noticed that a good bony fusion was obtained for 42 levels (95.5 %) from a total of 44 levels and a satisfactory fusion was achieved for 23 patients from 25 analyzed cases. Two patients needed revision surgeries to treat their induced pseudoarthrosis. It was concluded that PLIF, in combination with the Hydrosorb® implant, was a good choice for the patient's treatment, although some side effects were foreseen. Coe et al. [146] used the same type of spinal cage in the case of 31 patients but applied another type of surgical approach, TLIF. After a mean follow-up of about 18.4 months, it was concluded that solid bone fusion was obtained for about 30 patients (96.8 %), and good bone fusion was documented for 25 patients (81 %). Complications were reported for 3 patients with no direct relationship with polymeric material. However, in the case of one patient, implant mechanical failure was reported, highlighting that polymeric implants may not be suitable for all patients, particularly those with active lifestyles who engage in frequent upper body movements. A combined treatment including a Hydrosorb® Telamon (Medtronic Sofamor Danek) bioresorbable cage and infused bone graft (Medtronic Sofamor Danek) and rhBMP-2 was presented by Lanman and Hopkins [147]. 43 patients (57 levels) were treated with lumbar fusion for discogenic pain. After a follow-up of 6 months, the medical images obtained based on X-rays and CT scans revealed good bone fusion in 41 patients with an improved ODI score. The main conclusion of this study was that the bioresorbable implant exhibited no side effects, and at 1-year post-surgery, complete bone bridging has been achieved.
Taking into consideration the clinical success of Hydrosorb® fusion cages, one can expect that bioresorbable implants will represent the future. Unfortunately, some studies compared the efficacity of PLDLLA cages with those made of carbon fiber or PEEK and concluded that a high incidence of nonunion and complication were obtained for the first type [148]. For example, the study of Smith et al. [149] investigated the cage migration and nonunion side effects when bioabsorbable cages are used. The performance of Hydrosorb® (Medtronic Sofamor Danek) and Leopard CFRP (DePuy spine) were compared. The authors noticed a high incidence of nonunion (8 patients) in the case of Hydrosorb® cages compared to the cohort treated with carbon fiber implants (zero patients with nonunion). Cage migration was reported only for the PLDLLA cage (8 patients). It can be concluded that serious side effects were associated with the bioresorbable cages, demonstrating clear inferiority compared with their counterparts. Two investigations performed by Jiya et al. [145,150] analyzed the efficiency of PLIF surgical interventions, which were based on Telamon Hydrosorb® PLDLLA and Telamon PEEK cages from Medtronic Sofamor Danek, Memphis, TN. The findings of these studies underlined that PLDLLA bioresorbable cages were associated with reduced bone fusion and osteolysis (Fig. 3.III). The authors suggested that the use of bioresorbable cages remained questionable due to the reported serious side effects. They suggested that it is much safer to use permanent implants, such as those made of PEEK, due to better outcomes. Osteolysis formation was also reported in the study of Frost et al. [151], who treated 3 male and 6 female patients for degenerative spondylolisthesis and degenerative disc disease. All the patients did not take bisphosphonate-based medication and had bone-destructive illnesses. In all cases, a classical bioresorbable Telamon Hydrosorb® cage was used. After 1-year follow-up, osteolysis presence around the fusion cage was noticed in the CT scans for 4 patients. One patient needed a second surgical intervention for implant removal, and histological analyses revealed that the polymeric material generated bone loss.
Considering all the existing data in the literature, it can be concluded that the bioresorbable polymeric cages currently available on the market are unsuitable for treating spinal diseases compared to classical Ti-alloy, or PEEK alternatives. As stated in the previous subsection, these solutions have their merits but lack bioactive properties, as they are made from inert materials. However, the application of bioactive and osteoinductive coatings can enhance their biological performance. Table 2 includes some clinical investigations related to Hydrosorb® bioresorbable cages.
In 2024, Bioretec Ltd. received FDA breakthrough device designation status for the RemeOS™ spinal interbody cage dedicated to establishing normal vertebra height and facilitating spinal fusion in the cervical region [152]. The device is made of a hybrid composite that is based on the RemeOs™ (Mg – Balance, Ca 0.5 ÷ 0.7 wt%, Zn 0.4 ÷ 0.7 wt%) alloy (patent: US11969519B1) in combination with a bioresorbable polymer matrix. This composite has a special structure consisting of an inner RemeOS™ Mg alloy core embedded in a polymeric matrix of PLDLA 96/4 reinforced with bioresorbable glass fiber. One can immediately notice that this proprietary composite consists of a coating for Mg alloy that acts as an adhesive layer to the bioresorbable glass fiber-reinforced polymer matrix and/or behaves as a further layer, which reduces the hydrogen gas emission. The producers of this material stated that this complex coating could be considered a hydrogen trap, hindering the hydrogen release from the Mg alloy, and/or maintaining corrosion by-products on the Mg-Ca-Zn alloy surface and generating a corrosion-inhibiting layer [153]. In addition, preliminary studies have shown that Mg-Ca-Zn alloys have a high potential for promoting ossification at the site of a bone defect in approximately 6 weeks [154]. This solution is anticipated to be successful, and we hope it will be extended to the lumbar cage domain.
3. Biomaterials used, mechanical and biological matching
Today, it is still considered a challenge to find a perfect biomaterial whose mechanical properties and biological characteristics suit entirely the body part where the implant will be inserted. As stated in Ref. [16], an adequate bone substitute material must be compatible with the living tissues from a biological point of view and exhibit a similar value of Young's modulus to that of the human bone. Regarding other mechanical quantities, high stiffness, increased tensile strength, and high fatigue strength should be considered. In addition, low artifacts on medical images are a requirement.
3.1. Titanium and its alloys
As presented in Section 2, one of the most used materials for lumbar interbody cages is titanium and its alloy Ti6Al4V. The main advantages of this material are the high corrosion resistance and fracture toughness, low mass density, compatibility with magnetic resonance imaging, as well as good biocompatibility compared with other inert metals such as Co-based alloys or stainless steel. As important drawbacks, one should underline the increased elasticity modulus value of the metal (100–110 GPa), which can generate a stress shielding effect, and also the fact that obtaining a good osteointegration of the implant is difficult due to its inert character.
The mechanical attributes of Ti alloy cages were underlined in some publications. Fantigrossi et al. [156] performed a biomechanical analysis for PLIF surgery by reconstructing the post-operative medium based on computational analyses. The following hollow threaded titanium cages were considered: BAK™ (Zimmer Centerpulse, Warsaw, IN, USA), Interfix™, and Interfix Fly™ (Medtronic Sofamor Danek, Memphis, TN, USA). The numerical analysis was based on different loading conditions by assuming the sagittal plane as a symmetrical surface, and only one-half of the entire structure was analyzed. The authors modeled the cortical bone by considering Young's modulus (E) of 12 GPa and a 0.3 Poisson's ratio (ν). Trabecular vertebra bone was assumed to be anisotropic linear elastic, with posterior parts being homogenous and isotropic (elasticity modulus of 3.5 GPa and Poisson's ratio of 0.25). A model was developed for each type of implant, preloaded in axial compression with a force of 250 N, and subsequently subjected to independent loading conditions: extension (2 Nm), flexion (2 Nm), and anterior shear (100 N). The main conclusion of this study was based on a Finite Element Analysis (FEA) of the stress distribution at the interface between cage and bone. It was found that the Interfix Fly™ led to the most reduced stress value with a uniform distribution on the cancellous surface. This fact was linked to the possibility of preserving the native endplate, which is considered a success factor in generating a strong bony fusion. Lai et al. [157] proposed a 3D-printed porous Ti cage model for OLIF surgical intervention dedicated to osteoporotic patients. They performed two types of analyses, FEA and static/dynamic compression and compressive-shear test, which were carried out according to the ASTM F2077-14 standard. The FEA simulations were performed considering the following conditions: cage was implanted (i) along the L3-L4 disc (CA), (ii) in the L3-L4 region including two screws (diameter of 5.5 mm, length of 40 mm) (CES), (iii) in the L3-L4 zone using two screws and a lateral fixation plate (CLS). Ti6Al4V was assessed for all the implants' materials. After the FEA was made, von Misses stress distribution was analyzed in different testing conditions for CA, CES, and CLS models. It was noticed that the lowest values were obtained for the CES model (flexion – 43.94 MPa, extension – 13.94 MPa, lateral bending – 12.75 MPa, rotation – 15.82 MPa) compared to the other two geometries. Although the simulations yielded good results, the numerical measures revealed a stiffness value of 19,643 N/mm, about 9 times higher than FDA recommendations (2219 N/mm). In addition, the shear fatigue test evidenced a possibility of anterior edge damage during the dynamic fatigue compression-shear analysis exceeding 3500 N. An endurance limit of 2600 N was obtained. It can be concluded that this value is two times higher than that requested by the FDA (1225 N). The main conclusion of this study was that when a new model is developed, it must entirely respect the FDA requirements regarding the mechanical properties. Chen et al. [158] designed 3 bullet-type cages. The first one had a window, the second one exhibited windowless geometry, and the third one was a fenestrated type. All the cages were 3D printed based on Selective Laser Melting (SLM) using Ti6Al4V ELI powder. A fatigue test was performed, and it was noticed that no wear signs or fractures occurred in the case of the first model. Regarding the second geometry, one side of the solid piece was fractured, significantly influencing the deterioration of the porous structure, with important large defects. The third model exhibited shear failure at the middle hole edge and small debris. If one considers the number of cycles criteria in the dynamic fatigue test, it can be noticed that for the 1st and 3rd models, 5 million cycles were reached, while for the 2nd model, only 1.06 million cycles were possible. The main conclusion of this study was that the maximum load of the natural vertebral body is higher than that obtained in the study, and the 3D printed cages meet entirely the dynamic and static requirements. Pan et al. [159], based on the SLM process, made an interbody fusion cage from Ti6Al4V-ELI material with gradient porosity. It was designed as a hollow cylinder with variable porosity measured from center to top and bottom sides of 80-70-60-70-80 % and from outside to inside of 60-70-80 %. The human vertebra model was reconstructed based on InVesalius and Geomagic Studio 12 and analyzed in ANSYS Workbench 16 to establish the stress-strain states during the vertical position of the patient in extension, flexion, rotation, and lateral bending. A fatigue test based on ASTM F2077-17 was applied, and a mechanical simulation was performed to compare the developed model with those that already exist on the market from the following companies: Zimmer (ROI-A ALIF model, Zimmer Biomet Holdings. Inc., Warsaw. IN, USA), B. Braun (Arcadius XP, L model, B. Braun, Germany), and Ulrich (TEZO model, Ulrich, Germany). The developed porous structure was tested based on compression measurements with a strain rate of 1 × 10−4 1/s with an Instron 5582 (Instron Inc., Norwood, MA, USA) universal testing machine with a 100 kN load cell. In addition, a SHIMADZU HMV-2 (Shimadzu Corporation, Kyoto, Japan) was used to measure the implant Vickers hardness by applying a force of 4.903 N for 15 s. Regarding the fatigue test, an axial force-cycle of 25 % of the yield stress (2020 N) to 10 % of the maximum load (200 N) at 5 Hz frequency was applied approximately 1.5 million times. The fatigue characterization was performed with a Bose 3510. In the case of the numerical simulations, the IVD between L2 and L3 was removed and replaced by the cage. The bottom area of L4 was fixed, and a compression load of 280 N in correlation with a momentum of 7.5 Nm simulates different natural movements of the spine. The Vickers Hardness (HV) exhibited a maximum value of 400. After compression analysis, a Young's modulus of 13 GPa and a yield strength of 129 MPa were found. In the case of a 67 % porous sample, an elasticity modulus of 15 GPa and a yield stress of 129 MPa were experimentally determined, and it was concluded that these values are much closer to those of the human bone compared with non-porous Ti alloys. Regarding the fatigue test, all the specimens did not show signs of fracture or important and deep cracks. The numerical simulation showed that the developed implant with gradient porosity exhibited strain and stress values comparable with those obtained in the case of the native vertebra. In addition, by comparing the cage performance with the commercial models, it was concluded that the FDA-approved cages are characterized by lower strain and higher stress, which leads to stress concentration with an unfavorable outcome. Although many more analyses are necessary, it was concluded that the porous Ti alloy implant can potentially be used as a long-term cage. From the literature presented in this paragraph and detailed in Table S1 [[160], [161], [162]], it can be observed that Ti alloy-based interbody fusion cages must be porous in order to decrease their Young's modulus and adapt their mechanical properties to those of the human bone. Additionally, when pore sizes fall within certain regulated limits, they facilitate a strong connection between new bone and implant.
Despite the fact that Ti alloys are inert materials, they exhibit excellent biological matching with hard tissue if some conditions or bioactive factors are met [163]. For example, Arts et al. [164] compared the biological outcomes of a 3D-printed porous titanium cage with those of a commercial PEEK combined with (auto)graft in treating degenerative cervical radiculopathy. The fusion rates were measured at 3, 6, and 12 months for 49 patients (48 with commercial PEEK cages). It was noticed that VAS neck improved for all the patients, and 77.1 % of patients completely recovered after 12 months. Range Of Motion (ROM) decreased from 8.7° before surgery to 1.6° after the intervention, and fusion rates of 84 %, 89 %, and 91 % determined based on dynamic radiographs taken at the above-mentioned time intervals were reported for Ti cage. These results were very similar to those obtained in the case of PEEK implants combined with bone grafts (67 % - 3 months, 72 % - 6 months, 90 % - 12 months). One can conclude from this study that the 3D porous Ti cage exhibits important clinical improvements, and the fusion rate achieved for it is even better than that obtained for the PEEK implant. The faster consolidation showed the high biological matching of Ti alloys. Krafft et al. [165] proved that the subsidence rate obtained for the well-known 3D-printed titanium cage (Modulus, NuVasive, San Diego, CA) was lower than that determined in the case of patients who underwent LLIF surgical procedure with PEEK implants. Also, the study [165] evidenced the superior behavior of Ti alloy versus its counterparts in obtaining a good osteointegration process. The study developed by Mokawem et al. [166] demonstrated the undoubtedly bioactive character of a 3D-printed lamellar Ti cage K2M, (Leesburg, VA, USA) filled with SiCaP bone graft (Inductigraft Prep Syringe; Baxter Healthcare, Deerfield, IL, USA). The authors analyzed 93 adults and two types of surgical interventions: TLIF and LLIF. CT scans revealed, after 12 months, a solid fusion for 92 patients and important patient outcomes such as VAS score improvements. It was concluded that this bioactive implant was adequate and led to positive results in combination with both surgical approaches. Mobbs et al. [167] demonstrated that by modifying the implant's surface roughness, bone integration is facilitated, and better stability is offered to the implant (Fig. 4.I and Fig. 4.II). Cheng et al. [168] analyzed the properties of Ti coatings on PEEK and noticed that its negative valence charge can be beneficial to the proteins and osteoblast adhesion and proliferation, as well as to enhance the body fluid circulation.
Fig. 4.
Mechanical and biological investigations obtained for: Ti-alloy spinal cages – (4.I) Von Mises stress map obtained in accordance with ASTM F2267-04 standard for a 500 N axial compression load: (A)÷(C) generic device, (D)÷(F) patient anatomy adapted-device; (4.II) Intraoperative images taken for a 34-year old man: (A) discectomy, (B) implant preparation with allograft material, (C) implant insertion, (D) X-ray scan [167] (Reprinted from Ref. [167] Copyright (2025), with permission from Elsevier); PEEK and PEEK – Ti coated cages – (4.III) Average dorso-ventral stiffness at two different frequencies, (4.IV) interoperative images for merino sheep lumbar fusion model: (A) PEEK-Ti and (B) PEEK cages with autologous bone graft, (C) discectomy, (D) surgical placement, (E) cage implantation [175] (Reprinted from Ref. [175] Copyright (2025), with permission from Elsevier); Si3N4cages – (4.V) Comparison of mean subsidence as a function of loading cycles in the case of (A) low density and (B) high density foam substrate for Si3N4, PEEK, and Ti4Al4V cages [184] (Figure is licensed under CC BY-NC-ND 4.0), (4.VI) Histological sagittal analysis of the inside and outside of PEEK (a) ÷ (h) and Si3N4 (i) ÷ (p) cages in the case of caprine lumbar fusion model [185] (Reprinted from Ref. [185] Copyright (2025), with permission from John Wiley and Sons); Uncalcined hydroxyapatite/poly L-Lactide (F-u-HA/PLLA) cage – (4.VII) ROM of different surgical sheep groups: (A) flexion-extension, (B) lateral bending (loading ± 6 Nm) (Intact – no implant, AIB – corticocancellous grafts, carbon fiber cage (CFC) – Brandigan I/F cage, DePuy Acromed. Inc., Raynaham, MA, USA, F-u-HA/PLAA – high osteogenic cage); (4.VIII) Histologic interface between high osteogenic (A), and CFC (B) cages, and natural bone [231] (Reprinted from Ref. [231] Copyright (2025), with permission from Elsevier).
It can be concluded that Ti alloys are adequate materials for spinal cage manufacture, and their main biological drawback can be easily solved by using bioactive bone grafts to improve the osteointegration and osteoconduction, as well as bioactive coatings as some companies already have developed (Section 2). Alternatively, innovative alloys with novel chemical compositions incorporating new elements should be continually developed and explored. This is not an easy task because corrosion processes that usually occur between Ti or other metals must be avoided in order not to increase the medical side effects present at the implantation site.
3.2. Polyether ether ketone
Another material frequently used in lumbar fusion cage design is the polymer known as PEEK. This is a high-molecular-weight semi-crystalline linear polymer, which has a thermoplastic behavior. It exhibits a low value of mass density, a reduced corrosion rate, bone-like hardness, and radiation transmission, and it is biologically inert [169]. Since it exhibits Young's modulus (3.5 GPa) with a value very close to that of the human bone, the stress shielding phenomenon is prohibited, and the implant loosening phenomenon occurs very seldom. PEEK is radiolucent and can be highly compatible with medical image devices. Interbody PEEK fusion cages exhibit very low subsidence rates; however, this material has significant drawbacks, including its inability to support implant osteointegration and its susceptibility to colonization by multi-species biofilms [162]. Some studies have underlined the mechanical properties of commercial and laboratory-developed PEEK cages. Kiapour et al. [170] performed a biomechanical analysis of a carbon fiber-reinforced (CFR) PEEK cage produced by DePuy Spine based on FEA simulations and an experimental cadaveric study. For numerical investigations to simulate the human spine physiological loads, the authors applied a compressive load of about 400 N and a mechanical moment equal to 8 Nm. The finite element model was generated based on the CT scans of a 55-year-old non-osteoporotic woman and included the spine, intact vertebras, IVDs, ligaments, and posterior facet joints. Simulations were conducted using different types of CFR PEEK cages, fixed according to LIF, TLIF, and ALIF procedures, as well as stand-alone implants and a 360° design with posterior screw-rod fixation. The implants were positioned at the L4-L5 level. The simulations provide the in vitro ROM values for the intact model, such as 3.2° (extension), 5.2° (flexion), 5.0° (left and right lateral bending), and 3.4° (left and right axial rotation). The simulation results were compared with the experimental ones performed on cadaveric frozen L1-S1 spine segments that came from dead patients with age between 50 and 80 years old. Different mechanical moment values (0, 1.5, 3, 4.5, 6, and 8 Nm) were applied, and ROM values for the intact segment were measured (3.1° ± 0.9°, 7.1° ± 2.8°, (5.0° ± 1.7° and 5.0° ± 2.1°), (2.6° ± 1.8° and 2.4° ± 1.7°)). In addition, supplementary analyses were performed, and the following ROM values were obtained for the LIF cage: 1.7° ± 1.3°, 1.8° ± 1.0°, (1.7° ± 1.1° and 1.7° ± 1.0°), (0.9° ± 0.5° and 1.3° ± 0.9°). In the case of LIF 360° design cage ROM values were 0.8° ± 0.5°, 0.8° ± 0.7°, (0.9° ± 0.5° and 0.8° ± 0.4°), (0.5° ± 0.4° and 0.6° ± 0.3°). Some differences between the experimental and numerical results were noticed, and it was concluded that FEA simulations cannot fully replicate the actual movements of the spine but provide specialists with a valuable starting point for analysis. Yan et al. [171] made an FEA analysis and identified the maximum values of von Misses stress in the case of 3 types of lumbar spinal cages. They developed a triple periodic minimal surface (TPMS) dedicated to a porous PEEK cage and two bullet-type structures made of non-porous PEEK and Ti6Al4V. The loading conditions were similar to those applied in Ref. [170], except for the moment value, which was equal to 7.5 Nm. The maximum values of the von Misses stresses for the TPMS porous PEEK cage were 38 MPa (flexion), 10 MPa (extension), 23 MPa (left lateral bending), 19 MPa (right lateral bending), 20 MPa (left axial rotation), and 27 MPa (right axial rotation), while for the non-porous PEEK cage these values were equal to 37 MPa, 15 MPa, (25 MPa and 23 MPa), (29 MPa and 31 MPa), respectively. It was concluded that the porous topology exhibited better biomechanical behavior than the non-porous models and can be considered ideal for spinal disease treatment, but additional experimental analyses are needed. Fogel et al. [172] analyzed the mechanical properties of three different commercial cages from NuVasive (i.e., solid PEEK cage (CoRoent PEEK), roughened solid Ti (CoRoent Ti), and 3D-printed porous Ti) based on standardized methods to investigate the design influence on cage subsidence. The authors have chosen two static and one dynamic testing methods. The first analysis was performed according to ASTM F2077 standard and consisted of the cages' introduction between 2 stainless steel blocks. The system was compressed at 2 mm/min speed, and the implant stiffness was determined (Table S1). The CoRoent PEEK cage exhibited the lowest value of stiffness (37.1 kN/mm) since CoRoent Ti had the highest value of about 57.1 kN/mm. The second mechanical experiment was conducted in accordance with ASTM F2267. The cages were placed between 2 polyurethane foam blocks and then quasi-statically compressed at 6 mm/min. Also, the lowest value of block stiffness was attained for a solid PEEK cage (1750 N/mm). The dynamic subsidence test was made under a sinusoidal axial compression of a frequency of 4 Hz at a maximum force of 1550 N for a number of 216000 cycles. The lowest subsidence displacement was established for the porous Ti cage (0.195 mm), followed by the PEEK cage (0.328 mm). The main conclusion of that study was that material and geometry are of the utmost importance in establishing adequate mechanical properties and biological performance. All the cages investigated exhibited subsidence limits that were better than those imposed by the FDA [173]. Finally, it was concluded that the best mechanical attributes were associated with a 3D-printed porous Ti cage. In addition, it was estimated that this model could be suitable for faster implant osteointegration if bioactive factors are added.
As previously noted, PEEK material has a low Young's modulus, which significantly reduces the bone resorption process near the implant by minimizing the stress shielding effect. McGilvray et al. [163] showed the osteointegration capabilities of PEEK and 3D-printed porous Ti cages. These implants were inserted into 27 skeletally mature ovine at L2-L3 and L4-L5 levels. In all the cases, micro-computed tomography was used for bony fusion estimation. It was observed that the quantity of new bone was much more reduced than in the Ti cage vicinity. In addition, the histopathology results revealed that the PEEK cage was linked to a high amount of fibrous tissues with low vascularization in combination with an inflammatory response near the implant. Also, the “PEEK – halo” effect exhibited an important degree in this study. It was concluded that despite the adequate mechanical properties of the PEEK compared to its porous Ti counterparts, important disadvantages are present, and new solutions to increase the PEEK bioactivity should be searched. The biofilm formation on the PEEK surface was reported in some other studies [174,175]. In the first analysis [174], there were investigated PEEK cages (FORZA PEEK Spacer System; Orthofix, Inc., Lewisville, TX, USA) that were implanted between L2 and L3 vertebras of a sheep animal model. Exactly as in the previous investigation [163], new bone formation was seldom observed, being evidenced by the fact that biofilm appearance was confirmed by the laboratory analyses. The second investigation performed by Gunzburg et al. [175] analyzed the in vivo biomechanical properties of PEEK standard and PEEK nanosurfaced porous Ti interbody cages from Nanovis, Inc., Columbia City, IN. A higher stiffness was measured at 1-year post-implantation in an ovine model for the nanocoated PEEK cages (2 Hz-13.68 N/mm and 6 Hz-11.94 N/mm) compared to standard PEEK cages (2 Hz–13.24 N/mm and 6 Hz–10.82 N/mm). It was concluded that the coated cages exhibited better biomechanical behavior, and an increased osteointegration capability was foreseen (Fig. 4.III and Fig. 4.IV). The local inflammation phenomenon was reported also by Cheng et al. [168]. The authors [168] compared the osteointegrative potential of three PEEK cages: solid, Ti-coated, and 3D-printed. It was found that the 3D-printed porous PEEK implants exhibited superior bone ingrowth and reduced radiographic interference. The first observed property was attributed to the possibility of integrating artificial or natural grafts or bone-specific proteins into the implant. Although a high potential for porous PEEK cages in orthopedics was evident, inflammatory markers at the implantation site were determined based on histopathology analyses. To complete the observations of the previous study, Jain et al. [176] demonstrated that the bony fusion power rate of PEEK cages is similar to that obtained in the case of allogeneic bone grafts. Over time, solutions to improve the osteogenic character of PEEK have been continuously explored. Chu et al. [177] prepared a calcium silicate (CS)/polyetheretherketone (PEEK) composite material based on the injection molding method. The authors determined the main mechanical properties of the new composite and stated that a proper ratio for CS was 40 wt%. To analyze the osteogenic possibilities, implants prepared from this compound were investigated in in vivo conditions on a goat animal model. The histological assessment proved the mineralized bone formation around the implant, which sustained its rapid osteointegration. It was concluded that the new material can be successfully used in lumbar interbody fusion cage manufacture. An interesting analysis was performed by Dai and Jiang [178], who demonstrated that when β-tricalcium phosphate was inserted into a classical PEEK cage, better adhesion of the osteoblasts to implant occurred, and new bone formation was reported to appear faster and better. This idea of combining calcium phosphate with PEEK was earlier studied by Walsh et al. [179], who rhetorically asked if a combination of PEEK and hydroxyapatite could be directly linked to an enhanced bone formation in a sheep fusion model compared to standard PEEK. Undoubtedly, the addition of bioactive materials significantly enhanced bone fusion and overall implant osteointegration. The authors concluded that combining a polymer with calcium phosphate could offer a superior approach to spinal fusion treatment compared to 3D-porous Ti cages, as the latter lacks bioactive properties.
In conclusion, PEEK fusion cages have been on the market and have proven to be a viable alternative to Ti cages, although significant drawbacks may limit their use. However, incorporating bioactive materials to enhance their osteointegrative potential is always preferred.
3.3. Silicon nitride
Section 2 underlined the use of silicon nitride for manufacturing high-quality lumbar fusion cages. This material is a synthetic ceramic composite with a very interesting microstructure in which amorphous grain-boundary phases and crystalline grains are present [180]. In addition, it is perfectly compatible with MRI and CT scans because it does not have magnetic properties, and it is partially radiolucent. Regarding its mechanical properties, a study [181] demonstrated that the compressive strength of Si3N4 ceramic is higher than the values of metallic materials that were already used in clinical practice (i.e., Si3N4 – 4000 MPa, Co-Cr – 600 ÷ 1800 MPa, Ti6Al4V – 800 ÷ 970 MPa). Moreover, Si3N4 had a reduced friction coefficient, high wear resistance, flexural strength between 800 ÷ 1100 MPa, and Young's modulus in the range of 296 ÷ 313 GPa [181]. Another mechanical property considered of utmost importance in the case of ceramic materials is fracture toughness, which for Si3N4 was determined to vary between 5 ÷ 7 MPa/m2 as a function of the applied processing methods [182,183]. There are just a few literature studies that focus on the mechanical properties of lumbar interbody fusion cages made of Si3N4. Suh et al. [184] conducted an in vitro analysis to examine the influence of various factors on cage subsidence, including the material's chemical composition, morphology, and substrate density (Fig. 4.V). The authors have chosen open-core design commercially available cervical cages (Valeo C; Amedica) made of Si3N4, PEEK, and Ti6Al4V, with 8 implants for each material. All the cages had a 0° lordosis angle, a thickness of 10 mm, and were investigated with two geometrical sizes 16 mm × 12 mm (footprint area of 103.2 mm2) and 17 mm × 14 mm (footprint area of 125.5 mm2). The implants were mechanically tested with an empty central cavity that was dedicated to bone graft filling. In addition, the Si3N4 endplates were laser textured. Two supplementary smaller cages with smooth endplates were filled with dedicated porous material and were involved in the experiments to underline the plate texture and cavity-filling effects. The other implants were divided into two groups: the first group (8 samples) had the central cavity empty, while the second group (8 samples) exhibited a cavity filled with Si3N4 porous ceramic. The subsidence risk was experimentally investigated based on the ASTM F2267-04 standard. A cyclic sinusoidal compression with a maximum value of 250 N at a frequency of 1 Hz was applied. Five sets of different numbers of cycles with a maximum of 3600 were applied to each sample tested. Ramp-to-failure testing performed according to ASTM F2267-04 was used to estimate the assembly (foam-cage-foam) stiffness and yield point. Compressive loads were applied at a speed of 0.1 mm/s. It was found that the lowest subsidence was obtained for Si3N4 (at 3600 cycles low-density foam: small cage – 406 ± 91 μm, large cage – 189 ± 22 μm), while the highest values were determined in the case of small footprint cages for PEEK (468 ± 36 μm) and for large cages Ti6Al4V exhibited a value of about 233 ± 38 μm. The ramp-to-failure test provided approximately comparable values for all the analyzed materials (e.g., low-density foam substrate Si3N4 small cage – 259.8 ± 26.8 N/mm, large cage – 366.5 ± 7.4). One can conclude that silicon nitride had a reduced subsidence rate, and the above-described experimental program should also be extended for lumbar cage analysis since, at present, there are no investigations on this topic. Kersten et al. [185] made a biomechanical analysis of 4 lumbar spines (T13-L5) in the case of a goat animal model implanted with two types of spinal fusion cages 4 h after animal euthanasia. Human spacers made of Si3N4 and PEEK from Amedica Corporation, Salt Lake City, Utah, were used. The range of motion was determined for flexion/extension, lateral bending, and axial rotation and compared with the data obtained in the case of the control sample (non-operated segment) (7.87° ± 0.51/10.64° ± 4.38/1.05° ± 0.16). It can be noticed that higher ROM values were obtained for ceramic implant (3.38° ± 2.88/2.34° ± 1.24/0.40° ± 0.45) compared to PEEK cage (2.61° ± 3.26/2.24° ± 2.09/0.40° ± 0.17). The main conclusion of that study was that Si3N4 cages were not inferior to PEEK implants (Fig. 4.VI). In addition, the authors considered that ceramic cages were much more efficient in early arthrodesis, but in vivo studies are necessary. Unfortunately, few studies have investigated the mechanical performance of Si3N4 interbody fusion cages. At a global level, there is a recognized need for further material analyses to support the conclusions drawn from the clinical research presented in Section 2.
Regarding the biological compatibility of Si3N4, it has been confirmed that the unique properties of this material lie in its antibacterial and osteogenic dualism. Its surface promotes eukaryotic cell activity, facilitating a faster bone healing process. Additionally, its ability to inhibit the growth of various bacteria is crucial in the interbody fusion cage domain, where infections are a common concern both during and after the surgical procedure [183]. The Si3N4 cytotoxicity was investigated in a study [186] based on material extracts according to ISO 10993-5 [187], and the beneficial effects on cell development were proved. As presented in the previous paragraph, the investigation conducted by Kersten et al. [185] showed that the histological analyses in a goat animal model sustained a lack of adverse reaction after the silicon nitride cage implantation. Guedes e Silva et al. [188] introduced cylindrical Si3N4 scaffolds in rabbit tibia. After 8-week post-surgery, the animals were euthanized, and the tissue placed in the implant neighborhood was investigated. Various types of tissues were observed, including lamellar bone tissue with a high number of osteons and osteocytes, a non-calcified matrix containing osteoblasts, and a soft tissue component primarily composed of Collagen III, which can either transform into Collagen I or remain as fibrous tissue. It was concluded that Si3N4 exhibited a high biocompatibility and a positive influence on bone development due to the fact that bone growth was predominant in the cortical region and exhibited a certain variation in the distal or proximal zones of the cylindrical implant. It was observed that in the distal position, where the distance between the implant and the original compact bone was reduced, bone bridges formed, oriented from the original bone to the implant surface. This finding supports the osteoconductive properties of Si3N4 ceramic. Another in vitro and in vivo study by Howlett et al. [189] presented some important observations. The in vitro analysis showed that many Bone Marrow Stromal Cells (MSCs) were attached to the edges of the ceramic sample, with only a few remaining on the upper surface. None of the cells penetrated through the disc pores after 4 weeks of seeding. Regarding the in vivo investigation, intramedullary porous cylinders made of Si3N4 ceramic were inserted into the femoral marrow cavity of New Zealand rabbits. Initially, it was observed that woven bone surrounded the implant, and after 3 months, it developed into mature hard tissue, which was present even inside the implant pores. A pilot experiment was conducted and consisted of the fabrication of three Si3N4 endoprostheses that were inserted into adult rabbits, which were radiologically followed during the rest of their life. Autopsies revealed that the tissue placed adjacent to each prosthesis exhibited a normal morphological structure. It was concluded that Si3N4 ceramic has a bioactive character and great potential in bone tissue engineering. This conclusion was supported by a research paper [190], which determined the mechanical resistance of an implant that was introduced for 3 months in a rabbit animal model. Good bone attachment and growth processes were visible for ceramic samples compared to PEEK and Ti probes. Considering the inherent bioactive properties of Si3N4 material [191], it can be concluded that interbody fusion cages made from this material have significant potential for spinal arthrodesis. However, some studies have also explored methods to further enhance the osteoconductivity of Si3N4. These include surface functionalization with silanol groups (SiOH) [192], application of pressurized heat treatments, addition of sintering additives such as yttrium oxide (Y2O3) [192,193], control of surface wettability and roughness, and the development of Si3N4 porous cages with pores greater than 100 μm in diameter [194,195]. Additionally, the development of Si3N4-based composites containing hydroxyapatite [[196], [197], [198], [199]] or bioactive glass [200,201] could lead to increased bioactivity combined with good mechanical properties.
As stated in Section 1.2, an important problem that could occur in spinal cage implantation is infection. It is well known that for orthopedic implants, Gram-positive bacteria such as S. epidermidis and S. aureus and Gram-negative bacteria like E. coli, P. aeruginosa, and Enterococcus are primarily responsible for implant surface colonization and multi-species biofilm formation. This can lead to implant loosening, cage failure, or even the occurrence of secondary fractures, which may not heal without innovative treatments. Although antibiotic prophylaxis is applied, the risk of infections related to implants remains high. For example, some studies [202,203] underlined the fact that the infection rate is significant and induces a supplementary burden on the health system worldwide. It was estimated by The Review on Antimicrobial Resistance (AMR) that if no action is taken, a yearly death number of about 10 million can occur by 2050 [183]. In a study developed by Webster et al. [190], the antibacterial and bioactive properties of Si3N4 were compared to those of the so-called materials of choice in spinal surgeries, Ti and PEEK. This analysis was performed in vivo by generating a rat calvaria defect and introducing small implants made of these materials. Two animal groups were set: the first included animals infected with Staphylococcus epidermis, while the second one (control group) was populated with healthy subjects. Three months after the surgery, the animals were euthanized, and for the infected animal group, new bone formation was equal to 41 %, 26 %, and 21 % for Si3N4, Ti, and PEEK implants, respectively. In the case of the control group, a higher percentage of new bone formation was reported: 69 % (Si3N4), 36 % (Ti), and 24 % (PEEK). In addition, the percentage of histological bacterial counts was 0 % for Si3N4, 21 % for Ti, and 88 % for PEEK. Superior antibacterial behavior was attributed to Si3N4 ceramics. Ishikawa et al. [204] established that surface roughness is very important in providing an efficient antibacterial character. They tested the in vitro and in vivo properties of machined Si3N4 samples with a smooth surface and as-fired Si3N4 probes with a rough appearance. It was shown that the methicillin-resistant strain of Staphylococcus aureus was not able to adhere to the as-fired ceramic implants due to the non-regulated figure of the surface. Besides the surface roughness, some authors suggested that the antibacterial properties are influenced by surface energy and electrical charge. It is well-accepted that many bacterial genera exhibit a negative surface charge, so the materials with high negative zeta potential can inhibit the bacterial adhesion based on the electrostatic repulsion criterion [205]. A study performed by Gallo et al. [206] supported this observation by underlying that the oxidized silicon nitride sample had the highest electro-negative zeta potential of about – 70 mV in correlation with a very low value of the contact angle of 8 ± 1° and inhibited the highest amount of the E.coli and S.epidermis. It was concluded that hydrophilic surfaces do not permit pathogen adhesion since they absorb a high quantity of water, which is always unfavorable for bacteria adhesion. Other studies mentioned that the reduced friction coefficient of Si3N4 is of utmost importance in reducing the adhesion of bacteria due to a considerably lower number of abrasion particles that are even soluble in biological fluids [207,208]. Another important aspect to discuss regarding the antibacterial efficiency of Si3N4 is that this material disrupts cell membrane activity by generating reactive nitrogen species (RNS) simultaneously with surface-mediated chemical reactions. It is well known that this ceramic exhibits a surface chemical reaction in aqueous media, releasing nitrogen-based compounds that are transformed into reactive nitrogen species (RNS), such as nitric oxide (NO), which attack and damage the bacterial cell membrane. This topic was presented by Yang and Schoenfisch [209], which underlined the antibacterial activity of NO species. The authors [209] considered that its ability to exert oxidative stress, based on its byproducts such as dinitrogen trioxide, peroxynitrite, or nitrosative action, can affect bacterial membrane integrity. High-efficiency action was obtained against a large number of antibiotic-resistant bacteria [210,211]. By attacking and damaging the cell membrane, its contents leak, and a direct relationship with cell death can be expected [212,213]. The physical disruption processes characterize another mechanism of bacterial membrane disruption. The primary cause of this phenomenon is that Si3N4 has a rough surface, which physically damages the bacterial cell wall through mechanical processes. Pezzotti et al. [192] observed this finding using Raman and fluorescence spectroscopy to analyze nucleic acid degradation. The most important actions related to bacterial cell membrane damage led to cell lysis, generating the collapse of the cell structure, which interfered with nutrient uptake and waste removal in the bacteria and ultimately releases RNS, which makes a significant contribution to cell death. The anti-inflammatory signaling modulation was analyzed by Ma et al. [214]. The authors [214] established that a highly inflammatory medium generates abnormalities in epithelialization, revascularization, and cytokine control, which leads to significant delays in healing process. By developing a Si3N4-incorporated collagen/chitosan dressing patch, a rat diabetic wound model was investigated. A cutaneous wound of about 1.5 cm was created on the rat's back. The anti-inflammatory effect was attributed to the NH4+ and SiO44− ions resulting from the Si3N4 hydrolysis. Based on various tests comparing the developed ceramic patch to the control and other animal groups, a full recovery process was achieved with no signs of infection. Other aspects related to Si3N4 antibacterial properties can be found in the study of Du et al. [183].
As an overall conclusion, Si3N4 ceramics are suitable for interbody cage manufacture because of their unique chemistry. The controlled release kinetics of Si and N species enhance osteogenesis while providing a strong antibacterial effect [183].
3.4. Polylactic acid (PLA)-based materials
Bioresorbable interbody fusion cages are usually manufactured using different types of polymers, such as polycaprolactone (PCL) or PDLLA. The last one was used for the manufacture of the FDA-approved Hydrosorb™ cage. As highlighted in Section 2, bioresorbable polymeric interbody fusion devices have certain drawbacks that limit their clinical application. It is well known that bioresorbable cages are characterized by a much more reduced mechanical strength compared to metallic implants, and the degradation process is not entirely correlated with the increase of mechanical stability associated with new bone formation. In addition, inflammatory responses and foreign body effects were related to PLA-based cages [215,216]. For example, in vivo studies evidenced a high quantity of fibrous tissues containing foreign body giant cells [155,217]. Another investigation reported in vitro-observed negative effects of the irradiation and sterilization processes on the time-dependent mechanical properties of PLA-based materials [218]. Regarding the mechanical properties, the hollow-cylindrical shape of the Hydrosorb™ cage, which was initially dedicated to metallic material manufacture, has very thin walls that might collapse under natural physiological loads. In Ref. [219], the mechanical stability of Hydrosorb™ (Sofamor Danek, Memphis, TN) bioresorbable implants was analyzed. It was underlined that bioresorbable cages are safe for use in patients who are expected to achieve bony fusion about 2 years post-implantation. The result of this study was that strength retention decreased from 100 % (0 months) to 97 % (12 months), a fact that makes this material safe to be used in certain medical cases. Dijk et al. [220] investigated the effect of cage stiffness on lumbar interbody fusion rate in a goat animal model. The following types of interbody fusion cages manufactured by Stryker-Howmedica-Osteonics, Rutherford, NJ, with different axial compression stiffness, were tested: custom-designed Ti cage (700 kN/mm), stiff PLLA cage (4 kN/mm) and flexible PLLA cage (2 kN/mm). Autogenous iliac crest cancellous bone was used to fill the central cavity of the implants. At the 6-month follow-up, the PLLA cages exhibited a radiographic score of 2 with solid arthrodesis compared to the Ti implants, which were characterized by a radiographic score of 1. This result evidenced that PLLA material presented a good bone fusion, and it is a viable alternative to the classical Ti cages. In a study developed by Alexander et al. [221], it was shown and explained that implants, which have better mechanical properties such as Young's modulus or bending strength, are not easily resorbed into the human body, and dissolution times higher than 1-year post-surgery were reported for PLA-based materials. Smit et al. [218] attested a time-dependent failure mechanism of PLDLLA cages when biomechanical conditions are replicated. By analyzing the behavior of such materials in the case of continuous loads, it was concluded that a dynamic molecular segment rearrangement is present in PLA-based materials under the effect of torsional and rotational loads. To investigate the mechanical properties, the authors [218] used the uniaxial compression tests. The experimental procedure consisted of 70:30 (poly(L-lactide-co, D, L -lactide) laboratory spinal cages that were compressed at different strain rates of 10−4, 10−3, and 10−2 1/s between 2 parallel and flat SS plates. Two temperatures were chosen for tests (23 and 37 °C), and one cage was introduced in demineralized water at 37 °C with a presoaking time of about 24 h to analyze the influence of humidity. The strength tests revealed that the cage was stronger at higher loading velocity, dry conditions, and reduced temperatures. It was evidenced that by decreasing the loading velocity by a factor of 10, a decrease in cage strength by 0.86 was registered. In addition, at 37 °C, a cage strength decrease by 1.5 kN was measured, concomitantly with the reduction of cage strength by another 0.5 kN if humid medium conditions were considered. The long-term failure experiments showed that under a compression force of 4.5 kN, a complete cage collapse was seen at a maximum time of 5 min loading. The main conclusion of this study was that the mechanical strength of 70:30 PLDLLA was strongly influenced by environmental conditions such as temperature and humidity, and measuring standards that consider these variations should be developed.
The biological matching of PLA-based materials was intensively investigated and developed in various studies that focused on its safety profile and biocompatibility [215,[222], [223], [224], [225]]. In two different investigations developed by de Medinaceli et al. [222] and Gautier et al. [223], the influence of biodegradable polymers on neuronal and non-neuronal cell developments, peripheral nerves, and axonal growth were analyzed. In the first study, it was underlined that the regeneration of rat sciatic nerves was not negatively influenced by the PLA-based implants. It was noticed that when the tube was loosely fitted around the nerves, the regeneration process was enhanced by using a bioresorbable material that does not need a supplementary surgical intervention to remove it. In the second study, the biocompatibility and resorbability of poly(α-hydroxy acid) was analyzed, underlying its effects on spinal cord and adult rat Schwann cells. In vitro results proved that PLA25GA50 material did not exhibit adverse effects on the adhesion, proliferation, and development of Schwann rat cells. In vivo tests consisted of PLA25GA50 implants integration into the rat spinal tissue. After about 1-week post-surgery, growth-associated protein 43 signs were observed, a fact that proved that the axon regenerative process began. It was concluded that PLA-based materials are important in the damaged nerve regeneration process. The biocompatibility of PLA-based polymers with neurons and dura mater was underlined in the study of Lundgren et al. [226]. A rabbit animal model was used, and 2 circular bone defects with a diameter of 8 mm were created along the midline of the parietal and frontal bone of the calvarium. Some defects were supracalvarial covered with a PLA-based membrane, and others were treated by placing similar membranes on the inner and outer surfaces of the defect. At 6-week post-surgery, the animals were euthanized, and histological tests were performed. It was noticed that for the bone defects treated with two PLA-based implants, the thickness of the new bone layer was comparable with that measured in the case of healthy tissue. On the contrary, the bone defects covered with only one membrane exhibited a reduced thickness of the new bone. It was concluded that the bone regeneration process was obtained without bone grafts and that non-harmful by-products characterized the degradation process of the PLA-based membrane. Van der Elst et al. [225] proved that no changes in the pH of the medium placed near a PLA-based implant occurred. Taking into consideration all mentioned before, it can be noticed that PLA degrades via hydrolysis and is characterized by a high biocompatibility grade with bone tissue and nerves, making it a very good candidate for spinal fusion surgery. Numerous literature studies compared the outcomes of PLLA cages with those of similar implants made of titanium in the case of a Dutch goat model [220,[227], [228], [229]]. It was concluded that comparable or even higher fusion rates were achieved for the polymeric cages used in the lumbar zone of the animals. Van Dijk et al. [230] showed that the PLA-based cages maintained their mechanical integrity, and the bony fusion naturally occurred without cage subsidence. In addition, by filling the median cavity of the bioresorbable spinal cages with bone grafts, an interbody fusion of 86 % [227] was achieved, followed by a complete dissolution of the implant 3 years later [228]. Hojo et al. [231] developed a bioresorbable lumbar interbody cage made of uncalcined hydroxyapatite/poly L-Lactide (F-u-HA/PLLA) and tested its in vivo biocompatibility using sheep animal model in comparison with autologous iliac bone graft and carbon fiber cages (Fig. 4.VII). The histological analyses (Fig. 4.VIII) revealed that the cages were strongly linked to the neighboring hard tissue compared to carbon fiber cages that were surrounded by fibrous tissue. Supplementary, the fusion quality of the L2-L3 spinal level was very good and similar to bony fusion obtained in the case of other used fusion implants. It was concluded that this type of high osteogenic and bioresorbable material exhibited good osteoconductive properties combined with the possibility of implant degradation after a certain amount of time. One can conclude that combining bioactive calcium phosphates or bioactive glasses and bioresorbable polymers could be an alternative to the classical solution met in clinical practice, such as Ti or non-degradable PEEK. However, by analyzing the side effects associated with PLA-based spinal fusion cages, special attention should be devoted to improving the cage's mechanical properties to reduce the quantity of nanoparticles generated by the friction process and to increase the implant osteoconductive behavior.
Fig. 4 shows examples of mechanical investigations and biological matching results extracted from literature in the case of Ti alloy, PEEK, Si3N4, and PLA-based interbody fusion cages.
3.5. Long-term monitoring of biomaterial impact on spine local microenvironment
One can define long-term monitoring in the framework of spinal fusion procedures in a direct relationship with the influence of biomaterial on spinal microenvironment, taking into consideration also the biological aspects, as well as some negative drawbacks.
Firstly, one of the most important features is the patient's immune response and inflammation [232]. The immune response involves the formation of a fibrous capsule, which has a significant impact on the bone fusion rate and cage stability [233]. In the case of some patients, chronic pain or other complications could occur [234]. Foreign Body Reaction (FBR) typically occurs after cage implantation and is characterized by an inflammatory response, which can be defined as the recruitment of macrophages and neutrophils to the implantation site [235]. Based on cell phagocytosis, the cage degradation process begins, and cytokines and growth factors are released, leading to the differentiation and proliferation of fibroblasts [236]. In this way, an extracellular matrix produced by the fibroblast cells is formed, leading to the creation of a fibrous capsule around the cage that acts as a separation boundary between the cage and the surrounding microenvironment. For some patients, the fibrous capsule stabilizes the cage, promoting good fixation and an increased bone fusion rate. On the other hand, the fibrous capsule could also influence and hinder the circulation of nutritive substances to the cells necessary for fusion, and a cage failure is foreseen. As main biological mechanisms, the following signaling pathways Toll-Like Receptor (TLR) that recognize Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Linked Molecular Patterns (DAMPs) are present [237]. They activate transcription factors such as Nuclear Factor Kappa B (NF-κB) and Tumor Necrosis Factor Alpha (TNF-α) [232]. The first one modulates the expression of pro-inflammatory cytokines (interleukin-1 (IL-1), interleukin-6 (IL-6)), which collect immune cells (T and B type) at the implantation site. Immune system cells mount antigen-specific immune response [238]. The Transforming Growth Factor beta (TGF-β) pathway promotes fibrous capsule formation.
Other possible long-term complications include stress shielding, metallic ion emission from metallic cages such as Ti-alloys, and implant loosening in polymers due to their reduced mechanical properties, as well as particle debris leading to osteolysis [232]. Coating with bioactive ceramics must be carefully addressed because although they enhance bone fusion, they can also initiate a severe immune response [239,240]. Regarding inflammation and FBR, an equilibrium between immune system cells is of utmost importance in tissue repair and regeneration processes [232]. Metal ion or particle release from spinal cages is possible due to corrosion or degradation processes. The metallic ions can spread to the patient lymph nodes and other organs. The main mechanisms of corrosion for spinal implants were mentioned by Ganko et al. [241]. These could have a mechanical origin, such as fretting, which was present at the biomaterial interface following the application of a supplementary load. The electrochemical mechanisms considered in the study mentioned above included crevice corrosion, which resulted from local conditions such as reduced oxygen levels and medium pH and pitting corrosion caused by elemental impurities. Metal ion release should be continuously monitored for patients after the metallic cage insertion, as some clinical investigations proved [242,243]. In the case of PEEK and Si3N4 cages, wear particles occurred and could trigger a significant inflammatory response, as evidenced in a long-term study [244]. Thus, a correct understanding of the host reaction in relation to the type of wear debris should be established, and new solutions to the long-term consequences must be continuously sought.
Choosing the right orthobiologic materials could enhance and promote bone growth and fusion. As we have presented in previous sections, BMPs and other biological agents are successfully used, but not always potential risks are avoided. There are some clinical studies in the lumbar fusion domain [[245], [246], [247]] that proved that Cellular Mineralized Allografts (CBMs) that are manufactured from osteoconductive cadaveric bone combined with allogenic stem cells do not add a higher improvement in spinal fusion [248]. In addition, the preparation of stem cells is not standardized and can significantly impact the efficiency of the final product. Another important drawback is the higher price of orthobiologics compared to DBM. DBM is prepared from cortical bone, which is treated to yield a collagen matrix, non-collagenous proteins, residual calcium phosphates, and osteoinductive growth factors (BMPs). Today, some commercial companies manufacture DBMs, which were reviewed in the study of Cohen et al. [249]. Regarding the long-term benefits, a meta-analysis has shown that a combination of autografts and DBM used in PLIF or LLIF surgeries exhibits only a slightly higher fusion rate compared to the use of autograft as a single solution, with no statistically significant difference [250]. Some studies reported that increased age and osteoporosis should be considered as risk factors when DBM is used [251]. Another important drawback is the high price of the product compared to synthetic bone substitutes. Other biologically based approaches proposed in the literature include the use of autografts, pedicled Vascularized Bone Flaps (VBFs), free VBFs, allografts, and xenografts. All these solutions and bioactive factors are presented in a very detailed manner by Tian et al. [252]. To estimate the long-term effects of orthobiologics, further investigation is needed, as this research is relatively new (Section 5).
It can be concluded that long-term monitoring must always be associated with spinal fusion cages, as all scholars should consider the advantages and potential side effects that may appear after cage insertion at different times and attempt to mitigate them by developing innovative solutions and new materials.
In this section, we described in detail the biomaterials already used in clinical practice to design the interbody spinal fusion cages presented in Section 2. A comprehensive summary of the mechanical properties obtained from both numerical simulations and experimental tests is provided in Table S1. The biological properties of each biomaterial are presented, along with detailed examples of methods to enhance their bioactive characteristics. In the authors’ opinion, clinically approved materials possess certain drawbacks that, in some patients, could become significant and greatly impact their health (Section 3.5). The primary disadvantages identified include their inert behavior and lack of antibacterial properties—except for silicon nitride ceramics. Consequently, there is a need for a continuous development of new materials and innovative coatings, as well as the analysis of the long-term safety and effectiveness of these materials.
4. Prospective biomaterials and coatings for innovative spine fusion cages
Innovative and new solutions for spine fusion cage manufacture will be proposed in this section. Special attention will be devoted to the bioactive materials that have already been tested in in vivo conditions to demonstrate their effectiveness but have not yet been introduced or are limited in their use in clinical trials to offer an alternative to the traditional approaches. In addition, some bioactive and antibacterial surface coatings, which are supposed to overcome the limitations and drawbacks of the conventional biomaterials already used, presented in Section 3, will be detailed.
4.1. Bioactive materials for biodegradable or bioresorbable spinal cages
It is well known that when permanent, non-absorbable, and non-bioactive implants are used, they have some major complications such as local pain, breakage of the implants, and even infection present in the implant vicinity [[253], [254], [255]]. Although bioresorbable cages made of PLA-based biomaterials were proposed over time, it was noticed, as we underlined in Sections 2, 3, that these implants are characterized by acidic products, which occurred during the polymer degradation process and almost always were linked to bone resorption and osteolysis due to the polymer nanoparticles, which are a result of friction between human bone and spinal cage [256]. A direct consequence is the pursuit of new bioactive, biodegradable, or bioresorbable solutions that can be used to design a spinal cage. This spinal cage would have the ability to completely dissolve over a specific period, while its by-products promote continuous bone formation. These new biomaterials should enhance the bone's natural healing process and sustain its development. In addition, the synergistic relationship between material degradation and bone growth is of utmost importance to ensure that materials continue to provide appropriate mechanical support and a biologically active environment for new bone growth throughout the process of gradual degradation. Fusion failure can be caused by either too fast or too slow material degradation and must be avoided while promoting bone fusion. This aspect will be discussed in conjunction with the new proposed solutions in this section. Fig. 5 presents experimental results for two high osteogenic potential lumbar cages made of Mg-Zn-Nd-Zr alloy [257] and polycaprolactone, in which 20 % beta-tricalcium phosphate (mPCL-TCP) was incorporated [258].
Fig. 5.
High osteogenic potential lumbar cages: Mg-Zn-Nd-Zr – (5.I) Corrosion surface analysis after 28 days immersion in Hank's solution with an adjusted pH of 7.4 and EDS spectra for bare and MAO-coated Mg-alloy samples: (a) surface morphology and elemental composition, (b) cross-section morphology and EDS analysis; (5.II) RT-qPCR osteogenic differentiation genes (BMP2 – bone morphogenetic protein 2, OPG – osteoprotegerin, RUX2 – runt-related-transcription factor 2, COL-I – collagen I): (a) 7 days, (b) 14 days (statistical significance ∗ p < 0.05, ∗∗p < 0.01) [257] (Figure is licensed under CC BY-NC-ND 4.0); Poly (epsilon-caprolactone) with 20 % beta-tricalcium phosphate (mPCL-TCP) – (5.III) μ-CT images in a pig lumbar fusion model: (A)/(B) cage (scaffold + rhBMP-2) and (C)/(D) autograft bone at 3/6 months post-surgery, respectively, (E) Bone volume per tissue volume (BV/TV) ratio (∗p < 0.05); (5.IV) Typical variations of ROM construct rigidity at (A) 3 months, (B) 6 months, and (C) limitation of ROM (∗p < 0.05) [258] (Reprinted from Ref. [258] Copyright (2025), with permission from Elsevier).
4.1.1. Magnesium (Mg) and Mg-based alloys as metallic biodegradable material for spinal interbody fusion cages
As presented in Section 3, metallic materials such as Ti were extensively used in spinal fusion surgery since they have good mechanical properties and adequate energy absorption capacity. However, the high corrosion resistance of Ti alloys was linked to implant surface colonization with bacteria and inducing some unwanted infections. Supplementary, the compromise of their biocompatibility [259] can generate important inflammatory reactions, long-term endothelial malfunctions, and last but not least, a permanent physical irritation of the implantation site that could be linked to important cell modifications and even dangerous, life-threatening conditions such as osteosarcoma apparition [260]. Thus, the need for biodegradable metals to be used in general for the orthopedic domain and, in particular, in the case of spine surgery is considered today of utmost importance [253,261,262]. Due to the fact that many papers have already shown the clinical applications of Mg-based alloys [[263], [264], [265], [266]] and characterized their material properties [267], obtaining and processing technologies [268,269], and biodegradation behavior [270], in this review, only the studies in which Mg was considered one of the most promising bioactive solutions for spinal cage manufacture will be described.
To summarize some of magnesium's key advantages, we will highlight a few important aspects. In terms of mechanical properties, magnesium exhibits Young's modulus ranging from 41 to 45 GPa, which is close to that of human bone (elastic modulus 3–20 GPa) [253]. In addition, its yield strength is experimentally determined to be between 65 and 100 MPa, while the yield strength of the human bone is comprised between 104 and 121 MPa. One can easily notice that Mg alloy's mechanical properties exhibit values very close to those of the human bone, so the stress shielding effect is not expected to occur [253]. The general degradation processes of pure Mg and its alloys are very similar. Usually, magnesium hydroxide (Mg(OH)2) and hydrogen (H2) are produced, and specialists should pay attention to the fast corrosion speed of magnesium. It was established that a safe Mg ions (Mg2+) concentration has to be in the interval between 5 ÷ 10 mmol/L [[271], [272], [273]]. These values represent the tolerance range necessary for human muscle normal activity. A high survival rate of endothelial cells was observed when the Mg2+ concentration was below 20 mmol/L [274], and the human neurons functioned well at a concentration of about 30 mmol/L [275]. Hydrogen emission is an important issue in the clinical translation of the Mg-based alloy framework. Although some studies showed that H2 has a beneficial effect on osteoclast activity inhibition [276,277] and the control of oxidative stress [278,279], attention should be devoted to diminishing the hydrogen quantity because large amounts of H2 were associated with pulmonary embolism. Thus, the hydrogen emission should be carefully controlled and reduced as much as possible to avoid respiratory problems and cavity formation around the implant. If one analyzes the biological potential of Mg-based implants, one can see that these biomaterials meet the medical requests in a direct relationship with the bone-nerve-bone circuit, angiogenesis, and osteogenesis [280]. Many literature studies [[281], [282], [283], [284]] proved that Mg2+ is transported via Mg transporter 1 and transient receptor potential channel 7, that stimulate the Calcitonin Gene-Related Peptide (CGRP) and influence the bone marrow stem cell differentiation into osteoblasts. Supplementary, Mg ions based on Hypoxia-Inducible Factor 2a (HIF2a) and peroxisome proliferators activate receptors, enhance the Vascular Endothelial Growth Factor (VEGF) secretion. This phenomenon was associated with H-type capillary proliferation that always occurred when new bone formation was present at the defect site [[285], [286], [287]]. Usually, VEGF acts as an important stimulus for the Endothelial Cells (ECs) by controlling their mitogenic and chemotactic responses and binding to the cell receptors. Wang et al. [288] analyzed the impact of Mg2+ ions on ECs and proposed several mechanisms of their action. They demonstrated that Mg2+ induced the migration and invasion of ECs under in vitro conditions. Additionally, it was proved that Mg2+ enhanced the spreading of spheroidal-shaped ECs and increased the expression of endothelial cell-specific genes. The authors proposed that the main mechanisms driving cell differentiation involve the VEGFA-VEGFR2/Notch 1 signaling pathway cross-talk, which encourages migration and filopodia generation. Meanwhile, the proliferation of stalk cells is induced by YAP nuclear translocation, ultimately resulting in the formation of vascular networks. A much more detailed research focus on biological aspects was performed by He et al. [253] and Manescu (Paltanea) et al. [289] If someone aims to detail the effect of Mg2+ on osteogenesis, they should consider that the alkaline environment, which occurred after an Mg implant use, can reduce the action of the osteoclasts concomitantly with an acceleration of the mineral deposition [290,291]. Many studies [289,[292], [293], [294], [295]] proved and sustained the osteoconduction and osteogenesis properties of Mg-based alloys. An important issue that should be underlined is the correlation between magnesium degradation kinetics and bone formation, known as creeping substitution because the degradation rate of Mg alloys is directly linked to the quantity and quality of new bone formation at the defect site [296,297]. Usually, an ideal degradation rate determines implant adequate mechanical properties maintained for a longer time in relationship with good osseointegration and excellent bone formation, while a faster degradation rate is associated with implant failure and a low amount of new bone formation due to a rapid reduction of mechanical characteristics, inflammation, and tissue response, or incomplete tissue integration [298,299]. In the case of a too-slow degradation rate, the Mg alloy implant can act as a physical barrier, hindering angiogenesis and osteogenesis, or it could create conditions that favor the fibrotic encapsulation process. In many cases, chronic inflammation can occur. Okutan et al. [297] evidenced that the degradation of Mg alloy implants regulates the new bone formation via controlling the BMP2 and Osteoprotegerin (OPG) expression. Another aspect, which is related to an ideal biodegradation rate of Mg, consists of the circumventing process of Mg2+ accumulation that has a negative impact on osteogenesis due to unwanted immunomodulation in macrophages [300,301]. Some studies have proposed mathematical models to describe or estimate the kinetics of degradation based on environmental conditions or alloy properties [[302], [303], [304], [305]]. However, these models should always be correlated with in vitro and in vivo studies to accurately establish the relationship between magnesium degradation and osteogenesis. Taking into consideration all the above-mentioned aspects, one can easily consider the Mg and its alloys as ideal bioactive materials for groundbreaking products.
Some interesting studies in the literature consider the development of interbody fusion cages from Mg-based alloys. All of them concentrate on in vitro or in vivo analyses. Guo et al. [18] made a biodegradable High-Purity (HP) Mg (99.98 wt%) interbody cage and evaluated its in vivo biodegradation properties. The authors [18] implanted the cage in the cervical region of a goat animal model. The control group was treated with autologous iliac bone. Cervical samples were collected at different time intervals (3, 6, 12, and 24 weeks) to investigate the biocompatibility, degradation, quality of bony fusion, and histological examinations. Radiographic analysis revealed that at 3 and 6 weeks, comparable bone fusion was achieved between the Mg-cage and control groups. On the other hand, at 12 and 24 weeks, a better bony fusion was obtained for the control group. The Mg cage group exhibited a fusion rate lower than 30 %. Additionally, a faster degradation process was observed in the first 3 weeks post-surgery, but after that, the implant remained stable and slowly degradable. No systemic or important side effects were reported, and it was concluded that surface treatments would increase the corrosion resistance of the implant and could improve even its biocompatibility and osteointegration capabilities to achieve a better bony fusion. Yu et al. [306] analyzed a similar Mg HP spinal cage and tried to improve its interbody fusion potential by generating a fluoride conversion coating. Two test groups were established: the first one consisted of goats, which had a coated cage implanted in the cervical region, and for the second animal cohort, uncoated implants (control) were used. Initially, the hydrogen release was measured in a phosphate-buffered saline (PBS) solution, and weight loss was determined based on chromic acid. Regarding the in vitro degradation behavior, a reduced quantity of hydrogen release measured after 336 h of sample immersion was obtained for the cages with fluoride conversion coating compared to uncoated implants (H2 release: fluoride conversion coating – 0.0165 mmol/cm2, uncoated – 0.0285 mmol/cm2). This result was also sustained through mass measurements, which showed a very reduced mass decrease value for the MgF2-coated samples compared to the control group. After in vivo implantation in goat models, an increase in serum aspartate aminotransaminase level was noticed for the uncoated cage group, while a higher fusion rate of about 96 % was obtained for the coated cages. It was concluded that magnesium fluoride (MgF2) conversion coating exhibited a high osteogenic potential and could be successfully used for intervertebral Mg alloy cages due to its good biocompatibility. Wang et al. [307] proposed a biodegradable magnesium-magnesium phosphate cement (Mg-MPC) lumbar interbody fusion cage and investigated its feasibility based on a porcine animal model. The lumbar discectomy was made at L3-L4 and L4-L5 levels. The comparison group included animals with PEEK cages (Johnson & Johnson, Medical Devices Companies, USA), and the negative control group contained only healthy animals with no implants. Computed tomography was performed at 6, 12, and 24 weeks after surgery and was used to calculate the average Intervertebral Disc Space Height (DSH). Fusion rates were estimated based on micro-computed tomography. A mechanical test was performed on cadaveric lumbar spine specimens that included 6 motion segments. Flexion/extension, right/left axial rotation, and right/left lateral bending were determined. In addition, a histological investigation was conducted to check signs of inflammation. CT scans revealed similar values for the average DSH for both groups, and no gas accumulation was observed at 6, 12, and 24 weeks after the surgical intervention. At 24 weeks post-surgery, a fusion rate of about 83.3 % was registered for both animal groups. Regarding the Mg-based cage, the presence of cavities and cracks was evidenced for 6 weeks post-surgery samples, and it was concluded that the in vivo degradation process of the cage began. Mechanical tests proved that the PEEK group exhibits higher stiffness values in lateral bending and flexion/extension movements. Histology showed new bone formation concomitantly with empty zones at the interface between the Mg-based cage and bone or around the implant. These empty spaces were associated with a fast degradation process of the Mg. For PEEK cages, fibrous tissue formation was observed, creating a connection between the native endplates and the implant. No additional new bone cells were observed with PEEK implants. In both types of implants, neither osteolysis nor inflammatory cells were detected. The main conclusion of that study was that the Mg-based cage had a good osteointegration capacity, but it exhibited a reduced stiffness value, so improvements are necessary in order to obtain adequate mechanical properties. Chi et al. [257] manufactured a Mg-Zn-Nd-Zr lumbar cage and tested its biodegradation, mechanical behavior, and biocompatibility. Samples were prepared using the Micro-Arc Oxidation (MAO) technique for coating, along with uncoated samples for comparison. A PEEK cage was used as control. By analyzing the MAO-coated cage surface, it was established that the coating had a 55.8 ± 11.2 μm thickness (Fig. 5.I). A contact angle of about 10.4 ± 0.9° compared to the uncoated cage (61.6 ± 0.9°) proved that the implant became more hydrophilic after the surface treatment, and this characteristic was in direct correlation with an increased roughness. The electrochemical analysis and immersion test revealed that the MAO-treated samples had a higher corrosion resistance compared to the uncoated Mg-based group (i.e. corrosion current density icorr: MAO coated sample-1.2 μA/cm2, uncoated sample-233 μA/cm2). The mechanical compression test showed that both Mg-based spinal cages had ultimate load, yield load, and stiffness higher than the control group. For the in vitro investigation, the MC3T3-E1 cell line was selected, and mRNA sequencing, Western blotting, and RT-qPCR determined that Mg-Zn-Nd-Zr lumbar cages both MAO coated and uncoated had a higher osteogenic potential compared to PEEK cages (Fig. 5.II). For in vivo analyses, an ovine animal model was chosen. At 12 and 24 weeks post-implantation, the histological analyses and radiographs proved the presence of enhanced bone formation for both Mg-based spinal cages compared to the control group. It was concluded that the Mg rare-earths alloy was chosen correctly, and when coated by means of MAO technology, a decreased corrosion rate was registered. In addition, a higher osteointegrative potential compared to PEEK commercial cages was evidenced, making the proposed implant a good candidate for spinal fusion. Xu et al. [308] developed a porous interbody cage from Mg-Zn and implanted it into the cervical region of a goat spine model. The cage exhibited a rectangular shape and was designed to have a porosity of 45 %. This value was chosen in accordance with physiochemical characteristics established in a previous study [309]. The cage surface was changed by applying an MAO/silicon (Si)-containing coating. In vitro mechanical tests showed an elastic modulus of 42.64 ± 2.11 GPa and a compressive yield strength of 311.74 ± 14.20 MPa. The cohort of animals containing 24 goats was divided into 4 groups as a function of experiment duration time (3, 6, 12, and months). All the goats were implanted with a coated cage at one cervical spine level, and at another level, the clinicians used autologous iliac bone stabilized with a Ti plate. The autogenous bone considered the gold standard worldwide, was chosen as the control. The Mg-Zn-coated cages showed good mechanical properties after cadaveric tests. The CT scans revealed that the fusion rate was unacceptable, being lower than 30 %. This negative finding was associated with a very slow degradation process of the coated cage. It was concluded that the MAO/Si-coating is improper for a spinal cage because the biodegradability of the implant was reduced, and the authors [308] assimilated the implant behavior to that of commercial Ti and PEEK cages. It was confirmed that an autologous bone graft yielded better results than the newly developed implant. Zhang et al. [310] designed a Mg-3Al-1Zn (AZ31) cage coated with Si. A quantitative analysis of Mg accumulation in the area placed near the implant was performed using a goat cervical spine model. The cage was rectangular and similar to the Cervios (DePuy Synthes Spine, Raynham, MA, USA) model. The intervertebral Mg levels were measured at 3 weeks, and elevated values were obtained in the implant vicinity. At 24 weeks post-operatively, still high values were recorded for Mg, but compared to those measured at 6 and 12 weeks, these values were much reduced. Based on the CT scans and histological results analysis, it was established that adequate bony fusion was not achieved, exactly as described in the previously mentioned study [308]. It was concluded that Mg accumulation in the neighborhood of the implant determined a decreased Ca/P ratio and had an important contribution to the non-union result, as one could see from the CT scan images and negative histological analysis with reduced activity of the osteoblast in the contact zone between endplate and implant surface. A magnesium-polymer interbody cage was manufactured by Daenzer et al. [311], who designed a cylindrical skeleton made of Mg-3Al-1Zn (AZ31) covered with an absorbable polymer - polycaprolactone (PCL) - to reduce the corrosion speed of the Mg alloy. As previously discussed, 24 sheep were divided into 4 groups (3, 6, 12, and 24 weeks). All animals were implanted at two different cervical levels with Mg-PCL cage and autologous tricortical iliac bone grafts and Ti plates (control zone), respectively. The animals were euthanized, and cervical spines were mechanically tested and analyzed based on micro-CT scans to determine the degradation kinetics. Histological analyses were made to estimate the quality of bony fusion and the existence of foreign body reactions. The radiographical results revealed gas pocket presence in 50 % of the cage implanted places at 3-week post-surgery, with a complete absence of gas determined at 6 weeks. The most important result was that of 24 weeks, a good bony fusion of about 83.3 % was found in the case of interbody cages. This outcome was comparable to that obtained for an autologous bone graft. Biomechanical tests showed a higher stiffness value for the segment treated with a natural graft. A slight increase in the value of stiffness was noticed after 24 weeks post-operatively for the spinal levels treated with Mg alloy cages. The histological results proved no signs of necrosis or regenerative processes. In addition, the encapsulation of the cage with fibrous tissue was observed. New bone formation was evident at autologous graft levels, with no signs of fibrosis reported. A nonlinear degradation profile of the cage was observed based on micro-CT scans. The main result of this study was that, compared to the gold standard (autologous graft), the newly developed implant was inferior, exhibiting a reduced bony fusion and low stiffness values. Other research groups [253] developed an Mg-PEEK cage and analyzed the influence of the Mg part on the whole implant behavior. For example, it was stated that in the case of Mg powders introduced into PEEK, a high-speed corrosion rate accompanied by important hydrogen release occurred. Also, when a Mg alloy piece covered the PEEK entirely, a strong inhibition of osteogenesis was present at the surgery site. The research group found that the best shape in which Mg can be combined with polymeric implants is represented by Mg wires. This geometry offers the advantages of low hydrogen emission and enhanced osteogenesis in the early healing stages [312]. The main drawback of using such structures is represented by the reduced mechanical properties of the bioactive metal. However, with advancements and adaptations in processing technologies, this limitation can be overcome [253].
It can be concluded that although many studies analyzed the implantation site of Mg-based cages in the cervical zone of animals, these findings can also be extended to the lumbar spine. Taking into consideration all the positive effects of pure Mg and Mg-based alloys on osteointegration, along with their adequate mechanical properties, it is evident that with the development of suitable biocompatible coatings, these materials hold significant potential as bioactive implants in spinal cage surgery. Different surface treatments and coatings, exhaustively detailed by Antoniac et al. [269], could be applied, but there is currently a lack of in vivo or clinical trials related to bare Mg-based cages, and much more research should be undertaken to establish their outcomes.
Table 3 presents a synthesis of the literature studies examining the properties of Mg-based interbody spinal cages using various animal models with devoted attention to the synergistic relationship between material degradation and new bone formation.
Table 3.
Literature studies related to Mg-based spinal cages.
| Material | Implant geometry | Animal spine model characteristics | Control | Experimental approach | Comments | Synergistic relationship between material degradation and bone growth | Ref. |
|---|---|---|---|---|---|---|---|
| High purity Mg (99.982 wt% Mg; 0.0178 wt% Si; <0.001 wt% Fe; <0.001 wt% Al) | Parallelepipedal cage with 12 mm × 10 mm × 4–5 mm and a 7° wedge angle | Goat spine model/24 healthy animals, 2-year-old (12 male and 12 female), average weight 48.17 ± 5.48 kg | Autologous iliac bone with Ti plate and screws |
|
|
The Mg cage volume decrease: 9.40 % ± 2.75 % - 3 weeks,11.77 % ± 2.17 %- 6 weeks, 13.00 % ± 2.35 % - 12 weeks, 13.73 % ± 2.62 % - 24 weeks | [18] |
| New bone formation: total fusion area lower than 30 %, fibrous tissue occurred in a 300–400 μm space between cage and bone, ALT starting point/endpoint: 20 U/L/23 U/L – 3 weeks, 20 U/L/20 U/L – 24 weeks; Serum Mg concentration: 1 mmol/L/1.2 mmol/L – weeks, 0.8 mmol/L/1 mmol/L | |||||||
| Study drawbacks: reduced intervertebral blood supply, high Mg2+ concentration at the surgery place, reduced osteogenic activity, increased osteoclast activity | |||||||
| High purity Mg (99.982 wt% Mg; 0.0178 wt% Si; <0.001 wt% Fe; <0.001 wt% Al) uncoated and MgF2 conversion coating | Parallelepipedal cage with 14 mm × 11 mm × 4 mm and a 4° wedge angle | Goat spine model/10 healthy animals, 1.5 years old, average weight 38.78 ± 3.72 kg | Uncoated Mg cage |
|
|
Material degradation: Immersion test – mass before/after: 0.20 g/0.16 g (high purity Mg); 0.19 g/0.18 g (MgF2 coated Mg). The coating offers increased corrosion resistance | [306] |
| New bone formation: High purity Mg cages induced lesions to the upper and lower endplates and bone placed in the implant vicinity; cavity increased 24 weeks; continuous bone formation and good bony fusion noticed for coated cages; osteolysis and damaged bone tissue for non-coated cages; fusion score for coated cages - 2.8 ± 0.45) (P < 0.01), fusion score for uncoated cages (0.2 ± 0.45) (P < 0.01) | |||||||
| Coated cages: ALT starting point 3 weeks/endpoint 24 weeks: 25 U/L/-31 U/L; Serum Mg concentration: constant value 1.125 mmol/L | |||||||
| Magnesium and magnesium phosphate cement (Mg-MPC)/PEEK | Block cage, length of 20 mm, width of 8 mm, height of 6 mm/Box PEEK cage (Johnson & Johnson Medical Devices Companies, USA), length of 15 mm, width of 12 mm, height of 6 mm, 7 mm diameter central hole | Porcine lumbar fusion model/12 Ba-Ma mini pigs, 6 months, weight 25 ÷ 30 kg | Negative control (normal lumbar segments) for mechanical tests/Positive control PEEK cage |
|
|
Material degradation: μ-CT: cavities and cracks appeared on the surface of Mg-MPC cage | [307] |
| New bone formation: new bone was noticed by traversing the Mg-MPC cage to bridge, concomitantly with cage degradation | |||||||
| Study drawbacks: cage rotation for Mg-MPC and positive control cages due to a malfunction of the fixating system, leading to spinal failure | |||||||
| Mg-Zn-Nd-Zr (chemical composition 1.89 % Zn, 0.69 % Nd, 0.58 % Zr, Bal. Mg)/bioactive calcium phosphate Ca-P coating by MAO/PEEK | Mg-Zn-Nd-Zr and PEEK cages with a length of 12 mm, width of 6 mm, and height of 4.5 mm | Ovine lumbar spine model/12 female sheep, average age 23.83 ± 1.53 months, average weight 48.63 ± 3.93 kg | PEEK cage |
|
|
Material degradation: electrochemical test – Mg alloy (icorr = 233 μA/cm2, CR 621.7 mpy); Ca-P coated Mg (icorr = 1.2 μA/cm2, CR 3.1 mpy) | [257] |
| New bone formation: RT-qPCR (relative mRNA expression level): BMP2 (Mg – 1; Ca-P coated Mg – 1), RUNX2 (Mg – 2.2; Ca-P coated Mg – 1.3), COL -I (Mg – 1.8; Ca-P coated Mg – 1.4). Mg cages generated new bone and gas cavities at 12 and 24 weeks. BV/TV: Mg - 33.76 ± 16.93 %; Ca-P coated Mg - 12.00 ± 1.56 %). GV/TV: Mg - 33.76 ± 16.93 %, Ca-P coated Mg - (34.20 ± 2.46 %). | |||||||
| Study drawbacks: longer follow-up, values GV/TV were not satisfactory | |||||||
| Mg-Zn (3.5–4.5 wt% Zn, ≤0.02 wt% Ni, ≤0.02 wt% Zr, ≤0.01 wt% Fe, ≤0.01 wt% Si, ≤0.05 wt% Mn, ≤0.01 wt% Cu, ≤0.01 wt% Al)/Si coated through MAO | Rectangular geometry with a volume of 12 mm × 10 mm × 4.5–6 mm and a 7-degree wedge angle, porosity 45 % | Goat spine model/24 healthy goats, 2–3 years old | Autogenous bone from iliac crest |
|
|
Material degradation: fast degradation rate for Mg-based cages in the first 6 weeks and then a gradual decrease; the | [308] |
| average volume decrease of the cages was 11.29 ± 2.32–3 | |||||||
| weeks, 18.42 ± 3.8–6 weeks, 23.53 ± 4.17–12 weeks, 26.23 ± 5.33–24 weeks | |||||||
| New bone formation: Collapse of the DSH in segments with cages Bone (ΔDSH 0.75 ± 0.45, P = 0.0098–24 weeks); Cage (ΔDSH 0.07 ± 0.81, P = 0.8364). A number of 33.3 % of the goats exhibited gas accumulation in front of the implant. Fusion score increased from 12 weeks to 24 weeks (p = 0.0219 and p = 0.0002, respectively). Mg2+ serum concentration 23 mg/L (starting point) – 25 mg/L | |||||||
| Mg-3Al-1Zn (AZ31) (Al, 2.5 %–3.5 %, Zn, 0. 6 %–1.4 %, Mn, 0.2 %–1.0 %, Si, maximum 0. 3 %)/Si coated through MAO | Rectangular geometry similar to a commercial cage (Cervios; Synthes, DePuy Spine, Raynham, MA, USA) | Goat spine model/24 goats (12 females, 12 males), 2-year-old | Autologous iliac bone in combination with Ti plate |
|
|
Material degradation: the cage average volume decrease was: 3 weeks- 5.9 ± 3.2 %; at | [310] |
| 6 weeks - 19.82 ± 5. | |||||||
| 40 %; 12 weeks −24.47 ± 5.3 %; 24 - | |||||||
| 26.72 ± 4.11 % | |||||||
| New bone formation: control group reached a successful bony fusion; necrotic tissue was evidenced in the case of Mg-based cages. CT Fusion score (Si coated Mg alloy cage: 0.9 (p = 0.0035 and 0.0024); Control: 2.5 (p < 0.001) | |||||||
| Study drawbacks: Mg corrosion kinetic and excessive Mg ions accumulations led to implant failure | |||||||
| Composite material: Mg-3Al-1Zn (AZ31) (Al, 2.5 %–3.5 %, Zn, 0. 6 %–1.4 %, Mn, 0.2 %–1.0 %, Si, maximum 0. 3 %) and polycaprolactone (PCL) | Cylindrical Mg alloy skeleton covered by PCL | Sheep spine model/24 full-grown blackcap sheep, 2 ÷ 4 years | Autologous iliac bone graft |
|
|
Material degradation: 3 weeks post-surgery, the average volume decrease was 5.4 ± 4.0 %; 6 weeks - 21.0 ± 6.9 %; 12 weeks, 36.8 ± 13.1 %; 24 weeks, 45.3 ± 14.1 % | [311] |
| New bone formation: fusion rate of 83.3 % - 24 weeks post-surgery for both types of cages | |||||||
| Study drawbacks: lack of fusion tendency, inadequate biomechanical and histological examinations |
ALT - alanine aminotransferase, AST - aspartate aminotransferase, CREA - serum creatinine, UREA - urine creatinine, RBC - red blood cell, HGB – hemoglobin, WBC - white blood cell, RT-qPCR – quantitative reverse transcription polymerase chain reaction, messenger RNA – mRNA, BMP2 – bone morphogenetic protein 2, RUNX2 – runt-related transcription factor 2, COL-I – collagen type I, BV/TV bone volume versus the total volume of the fusion cage, GV/TV – residual graft, DSH – disc space height.
4.1.2. High osteogenic and bioactive polycaprolactone (PCL) as polymeric bioresorbable material for spinal interbody fusion cages
Compared to polylactic acid (PLA), which was already used in clinical applications, whose degradation process is a bulk one, PCL is considered biodegradable. It maintains its initial value of Young's modulus, and 95 % of its mass remains unchanged for about 12 months [313]. In addition, PCL has improved viscoelastic and rheological properties compared to other aliphatic polyesters [314,315]. Melt extrusion represents an adequate technology for PCL processing because its melting point is very low (60 °C). It has a negative glass transition of about −60 °C and a high decomposition temperature of 350 °C. One important disadvantage of PCL is that cell adhesion and proliferation are limited in the implant vicinity, so it is usually combined with bioactive ceramics. Due to the fact that PCL exhibits high biocompatibility [316], structural stability [317], biodegradability [318], but limited mechanical properties [319], it could be considered a potential material for spinal cage manufacture. In addition, it can be easily 3D printed with a large range of commercial equipment, and complex cage shapes that can be fast achieved [315]. As stated before, good mechanical matching between interbody cages and cancellous bone is of utmost importance, so a porosity higher than 60 % and pore size larger than 300 μm are considered prerequisites for the development of new models of spinal cages [320].
Many researchers consider that the composite materials composed of PCL and different calcium phosphates (Ca-P) have adequate mechanical properties and promote osteogenesis [321,322]. In addition, when combining PCL with hydroxyapatite (HA), a hydroxyl carbonate apatite layer formation occurs, enabling the formation of a strong bond between the implant and host tissue [323]. Bioresorbable and high osteogenic spinal implants were already used to achieve lumbar porcine [258] and thoracic ovine [324] spine fusion. Abbah et al. [258] prepared from medical grade poly (epsilon-caprolactone), in which they incorporated 20 % beta-tricalcium phosphate (mPCL-TCP), an innovative high osteogenic and bioresorbable implant used as a fusion cage. The bone ingrowth was quantified in a large animal model of lumbar interbody fusion (Fig. 5.III). Two animal groups were set as follows: 6 pigs underwent an ALIF surgical procedure, and an mPCL-TCP implant together with 0.6 mg rhBMP-2 was inserted at the L3-L4 or L5-L6 site, and another 4 similar pigs were treated with autogenous bone graft (control group). For the first animal group, CT scans revealed a full defect bridging for all 6 specimens achieved at 3 months post-surgery. Based on histological analyses, a continuous bone remodeling process was present at 6 months. Regarding the control group, it was noticed at 6 months post-operatively that only 50 % of segments achieved a complete defect bridging. In addition, graft fractures occurred for 25 % of segments, and signs of pseudoarthrosis and bone resorption were visible. These findings clearly demonstrated that the mPCL-TCP implant exhibited better outcomes compared to the worldwide accepted gold standard, and the proposed bi-material structure can be confidently used as a lumbar spine fusion implant (Fig. 5. IV). One of the main limitations of this study is that the authors reported only the short-term effects of the cages. As a result, the degradation process of the fully resorbable cages was not analyzed. Nevertheless, the overall conclusion was that the fusion grade achieved in the ALIF animal model was considered satisfactory. Li et al. [325] made a high osteogenic and biodegradable macro-porous polycaprolactone-tricalcium phosphate (PCL-TCP) implant to be used as an autograft-free spinal fusion implant. The authors [325] compared the outcomes of this implant with Ti cages, in which autografts were incorporated, by investigating their in vivo osteogenic capabilities in sheep animal models. CT scans demonstrated that although PCL-TCP implants showed significantly reduced osteoconduction at 6 months post-implantation, this limitation was overcome by 12 months, with bone fusion comparable to that observed with Ti cages. The bone volume versus the total volume of the fusion cage for PCL-TCP cage was estimated at 43 % (p < 0.05). Histology and micro-CT investigation determined a better bone ingrowth of a 2.6-fold higher bone/interspace ratio and a homogenous bone distribution than in the case of Ti cages. Cadaveric mechanical tests revealed comparable stiffness in all range motions for both types of implants. The degradation profile of PCL-TCP was analyzed based on μ-CT scans and exhibited a much-reduced volume at 12 months (17 % cage volume percentage) in comparison with that measured at 6 months (21 % cage volume percentage), proving a partial degradation process (p < 0.05). It was concluded that PCL-TCP implants could be considered promising solutions that can be used even without autografts. Yong et al. [324] investigated the efficiency of a bioactive solution consisting of a PCL-based developed implant in combination with 0.54 μg rhBMP-2 compared to a simple implant or autograft in a mini-thoracotomy surgical approach. An ovine thoracic spine fusion model was chosen, and each animal underwent the operation at 3 levels in the thoracal region. After 6 months, all the animals were euthanized. CT scans showed a higher fusion rate obtained for the PCL implant combined with an orthobiologic substance compared to the other cases. Histological investigations proved the new bone formation for the above-mentioned type of implant, putting in evidence also the biodegradation process of the PCL. It was concluded that the combination of PCL and orthobiologics could be a viable alternative to the bi-material systems, although the last mentioned seems to work more properly in establishing a better fusion rate.
Liu et al. [326] conducted a prospective cohort study to assess the short- and mid-term safety of a 3D-printed biodegradable cage manufactured of PCL and β-TCP with a mass ratio of 50:50. This implant had good mechanical strength and stable resorption pattern, being developed for PLIF surgery. In this single-arm pilot clinical trial, 22 patients were included, with a follow-up time of 1-, 3-, 6-, and 12-months post-surgery. The average age of patients was 53.5 years. One patient was lost during the follow-up period, and another was excluded from the investigation due to cage retropulsion. The clinical outcomes of this trial were established according to the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ) and VAS for low back and leg pain. Based on X-rays and CT scans, the Intervertebral Space Height (ISH) was measured, and the cage degradation and bony fusion were estimated. The VAS score for back pain varied from 5.89 ± 0.99 before surgery to 1.15 ± 0.86 at 12 months post-surgery, while the VAS score for leg pain decreased from 5.75 ± 1.11 to 1.05 ± 0.76. Japanese Orthopedic Association (JOA) score increased from 13.8 ± 2.64 to 26.45 ± 2.46. Regarding the ISH parameter, an increase from 11.01 ± 1.75 mm to 26.45 ± 2.46 was noticed. In addition, a bony fusion rate of 95.1 % was estimated at 12 months follow-up. It was concluded that this new type of high osteogenic and bioresorbable cage led to good results from short- and mid-term points of view, but a more extended follow-up time must be analyzed to investigate the cage degradation profile, and many more clinical trials are necessary to estimate its safe use on humans.
Adding calcium phosphates to biodegradable polymers leads to an increase in the mechanical properties of the composite material. In particular, hydroxyapatite exhibits a high stiffness value, and increasing the bioceramic content by 25 % resulted in a 40 % increase in the compressive modulus of the polymer-ceramic composite [327]. It can be expected that increasing the HA content in a polymeric matrix will lead to a proportional increase in mechanical strength. Relatively recently, the Resomer® composite was developed by Evonik Industries AG, Essen, Germany [328], characterized by a 70/30 (PCL/HA) ratio. The producers of this material stated that the composite mechanical properties suit very well those of the human bone, and the stress shielding effect does not occur in the case of medical implants manufactured from this PCL/HA composite polymer. In Ref. [329], it was shown that a PCL cage coated with Ca-P had an improved spinal fusion rate in a minipig animal model.
It can be concluded that PCL/Ca-P composite material has great potential in spinal fusion surgery, as proved by animal studies and a human study performed in China. Although, this solution must be further checked and analyzed to establish its long-term outcomes. In the author's opinion, the use of high osteogenic and bioresorbable polymer-based materials can help overcome the limitations of traditional non-degradable PEEK cages and bioresorbable Hydrosorb™ implants.
4.1.3. Limitations of biodegradable and bioresorbable biomaterials
We will discuss here the primary limitations that often arise with degradable or bioresorbable materials. For Mg-based alloys, one of the problems identified in clinical practice consists of early mechanical failure when the implant is affected by the corrosive living body fluids. In this direction, Bonithon et al. [330] manufactured Mg-Y-Zn-Mn (WZM211) porous scaffolds. They were introduced to a solution of hydrofluoric acid (HF) to generate a MgF2 protective chemical conversion coating. Then, the scaffold behavior was investigated under mechanical cyclic loading combined with sample immersion in Hank's Balanced Salt Solution (HBSS). The mechanical tests consisted of a cyclic compression (30N/1Hz, 15000 cycles applied over two days). Regarding the in vitro corrosion process, measurements were performed at human body temperature and 5 % CO2 conditions for 2, 8, and 14 days. The main finding of this interesting study was that mechanical compression could be applied to uncoated samples during the first two days, after which complete degradation of the scaffolds was observed. In addition, scaffolds with a MgF2 conversion coating exhibited a slower degradation profile under the same combined corrosion and mechanical stimuli. Another study that analyzed the link between mechanical property measurements and immersion in Revised Simulated Body Fluid (r-SBF) was that of Dong. et al. [331]. The authors prepared samples from Mg-based alloys uncoated and coated with MgF2-CaP and MgF2 and applied an uniaxial compression test under immersion in r-SBF for 1, 3, and 7 days. A higher Young's modulus value was achieved for MgF2 coated samples (350 MPa), maintained until day 3. A decrease to about 7 MPa was obtained on day 7. For the MgF2-CaP coated scaffolds, it varied between 1100 MPa (days 1 ÷ 3) and 400 MPa (day 7). An extensive analysis of the variations in mechanical properties of different orthopedic implants fabricated from Mg-based alloys in physiological fluids is presented in the paper of Antoniac et al. [269]. As a general conclusion, early mechanical failure should be carefully considered in the design and manufacturing of lumbar spinal cages, and various strategies to improve corrosion resistance should be explored. Another problem associated with Mg implants is the inflammatory reaction triggered by hydrogen emission during the Mg corrosion process, as mentioned earlier in Section 4.1.1. Xie et al. [332] demonstrated that the gas emission should be checked in conjunction with different pore sizes and scaffold geometry. Authors prepared Mg-Nd-Zn-Zr scaffolds that were immersed in SBF (23 °C, 7 days). A degradation rate of approximately 0.04 g/day was computed, and it was observed that the gas emission decreased in direct proportion to the immersion time. In Ref. [333], an in vivo study performed on Mg-Li-Al-RE (LAE442) scaffolds coated with MgF2 was described. Zika rabbits with surgically induced defects in the greater trochanter were involved. Hydrogen emission gas was present at different euthanasia times (6, 12, 24, and 36 weeks) inside and outside the scaffolds. For the scaffolds with a pore size of 500 μm, the H2 emission was constantly reduced, with only small bubbles observed at 36 weeks. A higher gas quantity was evidenced at 24 weeks with no variation until 36 weeks in the case of 400 μm pores size scaffolds. It can be concluded that the inflammatory response of healthy tissue can be significantly reduced by selecting an appropriate implant geometry and by applying biocompatible coatings, as previously suggested.
Compared to Mg-based alloys, PCL exhibited a much smaller decrease in mechanical properties when exposed to aqueous or physiological media. Franca et al. [334] investigated the hydrolytic degradation effect on the mechanical properties of PCL using injection-molded samples immersed in hot water at 40 °C. After 15, 30, and 45 days of immersion, mechanical tests were conducted. A decrease in Young's modulus of approximately 9.65 % and 13.65 % was observed for samples immersed in water for 15 and 45 days, respectively. On the other hand, a 3.43 % increase was observed for 30-day immersion samples. It was concluded that this peculiar behavior could be attributed to changes associated with the phase transition of PCL from amorphous to crystalline during the conventional hydrolytic degradation process. Deshpande et al. [335] prepared a multifilament yarn PCL sample that was introduced in Phosphate-Buffered Saline solution (PBS) at 37 °C and agitated at 45 rpm for 32 weeks. After this period, nearly all mechanical properties declined as follows: peak load at break decreased by 46.5 %, tenacity by 40 %, and elongation at break by 42 %. These changes correlated with a sample mass loss of 4.8 %. The authors concluded that the PCL multifilament yarn entirely dissolved in 14 months. This reduction was significantly faster compared to the typical resorption time of 2–3 years observed for bulk PCL. Regarding the side effects of PCL, Argentieri et al. [336] analyzed the drawbacks of various implants, including mesh for hernia treatment, suture wires, PCL packing with Nasopore for endoscopic sinus surgery, and PCL spacers (Artelon CMC) for arthritis treatment, as well as facial dermal filler injections (Ellanse/Ellanse-M). They identified complications such as chronic pain, foreign body reaction, hernia recurrence, seroma, hematoma, wound dehiscence, edema, inflammation, blood accumulation, and fluid retention. Although these side effects were rarely reported, close follow-up is recommended for patients with PCL implants. By analyzing these side effects of biodegradable and bioresorbable materials, it can be anticipated that, if the limitations are addressed using the solutions outlined at the end of 4.1, 4.1.1.2, these materials could represent the future of spinal fusion surgery.
4.2. Bioactive and antibacterial surface treatments for biomaterials available on the market
In this subsection, we will focus on the surface treatments used for conventional materials that have already been approved for use in humans. These methods can alter the surface properties without significantly changing the initial mechanical properties, provided no structural modifications, such as adding porosity or developing complex geometries.
A very interesting study performed by Rao et al. [337] suggested the most important treatments for Ti alloys to convert their inert surface into an osteointegrative or bioactive one. The following solutions can be observed: modification of surface topography, increase in surface roughness, application of alkali or heat treatments, removal of sodium (Na) ions, design of a porous structure, and coating with various calcium phosphates [338]. Regarding surface roughness, it is well known that when cells come into contact with a given material, they first attach and adhere to it and then spread and multiply. Surface roughness can control and promote cell adhesion while limiting their micromotion. It was shown that the bone apposition process is sustained by a motion of up to 50 μm, while fibrous integration occurs in the range between 40 μm and 150 μm and hinders the new bone on-/in-growth [339,340]. In Ref. [341], it has been proven that generating micro-scale roughness on the Ti surface enhances protein adhesion and increases alkaline phosphatase activity in cell culture. This fact was a clear proof of osteogenic cell differentiation, being favored by the nano modifications present at the material surface. Usually, the electron beam melting method or plasma spay is involved in such processes. Kroppenstedt et al. [342] applied this type of surface modification in the case of a Ti stand-alone lumbar cage coated with Plasma-pore (Prospace, B. Braun Aesculap AG, Tuttlingen, Germany), and a successful fusion rate was achieved. Nishiguchi et al. [343] proved that a combined heat and alkali surface treatment applied to Ti enhanced the formation of a strong link between Ti and bone in a rabbit animal model. In Ref. [344], it was observed that the Na ion removal process significantly contributed to sustaining a good osteogenesis process in relation to Ti alloys. Porosity is another key issue, which was detailed in Section 3. Briefly, it is well known that preparing spinal cages with a certain degree of porosity reduces the implant's Young's modulus, thereby minimizing the occurrence of stress shielding. Chemical bonding between Ti alloy and HA can be generated at high temperatures by means of techniques such as plasma spray. The basis of this process was described in a study by De Groot et al. [345], who prepared an HA coating with a thickness of 50 μm and obtained a material that exhibited Ti's mechanical strength in combination with HA's bioactive character. Titanium can form a titanium dioxide (TiO2) layer that is beneficial to bone development and increases alloy corrosion resistance. In addition, TiO2 can generate negatively charged hydroxide (OH−) ions in a humid environment, which can bind to ions such as Ca2+ and PO43−, and a bone-like apatite layer could be formed. There are just a few studies in the literature that underline the positive effect of Ca-P coating on Ti interbody cages. Sun et al. [346] investigated the outcomes of a bioactive porous interbody fusion cage, treated with micro-arc oxidation and hydrothermal treatments, on spinal fusion in sheep. A bioactive Ca-P layer exhibiting a micro/nano multilevel deposition was developed on a 3D porous Ti6Al4V cage. In vitro investigations confirmed the cage surface's ability to guide the pre-osteoblasts cells to develop into mature osteoblasts, proving the high osteogenic potential of the developed implant. Supplementary, the in vivo analysis proved a strong cage-bone link in the sheep spinal fusion model. The cage exhibited high stability, and no subsidence was reported. It was concluded that this type of new bioactive cage is a viable alternative to the traditional inert Ti cages.
A combined antibacterial and bioactive Ti cage was developed by Morimoto et al. [347]. They prepared a 2 mm thick, complex silver-hydroxyapatite (Ag-HA) coating based on powders of AgO2 and HA (Kyocera, Kyoto, Japan). This innovative implant was used in treating 48 patients using TLIF or PLIF surgical approaches. Initially, for one year, side effects related to Ag, such as argyria or neuropathy, were searched. However, no such drawbacks were noticed. In addition, in the case of 39 patients, clinical improvements were observed, along with bony fusion rates of approximately 88 % at 6 months post-operatively and 91 % at 12 months post-surgery. It was concluded that this particular cage can be successfully used to achieve a strong bony fusion, even in the case of immunosuppressed or osteoporotic patients, due to the dual action of the coating. A similar study was conducted by Nakashima et al. [348], who developed an Ag-HA coating applied on a Ti spinal cage. The efficiency of this implant was tested in vivo on a rat spinal model based on the ALIF surgical approach. Toe pinch and inclined plane tests were applied to investigate the silver side effects, and micro-computed tomography and histology analyses were carried out to assess the osteogenerative potential of the coated cage. A higher bone contact rate was noticed in the coated cages compared to the uncoated implants, and no necrosis or silver accumulation in the rat spinal cord was present. It was concluded that this type of interbody cage can be successfully applied in the ALIF surgical method. The antibacterial efficiency of chitosan coating applied on a 3D-printed Ti cage was analyzed by Kodama et al. [349]. The authors made an in vivo study, which consisted of cage implantation together with Staphylococcus aureus in rats’ caudal discs. The bacterial survival rate was estimated based on an innovative in vivo visualization system post-operatively at days 1, 3, and 5. Micro CT scans and histology analyses revealed the infection-related changes and wound healing status. It was noticed that chitosan behaves as an antibacterial substance limiting the spread of infection in the animal body compared to bare Ti cages. It was concluded that this approach of using a natural polymeric coating deserves to be further developed because it exhibited an important antibacterial property and prevented the spinal instability that was noticed for the Ti uncoated cages.
The overall investigation of Ti spinal cages demonstrated high efficiency in achieving an increased bone fusion rate, with many promising research directions. The most important one consists of developing innovative coatings that combine bioactivity with antibacterial effect, providing a mixed outcome regarding cage stability and osteointegration and preventing the biofilm formation on the implant surface or even healing the infection acquired during the surgical intervention.
As we mentioned in Section 3, the PEEK surface is hydrophobic, and due to its pronounced inert character, if not treated, it cannot bond to the natural bone. There are many cases of subsidence and migrations of spinal cages and pseudoarthrosis [16,350,351] in the literature. Luckily, some methods to increase the PEEK's osteointegrative potential were proposed to address these limitations. The first approach adopted consists of the development of PEEK composites. Walsh et al. [179] showed, based on a sheep spinal fusion model, that by incorporating HA in the PEEK matrix and designing an innovative implant, bone formation was stimulated. They compared their result with those obtained for a classical PEEK cage implantation. In the last-mentioned experiment, only fibrous tissue was present in the cage vicinity. Another example comes from Wu et al. [352], who prepared a nano-TiO2/PEEK composite and compared its in vitro and in vivo osteogenic potential with traditional PEEK material (control sample). For the in vitro investigations, discs were cut from both materials. It was noticed that osteoblasts' pseudopods (MG-63 cell line) were strongly linked to the part where nano-TiO2 was present at the composite surface. The cell attachment test proved that the rough surface of nano-TiO2/PEEK exhibited the highest attachment rate compared to the smooth surface of PEEK. In vivo tests were made based on cylindrical implants introduced in the tibia medial surface placed in the proximal diaphyseal region of beagle dogs (aged 1.5 years and 11.4 ± 2.1 kg weight). It was concluded that the composite material implant led to a double quantity of new bone formation compared to bare PEEK samples. The combination between nano-Ti and PEEK was analyzed by Gunzburg et al. [175], who coated a PEEK spinal cage with nano-Ti and achieved a higher spinal stiffness in the case of a goat animal model. It was concluded that by coating PEEK with nano-Ti, the mechanical properties of the substrate remain unchanged. In addition, the nano-Ti coating improved the osteogenesis and the osteointegration of the implant by stimulating the Transforming Growth Factor (TGF)-β1 osteoprotegerin, angiopoietin 1, Fibroblast Growth Factor (FGF), and Vascular Endothelial Growth Factor (VEGF) [353,354]. Liu et al. [355] developed a layer-by-layer self-assembled multilayers on PEEK implants. Polystyrene sulfonate and polyallylamine hydrochloride were used to obtain a complex coating. The bioactive implant proved to have good adhesion and proliferation of Bone Marrow Stromal Cells (BMSCs) and good bone regenerative properties in an osteoporotic rabbit animal model. As we stated, one of the most used solutions to enhance the bone cell response is represented by Ca-P coatings applied on the PEEK spinal cage surface. Zhu et al. [356] analyzed the fusion rate efficiency of the titanium interlayer-mediated hydroxyapatite-coated PEEK (PEEK-Ti-HA) cage in a TLIF surgical approach. The authors [356] compared the outcomes of PEEK-Ti-HA lumbar cages (32 patients - Group A) with those of uncoated PEEK cages (32 patients - Group B). A follow-up time of 2 years was established, while X-ray investigations helped to assess the Disc Height (DH), Regional Lordosis (RL), and fusion rate. The patient satisfaction and the surgery success were analyzed based on the JOA score and VAS for back and leg pain scores. The main advantage of the bioactive PEEK cages was that at 3 months postoperatively, a higher fusion rate of 93.7 % compared to 75.0 % for uncoated implants was noticed. After 2 years, similar results were obtained not only regarding the fusion rate (100 % for both patient groups) but also measuring radiographically the DH and RL, as well as the JOA and VAS scores. It was concluded that the bioactive PEEK cages were able to help in achieving a very strong bony fusion, ensuring faster healing of the patients and providing a larger range of motion, compared to classical PEEK lumbar cages. However, at the end of the follow-up time, both types of implants exhibited similar outcomes.
Regarding the antibacterial coatings applied to PEEK material, a very interesting study was developed by Ishihama et al. [357]. They produced a new coating comprised of an HA film, in which ionic silver was trapped based on inositol hexaphosphate chelation performed at different immersion times and at a reduced temperature in order not to damage the polymeric substrate. In this way, it hindered the liberation of supplementary reactive oxidative species and avoided high working temperatures. In vitro tests proved the absence of a biofilm on the developed coated material, showing its strong bacteria-resistance feature. In vivo analyses performed on infected mice showed an important reduction of infection signs for the coated samples, while the animals with uncoated PEEK implants remained ill. It was concluded that such a bioactive and antibacterial coating could be successfully used in spinal surgery and has a promising future as an implant in orthopedics. An innovative solution combining antibacterial behavior with osteoconductive properties was developed by Pezzotti et al. [358], who incorporated Si3N4 into PEEK to obtain radiolucent spinal cages. The composite was prepared by dispersing a minor fraction of about 15 vol% of Si3N4 in the PEEK matrix in three different forms: β-Si3N4, α-Si3N4, and β-SiYAION. In vitro tests investigated the osteogenic potential of this new composite using SaOS-2 cells and proved an important proliferation rate, cell attachment, and differentiation, while antibacterial investigations analyzed the material bacteriostatic character against Staphylococcus epidermis. The most reduced number of bacteria was noticed for PEEK/β-SiYAION samples, and the highest number of pathogens was reported for PEEK/α-Si3N4. It was concluded that the type of bioactive ceramic is of utmost importance in developing such complex antibacterial spinal cages.
This summary regarding the PEEK bioinertia improvements suggests that creating composite materials or using bioactive and antibacterial coatings could represent promising approaches to increase the osteointegration of PEEK cages in spinal fusion surgery. One can observe that, despite these solutions demonstrating good results in in vitro and in vivo studies, a limited number of published clinical trials are available to confirm their efficacy and safety in humans. It is recommended to search for such innovative solutions only after these already discovered approaches are thoroughly tested because they exhibit a high potential to improve patients' well-being.
Fig. 6 presents in vitro and in vivo results obtained for Ti6Al4V or PEEK cages with different surface treatments or coatings.
Fig. 6.
In vitro and in vivo analysis results obtained in the case of different lumbar cages with surface treatment or coatings. Sun et al., 2024 [346]: (6.I) In vitro results on hBMSCs: (a) Life/Dead assay, (b) CCK-8 assay, (c) CLSM images of cell cytoskeleton, (d) ALP activity; (6.II) In vivo results: (a) 2D and 3D μ-CT images, (b) quantitative analysis for new bone formation (BV/TV and Tb.Th), (c) intervertebral fusion status analysis (size bar = 1 mm) (Figure is licensed under CC BY-NC 3.0); Wu et al., 2023 [359]: (6.III) (a)/(b) Surface morphology/Contact angle values: (a-1)/(b-1) 800 °C, (a-2)/(b-2) 800 °C and SB, (a-3)/(b-3) 800 °C, acid-etching, and alkali-etching after SB (AAS); (6.IV) In vitro tests: (a) MC3T3-E1 culture on Day 1 (a-1) and Day 5 (a-2), (b) osteogenic ALP (b-1) and mineralized ARS (b-2) (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001) (Reprinted from Ref. [359] Copyright (2025), with permission from Elsevier); Kodama et al., 2021 [349]: (6.V) Antibacterial in vitro test: (A) Agar plates showing inhibition zone, (B) Size of bacteria inhibition zone, (C) Colonies on agar plates, (D) Number of colonies ∗∗p < 0.01, (E) Crystal violet staining at 24 and 48 h, (F) Spectrometer data (average ± standard deviation, n = 3, ∗p < 0.05 T-test (scale bars = 1 mm); (6.VI) In vivo test: Wound image and μ-CT evaluation: (A) Surgical images, (B) Wound scoring, (C) μ-CT image of Ti cage, (D) μ-CT image of Ti-HACC cage, (E) CT score (average ± standard deviation, n = 6 - Ti cage and n = 7 – Ti-HACC, ∗p < 0.05 Mann-Whitney U test (scale bars = 0.5 mm) (Reprinted from Ref. [349] Copyright (2025), with permission from Elsevier); Ishihama et al., 2021 [357]: (6.VII) Antibacterial in vitro test: (A) High bacterial bioluminescent signals for PEEK cage and absence of bacterial signals for PEEK-Ag+, (B) Bacterial photon intensity (p = 0.0032), (C) SEM images indicated the presence of S. aureus and biofilm existence – PEEK cage, (D) SEM images with no pathogens – PEEK-Ag+ cage; (6.VIII) Histopathology results: (A) PEEK cage – large abscess presence (∗), (B) - PEEK-Ag+ cage, (C) PEEK – cage with enlarged images (D) and (E) revealing bacterial clots and inflammatory cells (bars: A,B – 2000 μm, C – 1000 μm, D – 400 μm, E − 200 μm (Figure is licensed under CC BY-4.0); Shimizu et al., 2017 [360]: (6.IX) In vivo test: (A) μ-CT images: (a), (b) uncoated PEEK cage, (c), (d) TiO2-coated PEEK cage, (B) Bony union rate; (6.X) In vivo results: (A) Histology: (a) uncoated PEEK cage, (b) TiO2-coated PEEK cage, (c) magnified image, (B) Histomorphometry measurement of bone-implant contact ratio (Figure is licensed under CC BY-4.0).
In this subsection, we presented only the studies directly related to the most used commercial biomaterials, such as Ti alloys and PEEK, in spinal fusion surgery. The literature describes numerous methods to enhance the bioactivity and antibacterial properties of these materials in general, but only a limited number of studies have evaluated their potential for use in spinal cages. In the authors’ opinion, these solutions must be carefully checked to see if they fit correctly into the interbody fusion domain.
Table S2 provides a literature selection, which presents some examples of osteointegration, bioactive, or antibacterial surface treatments or coatings used for spinal cages in clinical trials or in vitro and in vivo tests [[359], [360], [361], [362], [363]].
5. Current limitations, future perspectives, and conclusions
From the outset, it is important to notice several critical technical bottlenecks directly related to the materials involved in lumbar cage manufacturing. To underscore these limitations, attention will be focused on the mechanical attenuation law and degradation behavior, toxicity of degradation products, and long-term imaging performance of the two proposed bioactive materials (Section 4.1), compared to traditional Ti alloys and PEEK cages, which are the most commonly used biomaterials in clinical practice (Table 4). It is evident that a feasible approach for the clinical application of lumbar cages made from Mg-based alloys involves controlling their degradation rate while enhancing their mechanical properties.
Table 4.
Comparison between bioactive and traditional materials used in spinal cage fabrication.
| Material | Main mechanical properties/Mechanical attenuation law | Degradation product toxicity | Long-term imaging performance |
|---|---|---|---|
| Bioactive Mg-based alloys | Young's modulus: 41 ÷ 45 GPa, Yield Strength: 65 ÷ 100 MPa, Main Advantage: mechanical properties with values very close to those of the human bone (Section 4.1.1.) Mechanical attenuation law is usually characterized by a good damping capacity, which is influenced by alloying chemical elements, processing methods, and surface quality [364,365]. When introduced as an implant inside the human body due to various conditions, it began to degrade, but it must maintain its mechanical properties for about 4 ÷ 8 weeks. Main disadvantage: Mg-based alloy mechanical properties should be tuned to offer the human body sufficient time to regenerate | Biocompatible product toxicity: magnesium ions Mg2+, magnesium hydroxide Mg(OH)2, and hydrogen gas H2. The quantity of hydrogen emission should be carefully checked in order to avoid hydrogen accumulation and necrosis | Mg-based alloys are biodegradable metals that are compatible with medical images techniques (MRI and CT scans), and regarding their long-term performance they will dissolve entirely in a certain amount of time |
| Bioresorbable PCL | Bulk structure: tensile yield strength 8.2 ÷ 10.1 MPa, compressive strength 38.7 MPa, porous structure 3.2 ÷ 0.76 MPa [366]. Main advantage: good mechanical property, main disadvantage: material inertia. Regarding the mechanical attenuation law, PCL has the same Young's modulus value for 12 months, and 95 % of his mass was maintained for about 1 year, with a complete resorption higher than 3 years [335]. It is advisable to increase the material osteogenic capabilities as presented in Section 4.1.2. | Low product toxicity: PCL degrades inside the human body based on hydrolysis and results in carbon dioxide and water. Attention should be devoted to maintaining the CO2 level in biological limits to avoid hypercapnia [367] | PCL is radiolucent, and without using a contrast agent, it can become difficult to monitor its state based on X-ray investigations [368] |
| Traditional Ti alloys | Young's modulus: 100 ÷ 110 GPa, tensile strength 600 MPa ÷ 1250 MPa [369]. Main disadvantage: stress shielding effect. Ti alloys are not biodegradable metals, so as a direct consequence, their mechanical properties are not affected by the corrosive media inside the living bodies | Ti alloy toxicity is due to metallic ions and particle debris that can determine the metalosis onset as well as the hard tissue necrosis | Compatible with MRI images and CT scans, due to their non-magnetic nature [370] |
| Traditional PEEK | Young's modulus: 3.5 GPa, carbon fiber reinforced PEEK tensile strength 120 MPa. Main advantage: mechanical properties with values close to those of the human bone Main disadvantage: PEEK bioinertia. Exactly as in the case of Ti alloys PEEK did not lose in time its mechanical properties due to the fact that it is not bioresorbable | The main decomposition PEEK products are carbon monoxide CO, aromatic ethers, carbon dioxide (CO2), and phenols. Another harmful condition to bone health is linked to polymer debris due to friction and wear processes [371] | PEEK is highly compatible with MRI and CT investigations, allowing a good monitoring of the implant integration and healing process [372] |
Spinal fusion failure cases are usually associated with non-union at the surgery place and loss of device fixation. The last category includes changes in implant position, cage subsidence, cage migration, or a combination of subsidence and migration [373]. Yu et al. [374] performed a very detailed and interesting study that showed that anatomical and surgical factors are very important in establishing a good outcome of lumbar fusion surgery by selecting and analyzing 29 publications in the field. They found that the highest subsidence rate, which varied between 7.4 and 62.2 %, was detected when the cage was inserted at distal levels (L5-S1 and L4-L5). In addition, cage migration exhibited a similar trend, with an incidence of about 54.4 % for the L4-L5 level and 36.9 % for the case of L5-S1. One can notice that these two levels seem to be more affected by DDD. It was established that when surgical intervention is made at distal levels, a high risk of cage subsidence or migration should be considered [[375], [376], [377]]. Another analyzed aspect was the number of fusion levels. By performing the fusion surgery at two spine levels, a higher number of cage subsidence and migration was observed (cage subsidence: 15.5 % - one level, 26.2 % - multi-level; cage migration: 4.9 % - one level, 5.3 % - multi-level). The patient Bone Mineral Density (BMD) is another key factor. Some studies by Park et al. [121] and Cho et al. [378] concluded that implant failure occurred mainly in the case of osteoporotic patients (18.1 % compared to 9.7 % for patients with good BMD in Ref. [121] and 65.4 % compared to 17.6 % for non-osteoporotic patients in Ref. [378]). Supplementary, a shorter disk height before the surgery was linked to an increased risk of subsidence, as underlined by Yao et al. [379]. Also, pear-shaped/irregular discs were associated in the literature with high subsidence and/or migration cage rate [121,380,381]. Other anatomical factors reported in different clinical research were lordosis angle, range of motion, and screw fixation. Regarding the lordosis, Marchi et al. [382] noticed that cage subsidence was present at a lordosis angle higher than 49.1°, the range of motion was slightly decreased when cage failure occurred [383,384], and cage subsidence was associated with unilateral screw fixation in Refs. [115,385,386].
As stated before, cage attributes such as material, size, shape, and position are important aspects that must be considered when a successful fusion is followed in clinical practice. Many cage subsidence and migration were noticed in the case of PEEK material [380,383,385,387], compared to Ti or 3D-printed porous Ti cages [388]. A thorough review of the literature indicates that a detailed comparison between Ti and PEEK cages is essential to effectively address concerns related to fusion rates and the incidence of postoperative infections. In this direction, Kim et al. [389] analyzed 3D–printed Ti and PEEK cages after 1-year outcome. In all cases, it was used as a surgical approach the MIS-TLIF technique. For Ti cages, the fusion grades were as follows: grade I – 77.5 %, grade II – 17.5 %, grade III – 5 %, while for PEEK cages: grade I – 51.2 %, grade II – 17.5 %, grade III – 7.0 % were found. The main conclusion of this study was that no significant differences in fusion rates were achieved; however, in the case of Ti cages, slightly increased values were reported. A similar investigation was performed by Yang et al. [390], who compared fusion rates, subsidence rates, and clinical outcomes between 3D-printed porous titanium and PEEK cages in 150 patients who underwent PLIF surgery, with a minimum follow-up period of two years. After one year, the fusion rates were 86.9 % for titanium cages and 67.7 % for PEEK cages. However, after a 2-year follow-up, higher percentages, such as 92.9 % (Ti cages) and 82.3 % (PEEK cages), were achieved. Regarding the subsidence rate, no important changes were reported (Ti cage – 17.9 %, PEEK cage – 23.4 %). Similar outcomes were obtained for VAS score for back pain and leg pain in the case of the two investigated cages. A recent study [391] compared the radiographic fusion rates and subsidence for Ti cages and Ti-coated PEEK cages (PEEK-Ti). Based on the TLIF surgical approach, 48 patients were treated with PEEK-Ti and Ti cages. At the 6-month follow-up, subsidence was significantly reduced for PEEK-Ti cages (4.8 %) compared to 27.9 % for Ti cages. A fusion rate of 100 % for PEEK and 95.5 % for Ti cages was reported. It was concluded that an important reduced subsidence rate was associated with PEEK-Ti cages. It can be noticed that this type of lumbar spinal cage could be successfully applied in fusion surgery treatments. A contradictory result was presented in Ref. [392], where 3D-printed Ti cages exhibited a lower subsidence rate than PEEK cages for the LLIF surgical approach. To determine which cage material yields better clinical outcomes—such as reduced subsidence or higher fusion rates—long-term clinical studies with extended follow-up periods are necessary. A lack of detailed clinical trials regarding antibacterial coatings on lumbar spinal cages and extensive comparisons between different materials can be identified in the literature. Although Basgul et al. [393] demonstrated the antimicrobial performance of a Si3N4–PEEK composite—showing a 93.9 % reduction in bacterial growth against Escherichia coli and Staphylococcus epidermidis compared to conventional PEEK—there remains a clear need for further clinical studies. There are also other factors that influence infection rates, including the surgical approach, the patient's general health state, and post-surgical care [394]. Another important aspect that is worth mentioning is the fact that cage size should also be investigated. Zhao et al. [395] analyzed the cage migration in the case of large cages (31 × 18 × 11 mm3) compared to small cages (28 × 14 × 9 mm3) and found a higher failure rate for small cages. The cage shape was associated with the possibility of failure. Some studies [[383], [384], [385]] proved that cage migration occurred mainly in the case of straight cages (bullet – or rectangular-shaped) compared to curved cages (banana- or wedge-shaped). Regarding cage position, a higher risk of subsidence and migration was noticed for the medial insertion, followed by anterior and posterior localization [121,[396], [397], [398]].
It can be concluded that the present cage solutions are associated in some cases with an increased failure risk or even non-union and infections, and improved solutions should be continuously searched (Fig. 7).
Fig. 7.
Current clinical limitations and key factors in lumbar fusion surgery: (7.I) Example of cage migration: CT scan image showing displacement and cage position modification generating central canal stenosis [386] (Figure is licensed under CC BY-3.0); (7.II) Example of cage subsidence: (a) lateral view, (b) anterior-posterior view exhibiting pseudoarthrosis, (c) lateral view presenting cage breakage, (d) lateral view evidencing cage retropulsion [388] (Figure is licensed under CC BY-4.0); (7.III) Example of cage subsidence and influence of spinal fusion level number after OLIF: (a) pre-operatory image for OLIF at L3-L4-L5 levels, (b) postoperative image taken immediately after surgical intervention, (c) 6 months post-surgery image showing an important cage subsidence [48] (Figure is licensed under CC BY-NC 4.0); (7.IV) Example of CT scan presenting non-union at L5-S1 level at 1 year post-surgery: (a) coronal view, (b) sagittal view [166] (Figure is licensed under CC BY-NC-ND 4.0); (7.V) Example of migration cage and pedicle screw loosening influence: (a) lateral view of L4-L5 TLIF and L3-L5 bilateral posterior instrumentation, (b) PCM at L4-L5 level at 13 days post-operatively, (3) lateral view at 2 days after revision surgery (cage was removed and the right pedicle screw at L5 was improved [387] (Figure is licensed under CC BY-4.0); (7.VI) Example of measurements on plan radiograph [397] (Figure is licensed under CC BY-NC-ND 4.0); (7.VII) Example of aggravation of sagittal balance after PLIF at L5-S1 level with 8° lordotic cage: (A) preoperative image, (B) MRI image presenting stenosis at L5-S1 (white arrow), (S) Sagittal parameters did not improve even after 2 years post-surgery [398] (Figure is licensed under CC BY-NC-4.0). Fig. 7 was generated using an image from www.freepik.com, accessed on February 3, 2025.
One future trend supposes the development of an ideal surface with osteoprogenitive, bioactive, and antibacterial properties. The integration of biologics into spinal surgery could optimize surgical operation outcomes by enhancing the bone regeneration process [252,399]. One of the most discussed bioactive factors in the clinical practice are Bone Morphogenetic Proteins (BMPs) [400,401], inorganic Bovine-derived Hydroxyapatite Matrix (ABM) in combination with 15 amino acid sequence (P-15) [402], Platelet-Rich Plasma (PRP), Recombinant Human Platelet-derived Growth Factor B homodimer (rhPDGF-BB), and prostaglandin E2 receptor EP4 [402]. Innovative coatings are still being researched worldwide, and clinical trials are ongoing. Attention should be devoted firstly to reducing bacterial adhesion, colonization, and biofilm formation [403]. In addition, the ideal coating should promote implant osteointegration by providing bioactive inorganic or organic components to bone. In our opinion, the most adequate solutions are the use of calcium phosphates and biodegradable polymers in order to obtain complex hybrid and bioactive coatings. Unfortunately, there is a lack in the literature regarding these aspects.
Another direction comes from biodegradable metals like magnesium-based alloys that can be successfully used to design new biodegradable spinal cages with better mechanical properties. These types of implants facilitate a good osteointegration process and gradually dissolve over time, preventing biofilm formation on their surface. In addition, they have demonstrated promising outcomes in in vivo testing on various animal models and can be further developed to be applied in clinical trials. In our opinion, this approach represents the future of spinal cage fusion, combining advanced materials with natural solutions to inhibit biofilm formation. In this way, it will be possible for the patients to heal themselves starting from the disease place and to fight the pathogens that exist in every hospital.
As a final conclusion drawn after analyzing the advantages and disadvantages of traditional and bioactive solutions proposed in the present paper, a clear transformation strategy can be proposed. First, the biological requirements of the natural bone healing process must align with the biodegradability or bioresorbability of the spinal cage to ensure that the implant provides adequate mechanical support while releasing bioactive substances that promote bone regeneration. By incorporating orthobiologics or metallic ions commonly found as trace elements in the human body, it may be possible to surpass the current gold standard for spinal cages, which consists of titanium alloys and PEEK. In addition, the transition to these newly proposed solutions must be carefully managed to decrease the rapid corrosion process of Mg-based alloys, reduce H2 emission, enhance the biocompatibility of PCL, and minimize its associated inflammatory response. All these compulsory requirements should be considered to develop a highly efficient spinal cage that supports natural vertebral bone regeneration, eliminating the need for traditional cages that are often regarded as foreign bodies.
The main objective of the current review was to raise awareness among specialists interested in lumbar spine fusion about bioactive, high osteogenic, and antibacterial solutions, focusing on the development of new biodegradable or bioresorbable materials and the enhancement of existing ones to address the complex challenge of this clinical problem, a globally significant objective.
CRediT authorship contribution statement
Iulian Antoniac: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Veronica Manescu: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Gheorghe Paltanea: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Aurora Antoniac: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Marco Fosca: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Dan Laptoiu: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Julietta V. Rau: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization.
Ethics approval and consent to participate
Not applicable.
Founding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Julietta V. Rau is an associate editor board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article.
Iulian Antoniac is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article.
All authors declare that there are no competing interests.
5. Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under the responsibility of editorial board of Bioactive Materials.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2025.07.035.
Contributor Information
Iulian Antoniac, Email: antoniac.iulian@gmail.com.
Veronica Manescu (Paltanea), Email: veronica.paltanea@upb.ro.
Gheorghe Paltanea, Email: gheorghe.paltanea@upb.ro.
Aurora Antoniac, Email: antoniac.aurora@gmail.com.
Marco Fosca, Email: marco.fosca@ism.cnr.it.
Dan Laptoiu, Email: danlaptoiu@yahoo.com.
Julietta V. Rau, Email: giulietta.rau@ism.cnr.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chong E., Mobbs R.J., Pelletier M.H., Walsh W.R. Titanium/polyetheretherketone cages for cervical arthrodesis with degenerative and traumatic pathologies: early clinical outcomes and fusion rates. Orthop. Surg. 2016;8:19–26. doi: 10.1111/os.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim K.-M., Park T.-H., Lee S.-J., Park S.-J. Design and biomechanical verification of additive manufactured composite spinal cage composed of porous titanium cover and PEEK body. Appl. Sci. 2019;9:4258. doi: 10.3390/app9204258. [DOI] [Google Scholar]
- 3.Warren J.M., Hey L.A., Mazzoleni A.P. A finite element study of the relationship between upper body weight and the loads experienced by the human Lumbosacral spine, and fusion instrumentation, in a standing upright posture. Biomed. Eng. Adv. 2021;2 doi: 10.1016/j.bea.2021.100023. [DOI] [Google Scholar]
- 4.Parenteau C.S., Lau E.C., Campbell I.C., Courtney A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using medicare data. Sci. Rep. 2021;11:5389. doi: 10.1038/s41598-021-84724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinjikji W., Luetmer P.H., Comstock B., Bresnahan B.W., Chen L.E., Deyo R.A., Halabi S., Turner J.A., Avins A.L., James K., et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J. Neuroradiol. 2015;36:811–816. doi: 10.3174/ajnr.A4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theodore N. Degenerative cervical spondylosis. N. Engl. J. Med. 2020;383:159–168. doi: 10.1056/NEJMra2003558. [DOI] [PubMed] [Google Scholar]
- 7.Weiss H.-R., Goodall D. Rate of complications in scoliosis surgery – a systematic review of the pub med literature. Scoliosis. 2008;3:9. doi: 10.1186/1748-7161-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T., Zhang N., Chen L., Li J., Chen Q., Li F. Posterior spinal fusion for severe kyphosis in a child with gaucher disease: a case report and review of the literature. Surgeries. 2024;5:619–626. doi: 10.3390/surgeries5030049. [DOI] [Google Scholar]
- 9.Crawford C.H., Carreon L.Y., Djurasovic M., Glassman S.D. Lumbar fusion outcomes in patients with rheumatoid arthritis. Eur. Spine J. 2008;17:822–825. doi: 10.1007/s00586-008-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Wang Z., Wang Y., Li Z., Chao B., Liu S., Luo W., Jiao J., Wu M. Biomaterials for interbody fusion in bone tissue engineering. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.900992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanek P., Bradac O., DeLacy P., Saur K., Belsan T., Benes V. Comparison of 3 fusion techniques in the treatment of the degenerative cervical spine disease. Is stand-alone autograft really the “gold standard?”: prospective study with 2-Year Follow-Up. Spine. 2012;37:1645. doi: 10.1097/BRS.0b013e31825413fe. [DOI] [PubMed] [Google Scholar]
- 12.Farrokhi M.R., Nikoo Z., Gholami M., Hosseini K. Comparison between acrylic cage and polyetheretherketone (PEEK) cage in single-level anterior cervical discectomy and fusion: a randomized clinical trial. Clin. Spine Surg. 2017;30:38. doi: 10.1097/BSD.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 13.Huang H., Jiang C., Feng Z., Jiang X. Comparing the process of creeping substitution between allograft bone and local bone grafting in lumbar interbody fusion. Eur. Spine J. 2014;23:2068–2074. doi: 10.1007/s00586-014-3388-6. [DOI] [PubMed] [Google Scholar]
- 14.Grgurevic L., Erjavec I., Gupta M., Pecin M., Bordukalo-Niksic T., Stokovic N., Vnuk D., Farkas V., Capak H., Milosevic M., et al. Autologous blood coagulum containing rhBMP6 induces new bone formation to promote anterior lumbar interbody fusion (ALIF) and posterolateral lumbar fusion (PLF) of spine in sheep. Bone. 2020;138 doi: 10.1016/j.bone.2020.115448. [DOI] [PubMed] [Google Scholar]
- 15.Virk S.S., Coble D., Bertone A.L., Hussein H.H., Khan S.N. Experimental design and surgical approach to create a spinal fusion model in a New Zealand white rabbit (oryctolagus cuniculus) J. Invest. Surg. 2017;30:226–234. doi: 10.1080/08941939.2016.1235748. [DOI] [PubMed] [Google Scholar]
- 16.Warburton A., Girdler S.J., Mikhail C.M., Ahn A., Cho S.K. Biomaterials in spinal implants: a review. Neurospine. 2020;17:101–110. doi: 10.14245/ns.1938296.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma R., Virk S., Qureshi S. Interbody fusions in the lumbar spine: a review. HSS J. 2020;16:162–167. doi: 10.1007/s11420-019-09737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X., Xu H., Zhang F., Lu F. Bioabsorbable high-purity magnesium interbody cage: degradation, interbody fusion, and biocompatibility from a goat cervical spine model. Ann. Transl. Med. 2020;8:1054. doi: 10.21037/atm-20-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park P.J., Lehman R.A. Optimizing the spinal interbody implant: current advances in material modification and surface treatment technologies. Curr. Rev. Musculoskelet Med. 2020;13:688–695. doi: 10.1007/s12178-020-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Kunder S.L., Rijkers K., Caelers I.J.M.H., de Bie R.A., Koehler P.J., van Santbrink H. Lumbar interbody fusion: a historical overview and a future perspective. Spine. 2018;43:1161. doi: 10.1097/BRS.0000000000002534. [DOI] [PubMed] [Google Scholar]
- 21.Hibbs R.A. A report of fifty-nine cases of scoliosis treated by the FUSION operation. JBJS. 1924;6:3. [PubMed] [Google Scholar]
- 22.Albee F.H. The classic: transplantation of a portion of the tibia into the spine for pott's disease: a preliminary report. Clin. Orthop. Relat. Res. 2007;460:14. doi: 10.1097/BLO.0b013e3180686a0f. [DOI] [PubMed] [Google Scholar]
- 23.Cloward R.B. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. J. Neurosurg. 1953;10:154–168. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 24.Cloward R.B. The anterior approach for removal of ruptured cervical disks. J. Neurosurg. 1958;15:602–617. doi: 10.3171/jns.1958.15.6.0602. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald R.L., Fehlings M.G., Tator C.H., Lozano A., Fleming J.R., Gentili F., Bernstein M., Wallace M.C., Tasker R.R. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J. Neurosurg. 1997;86:990–997. doi: 10.3171/jns.1997.86.6.0990. [DOI] [PubMed] [Google Scholar]
- 26.Rieger J.H. Hypersurfaces of extremal slope. Proc. R. Soc. Edinb. Sect. A (Math. Phys. Sci.): Mathematics. 2003;133:449–465. doi: 10.1017/S030821050000247X. [DOI] [Google Scholar]
- 27.Lewandrowski K.-U., Hecht A.C., DeLaney T.F., Chapman P.A., Hornicek F.J., Pedlow F.X. Anterior spinal arthrodesis with structural cortical allografts and instrumentation for spine tumor surgery. Spine (Phila Pa 1976) 2004;29:1150–1158. doi: 10.1097/00007632-200405150-00019. ; discussion 1159. [DOI] [PubMed] [Google Scholar]
- 28.Bridwell K.H., Lenke L.G., McEnery K.W., Baldus C., Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410–1418. [PubMed] [Google Scholar]
- 29.Couture D.E., Branch C.L. Posterior lumbar interbody fusion with bioabsorbable spacers and local autograft in a series of 27 patients. Neurosurg. Focus. 2004;16:E8. doi: 10.3171/foc.2004.16.3.9. [DOI] [PubMed] [Google Scholar]
- 30.Kabins M.B., Weinstein J.N. The history of vertebral screw and pedicle screw fixation. Iowa Orthop. J. 1991;11:127–136. [Google Scholar]
- 31.Steffee A.D., Sitkowski D.J. Posterior lumbar interbody fusion and plates. Clin. Orthop. Relat. Res. 1988;227:99–102. [PubMed] [Google Scholar]
- 32.Blume H.G. Unilateral posterior lumbar interbody fusion: simplified dowel technique. Clin. Orthop. Relat. Res. 1985:75–84. [PubMed] [Google Scholar]
- 33.Harms J. True spondylolisthesis reduction and monosegmental fusion in spondylolisthesis. Textbook Spinal Surg. 1997:1337–1347. [Google Scholar]
- 34.Heiple K.G., Goldberg V.M., Powell A.E., Bos G.D., Zika J.M. Biology of cancellous bone grafts. Orthop. Clin. N. Am. 1987;18:179–185. doi: 10.1016/S0030-5898(20)30381-3. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein J.N., Tosteson T.D., Lurie J.D., Tosteson A.N.A., Hanscom B., Skinner J.S., Abdu W.A., Hilibrand A.S., Boden S.D., Deyo R.A. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilyas H., Udo-Inyang I., Savage J. Lumbar spinal stenosis and degenerative spondylolisthesis: a review of the SPORT literature. Clin. Spine Surg. 2019;32:272–278. doi: 10.1097/BSD.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 37.Abdu W.A., Sacks O.A., Tosteson A.N.A., Zhao W., Tosteson T.D., Morgan T.S., Pearson A., Weinstein J.N., Lurie J.D. Long-term results of surgery compared with nonoperative treatment for lumbar degenerative spondylolisthesis in the spine patient outcomes research trial (SPORT) Spine (Phila Pa 1976) 2018;43:1619–1630. doi: 10.1097/BRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provaggi E., Capelli C., Leong J.J.H., Kalaskar D.M. A UK-Based pilot study of current surgical practice and implant preferences in lumbar fusion surgery. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grotle M., Småstuen M.C., Fjeld O., Grøvle L., Helgeland J., Storheim K., Solberg T.K., Zwart J.-A. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyer F., Yagdiran A., Eysel P., Bredow J. Vergleich der Lebensqualität nach operativer Therapie einer vertebralen Osteomyelitis und degenerativer Spondylolisthesis. Z. für Orthop. Unfallchirurgie. 2023;162:487–492. doi: 10.1055/a-2151-5022. [DOI] [PubMed] [Google Scholar]
- 41.Kim P., Kurokawa R., Itoki K. Technical advancements and utilization of spine Surgery--International disparities in trend-dynamics between Japan, Korea, and the USA. Neurol. Med.-Chir. 2010;50:853–858. doi: 10.2176/nmc.50.853. [DOI] [PubMed] [Google Scholar]
- 42.Deyo R.A., Nachemson A., Mirza S.K. Spinal-fusion surgery - the case for restraint. N. Engl. J. Med. 2004;350:722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 43.Rajaee S.S., Bae H.W., Kanim L.E.A., Delamarter R.B. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh S.R., Thompson N.R., Benzel E., Steinmetz M., Mroz T., Tomic D., Machado A., Jehi L. Can we justify it? Trends in the utilization of spinal fusions and associated reimbursement. Neurosurgery. 2020;86:E193–E202. doi: 10.1093/neuros/nyz400. [DOI] [PubMed] [Google Scholar]
- 45.Taba H.A., Williams S.K. Lateral lumbar interbody fusion. Neurosurg. Clin. 2020;31:33–42. doi: 10.1016/j.nec.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Yingsakmongkol W., Jitpakdee K., Varakornpipat P., Choentrakool C., Tanasansomboon T., Limthongkul W., Singhatanadgige W., Kotheeranurak V. Clinical and radiographic comparisons among minimally invasive lumbar interbody fusion: a comparison with three-way matching. Asian Spine J. 2022;16:712–722. doi: 10.31616/asj.2021.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee W.-M., You K.-H., Kang M.-S., Kim J.-H., Park H.-J. Oblique lumbar interbody fusion with selective biportal endoscopic posterior decompression for multilevel lumbar degenerative diseases. Asian Spine J. 2023;17:392–400. doi: 10.31616/asj.2022.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y.-H., Ha K.-Y., Rhyu K.-W., Park H.-Y., Cho C.-H., Kim H.-C., Lee H.-J., Kim S.-I. Lumbar interbody fusion: techniques, pearls and pitfalls. Asian Spine J. 2020;14:730–741. doi: 10.31616/asj.2020.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S.-B., Yoon J., Park S.-J., Chae D.-S. Expandable cages for lumbar interbody fusion: a narrative review. J. Clin. Med. 2024;13:2889. doi: 10.3390/jcm13102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frisch R.F., Luna I.Y., Brooks D.M., Joshua G., O'Brien J.R. Clinical and radiographic analysis of expandable versus static lateral lumbar interbody fusion devices with two-year Follow-Up. J. Spine Surg. 2018;4:62–71. doi: 10.21037/jss.2018.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawasli A.H., Khalifeh J.M., Chatrath A., Yarbrough C.K., Ray W.Z. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg. Focus. 2017;43:E10. doi: 10.3171/2017.5.FOCUS17197. [DOI] [PubMed] [Google Scholar]
- 52.Kersten R.F.M.R., Öner F.C., Arts M.P., Mitroiu M., Roes K.C.B., De Gast A., Van Gaalen S.M. The SNAP trial: 2-year results of a double-blind multicenter randomized controlled trial of a silicon nitride versus a PEEK cage in patients after lumbar fusion surgery. Glob. Spine J. 2022;12:1687–1695. doi: 10.1177/2192568220985472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bereczki F., Turbucz M., Kiss R., Eltes P.E., Lazary A. Stability evaluation of different oblique lumbar interbody fusion constructs in normal and osteoporotic condition – a finite element based study. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.749914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buranakarl T., Jaisanuk K. Preliminary clinical and radiographic outcomes of anterior lumbar interbody fusion (ALIF) with stand alone PEEK cage and anterior plate construct at the Bangkok spine academy. bkkmedj. 2013;6:1–11. doi: 10.31524/bkkmedj.2013.09.001. [DOI] [Google Scholar]
- 55.Mobbs R.J., Phan K., Malham G., Seex K., Rao P.J. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DiPaola C.P., Molinari R.W. Posterior lumbar interbody fusion. J. Am. Acad. Orthop. Surg. 2008;16:130–139. doi: 10.5435/00124635-200803000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Krishna M., Pollock R.D., Bhatia C. Incidence, etiology, classification, and management of neuralgia after posterior lumbar interbody fusion surgery in 226 patients. Spine J. 2008;8:374–379. doi: 10.1016/j.spinee.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q., Yuan Z., Zhou M., Liu H., Xu Y., Ren Y. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Muscoskelet. Disord. 2014;15:367. doi: 10.1186/1471-2474-15-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.AlShazli A.B.A.D., Amer A.Y., Sultan A.M., Barakat A.S., Koptan W., ElMiligui Y., Shaker H. Minimally invasive transforaminal lumbar interbody fusion for the surgical management of post-discectomy syndrome. Asian Spine J. 2020;14:148–156. doi: 10.31616/asj.2019.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammad A., Wirries A., Ardeshiri A., Nikiforov O., Geiger F. Open versus minimally invasive TLIF: literature review and meta-analysis. J. Orthop. Surg. Res. 2019;14:229. doi: 10.1186/s13018-019-1266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y.-H., Ha K.-Y., Kim Y.-S., Kim K.-W., Rhyu K.-W., Park J.-B., Shin J.-H., Kim Y.-Y., Lee J.-S., Park H.-Y., et al. Lumbar interbody fusion and osteobiologics for lumbar fusion. Asian Spine J. 2022;16:1022–1033. doi: 10.31616/asj.2022.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley M.R., Doan A.T., Vogel R.W., Aguirre A.O., Pieri K.S., Scheid E.H. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative deficits. Spine J. 2018;18:1763–1778. doi: 10.1016/j.spinee.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 63.Park H.-Y., Kim Y.-H., Ha K.-Y., Kim S.-I., Min H.-K., Oh I.-S., Seo J.-Y., Chang D.-G., Park J.-T. Minimally invasive lateral lumbar interbody fusion for clinical adjacent segment pathology: a comparative study with conventional posterior lumbar interbody fusion. Clin. Spine Surg. 2019;32:E426–E433. doi: 10.1097/BSD.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 64.Bateman D.K., Millhouse P.W., Shahi N., Kadam A.B., Maltenfort M.G., Koerner J.D., Vaccaro A.R. Anterior lumbar spine surgery: a systematic review and meta-analysis of associated complications. Spine J. 2015;15:1118–1132. doi: 10.1016/j.spinee.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 65.Manunga J., Alcala C., Smith J., Mirza A., Titus J., Skeik N., Senthil J., Stephenson E., Alexander J., Sullivan T. Technical approach, outcomes, and exposure-related complications in patients undergoing anterior lumbar interbody fusion. J. Vasc. Surg. 2021;73:992–998. doi: 10.1016/j.jvs.2020.06.129. [DOI] [PubMed] [Google Scholar]
- 66.Spiessberger A., Arvind V., Dietz N., Grueter B., Huber F., Guggenberger R., Moriggl B., Varma V., Cho S.K. A comparison of complications and clinical and radiologic outcome between the mini-open prepsoas and mini-open transpsoas approaches for lumbar interbody fusion: a meta-analysis. Clin. Spine Surg. 2020;33:271–279. doi: 10.1097/BSD.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 67.Lenz M., Mohamud K., Bredow J., Oikonomidis S., Eysel P., Scheyerer M.J. Comparison of different approaches in Lumbosacral spinal fusion surgery: a systematic review and meta-analysis. Asian Spine J. 2022;16:141–149. doi: 10.31616/asj.2020.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nourian A.A., Harrington J., Pulido P.A., McCauley J.C., Bruffey J.D., Eastlack R.K. Fusion rates of lateral lumbar interbody fusion using recombinant human bone morphogenetic Protein-2. Glob. Spine J. 2019;9:398–402. doi: 10.1177/2192568218797097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han S.-H., Hyun S.-J., Jahng T.-A., Kim K.-J. A comparative radiographic analysis of fusion rate between L4-5 and L5-S1 in a single level posterior lumbar interbody fusion. Korean J. Spine. 2015;12:60–67. doi: 10.14245/kjs.2015.12.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Girasole G., Muro G., Mintz A., Chertoff J. Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge. Int. J. Spine Surg. 2013;7:e95–e100. doi: 10.1016/j.ijsp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo S.-J., Kim K.-H., Chin D.-K., Kim K.-S., Cho Y.-E., Park J.-Y. Minimally invasive versus conventional lumbar interbody fusion at L5–S1: a retrospective comparative study. J. Minim Invasive Spine Surg. Tech. 2022;7:37–45. doi: 10.21182/jmisst.2022.00472. [DOI] [Google Scholar]
- 72.Zhang X., Zhang Y., Gu Z., Li G. Comparison of midline lumbar interbody fusion and minimally invasive transforaminal lumbar interbody fusion for treatment of lumbar degeneration disease. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-73213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manzur M.K., Steinhaus M.E., Virk S.S., Jivanelli B., Vaishnav A.S., McAnany S.J., Albert T.J., Iyer S., Gang C.H., Qureshi S.A. Fusion rate for stand-alone lateral lumbar interbody fusion: a systematic review. Spine J. 2020;20:1816–1825. doi: 10.1016/j.spinee.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Manzur M., Virk S.S., Jivanelli B., Vaishnav A.S., McAnany S.J., Albert T.J., Iyer S., Gang C.H., Qureshi S. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J. 2019;19:1294–1301. doi: 10.1016/j.spinee.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Xu Q., Lu Z., Chen P., Li B., Zheng X., Jiang S., Jiang L. Acceptable fusion rate of single‐level OLIF using pure allograft combined with posterior instrumentation through the wiltse approach: a 2‐Year follow‐up study. Orthop. Surg. 2023;15:801–809. doi: 10.1111/os.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung H.-W., Park K.-H., Lee H.-D., Jeon C.-H., Jeon J.-M., Chung N.-S. Risk factors for nonunion in oblique lateral interbody fusion. J. Orthop. Sci. 2024;29:59–63. doi: 10.1016/j.jos.2022.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Fosca M., Streza A., Antoniac I.V., Vadalà G., Rau J.V. Ion-doped calcium phosphate-based coatings with antibacterial properties. J. Funct. Biomater. 2023;14:250. doi: 10.3390/jfb14050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darouiche R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 79.Wimmer C., Nogler M., Frischhut B. Influence of antibiotics on infection in spinal surgery: a prospective study of 110 patients. J. Spinal Disord. 1998;11:498–500. [PubMed] [Google Scholar]
- 80.Olsen M.A., Mayfield J., Lauryssen C., Polish L.B., Jones M., Vest J., Fraser V.J. Risk factors for surgical site infection in spinal surgery. J. Neurosurg. 2003;98:149–155. [PubMed] [Google Scholar]
- 81.Nagashima H., Yamane K., Nishi T., Nanjo Y., Teshima R. Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int. Orthop. 2010;34:395–399. doi: 10.1007/s00264-009-0741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akbar M., Sobottke R., Lehner B., Eichler M., Wang H., Carstens C., Wiedenhöfer B. Pyogene spondylodiszitis. Orthopä. 2012;41:749–758. doi: 10.1007/s00132-012-1998-4. [DOI] [PubMed] [Google Scholar]
- 83.Fantoni M., Trecarichi E.M., Rossi B., Mazzotta V., Di Giacomo G., Nasto L.A., Di Meco E., Pola E. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur. Rev. Med. Pharmacol. Sci. 2012;16(Suppl 2):2–7. [PubMed] [Google Scholar]
- 84.Vettivel J., Bortz C., Passias P.G., Baker J.F. Pyogenic vertebral column osteomyelitis in adults: analysis of risk factors for 30-Day and 1-Year mortality in a single center cohort study. Asian Spine J. 2019;13:608–614. doi: 10.31616/asj.2018.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fang A., Hu S.S., Endres N., Bradford D.S. Risk factors for infection after spinal surgery. Spine. 2005;30:1460. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 86.Levi A.D.O., Dickman C.A., Sonntag V.K.H. Management of postoperative infections after spinal instrumentation. J. Neurosurg. 1997;86:975–980. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- 87.Weinstein M.A., McCabe J.P., Cammisa F.P. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J. Spinal Disord. 2000;13:422–426. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Hegde V., Meredith D.S., Kepler C.K., Huang R.C. Management of postoperative spinal infections. World J. Orthoped. 2012;3:182–189. doi: 10.5312/wjo.v3.i11.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marcut L., Manescu Paltanea V., Antoniac A., Paltanea G., Robu A., Mohan A.G., Grosu E., Corneschi I., Bodog A.D. Antimicrobial solutions for endotracheal tubes in prevention of ventilator-associated pneumonia. Materials (Basel) 2023;16:5034. doi: 10.3390/ma16145034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Broggini N., McManus L.M., Hermann J.S., Medina R., Schenk R.K., Buser D., Cochran D.L. Peri-implant inflammation defined by the implant-abutment interface. J. Dent. Res. 2006;85:473–478. doi: 10.1177/154405910608500515. [DOI] [PubMed] [Google Scholar]
- 91.Wang R., Neoh K.G., Shi Z., Kang E.-T., Tambyah P.A., Chiong E. Inhibition of Escherichia coli and Proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2012;109:336–345. doi: 10.1002/bit.23342. [DOI] [PubMed] [Google Scholar]
- 92.Campoccia D., Montanaro L., Arciola C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 93.Prinz V., Vajkoczy P. Surgical revision strategies for postoperative spinal implant infections (PSII) J. Spine Surg. 2020;6:777–784. doi: 10.21037/jss-20-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glassman S.D., Dimar J.R., Puno R.M., Johnson J.R. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163–2169. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- 95.Weiss L.E., Vaccaro A.R., Scuderi G., McGuire M., Garfin S.R. Pseudarthrosis after postoperative wound infection in the lumbar spine. J. Spinal Disord. 1997;10:482–487. [PubMed] [Google Scholar]
- 96.Derman P.B., Yusufbekov R., Braaksma B. Device profile of the FlareHawk interbody fusion system, an endplate-conforming multi-planar expandable lumbar interbody fusion cage. Expet Rev. Med. Dev. 2023;20:357–364. doi: 10.1080/17434440.2023.2198123. [DOI] [PubMed] [Google Scholar]
- 97.Park D.Y., Heo D.H. The use of dual direction expandable titanium cage with biportal endoscopic transforaminal lumbar interbody fusion: a technical consideration with preliminary results. Neurospine. 2023;20:110–118. doi: 10.14245/ns.2346116.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitsopoulos K., Wiedenhoefer B., Hemmer S., Fleege C., Arabmotlagh M., Rauschmann M., Rickert M. Preliminary results of expandable transforaminal lumbar interbody fusion cages. TOORTHJ. 2021;15:35–40. doi: 10.2174/1874325002115010035. [DOI] [Google Scholar]
- 99.Segi N., Nakashima H., Shinjo R., Kagami Y., Machino M., Ito S., Ouchida J., Morishita K., Oishi R., Yamauchi I., et al. Vertebral endplate concavity in lateral lumbar interbody fusion: tapered 3D-Printed porous titanium cage versus squared PEEK cage. Medicina. 2023;59:372. doi: 10.3390/medicina59020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.SPINEMarketGroup accelus announces global expansion of its FlareHawk interbody fusion system. https://thespinemarketgroup.com/accelus-announces-global-expansion-of-its-flarehawk-interbody-fusion-system/ Available online:
- 101.Cheng B.C., Swink I., Yusufbekov R., Birgelen M., Ferrara L., Coric D. Current concepts of contemporary expandable lumbar interbody fusion cage designs, part 2: feasibility assessment of an endplate conforming bidirectional expandable interbody cage. Int. J. Spine Surg. 2020;7129 doi: 10.14444/7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coric D., Roybal R.R., Grubb M., Rossi V., Yu A.K., Swink I.R., Long J., Cheng B.C., Inzana J.A. Bidirectional expandable technology for transforaminal or posterior lumbar interbody fusion: a retrospective analysis of safety and performance. Int. J. Spine Surg. 2020;14:S22–S30. doi: 10.14444/7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan L.A., Rivera J., Tan X.A., Le V.P., Khoo L.T., Berven S.H. Clinical and radiographic outcomes after minimally invasive transforaminal lumbar interbody fusion—early experience using a biplanar expandable cage for lumbar spondylolisthesis. Int. J. Spine Surg. 2020;14:S39–S44. doi: 10.14444/7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zygogiannis K., Chatzikomninos I., Moschos S., Palavos I., Thivaios G.C., Kalampokis A. Lumbar spinal rahisynthesis with plif: a retrospective study of 58 patients demonstrating imaging and clinical outcomes with one year Follow-Up. Maedica. 2024;19 doi: 10.26574/maedica.2024.19.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adl Amini D., Moser M., Oezel L., Shue J., Pumberger M., Sama A.A., Cammisa F.P., Girardi F.P., Hughes A.P. Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages. J. Spine Surg. 2022;8:323–332. doi: 10.21037/jss-22-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Velluto C., Mundis G., Scaramuzzo L., Perna A., Capece G., Cruciani A., Inverso M., Borruto M.I., Proietti L. Radiological evaluation of fusion patterns after lateral lumbar interbody fusion with 3D-Printed porous titanium cages vs. conventional titanium cages. Front. Surg. 2024;11 doi: 10.3389/fsurg.2024.1446792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pathria M., Sartoris D.J., Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227–230. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 108.SPINEMarketGroup valeo® II TL available online. https://thespinemarketgroup.com/valeo-ii-tl/
- 109.Youssef J.A. Radiographic Follow-up of transforaminal lumbar fusion with silicon nitride spacers: a case report of two patients. J. Musculoskelet Disord. Treat. 2016;2 doi: 10.23937/2572-3243.1510009. [DOI] [Google Scholar]
- 110.Calvert G.C., Iii G.V.H., Jr W.M.R., Smith M.W., McEntire B.J., Bal B.S. Clinical outcomes for lumbar fusion using silicon nitride versus other biomaterials. J. Spine Surg. 2020;6:33–48. doi: 10.21037/jss.2019.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McEntire B.J., Maslin G., Bal B.S. Two-year results of a double-blind multicenter randomized controlled non-inferiority trial of polyetheretherketone (PEEK) versus silicon nitride spinal fusion cages in patients with symptomatic degenerative lumbar disc disorders. J. Spine Surg. 2020;6:523–540. doi: 10.21037/jss-20-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lumbar Disc replacement, fusion. https://signus.com/intl/products/portfolio/mobis-ii-lumbar-interbody-fusion.html TLIF Available online:
- 113.Jung S., Breitenfelder M., Niggemann H., Stove J., Hasan A. Fusion rate of a PEEK TLIF cage according to the addressed spinal segment - a retrospective analysis. Neuro Neurosurg. 2018;1 doi: 10.15761/NNS.1000105. [DOI] [Google Scholar]
- 114.Häckel S., Gaff J., Celenza A., Cunningham G., Kern M., Taylor P., Miles A. 2024. Structured Titanium TLIF Cage with and Without Adjacent Level Dynamic Stabilization - a Retrospective 1-Year Follow-Up Study. [Google Scholar]
- 115.Duncan J.W., Bailey R.A. An analysis of fusion cage migration in unilateral and bilateral fixation with transforaminal lumbar interbody fusion. Eur. Spine J. 2013;22:439–445. doi: 10.1007/s00586-012-2458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brase A., Ringel F., Stüer C., Meyer B., Stoffel M. Debridement and fusion with polyetheretherketone implants in purulent spondylodiscitis: a clinical experience with nine patients. Acta Neurochir. 2010;152:2001–2004. doi: 10.1007/s00701-010-0798-z. [DOI] [PubMed] [Google Scholar]
- 117.Sorge O., Günther L., Strasser E., Gahr R.H. Two times unlucky: treatment of repeated adjacent vertebral fractures following posterolateral interbody fusion. Arch. Orthop. Trauma. Surg. 2006;126:346–349. doi: 10.1007/s00402-006-0115-8. [DOI] [PubMed] [Google Scholar]
- 118.Morgenstern C., Morgenstern R. Full-endoscopic removal of migrated and pseudoarthrotic lumbar interbody cages: case reports and technical note. Int. J. Spine Surg. 2023;17:370–379. doi: 10.14444/8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou Q., Chen X., Xu L., Li S., Du C., Sun X., Wang B., Zhu Z., Qiu Y. Does vertebral end plate morphology affect cage subsidence after transforaminal lumbar interbody fusion? World Neurosurg. 2019;130:e694–e701. doi: 10.1016/j.wneu.2019.06.195. [DOI] [PubMed] [Google Scholar]
- 120.Mun H.Y., Ko M.J., Kim Y.B., Park S.W. Usefulness of oblique lateral interbody fusion at L5–S1 level compared to transforaminal lumbar interbody fusion. J. Korean Neurosurg. Soc. 2020;63:723–729. doi: 10.3340/jkns.2018.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park M.-K., Kim K.-T., Bang W.-S., Cho D.-C., Sung J.-K., Lee Y.-S., Lee C.K., Kim C.H., Kwon B.K., Lee W.-K., et al. Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J. 2019;19:437–447. doi: 10.1016/j.spinee.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Lee N., Kim K.N., Yi S., Ha Y., Shin D.A., Yoon D.H., Kim K.S. Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg. 2017;101:216–226. doi: 10.1016/j.wneu.2017.01.114. [DOI] [PubMed] [Google Scholar]
- 123.Lin G.-X., Akbary K., Kotheeranurak V., Quillo-Olvera J., Jo H.-J., Yang X.-W., Mahatthanatrakul A., Kim J.-S. Clinical and radiologic outcomes of direct versus indirect decompression with lumbar interbody fusion: a matched-pair comparison analysis. World Neurosurg. 2018;119:e898–e909. doi: 10.1016/j.wneu.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Liu J., Feng H. Oblique lateral interbody fusion (OLIF) with supplemental anterolateral screw and rod instrumentation: a preliminary clinical study. World Neurosurg. 2020;134:e944–e950. doi: 10.1016/j.wneu.2019.11.046. [DOI] [PubMed] [Google Scholar]
- 125.Woods K.R.M., Billys J.B., Hynes R.A. Technical description of oblique lateral interbody fusion at L1–L5 (OLIF25) and at L5–S1 (OLIF51) and evaluation of complication and fusion rates. Spine J. 2017;17:545–553. doi: 10.1016/j.spinee.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 126.Choi W.-S., Kim J.-S., Hur J.-W., Seong J.-H. Minimally invasive transforaminal lumbar interbody fusion using banana-shaped and straight cages: radiological and clinical results from a prospective randomized clinical trial. Neurosurgery. 2018;82:289–298. doi: 10.1093/neuros/nyx212. [DOI] [PubMed] [Google Scholar]
- 127.Choi W.-S., Kim J.-S., Ryu K.-S., Hur J.-W., Seong J.-H. Minimally invasive transforaminal lumbar interbody fusion at L5-S1 through a unilateral approach: technical feasibility and outcomes. BioMed Res. Int. 2016;2016:1–8. doi: 10.1155/2016/2518394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rentenberger C., Okano I., Salzmann S.N., Winter F., Plais N., Burkhard M.D., Shue J., Sama A.A., Cammisa F.P., Girardi F.P., et al. Perioperative risk factors for early revisions in stand-alone lateral lumbar interbody fusion. World Neurosurg. 2020;134:e657–e663. doi: 10.1016/j.wneu.2019.10.164. [DOI] [PubMed] [Google Scholar]
- 129.Stosch-Wiechert K., Wuertz-Kozak K., Hitzl W., Szeimies U., Stäbler A., Siepe C.J. Clinical and radiological Mid- to long-term investigation of anterior lumbar stand-alone fusion: incidence of reoperation and adjacent segment degeneration. Brain and Spine. 2022;2 doi: 10.1016/j.bas.2022.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Phan K., Ramachandran V., Tran T., Phan S., Rao P.J., Mobbs R.J. Impact of elderly age on complications and clinical outcomes following anterior lumbar interbody fusion surgery. World Neurosurg. 2017;105:503–509. doi: 10.1016/j.wneu.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 131.Rao P.J., Phan K., Giang G., Maharaj M.M., Phan S., Mobbs R.J. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J. Spine Surg. 2017;3:168–175. doi: 10.21037/jss.2017.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hydroxyapatite H.A. 2024. HAPPE Spine. [Google Scholar]
- 133.HIMED — Polyetheretherketone (PEEK) Modifications white paper available online. https://www.himed.com/polyetheretherketone-peek-properties-white-paper
- 134.Roeder R.K., Conrad T.L. In: PEEK Biomaterials Handbook. Kurtz S.M., editor. William Andrew Publishing; Oxford: 2012. Chapter 11 - bioactive polyaryletherketone composites; pp. 163–179. (Plastics Design Library). ISBN 978-1-4377-4463-7. [Google Scholar]
- 135.Roeder R.K., Sproul M.M., Turner C.H. Hydroxyapatite whiskers provide improved mechanical properties in reinforced polymer composites. J. Biomed. Mater. Res. 2003;67A:801–812. doi: 10.1002/jbm.a.10140. [DOI] [PubMed] [Google Scholar]
- 136.Kane R.J., Converse G.L., Roeder R.K. Effects of the reinforcement morphology on the fatigue properties of hydroxyapatite reinforced polymers. J. Mech. Behav. Biomed. Mater. 2008;1:261–268. doi: 10.1016/j.jmbbm.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spine V. Vy spine. https://vyspine.com/osteovy%E2%84%A2-ha Available online:
- 138.Oh K.W., Lee J.H., Lee J.-H., Lee D.-Y., Shim H.J. The correlation between cage subsidence, bone mineral density, and clinical results in posterior lumbar interbody fusion. Clin. Spine Surg.: A Spine Publ. 2017;30:E683–E689. doi: 10.1097/BSD.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 139.Zhao Y., Jia J., Liu W., Chen X., Mai R., Tian Y., Zhao J., Liu X. Influence of contoured versus straight rod on clinical outcomes and sagittal parameters in minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) at L4/5 level-more than 5 years Follow-Up. J. Orthop. Sci. 2020;25:89–95. doi: 10.1016/j.jos.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 140.Kim D.-H., Jeong S.-T., Lee S.-S. Posterior lumbar interbody fusion using a unilateral single cage and a local morselized bone graft in the degenerative lumbar spine. Clin. Orthop. Surg. 2009;1:214. doi: 10.4055/cios.2009.1.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Omosor E., Edelbach B.M., Amer H., Hussain N.S. Utilization of dual expandable cages in lateral lumbar interbody fusion surgery. Cureus. 2023 doi: 10.7759/cureus.41455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Obeidallah M., Hamad M.K., Holland R., Mammis A. The use of a transforaminal lumbar interbody fusion (TLIF) cage following inadequate access to the disc space during an anterior lumbar interbody fusion procedure. Cureus. 2022 doi: 10.7759/cureus.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tu Z., Li L., Wang B., Li Y., Lv G., Dai Y. Stand-alone anterolateral interbody fusion versus extended posterior fusion for symptomatic adjacent-segment degeneration: a retrospective study of 2 years' Follow-Up. World Neurosurg. 2018;115:e748–e755. doi: 10.1016/j.wneu.2018.04.165. [DOI] [PubMed] [Google Scholar]
- 144.Kuang L., Wang B., Lü G. Transforaminal lumbar interbody fusion versus mini-open anterior lumbar interbody fusion with oblique self-anchored stand-alone cages for the treatment of lumbar disc herniation: a retrospective study with 2-Year Follow-Up. Spine. 2017;42:E1259–E1265. doi: 10.1097/BRS.0000000000002145. [DOI] [PubMed] [Google Scholar]
- 145.Jiya T.U., Smit T., Van Royen B.J., Mullender M. Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable Poly-l-Lactide-Co-d,l-Lactide fusion devices. Clinical outcome at a minimum of 2-Year Follow-Up. Eur. Spine J. 2011;20:618–622. doi: 10.1007/s00586-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coe J.D. Instrumented transforaminal lumbar interbody fusion with bioabsorbable polymer implants and iliac crest autograft. FOC. 2004;16:1–9. doi: 10.3171/foc.2004.16.3.12. [DOI] [PubMed] [Google Scholar]
- 147.Lanman T.H., Hopkins T.J. Lumbar interbody fusion after treatment with recombinant human bone morphogenetic Protein–2 added to 70:30 Poly(L-Lactide-Co-D,L-Lactide) bioresorbable implants. FOC. 2004;16:1–4. doi: 10.3171/foc.2004.16.3.10. [DOI] [PubMed] [Google Scholar]
- 148.Koutserimpas C., Alpantaki K., Chatzinikolaidou M., Chlouverakis G., Dohm M., Hadjipavlou A.G. The effectiveness of biodegradable instrumentation in the treatment of spinal fractures. Injury. 2018;49:2111–2120. doi: 10.1016/j.injury.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 149.Smith A.J., Arginteanu M., Moore F., Steinberger A., Camins M. Increased incidence of cage migration and nonunion in instrumented transforaminal lumbar interbody fusion with bioabsorbable cages. SPI. 2010;13:388–393. doi: 10.3171/2010.3.SPINE09587. [DOI] [PubMed] [Google Scholar]
- 150.Jiya T., Smit T., Deddens J., Mullender M. Posterior lumbar interbody fusion using nonresorbable poly-ether-ether-ketone versus resorbable Poly-l-Lactide-Co-d,l-Lactide fusion devices: a prospective, randomized study to assess fusion and clinical outcome. Spine. 2009;34:233–237. doi: 10.1097/BRS.0b013e318194ed00. [DOI] [PubMed] [Google Scholar]
- 151.Frost A., Bagouri E., Brown M., Jasani V. Osteolysis following resorbable Poly-l-Lactide-Co-d, l-Lactide PLIF cage use: a review of cases. Eur. Spine J. 2012;21:449–454. doi: 10.1007/s00586-011-2002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bioretec: bioabsorbable implants for orthopaedics - bioretec ltd. https://bioretec.com/ Available online:
- 153.Lähteenkorva K., Numminen T. vol. 27. 2022. Composite material. (IMPLANT COMPRISING THEREOF, USE OF THE COMPOSITE MATERIAL AND METHOD FOR PREPARING A MEDICAL DEVICE). [Google Scholar]
- 154.Kassai T., Krupa Z., Józsa G., Hanna D., Varga M. Comparison of biodegradable and metallic tension-band fixation for paediatric lateral condyle fracture of the elbow. Injury. 2024;55(Suppl 3) doi: 10.1016/j.injury.2024.111403. [DOI] [PubMed] [Google Scholar]
- 155.Kuklo T.R., Rosner M.K., Polly D.W. Computerized tomography evaluation of a resorbable implant after transforaminal lumbar interbody fusion. FOC. 2004;16:1–6. doi: 10.3171/foc.2004.16.3.11. [DOI] [PubMed] [Google Scholar]
- 156.Fantigrossi A., Galbusera F., Raimondi M.T., Sassi M., Fornari M. Biomechanical analysis of cages for posterior lumbar interbody fusion. Med. Eng. Phys. 2007;29:101–109. doi: 10.1016/j.medengphy.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 157.Lai P.-L., Huang S.-F., Wang H.-W., Liu P.-H., Lin C.-L. Designing an anatomical contour titanium 3D-Printed oblique lumbar interbody fusion cage with porous structure and embedded fixation screws for patients with osteoporosis. IJB. 2023;9:772. doi: 10.18063/ijb.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Y., Ge X., Wang J., Zeng D., Wang J., Chu B. Comparison of mechanical properties of interbody fusion cage with three general structures. J. Phys.: Conf. Ser. 2023;2541 doi: 10.1088/1742-6596/2541/1/012052. [DOI] [Google Scholar]
- 159.Pan C.-T., Lin C.-H., Huang Y.-K., Jang J.S.C., Lin H.-K., Kuo C.-N., Lin D.-Y., Huang J.C. Design of customize interbody fusion cages of Ti64ELI with gradient porosity by selective laser melting process. Micromachines. 2021;12:307. doi: 10.3390/mi12030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shuai H., Xuhui J., Lei L., Shujun L., Cheng L. Biomechanical analysis of 3D printed porous extremely-low modulus Ti-24Nb-4Zr-8Sn lumbar interbody fusion Cage-A finite element study. Mater. Technol. 2024;39 doi: 10.1080/10667857.2024.2345960. [DOI] [Google Scholar]
- 161.Loenen A.C.Y., Noailly J., Ito K., Willems P.C., Arts J.J., Van Rietbergen B. Patient-specific variations in local strain patterns on the surface of a trussed titanium interbody cage. Front. Bioeng. Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.750246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumar P., Bhardwaj R., Matharu A.L., Meena V.K. Comparative analysis of porous titanium spinal cage with conventional spinal cages: a finite element study. J. Sci. Ind. Res. 2023;82 doi: 10.56042/jsir.v82i11.5506. [DOI] [Google Scholar]
- 163.McGilvray K.C., Easley J., Seim H.B., Regan D., Berven S.H., Hsu W.K., Mroz T.E., Puttlitz C.M. Bony ingrowth potential of 3D-Printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018;18:1250–1260. doi: 10.1016/j.spinee.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Arts M., Torensma B., Wolfs J. Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-Year results of a prospective controlled trial. Spine J. 2020;20:1065–1072. doi: 10.1016/j.spinee.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 165.Krafft P.R., Osburn B., Vivas A.C., Rao G., Alikhani P. Novel titanium cages for minimally invasive lateral lumbar interbody fusion: first assessment of subsidence. Spine Surg. Relat Res. 2020;4:171–177. doi: 10.22603/ssrr.2019-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mokawem M., Katzouraki G., Harman C.L., Lee R. Lumbar interbody fusion rates with 3D-Printed lamellar titanium cages using a silicate-substituted calcium phosphate bone graft. J. Clin. Neurosci. 2019;68:134–139. doi: 10.1016/j.jocn.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 167.Mobbs R.J., Parr W.C.H., Choy W.J., McEvoy A., Walsh W.R., Phan K. Anterior lumbar interbody fusion using a personalized approach: is custom the future of implants for anterior lumbar interbody fusion surgery? World Neurosurg. 2019;124:452–458.e1. doi: 10.1016/j.wneu.2018.12.144. [DOI] [PubMed] [Google Scholar]
- 168.Cheng B.C., Jaffee S., Averick S., Swink I., Horvath S., Zhukauskas R. A comparative study of three biomaterials in an ovine bone defect model. Spine J. 2020;20:457–464. doi: 10.1016/j.spinee.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 169.Basgul C., Yu T., MacDonald D.W., Siskey R., Marcolongo M., Kurtz S.M. Does annealing improve the interlayer adhesion and structural integrity of FFF 3D printed PEEK lumbar spinal cages? J. Mech. Behav. Biomed. Mater. 2020;102 doi: 10.1016/j.jmbbm.2019.103455. [DOI] [PubMed] [Google Scholar]
- 170.Kiapour A., Massaad E., Joukar A., Hadzipasic M., Shankar G.M., Goel V.K., Shin J.H. Biomechanical analysis of stand-alone lumbar interbody cages versus 360° constructs: an in vitro and finite element investigation. J. Neurosurg. Spine. 2022;36:928–936. doi: 10.3171/2021.9.SPINE21558. [DOI] [PubMed] [Google Scholar]
- 171.Yan Y., Yu J., Wang Y., Dong H., Zhang K., Wang Y., Xue Y., Wu X., He L., Feng H., et al. A newly designed personalized interbody fusion cage and its biomechanical analysis. Acta Mech. Sin. 2023;39 doi: 10.1007/s10409-023-23047-x. [DOI] [Google Scholar]
- 172.Fogel G., Martin N., Williams G.M., Unger J., Yee-Yanagishita C., Pelletier M., Walsh W., Peng Y., Jekir M. Choice of spinal interbody fusion cage material and design influences subsidence and osseointegration performance. World Neurosurg. 2022;162:e626–e634. doi: 10.1016/j.wneu.2022.03.087. [DOI] [PubMed] [Google Scholar]
- 173.Peck J.H., Kavlock K.D., Showalter B.L., Ferrell B.M., Peck D.G., Dmitriev A.E. Mechanical performance of lumbar intervertebral body fusion devices: an analysis of data submitted to the food and drug administration. J. Biomech. 2018;78:87–93. doi: 10.1016/j.jbiomech.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 174.McGilvray K.C., Waldorff E.I., Easley J., Seim H.B., Zhang N., Linovitz R.J., Ryaby J.T., Puttlitz C.M. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses. Spine J. 2017;17:1907–1916. doi: 10.1016/j.spinee.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 175.Gunzburg R., Colloca C.J., Jones C.F., Hall D.J., McAviney J., Callary S., Hegazy M.A., Szpalski M., Freeman B.J.C. Does nanoscale porous titanium coating increase lumbar spinal stiffness of an interbody fusion cage? An in vivo biomechanical analysis in an ovine model. Clin. BioMech. 2019;67:187–196. doi: 10.1016/j.clinbiomech.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 176.Jain A., Marrache M., Harris A., Puvanesarajah V., Neuman B.J., Buser Z., Wang J.C., Yoon S.T., Meisel H.J. Structural allograft versus PEEK implants in anterior cervical discectomy and fusion: a systematic review. Glob. Spine J. 2020;10:775–783. doi: 10.1177/2192568219883256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chu L., Li R., Liao Z., Yang Y., Dai J., Zhang K., Zhang F., Xie Y., Wei J., Zhao J., et al. Highly effective bone fusion induced by the interbody cage made of calcium silicate/polyetheretherketone in a goat model. ACS Biomater. Sci. Eng. 2019;5:2409–2416. doi: 10.1021/acsbiomaterials.8b01193. [DOI] [PubMed] [Google Scholar]
- 178.Dai L.-Y., Jiang L.-S. Anterior cervical fusion with interbody cage containing β-Tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-Year Follow-Up. Eur. Spine J. 2008;17:698–705. doi: 10.1007/s00586-008-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Walsh W.R., Pelletier M.H., Bertollo N., Christou C., Tan C. Does PEEK/HA enhance bone formation compared with PEEK in a sheep cervical fusion model? Clin. Orthop. Relat. Res. 2016;474:2364–2372. doi: 10.1007/s11999-016-4994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mantripragada V.P., Lecka-Czernik B., Ebraheim N.A., Jayasuriya A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. 2013;101:3349–3364. doi: 10.1002/jbm.a.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McEntire B.J., Bal B.S., Rahaman M.N., Chevalier J., Pezzotti G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2015;35:4327–4369. doi: 10.1016/j.jeurceramsoc.2015.07.034. [DOI] [Google Scholar]
- 182.Turon-Vinas M., Anglada M. Assessment in Si3N4 of a new method for determining the fracture toughness from a surface notch micro-machined by ultra-short pulsed laser ablation. J. Eur. Ceram. Soc. 2015;35:1737–1741. doi: 10.1016/j.jeurceramsoc.2014.12.024. [DOI] [Google Scholar]
- 183.Du X., Lee S.S., Blugan G., Ferguson S.J. Silicon nitride as a biomedical material: an overview. Int. J. Mol. Sci. 2022;23:6551. doi: 10.3390/ijms23126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suh P.B., Puttlitz C., Lewis C., Bal B.S., McGilvray K. The effect of cervical interbody cage morphology, material composition, and substrate density on cage subsidence. J. Am. Acad. Orthop. Surg. 2017;25:160–168. doi: 10.5435/JAAOS-D-16-00390. [DOI] [PubMed] [Google Scholar]
- 185.Kersten R.F.M.R., Wu G., Pouran B., Van Der Veen A.J., Weinans H.H., De Gast A., Öner F.C., Van Gaalen S.M. Comparison of polyetheretherketone versus silicon nitride intervertebral spinal spacers in a caprine model. J. Biomed. Mater. Res. 2019;107:688–699. doi: 10.1002/jbm.b.34162. [DOI] [PubMed] [Google Scholar]
- 186.Guedes e Silva C.C., Higa O.Z., Bressiani J.C. Cytotoxic evaluation of silicon nitride-based ceramics. Mater. Sci. Eng. C. 2004;24:643–646. doi: 10.1016/j.msec.2004.08.007. [DOI] [Google Scholar]
- 187.ISO 10993-5:2009(En) Biological evaluation of medical devices — part 5: tests for in vitro cytotoxicity. https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en Available online:
- 188.Guedes e Silva C.C., König Jr., B., Carbonari M.J., Yoshimoto M., Allegrini Jr., S., Bressiani J.C. Tissue response around silicon nitride implants in rabbits. J. Biomed. Mater. Res. 2008;84A:337–343. doi: 10.1002/jbm.a.31363. [DOI] [PubMed] [Google Scholar]
- 189.Howlett C.R., Mccartney E., Ching W. The effect of silicon nitride ceramic on rabbit skeletal cells and tissue: an: in vitro: and: in vivo: investigation. Clin. Orthop. Relat. Res. 1989;244:293. [PubMed] [Google Scholar]
- 190.Webster T.J., Patel A.A., Rahaman M.N., Sonny Bal B. Anti-infective and osteointegration properties of silicon nitride, Poly(Ether ether ketone), and titanium implants. Acta Biomater. 2012;8:4447–4454. doi: 10.1016/j.actbio.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 191.Pezzotti G., Marin E., Adachi T., Rondinella A., Boschetto F., Zhu W., Sugano N., Bock R.M., McEntire B., Bal S.B. Bioactive silicon nitride: a new therapeutic material for osteoarthropathy. Sci. Rep. 2017;7 doi: 10.1038/srep44848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pezzotti G., Bock R.M., Adachi T., Rondinella A., Boschetto F., Zhu W., Marin E., McEntire B., Bal B.S., Mazda O. Silicon nitride surface chemistry: a potent regulator of mesenchymal progenitor cell activity in bone formation. Appl. Mater. Today. 2017;9:82–95. doi: 10.1016/j.apmt.2017.05.005. [DOI] [Google Scholar]
- 193.Lange F.F., Singhal S.C., Kuznicki R.C. Phase relations and stability studies in the Si3N4-SiO2-Y2O3 pseudoternary system. J. Am. Ceram. Soc. 1977;60:249–252. doi: 10.1111/j.1151-2916.1977.tb14118.x. [DOI] [Google Scholar]
- 194.Du X., Yu B., Pei P., Ding H., Yu B., Zhu Y. 3D printing of Pearl/CaSO4 composite scaffolds for bone regeneration. J. Mater. Chem. B. 2018;6:499–509. doi: 10.1039/C7TB02667F. [DOI] [PubMed] [Google Scholar]
- 195.Anderson M.C., Olsen R. Bone ingrowth into porous silicon nitride. J. Biomed. Mater. Res. 2010;92:1598–1605. doi: 10.1002/jbm.a.32498. [DOI] [PubMed] [Google Scholar]
- 196.Precnerová M., Bodišová K., Frajkorová F., Galusková D., Varchulová Nováková Z., Vojtaššák J., Lenčéš Z., Šajgalík P. In vitro bioactivity of silicon nitride–hydroxyapatite composites. Ceram. Int. 2015;41:8100–8108. doi: 10.1016/j.ceramint.2015.03.011. [DOI] [Google Scholar]
- 197.Silva C.C.G. e, Rigo E.C. da S., Marchi J., Bressiani A.H. de A., Bressiani J.C. Hydroxyapatite coating on silicon nitride surfaces using the biomimetic method. Mat. Res. 2008;11:47–50. doi: 10.1590/S1516-14392008000100009. [DOI] [Google Scholar]
- 198.Dorozhkin S.V. Calcium orthophosphate (CaPO4) containing composites for biomedical applications: formulations, properties, and applications. J. Composites Sci. 2024;8:218. doi: 10.3390/jcs8060218. [DOI] [Google Scholar]
- 199.Dorozhkin S.V. In: Handbook of Bioceramics and Biocomposites. Antoniac I.V., editor. Springer International Publishing; Cham: 2016. Calcium phosphates; pp. 91–118. ISBN 978-3-319-12460-5. [Google Scholar]
- 200.Frajkorová F., Bodišová K., Boháč M., Bartoníčková E., Sedláček J. Preparation and characterisation of porous composite biomaterials based on silicon nitride and bioglass. Ceram. Int. 2015;41:9770–9778. doi: 10.1016/j.ceramint.2015.04.049. [DOI] [Google Scholar]
- 201.Amaral M., Costa M.A., Lopes M.A., Silva R.F., Santos J.D., Fernandes M.H. Si3N4-Bioglass composites stimulate the proliferation of MG63 osteoblast-like cells and support the osteogenic differentiation of human bone marrow cells. Biomaterials. 2002;23:4897–4906. doi: 10.1016/S0142-9612(02)00249-1. [DOI] [PubMed] [Google Scholar]
- 202.Gbejuade H.O., Lovering A.M., Webb J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015:147–158. doi: 10.3109/17453674.2014.966290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sewick A., Makani A., Wu C., O'Donnell J., Baldwin K.D., Lee G.-C. Does dual antibiotic prophylaxis better prevent surgical site infections in total joint arthroplasty? Clin. Orthop. Relat. Res. 2012;470:2702. doi: 10.1007/s11999-012-2255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ishikawa M., de Mesy Bentley K.L., McEntire B.J., Bal B.S., Schwarz E.M., Xie C. Surface topography of silicon nitride affects antimicrobial and osseointegrative properties of tibial implants in a murine model. J. Biomed. Mater. Res. 2017;105:3413–3421. doi: 10.1002/jbm.a.36189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.An Y.H., Friedman R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 206.Gallo J., Holinka M., Moucha C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014;15:13849–13880. doi: 10.3390/ijms150813849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stimmelmayr M., Edelhoff D., Güth J.-F., Erdelt K., Happe A., Beuer F. Wear at the titanium–titanium and the titanium–zirconia implant–abutment interface: a comparative in vitro study. Dent. Mater. 2012;28:1215–1220. doi: 10.1016/j.dental.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 208.Olofsson J., Grehk T.M., Berlind T., Persson C., Jacobson S., Engqvist H. Evaluation of silicon nitride as a wear resistant and resorbable alternative for total hip joint replacement. Biomatter. 2012;2:94–102. doi: 10.4161/biom.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang L., Schoenfisch M.H. Nitric oxide-releasing hyperbranched polyaminoglycosides for antibacterial therapy. ACS Appl. Bio Mater. 2018;1:1066–1073. doi: 10.1021/acsabm.8b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park J., Kim J., Singha K., Han D.-K., Park H., Kim W.J. Nitric oxide integrated polyethylenimine-based tri-block copolymer for efficient antibacterial activity. Biomaterials. 2013;34:8766–8775. doi: 10.1016/j.biomaterials.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 211.Dong K., Ju E., Gao N., Wang Z., Ren J., Qu X. Synergistic eradication of antibiotic-resistant bacteria based biofilms in vivo using a NIR-sensitive nanoplatform. Chem Commun (Camb) 2016;52:5312–5315. doi: 10.1039/c6cc00774k. [DOI] [PubMed] [Google Scholar]
- 212.Abdul-Nabi R.A., Al-Bermany E. Antibacterial and anticancer potentials of graphene-silicon nitride nanomaterials-enhanced polymer nanocomposites: advanced characterization and optical behavior insights. J. Biosafety Biosec. 2025;7:55–68. doi: 10.1016/j.jobb.2025.04.001. [DOI] [Google Scholar]
- 213.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015;5 doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ma P., Yang C.-Y., Li C., Hu P., Yang F., Lu J., Huang Y.-Y., Wu H., Wu Q., Pan Y., et al. Blow-spun Si3N4-Incorporated nanofibrous dressing with antibacterial, anti-inflammatory, and angiogenic activities for chronic wound treatment. Adv. Fiber Mater. 2024;6:543–560. doi: 10.1007/s42765-023-00361-w. [DOI] [Google Scholar]
- 215.Toth J.M., Estes B.T., Wang M., Seim H.B., Scifert J.L., Turner A.S., Cornwall G.B. 2002. Evaluation of 70/30 Poly (l-Lactide-Co-d,l-Lactide) for Use as a Resorbable Interbody Fusion Cage. [DOI] [PubMed] [Google Scholar]
- 216.Robbins M.M., Vaccaro A.R., Madigan L. The use of bioabsorbable implants in spine surgery. Neurosurg. Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.3.2. [DOI] [PubMed] [Google Scholar]
- 217.Wuisman P.I.J.M., van Dijk M., Smit T.H. Resorbable cages for spinal fusion: an experimental goat model. J. Neurosurg. 2002;97:433–439. doi: 10.3171/spi.2002.97.4.0433. [DOI] [PubMed] [Google Scholar]
- 218.Smit T.H., Engels T.A.P., Wuisman P.I.J.M., Govaert L.E. Time-dependent mechanical strength of 70/30 Poly(L, DL-lactide): shedding light on the premature failure of degradable spinal cages. Spine (Phila Pa 1976) 2008;33:14–18. doi: 10.1097/BRS.0b013e31815e39df. [DOI] [PubMed] [Google Scholar]
- 219.Kim D.H., Vaccaro A.R., Fessler R.G., editors. Spinal Instrumentation: Surgical Techniques. Georg Thieme Verlag; Stuttgart: 2005. b-002-76305; ISBN 978-1-58890-375-4. [Google Scholar]
- 220.Van Dijk M., Smit T.H., Sugihara S., Burger E.H., Wuisman P.I. The effect of cage stiffness on the rate of lumbar interbody fusion: an in vivo model using Poly(L-Lactic acid) and titanium cages. Spine. 2002;27:682–688. doi: 10.1097/00007632-200204010-00003. [DOI] [PubMed] [Google Scholar]
- 221.Alexander J.T., Branch C.L., Subach B.R., Haid R.W. Applications of a resorbable interbody spacer in posterior lumbar interbody fusion. J. Neurosurg. Spine. 2002;97:468–472. doi: 10.3171/spi.2002.97.4.0468. [DOI] [PubMed] [Google Scholar]
- 222.De Medinaceli L., Khoury R., Merle M. Large amounts of polylactic acid in contact with divided nerve sheaths have no adverse effects on regeneration. J. Reconstr. Microsurg. 1995;11:43–49. doi: 10.1055/s-2007-1006510. [DOI] [PubMed] [Google Scholar]
- 223.Gautier S.E., Oudega M., Fragoso M., Chapon P., Plant G.W., Bunge M.B., Parel J.M. Poly(Alpha-Hydroxyacids) for application in the spinal cord: resorbability and biocompatibility with adult rat schwann cells and spinal cord. J. Biomed. Mater. Res. 1998;42:642–654. doi: 10.1002/(sici)1097-4636(19981215)42:4<642::aid-jbm22>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 224.Hollinger J.O., Battistone G.C. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin. Orthop. Relat. Res. 1986:290–305. [PubMed] [Google Scholar]
- 225.van der Elst M., Dijkema A.R.A., Klein C.P.A.T., Patka P., Haarman H.J.ThM. Tissue reaction on PLLA versus stainless steel interlocking nails for fracture fixation: an animal study. Biomaterials. 1995;16:103–106. doi: 10.1016/0142-9612(95)98270-O. [DOI] [PubMed] [Google Scholar]
- 226.Lundgren D., Nyman S., Mathisen T., Isaksson S., Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J. Cranio-Maxillofacial Surg. 1992;20:257–260. doi: 10.1016/S1010-5182(05)80438-X. [DOI] [PubMed] [Google Scholar]
- 227.van Dijk M., Smit T.H., Burger E.H., Wuisman P.I. Bioabsorbable Poly-L-Lactic acid cages for lumbar interbody fusion: three-year Follow-up radiographic, histologic, and histomorphometric analysis in goats. Spine (Phila Pa 1976) 2002;27:2706–2714. doi: 10.1097/00007632-200212010-00010. [DOI] [PubMed] [Google Scholar]
- 228.van Dijk M., van Diest P.J., Smit T.H., Berkhof H., Burger E.H., Wuisman P.I.J.M. Four-year Follow-up of Poly-L-Lactic acid cages for lumbar interbody fusion in goats. J. Long Term Eff. Med. Implants. 2005;15:125–138. doi: 10.1615/jlongtermeffmedimplants.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- 229.Smit T.H., Krijnen M.R., van Dijk M., Wuisman P.I.J.M. Application of polylactides in spinal cages: studies in a goat model. J. Mater. Sci. Mater. Med. 2006;17:1237–1244. doi: 10.1007/s10856-006-0597-5. [DOI] [PubMed] [Google Scholar]
- 230.van Dijk M., Tunc D.C., Smit T.H., Higham P., Burger E.H., Wuisman P.I.J.M. In vitro and in vivo degradation of bioabsorbable PLLA spinal fusion cages. J. Biomed. Mater. Res. 2002;63:752–759. doi: 10.1002/jbm.10466. [DOI] [PubMed] [Google Scholar]
- 231.Hojo Y., Kotani Y., Ito M., Abumi K., Kadosawa T., Shikinami Y., Minami A. A biomechanical and histological evaluation of a bioresorbable lumbar interbody fusion cage. Biomaterials. 2005;26:2643–2651. doi: 10.1016/j.biomaterials.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 232.Cai J., Wang W., Cai P., Cao B. Immune response to foreign materials in spinal fusion surgery. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eslami-Kaliji F., Hedayat Nia N., Lakey J.R.T., Smink A.M., Mohammadi M. Mechanisms of foreign body giant cell formation in response to implantable biomaterials. Polymers. 2023;15:1313. doi: 10.3390/polym15051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lahousse A., Roose E., Leysen L., Yilmaz S.T., Mostaqim K., Reis F., Rheel E., Beckwée D., Nijs J. Lifestyle and pain following cancer: state-Of-The-art and future directions. J. Clin. Med. 2021;11:195. doi: 10.3390/jcm11010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang B., Su Y., Zhou J., Zheng Y., Zhu D. Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Adv. Sci. (Weinh.) 2021;8 doi: 10.1002/advs.202100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jimi S., Jaguparov A., Nurkesh A., Sultankulov B., Saparov A. Sequential delivery of cryogel released growth factors and cytokines accelerates wound healing and improves tissue regeneration. Front. Bioeng. Biotechnol. 2020;8:345. doi: 10.3389/fbioe.2020.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gupta A., Burgess J.K., Borghuis T., de Vries M.P., Kuipers J., Permentier H.P., Bischoff R., Slebos D.-J., Pouwels S.D. Identification of damage associated molecular patterns and extracellular matrix proteins as major constituents of the surface proteome of lung implantable silicone/nitinol devices. Acta Biomater. 2022;141:209–218. doi: 10.1016/j.actbio.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 238.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liu Z., He X., Chen S., Yu H. Advances in the use of calcium silicate-based materials in bone tissue engineering. Ceram. Int. 2023;49:19355–19363. doi: 10.1016/j.ceramint.2023.03.063. [DOI] [Google Scholar]
- 240.Li X., Zhou Q., Wu Y., Feng C., Yang X., Wang L., Xiao Y., Zhang K., Zhu X., Liu L., et al. Enhanced bone regenerative properties of calcium phosphate ceramic granules in rabbit posterolateral spinal fusion through a reduction of grain size. Bioact. Mater. 2022;11:90–106. doi: 10.1016/j.bioactmat.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ganko R., Madhavan A., Hamouda W., Muthu S., Jain A., Yoon S.T., El-Rozz H., Cyril D., Pabbruwe M., Tipper J.L., et al. Spinal implant wear particles: generation, characterization, biological impacts, and future considerations. iScience. 2025;28 doi: 10.1016/j.isci.2025.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bances I.F., Aparicio J.P., Vega M.A.A., Bances I.F., Aparicio J.P., Vega M.A.A. Evaluation of titanium serum levels in patients after spine instrumentation: Comparison between posterolateral and 360o spinal fusion surgery. Cureus. 2019;11 doi: 10.7759/cureus.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fell D., Diarbakerli E., Gerdhem P. Serum metal ion levels following spinal deformity surgery: a case-control study of 182 individuals. Eur. Spine J. 2022;31:3036–3041. doi: 10.1007/s00586-022-07341-5. [DOI] [PubMed] [Google Scholar]
- 244.Ianuzzi A., Kurtz S.M., Kane W., Shah P., Siskey R., van Ooij A., Bindal R., Ross R., Lanman T., Büttner-Janz K., et al. Vivo deformation, surface damage, and biostability of retrieved dynesys systems. Spine (Phila Pa 1976) 2010;35:E1310–E1316. doi: 10.1097/BRS.0b013e3181d6f84f. [DOI] [PubMed] [Google Scholar]
- 245.Wind J., Park D., Lansford T., Nunley P., Peppers T., Russo A., Hassanzadeh H., Sembrano J.N., Yoo J., Sales J. Twelve-month results from a prospective clinical study evaluating the efficacy and safety of cellular bone allograft in subjects undergoing lumbar spinal fusion. Neurol. Int. 2022;14:875–883. doi: 10.3390/neurolint14040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tally W.C., Temple H.T., Burkus J.K. Lateral lumbar interbody fusion using a cellular allogeneic bone matrix in the treatment of symptomatic degenerative lumbar disc disease and lumbar spinal instability. J. Spine Surg. 2021;7:310–317. doi: 10.21037/jss-21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Park D.K., Wind J.J., Lansford T., Nunley P., Peppers T.A., Russo A., Hassanzadeh H., Sembrano J., Yoo J., Sales J. Twenty-four-month interim results from a prospective, single-arm clinical trial evaluating the performance and safety of cellular bone allograft in patients undergoing lumbar spinal fusion. BMC Muscoskelet. Disord. 2023;24:895. doi: 10.1186/s12891-023-06996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Abedi A., Formanek B., Russell N., Vizesi F., Boden S.D., Wang J.C., Buser Z. Examination of the role of cells in commercially available cellular allografts in spine fusion: an in vivo animal study. JBJS. 2020;102 doi: 10.2106/JBJS.20.00330. [DOI] [PubMed] [Google Scholar]
- 249.Cohen J.D., Kanim L.E., Trontis A.J., Bae H.W. Allografts and spinal fusion. Int. J. Spine Surg. 2021;15:68–93. doi: 10.14444/8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Han S., Park B., Lim J.-W., Youm J.-Y., Choi S.-W., Kim D.H., Ahn D.K. Comparison of fusion rate between demineralized bone matrix versus autograft in lumbar fusion : meta-analysis. J. Korean Neurosurg. Soc. 2020;63:673–680. doi: 10.3340/jkns.2019.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shepard N.A., Rush A.J., Scarborough N.L., Carter A.J., Phillips F.M. Demineralized bone matrix in spine surgery: a review of current applications and future trends. Int. J. Spine Surg. 2021;15:113–119. doi: 10.14444/8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tian X., Liu Y., Liu S., Tian Q., Raina D.B., Gelinsky M., Zwingenberger S. Transforming spinal surgery with innovations in biologics and additive manufacturing. Mater. Today Bio. 2025;32 doi: 10.1016/j.mtbio.2025.101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.He X., Li Y., Zou D., Zu H., Li W., Zheng Y. An overview of magnesium-based implants in orthopaedics and a prospect of its application in spine fusion. Bioact. Mater. 2024;39:456–478. doi: 10.1016/j.bioactmat.2024.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Reith G., Schmitz-Greven V., Hensel K.O., Schneider M.M., Tinschmann T., Bouillon B., Probst C. Metal implant removal: benefits and Drawbacks--a patient survey. BMC Surg. 2015;15:96. doi: 10.1186/s12893-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Williams B.R., McCreary D.L., Chau M., Cunningham B.P., Pena F., Swiontkowski M.F. Functional outcomes of symptomatic implant removal following ankle fracture open reduction and internal fixation. Foot Ankle Int. 2018;39:674–680. doi: 10.1177/1071100718757719. [DOI] [PubMed] [Google Scholar]
- 256.Bose S., Banerjee D., Bandyopadhyay A. In: Materials for Bone Disorders. Bose S., Bandyopadhyay A., editors. Academic Press; 2017. Chapter 1 - introduction to biomaterials and devices for bone disorders; pp. 1–27. ISBN 978-0-12-802792-9. [Google Scholar]
- 257.Chi P., Yu W., Wu B., Gao M., Song K., Mao K., Li B., Liu X., Liu H., Zhang C., et al. A novel mg-zn-nd-zr alloy lumbar interbody fusion cage: an in vitro and in vivo study. J. Magnesium Alloys. 2024 doi: 10.1016/j.jma.2024.06.017. [DOI] [Google Scholar]
- 258.Abbah S.A., Lam C.X.L., Hutmacher D.W., Goh J.C.H., Wong H.-K. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials. 2009;30:5086–5093. doi: 10.1016/j.biomaterials.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 259.van Hengel I.A.J., Riool M., Fratila-Apachitei L.E., Witte-Bouma J., Farrell E., Zadpoor A.A., Zaat S.A.J., Apachitei I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017;140:1–15. doi: 10.1016/j.biomaterials.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 260.Mohsin F., Zubairi M.B.A., Fatima K., Diwan M.A. Metallic implant-related osteosarcoma. Radiol Case Rep. 2023;18:1311–1315. doi: 10.1016/j.radcr.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Laubach M., Kobbe P., Hutmacher D.W. Biodegradable interbody cages for lumbar spine fusion: current concepts and future directions. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121699. [DOI] [PubMed] [Google Scholar]
- 262.Dragomir (Nicolescu) L., Antoniac A., Manescu (Paltanea) V., Robu A., Dinu M., Pana I., Cotrut C.M., Kamel E., Antoniac I., Rau J.V., et al. Preparation and characterization of hydroxyapatite coating by magnetron sputtering on mg–zn–ag alloys for orthopaedic trauma implants. Ceram. Int. 2023 doi: 10.1016/j.ceramint.2023.05.116. [DOI] [Google Scholar]
- 263.Zhao D., Huang S., Lu F., Wang B., Yang L., Qin L., Yang K., Li Y., Li W., Wang W., et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 264.Sun J., Li Z., Liu S., Xia T., Shen J. Biodegradable magnesium screw, titanium screw and direct embedding fixation in pedicled vascularized iliac bone graft transfer for osteonecrosis of the femoral head: a randomized controlled study. J. Orthop. Surg. Res. 2023;18:523. doi: 10.1186/s13018-023-04012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U., Schavan R., Stukenborg-Colsman C., Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Antoniac I.V., Antoniac A., Vasile E., Tecu C., Fosca M., Yankova V.G., Rau J.V. In vitro characterization of novel nanostructured collagen-hydroxyapatite composite scaffolds doped with magnesium with improved biodegradation rate for hard tissue regeneration. Bioact. Mater. 2021;6:3383–3395. doi: 10.1016/j.bioactmat.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Antoniac I., Miculescu M., Mănescu (Păltânea) V., Stere A., Quan P.H., Păltânea G., Robu A., Earar K. Magnesium-based alloys used in orthopedic surgery. Materials. 2022;15:1148. doi: 10.3390/ma15031148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Antoniac I., Manescu (Paltanea) V., Paltanea G., Antoniac A., Nemoianu I.V., Petrescu M.I., Dura H., Bodog A.D. Additive manufactured magnesium-based scaffolds for tissue engineering. Materials. 2022;15:8693. doi: 10.3390/ma15238693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Antoniac I., Manescu (Paltanea) V., Antoniac A., Paltanea G. Magnesium-based alloys with adapted interfaces for bone implants and tissue engineering. Regen. Biomater. 2023;10 doi: 10.1093/rb/rbad095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Streza A., Antoniac A., Manescu (Paltanea) V., Paltanea G., Robu A., Dura H., Verestiuc L., Stanica E., Voicu S.I., Antoniac I., et al. Effect of filler types on cellulose-acetate-based composite used as coatings for biodegradable magnesium implants for trauma. Materials. 2023;16:554. doi: 10.3390/ma16020554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhen Z., Liu X., Huang T., Xi T., Zheng Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C. 2015;46:202–206. doi: 10.1016/j.msec.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 272.Durlach J., Durlach V., Bac P., Bara M., Guiet-Bara A. Magnesium and therapeutics. Magnes. Res. 1994;7:313–328. [PubMed] [Google Scholar]
- 273.Kim K.-J., Choi S., Sang Cho Y., Yang S.-J., Cho Y.-S., Kim K.K. Magnesium ions enhance infiltration of osteoblasts in scaffolds via increasing cell motility. J. Mater. Sci. Mater. Med. 2017;28:96. doi: 10.1007/s10856-017-5908-5. [DOI] [PubMed] [Google Scholar]
- 274.Ye Li, Xu J., Mi J., He X., Pan Q., Zheng L., Zu H., Chen Z., Dai B., Li X., et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120984. [DOI] [PubMed] [Google Scholar]
- 275.Yao Z., Yuan W., Xu J., Tong W., Mi J., Ho P.-C., Chow D.H.K., Li Y., Yao H., Li X., et al. Magnesium-encapsulated injectable hydrogel and 3D-Engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv. Sci. (Weinh.) 2022;9 doi: 10.1002/advs.202202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2017;112:287–302. doi: 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 277.Gu X.N., Xie X.H., Li N., Zheng Y.F., Qin L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012;8:2360–2374. doi: 10.1016/j.actbio.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 278.Fukuda K., Asoh S., Ishikawa M., Yamamoto Y., Ohsawa I., Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 279.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 280.Antoniac I., Miculescu F., Cotrut C., Ficai A., Rau J.V., Grosu E., Antoniac A., Tecu C., Cristescu I. Controlling the degradation rate of biodegradable Mg–Zn-Mn alloys for orthopedic applications by electrophoretic deposition of hydroxyapatite coating. Materials. 2020;13:263. doi: 10.3390/ma13020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22:1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tian L., Sheng Y., Huang L., Chow D.H.-K., Chau W.H., Tang N., Ngai T., Wu C., Lu J., Qin L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. doi: 10.1016/j.biomaterials.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 283.Zheng N., Xu J., Ruan Y.C., Chang L., Wang X., Yao H., Wang J., Zhang R., Xue Q., Tang N., et al. Magnesium facilitates the healing of atypical femoral fractures: a single-cell transcriptomic study. Mater. Today. 2022;52:43–62. doi: 10.1016/j.mattod.2021.11.028. [DOI] [Google Scholar]
- 284.Wang J., Xu J., Wang X., Sheng L., Zheng L., Song B., Wu G., Zhang R., Yao H., Zheng N., et al. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120576. [DOI] [PubMed] [Google Scholar]
- 285.Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: degradation, application, and alloying elements. Interv. Med. Appl. Sci. 9, 27–38, doi:10.1556/1646.9.2017.1.04. [DOI] [PMC free article] [PubMed]
- 286.Maier J.A.M., Bernardini D., Rayssiguier Y., Mazur A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2004;1689:6–12. doi: 10.1016/j.bbadis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 287.Bernardini D., Nasulewic A., Mazur A., Maier J.A.M. Magnesium and microvascular endothelial cells: a role in inflammation and angiogenesis. FBL. 2005;10:1177–1182. doi: 10.2741/1610. [DOI] [PubMed] [Google Scholar]
- 288.Wang L., Wang X., Wu J., Chen J., He Z., Wang J., Zhang X. Magnesium ions induce endothelial cell differentiation into tip cell and enhance vascularized bone regeneration. Adv. Healthcare Mater. 2025 doi: 10.1002/adhm.202500274. [DOI] [PubMed] [Google Scholar]
- 289.Manescu (Paltanea) V., Antoniac I., Antoniac A., Laptoiu D., Paltanea G., Ciocoiu R., Nemoianu I.V., Gruionu L.G., Dura H. Bone regeneration induced by patient-adapted Mg alloy-based scaffolds for bone defects: present and future perspectives. Biomimetics (Basel) 2023;8:618. doi: 10.3390/biomimetics8080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Galow A.-M., Rebl A., Koczan D., Bonk S.M., Baumann W., Gimsa J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem. Biophys.Rep. 2017;10:17–25. doi: 10.1016/j.bbrep.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kim J.-W., Alfafara A.M.D., Kim H.-Y., Kim S.-Y., Kim S.-J. Effects of pH alteration on the pathogenesis of medication-related osteonecrosis of the jaw. Bone. 2019;122:45–51. doi: 10.1016/j.bone.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 292.Wang J., Xu J., Song B., Chow D.H., Shu-hang Yung P., Qin L. Magnesium (mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 293.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J., Hou P., Zhang Y., Liu J., Xu H., et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 294.Hung C.-C., Chaya A., Liu K., Verdelis K., Sfeir C. The role of magnesium ions in bone regeneration involves the canonical wnt signaling pathway. Acta Biomater. 2019;98:246–255. doi: 10.1016/j.actbio.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 295.Hamushan M., Cai W., Zhang Y., Ren Z., Du J., Zhang S., Zhao C., Cheng P., Zhang X., Shen H., et al. High-purity magnesium pin enhances bone consolidation in distraction osteogenesis via regulating ptch protein activating hedgehog-alternative wnt signaling. Bioact. Mater. 2021;6:1563–1574. doi: 10.1016/j.bioactmat.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chen L., Yan Z., Qiu T., Zhu J., Liu G., Han J., Guo C. Long-term temporospatial complementary relationship between degradation and bone regeneration of Mg–Al alloy. ACS Appl. Bio Mater. 2023;6:4703–4713. doi: 10.1021/acsabm.3c00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Okutan B., Schwarze U.Y., Habisch H., Iskhakova K., Ćwieka H., Ribeiro-Machado C., Moosmann J.P., Blanchet C., Brcic I., Santos S.G., et al. Biodegradable ultrahigh-purity magnesium and its alloy ZX00 promote osteogenesis in the medullary cavity and glycogenolysis in the liver. Acta Biomater. 2025;195:599–613. doi: 10.1016/j.actbio.2025.02.007. [DOI] [PubMed] [Google Scholar]
- 298.Kaur S., Grover V., Kaur H., Malhotra R. Evaluation of bone morphogenic proteins in periodontal practice. Indian J. Dent. 2016;7:28–37. doi: 10.4103/0975-962X.179379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gao Y., Huang H., Jiang X., Ha T., Li Y., Zhang K., Wang C., Wang L., Fan Y. Effect of mechanical loading on osseointegration combined with degradation behavior of magnesium bone screw in vivo. Appl. Mater. Today. 2023;32 doi: 10.1016/j.apmt.2023.101793. [DOI] [Google Scholar]
- 300.Guan Q., Hu T., Zhang L., Yu M., Niu J., Ding Z., Yu P., Yuan G., An Z., Pei J. Concerting magnesium implant degradation facilitates local chemotherapy in tumor-associated bone defect. Bioact. Mater. 2024;40:445–459. doi: 10.1016/j.bioactmat.2024.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li C., Guo C., Fitzpatrick V., Ibrahim A., Zwierstra M.J., Hanna P., Lechtig A., Nazarian A., Lin S.J., Kaplan D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020;5:61–81. doi: 10.1038/s41578-019-0150-z. [DOI] [Google Scholar]
- 302.Li X., Qi C., Han L., Chu C., Bai J., Guo C., Xue F., Shen B., Chu P.K. Influence of dynamic compressive loading on the In vitro degradation behavior of pure PLA and Mg/PLA composite. Acta Biomater. 2017;64:269–278. doi: 10.1016/j.actbio.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 303.Fernandes M.T., Martins da Silva L., Nilo Mendes P.S., Vergílio de Queiroz A., Adilson de Castro J., Santos C. Dos A degradation kinetics model of mg–zn–mn–ca alloys in kokubo solution. J. Mater. Res. Technol. 2021;11:887–895. doi: 10.1016/j.jmrt.2021.01.063. [DOI] [Google Scholar]
- 304.Rahman Z.U., Deen K.M., Haider W. Controlling corrosion kinetics of magnesium alloys by electrochemical anodization and investigation of film mechanical properties. Appl. Surf. Sci. 2019;484:906–916. doi: 10.1016/j.apsusc.2019.02.168. [DOI] [Google Scholar]
- 305.Cao B., Cao L., Kallmes D.F. Degradation rate assessment of biodegradable magnesium alloys. Mater. Sci. Appl. 2024;15:245–252. doi: 10.4236/msa.2024.158017. [DOI] [Google Scholar]
- 306.Yu L., Sun Y., Wang M., Wu Y., Zhang X., Xu J. Fluoride-coated high-purity magnesium cage promotes bone fusion in goat models. Ann. Transl. Med. 2022;10 doi: 10.21037/atm-22-2098. 537–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wang X., Zhang Y., Wang Y., Liu Y., Li X., Han Z., Zhao Y., Wang B., Liu J., Wang R., et al. Biological performance of a bioabsorbable magnesium–magnesium phosphate cement interbody fusion cage in a porcine lumbar interbody fusion model: a feasibility study. Eur. Spine J. 2024;33:3324–3333. doi: 10.1007/s00586-024-08387-3. [DOI] [PubMed] [Google Scholar]
- 308.Xu H., Zhang F., Wang H., Geng F., Shao M., Xu S., Xia X., Ma X., Lu F., Jiang J. Evaluation of a porous bioabsorbable interbody Mg-Zn alloy cage in a goat cervical spine model. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/7961509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Geng F., Tan L., Zhang B., Wu C., He Y., Yang J., Yang K. Study on β-TCP coated porous Mg as a bone tissue engineering scaffold material. J. Mater. Sci. Technol. 2009;25:123. [Google Scholar]
- 310.Zhang F., Xu H., Wang H., Geng F., Ma X., Shao M., Xu S., Lu F., Jiang J. Quantitative analysis of near-implant magnesium accumulation for a Si-Containing coated AZ31 cage from a goat cervical spine fusion model. BMC Muscoskelet. Disord. 2018;19:105. doi: 10.1186/s12891-018-2027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Daentzer D., Willbold E., Kalla K., Bartsch I., Masalha W., Hallbaum M., Hurschler C., Kauth T., Kaltbeitzel D., Hopmann C., et al. Bioabsorbable interbody magnesium-polymer cage: degradation kinetics, biomechanical stiffness, and histological findings from an ovine cervical spine fusion model. Spine. 2014;39:E1220–E1227. doi: 10.1097/BRS.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 312.Seelig M.G. A study of magnesium wire as an absorbable suture and ligature material. Arch. Surg. 1924;8:669. doi: 10.1001/archsurg.1924.01120050210011. [DOI] [Google Scholar]
- 313.Pitt C.G., Chasalow F.I., Hibionada Y.M., Klimas D.M., Schindler A., Aliphatic Polyesters I. The degradation of poly(ϵ-caprolactone) in vivo. J. Appl. Polym. Sci. 1981;26:3779–3787. doi: 10.1002/app.1981.070261124. [DOI] [Google Scholar]
- 314.Suggs L.J., Moore S.A., Mikos A.G. In: Physical Properties of Polymers Handbook. Mark J.E., editor. Springer; New York, NY: 2007. Synthetic biodegradable polymers for medical applications; pp. 939–950. [Google Scholar]
- 315.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- 316.Wong H.M., Zhao Y., Leung F.K.L., Xi T., Zhang Z., Zheng Y., Wu S., Luk K.D.K., Cheung K.M.C., Chu P.K., et al. Functionalized polymeric membrane with enhanced mechanical and biological properties to control the degradation of magnesium alloy. Adv. Healthcare Mater. 2017;6 doi: 10.1002/adhm.201601269. [DOI] [PubMed] [Google Scholar]
- 317.Domingos M., Intranuovo F., Gloria A., Gristina R., Ambrosio L., Bártolo P.J., Favia P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013;9:5997–6005. doi: 10.1016/j.actbio.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 318.Vandrovcova M., Douglas T.E.L., Mróz W., Musial O., Schaubroeck D., Budner B., Syroka R., Dubruel P., Bacakova L. Pulsed laser deposition of magnesium-doped calcium phosphate coatings on porous polycaprolactone scaffolds produced by rapid prototyping. Mater. Lett. 2015;148:178–183. doi: 10.1016/j.matlet.2015.02.074. [DOI] [Google Scholar]
- 319.Dash T.K., Konkimalla V.B. Poly-є-Caprolactone based formulations for drug delivery and tissue engineering: a review. J. Contr. Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 320.Kumar N., Alathur Ramakrishnan S., Lopez K.G., Chin B.Z., S D., Kumar L., Baskar S., Vellayappan B.A., Fuh J.Y.H., Anantharajan S.K. Current trends and future scope in 3D printing for surgical management of spine pathologies. Bioprinting. 2022;26 doi: 10.1016/j.bprint.2022.e00197. [DOI] [Google Scholar]
- 321.Hajiali F., Tajbakhsh S., Shojaei A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym. Rev. 2018 [Google Scholar]
- 322.Bartnikowski M., Dargaville T.R., Ivanovski S., Hutmacher D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019;96:1–20. doi: 10.1016/j.progpolymsci.2019.05.004. [DOI] [Google Scholar]
- 323.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 324.Yong M.R.N.O., Saifzadeh S., Askin G.N., Labrom R.D., Hutmacher D.W., Adam C.J. Establishment and characterization of an open mini-thoracotomy surgical approach to an ovine thoracic spine fusion model. Tissue Eng. C Methods. 2014;20:19–27. doi: 10.1089/ten.tec.2012.0746. [DOI] [PubMed] [Google Scholar]
- 325.Li Y., Wu Z., Li X., Guo Z., Wu S., Zhang Y., Shi L., Teoh S., Liu Y., Zhang Z. A polycaprolactone-tricalcium phosphate composite scaffold as an autograft-free spinal fusion cage in a sheep model. Biomaterials. 2014;35:5647–5659. doi: 10.1016/j.biomaterials.2014.03.075. [DOI] [PubMed] [Google Scholar]
- 326.Liu Y., Wu H., Bao S., Huang H., Tang Z., Dong H., Liu J., Chen S., Wang N., Wu Z., et al. Clinical application of 3D-Printed biodegradable lumbar interbody cage (Polycaprolactone/β-Tricalcium phosphate) for posterior lumbar interbody fusion. J. Biomed. Mater. Res. B Appl. Biomater. 2023;111:1398–1406. doi: 10.1002/jbm.b.35244. [DOI] [PubMed] [Google Scholar]
- 327.Shor L., Güçeri S., Wen X., Gandhi M., Sun W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions In vitro. Biomaterials. 2007;28:5291–5297. doi: 10.1016/j.biomaterials.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 328.Bioresorbable polymers developed for use in the production of bone fixation devices available online. https://medical.evonik.com/en/materials-and-solutions/resomer-bioresorbable-polymers/osteoconductive-polymers
- 329.LaMarca F., Flanagan C.L., Tseng W.-J., Suarez-Gonzalez D., Murphy W.L., Lin C.-Y., Hollister S.J. 2012. A New Resorbable Integrated Cervical Plate/Cage Fusion Device Modified with Osteoconductive Coating or Osteoinductive Factors: Preliminary Results in a Pre-clinical Yucatan Minipig Model. San Diego, CA. [Google Scholar]
- 330.Bonithon R., Lupton C., Roldo M., Dunlop J.N., Blunn G.W., Witte F., Tozzi G. Open-porous magnesium-based scaffolds withstand in vitro corrosion under cyclic loading: a mechanistic study. Bioact. Mater. 2023;19:406–417. doi: 10.1016/j.bioactmat.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dong J., Tümer N., E. Putra N., Zhu J., Li Y., A. Leeflang M., Taheri P., Fratila-Apachitei E.L., C. Mol J.M., A. Zadpoor A., et al. Extrusion-based 3D printed magnesium scaffolds with multifunctional MgF 2 and MgF 2 –CaP coatings. Biomater. Sci. 2021;9:7159–7182. doi: 10.1039/D1BM01238J. [DOI] [PubMed] [Google Scholar]
- 332.Xie K., Wang N., Guo Y., Zhao S., Tan J., Wang L., Li G., Wu J., Yang Y., Xu W., et al. Additively manufactured biodegradable porous magnesium implants for elimination of implant-related infections: an in vitro and in vivo study. Bioact. Mater. 2021;8:140–152. doi: 10.1016/j.bioactmat.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Augustin J., Feichtner F., Waselau A.-C., Julmi S., Klose C., Wriggers P., Maier H.J., Meyer-Lindenberg A. Effect of pore size on tissue ingrowth and osteoconductivity in biodegradable Mg alloy scaffolds. J. Appl. Biomater. Funct. Mater. 2022;20 doi: 10.1177/22808000221078168. [DOI] [PubMed] [Google Scholar]
- 334.França D.C., Bezerra E.B., Morais D.D.S., Araújo E.M., Wellen R.M.R. Effect of hydrolytic degradation on mechanical properties of PCL. MSF. 2016;869:342–345. doi: 10.4028/www.scientific.net/MSF.869.342. [DOI] [Google Scholar]
- 335.Deshpande M.V., Girase A., King M.W. Degradation of Poly(ε-Caprolactone) resorbable multifilament yarn under physiological conditions. Polymers. 2023;15:3819. doi: 10.3390/polym15183819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Argentieri, M.; Schabowsky, C.N.; Kommala, D. ECRI Corporate Governance.
- 337.Rao P.J., Pelletier M.H., Walsh W.R., Mobbs R.J. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014;6:81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kharissova O.V., Méndez Y.P., Kharisov B.I., Nikolaev A.L., Dorozhkin S.V., Mena D.N., García B.O. Biomineralization of calcium phosphates in nature. Nano-Struct. Nano-Objects. 2025;41 doi: 10.1016/j.nanoso.2024.101425. [DOI] [Google Scholar]
- 339.Jasty M., Bragdon C., Burke D., O'Connor D., Lowenstein J., Harris W.H. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J. Bone Joint Surgl. Am. 1997;79:707–714. doi: 10.2106/00004623-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 340.Leucht P., Kim J.-B., Wazen R., Currey J.A., Nanci A., Brunski J.B., Helms J.A. Effect of mechanical stimuli on skeletal regeneration around implants. Bone. 2007;40:919–930. doi: 10.1016/j.bone.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rosa A.L., Beloti M.M. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz. Dent. J. 2003;14:16–21. doi: 10.1590/s0103-64402003000100003. [DOI] [PubMed] [Google Scholar]
- 342.Kroppenstedt S., Gulde M., Schönmayr R. Radiological comparison of instrumented posterior lumbar interbody fusion with one or two closed-box plasmapore coated titanium cages: follow-Up study over more than seven years. Spine. 2008;33:2083. doi: 10.1097/BRS.0b013e31818448a9. [DOI] [PubMed] [Google Scholar]
- 343.Nishiguchi S., Kato H., Fujita H., Kim H.M., Miyaji F., Kokubo T., Nakamura T. Enhancement of bone-bonding strengths of titanium alloy implants by alkali and heat treatments. J. Biomed. Mater. Res. 1999;48:689–696. doi: 10.1002/(sici)1097-4636(1999)48:5<689::aid-jbm13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 344.Fujibayashi S., Nakamura T., Nishiguchi S., Tamura J., Uchida M., Kim H.M., Kokubo T. Bioactive titanium: effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J. Biomed. Mater. Res. 2001;56:562–570. doi: 10.1002/1097-4636(20010915)56:4<562::aid-jbm1128>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 345.de Groot K., Geesink R., Klein C.P., Serekian P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987;21:1375–1381. doi: 10.1002/jbm.820211203. [DOI] [PubMed] [Google Scholar]
- 346.Sun J., Liu S.-S., Zou D., Ni R.-H., Wei C.-B., Wang H., Li W.-S. A novel porous interbody fusion cage modified by microarc oxidation and hydrothermal treatment technology accelerate osseointegration and spinal fusion in sheep. RSC Adv. 2024;14:31966–31978. doi: 10.1039/D3RA08185K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Morimoto T., Tsukamoto M., Aita K., Fujita N., Mawatari M. First clinical experience with posterior lumbar interbody fusion using a thermal-sprayed silver-containing hydroxyapatite-coated cage. J. Orthop. Surg. Res. 2023;18:392. doi: 10.1186/s13018-023-03882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nakashima T., Morimoto T., Hashimoto A., Kii S., Tsukamoto M., Miyamoto H., Todo M., Sonohata M., Mawatari M. Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats. JOR SPINE. 2023;6 doi: 10.1002/jsp2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kodama J., Chen H., Zhou T., Kushioka J., Okada R., Tsukazaki H., Tateiwa D., Nakagawa S., Ukon Y., Bal Z., et al. Antibacterial efficacy of quaternized chitosan coating on 3D printed titanium cage in rat intervertebral disc space. Spine J. 2021;21:1217–1228. doi: 10.1016/j.spinee.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 350.Chan J.L., Bae H.W., Harrison Farber S., Uribe J.S., Eastlack R.K., Walker C.T. Evolution of bioactive implants in lateral interbody fusion. Int. J. Spine Surg. 2022;16:S61–S68. doi: 10.14444/8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Croft A.J., Chanbour H., Chen J.W., Young M.W., Stephens B.F. Implant surface technologies to promote spinal fusion: a narrative review. Int. J. Spine Surg. 2023;17:S35–S43. doi: 10.14444/8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wu X., Liu X., Wei J., Ma J., Deng F., Wei S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies. Int. J. Nanomed. 2012;7:1215–1225. doi: 10.2147/IJN.S28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bassous N.J., Jones C.L., Webster T.J. 3-D printed Ti-6Al-4V scaffolds for supporting osteoblast and restricting bacterial functions without using drugs: predictive equations and experiments. Acta Biomater. 2019;96:662–673. doi: 10.1016/j.actbio.2019.06.055. [DOI] [PubMed] [Google Scholar]
- 354.Ohanisian L., Dorsi M.J., Ohanisian L., Dorsi M.J. A novel 3D printed titanium implant for anterior cervical discectomy and fusion. Cureus. 2019;11 doi: 10.7759/cureus.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liu X., Han F., Zhao P., Lin C., Wen X., Ye X. Layer-by-Layer self-assembled multilayers on PEEK implants improve osseointegration in an osteoporosis rabbit model. Nanomed. Nanotechnol. Biol. Med. 2017;13:1423–1433. doi: 10.1016/j.nano.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 356.Zhu C., He M., Mao L., Yang H., Hu B., Zhang L., Feng G., Liu L., Song Y. Titanium interlayer-mediated hydroxyapatite-coated polyetheretherketone cage in transforaminal lumbar interbody fusion surgery. BMC Muscoskelet. Disord. 2021;22:918. doi: 10.1186/s12891-021-04803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ishihama H., Ishii K., Nagai S., Kakinuma H., Sasaki A., Yoshioka K., Kuramoto T., Shiono Y., Funao H., Isogai N., et al. An antibacterial coated polymer prevents biofilm formation and implant-associated infection. Sci. Rep. 2021;11:3602. doi: 10.1038/s41598-021-82992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pezzotti G., Marin E., Adachi T., Lerussi F., Rondinella A., Boschetto F., Zhu W., Kitajima T., Inada K., McEntire B.J., et al. Incorporating Si3 N4 into PEEK to produce antibacterial, osteocondutive, and radiolucent spinal implants. Macromol. Biosci. 2018;18 doi: 10.1002/mabi.201800033. [DOI] [PubMed] [Google Scholar]
- 359.Wu Y.-J., Wang C.-Y., Feng K.-C., Chien R.R., Mana-ay H., Kung S.-Y., Hou K.-H., Tu C.-S., Chen P.-Y., Lai P.-L. Ti-6Al-4V intervertebral fusion cage with compatible stiffness, enhanced fatigue life, and osteogenic differentiation. J. Alloys Compd. 2023;957 doi: 10.1016/j.jallcom.2023.170450. [DOI] [Google Scholar]
- 360.Shimizu T., Fujibayashi S., Yamaguchi S., Otsuki B., Okuzu Y., Matsushita T., Kokubo T., Matsuda S. In vivo experimental study of anterior cervical fusion using bioactive polyetheretherketone in a canine model. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Liu C., Zhang Y., Xiao L., Ge X., Öner F.C., Xu H. Vacuum plasma sprayed porous titanium coating on polyetheretherketone for ACDF improves the osteogenic ability: an in vitro and in vivo study. Biomed. Microdevices. 2021;23:21. doi: 10.1007/s10544-021-00559-y. [DOI] [PubMed] [Google Scholar]
- 362.Kashii M., Kitaguchi K., Makino T., Kaito T. Comparison in the same intervertebral space between titanium-coated and uncoated PEEK cages in lumbar interbody fusion surgery. J. Orthop. Sci. 2020;25:565–570. doi: 10.1016/j.jos.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 363.Lee J.H., Jang H.L., Lee K.M., Baek H.-R., Jin K., Noh J.H. Cold-spray coating of hydroxyapatite on a three-dimensional polyetheretherketone implant and its biocompatibility evaluated by In vitro and In vivo minipig model: cold-spray coated ha-peek implant and evaluation BY IN vitro and IN VIVO minipig model. J. Biomed. Mater. Res. 2017;105:647–657. doi: 10.1002/jbm.b.33589. [DOI] [PubMed] [Google Scholar]
- 364.Wang T., Liu F. Optimizing mechanical properties of magnesium alloys by philosophy of thermo-kinetic synergy: review and outlook. J. Magnesium Alloys. 2022;10:326–355. doi: 10.1016/j.jma.2021.12.016. [DOI] [Google Scholar]
- 365.Johanes M., Bin Gombari A.A., Gupta M. Enhancing multiple properties of a multicomponent Mg-Based alloy using a sinterless turning-induced deformation technique. Technologies. 2023;11:181. doi: 10.3390/technologies11060181. [DOI] [Google Scholar]
- 366.Eshraghi S., Das S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010;6:2467–2476. doi: 10.1016/j.actbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Permentier K., Vercammen S., Soetaert S., Schellemans C. Carbon dioxide poisoning: a literature review of an often forgotten cause of intoxication in the emergency department. Int. J. Emerg. Med. 2017;10:14. doi: 10.1186/s12245-017-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Emonde C.K., Eggers M.-E., Wichmann M., Hurschler C., Ettinger M., Denkena B. Radiopacity enhancements in polymeric implant biomaterials: a comprehensive literature review. ACS Biomater. Sci. Eng. 2024;10:1323–1334. doi: 10.1021/acsbiomaterials.3c01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Elshalakany A.B., Abdel-Mottaleb M.M., Salunkhe S., Alqahtani B. In: Advances in Metal Additive Manufacturing. Salunkhe S., Amancio-Filho S.T., Davim J.P., editors. Woodhead Publishing; 2023. 7 - mechanical properties of titanium alloys additive manufacturing for biomedical applications; pp. 219–231. (Woodhead Publishing Reviews: Mechanical Engineering Series). ISBN 978-0-323-91230-3. [Google Scholar]
- 370.Marin E., Lanzutti A. Biomedical applications of titanium alloys: a comprehensive review. Materials (Basel) 2023;17:114. doi: 10.3390/ma17010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ramgobin A., Fontaine G., Bourbigot S. A case study of polyether ether ketone (I): investigating the thermal and fire behavior of a high-performance material. Polymers. 2020;12:1789. doi: 10.3390/polym12081789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Akhdar H. Theoretical investigation of photon interaction and X-Ray imaging performance of PEEK-based composites for medical implants. Polymers. 2025;17:996. doi: 10.3390/polym17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Chen D.-J., Yao C., Song Q., Tang B., Liu X., Zhang B., Dai M., Nie T., Wan Z. Unilateral versus bilateral pedicle screw fixation combined with transforaminal lumbar interbody fusion for the treatment of low lumbar degenerative disc diseases: analysis of clinical and radiographic results. World Neurosurg. 2018;115:e516–e522. doi: 10.1016/j.wneu.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 374.Yu Y., Robinson D.L., Ackland D.C., Yang Y., Lee P.V.S. Influence of the geometric and material properties of lumbar endplate on lumbar interbody fusion failure: a systematic review. J. Orthop. Surg. Res. 2022;17:224. doi: 10.1186/s13018-022-03091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Beutler W.J., Peppelman W.C. Anterior lumbar fusion with paired BAK standard and paired BAK proximity cages: subsidence incidence, subsidence factors, and clinical outcome. Spine J. 2003;3:289–293. doi: 10.1016/s1529-9430(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 376.Kim M.-C., Chung H.-T., Cho J.-L., Kim D.-J., Chung N.-S. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. Clin. Spine Surg. 2013;26:87. doi: 10.1097/BSD.0b013e318237b9b1. [DOI] [PubMed] [Google Scholar]
- 377.Pan F.-M., Wang S.-J., Yong Z.-Y., Liu X.-M., Huang Y.-F., Wu D.-S. Risk factors for cage retropulsion after lumbar interbody fusion surgery: series of cases and literature review. Int. J. Surg. 2016;30:56–62. doi: 10.1016/j.ijsu.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 378.Cho J.H., Hwang C.J., Kim H., Joo Y.-S., Lee D.-H., Lee C.S. Effect of osteoporosis on the clinical and radiological outcomes following one-level posterior lumbar interbody fusion. J. Orthop. Sci. 2018;23:870–877. doi: 10.1016/j.jos.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 379.Yao Y.-C., Chou P.-H., Lin H.-H., Wang S.-T., Liu C.-L., Chang M.-C. Risk factors of cage subsidence in patients received minimally invasive transforaminal lumbar interbody fusion. Spine. 2020;45 doi: 10.1097/BRS.0000000000003557. [DOI] [PubMed] [Google Scholar]
- 380.Kimura H., Shikata J., Odate S., Soeda T., Yamamura S. Risk factors for cage retropulsion after posterior lumbar interbody fusion: analysis of 1070 cases. Spine (Phila Pa 1976) 2012;37:1164–1169. doi: 10.1097/BRS.0b013e318257f12a. [DOI] [PubMed] [Google Scholar]
- 381.Lee D.-Y., Park Y.-J., Song S.-Y., Jeong S.-T., Kim D.-H. Risk factors for posterior cage migration after lumbar interbody fusion surgery. Asian Spine J. 2018;12:59–68. doi: 10.4184/asj.2018.12.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Marchi L., Abdala N., Oliveira L., Amaral R., Coutinho E., Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J. Neurosurg. Spine. 2013;19:110–118. doi: 10.3171/2013.4.SPINE12319. [DOI] [PubMed] [Google Scholar]
- 383.Aoki Y., Yamagata M., Nakajima F., Ikeda Y., Shimizu K., Yoshihara M., Iwasaki J., Toyone T., Nakagawa K., Nakajima A., et al. Examining risk factors for posterior migration of fusion cages following transforaminal lumbar interbody fusion: a possible limitation of unilateral pedicle screw fixation. J. Neurosurg. Spine. 2010;13:381–387. doi: 10.3171/2010.3.SPINE09590. [DOI] [PubMed] [Google Scholar]
- 384.Zhou Z.-J., Xia P., Zhao F.-D., Fang X.-Q., Fan S.-W., Zhang J.-F. Endplate injury as a risk factor for cage retropulsion following transforaminal lumbar interbody fusion: an analysis of 1052 cases. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000024005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Choi U.Y., Park J.Y., Kim K.H., Kuh S.U., Chin D.K., Kim K.S., Cho Y.E. Unilateral versus bilateral percutaneous pedicle screw fixation in minimally invasive transforaminal lumbar interbody fusion. Neurosurg. Focus. 2013;35:E11. doi: 10.3171/2013.2.FOCUS12398. [DOI] [PubMed] [Google Scholar]
- 386.Moisi M., Page J., Paulson D., Oskouian R.J. Technical note – lateral approach to the lumbar spine for the removal of interbody cages. Cureus. 2015 doi: 10.7759/cureus.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hu Y.-H., Niu C.-C., Hsieh M.-K., Tsai T.-T., Chen W.-J., Lai P.-L. Cage positioning as a risk factor for posterior cage migration following transforaminal lumbar interbody fusion – an analysis of 953 cases. BMC Muscoskelet. Disord. 2019;20:260. doi: 10.1186/s12891-019-2630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kim S.-K., Elbashier O.M., Lee S., Choi W.-J. Can posterior stand-alone expandable cages safely restore lumbar lordosis? A minimum 5-Year Follow-up study. J. Orthop. Surg. Res. 2020;15:442. doi: 10.1186/s13018-020-01866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kim D.-Y., Kwon O.-H., Park J.-Y. Comparison between 3-Dimensional-Printed titanium and polyetheretherketone cages: 1-year outcome after minimally invasive transforaminal interbody fusion. Neurospine. 2022;19:524–532. doi: 10.14245/ns.2244140.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Yang J.J., Kim D.-M., Park S. Comparison of fusion, subsidence, and clinical results between 3D-Printed porous titanium cage and polyetheretherketone cage in posterior lumbar interbody fusion: a minimum of 2 years Follow-Up. World Neurosurg. 2023;177:e732–e741. doi: 10.1016/j.wneu.2023.06.132. [DOI] [PubMed] [Google Scholar]
- 391.Chahlavi A. Reduced subsidence with PEEK-titanium composite versus 3D titanium cages in a retrospective, self-controlled study in transforaminal lumbar interbody fusion. Glob. Spine J. 2025;15:1598–1607. doi: 10.1177/21925682241253168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Liu S.-X., Zeng T.-H., Chen C.-M., He L.-R., Feng A.-P., Jhang S.-W., Lin G.-X. 3D-Printed porous titanium versus polyetheretherketone cages in lateral lumbar interbody fusion: a systematic review and meta-analysis of subsidence. Front. Med. 2024;11 doi: 10.3389/fmed.2024.1389533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Basgul C., DeSantis P., Derr T., Hickok N.J., Bock R.M., Kurtz S.M. Exploring the mechanical strength, antimicrobial performance, and bioactivity of 3D-Printed silicon Nitride-PEEK composites in cervical spinal cages. Int. J. Bioprinting. 2024;10:2124. doi: 10.36922/ijb.2124. [DOI] [Google Scholar]
- 394.Hughes, E.B.; Alfarone, J.; Chernov, E.S.; Debick, N.A.; Jalal, M.; Kim, Y.; Suryadevara, A.; Krishnamurthy, S. Polyetheretherketone (PEEK) into the future: lowering infection rates in cranioplasty. Cureus 16, e72060, doi:10.7759/cureus.72060. [DOI] [PMC free article] [PubMed]
- 395.Zhao F., Yang W., Shan Z., Wang J., Chen H., Hong Z., Qian Y., He D., Fan S. Cage migration after transforaminal lumbar interbody fusion and factors related to it. Orthop. Surg. 2012;4:227–232. doi: 10.1111/os.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Xi Z., Mummaneni P.V., Wang M., Ruan H., Burch S., Deviren V., Clark A.J., Berven S.H., Chou D. 2020. The Association Between Lower Hounsfield Units on Computed Tomography and Cage Subsidence After Lateral Lumbar Interbody Fusion. [DOI] [PubMed] [Google Scholar]
- 397.Chen L., Han Z., Wei J., Sun Y., Liu L., Liu H., Wang D. Accuracy of the cage placement in oblique lumbar interbody fusion and its effects on the radiological outcome in lumbar degenerative disease. Glob. Spine J. 2025;15:127–135. doi: 10.1177/21925682241226956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Cho J.H., Hwang C.J., Lee D.-H., Lee C.S. Using lordotic cages at the L5-S1 level does not guarantee the improvement of sagittal alignment in patients who underwent posterior lumbar interbody fusion. Asian Spine J. 2023;17:477–484. doi: 10.31616/asj.2022.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Loenen A.C.Y., Peters M.J.M., Wierts R., Bevers R.T.J., van Rhijn L.W., Arts J.J., Willems P.C. Local bone metabolism during the consolidation process of spinal interbody fusion. J. Bone Miner. Metabol. 2022;40:220–228. doi: 10.1007/s00774-021-01281-8. [DOI] [PubMed] [Google Scholar]
- 400.Weinberg D.S., Eoh J.H., Manz W.J., Fakunle O.P., Dawes A.M., Park E.T., Rhee J.M. Off-label usage of RhBMP-2 in posterior cervical fusion is not associated with early increased complication rate and has similar clinical outcomes. Spine J. 2022;22:1079–1088. doi: 10.1016/j.spinee.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 401.Malham G.M., Louie P.K., Brazenor G.A., Mobbs R.J., Walsh W.R., Sethi R.K. Recombinant human bone morphogenetic Protein-2 in spine surgery: recommendations for use and alternative bone Substitutes—A narrative review. J. Spine Surg. 2022;8:477–490. doi: 10.21037/jss-22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Gillman C.E., Jayasuriya A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C. 2021;130 doi: 10.1016/j.msec.2021.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Chang S.Y., Kang D.-H., Cho S.K. Innovative developments in lumbar interbody cage materials and design: a comprehensive narrative review. Asian Spine J. 2024;18:444–457. doi: 10.31616/asj.2023.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.