Abstract
The objective of this study was to determine the effect of receptor-ligand affinity on the strength of endothelial cell adhesion. Linear and cyclic forms of the fibronectin (Fn) cell-binding domain peptide Arg-Gly-Asp (RGD) were covalently immobilized to glass, and Fn was adsorbed onto glass slides. Bovine aortic endothelial cells attached to the surfaces for 15 min. The critical wall shear stress at which 50% of the cells detached increased nonlinearly with ligand density and was greater with immobilized cyclic RGD than with immobilized linear RGD or adsorbed Fn. To directly compare results for the different ligand densities, the receptor-ligand dissociation constant and force per bond were estimated from data for the critical shear stress and contact area. Total internal reflection fluorescence microscopy was used to measure the contact area as a function of separation distance. Contact area increased with increasing ligand density. Contact areas were similar for the immobilized peptides but were greater on surfaces with adsorbed Fn. The dissociation constant was determined by nonlinear regression of the net force on the cells to models that assumed that bonds were either uniformly stressed or that only bonds on the periphery of the contact region were stressed (peeling model). Both models provided equally good fits for cells attached to immobilized peptides whereas the peeling model produced a better fit of data for cells attached to adsorbed Fn. Cyclic RGD and linear RGD both bind to the integrin alpha v beta 3, but immobilized cyclic RGD exhibited a greater affinity than did linear RGD. Receptor affinities of Fn adsorbed to glycophase glass and Fn adsorbed to glass were similar. The number of bonds was calculated assuming binding equilibrium. The peeling model produced good linear fits between bond force and number of bonds. Results of this study indicate that 1) bovine aortic endothelial cells are more adherent on immobilized cyclic RGD peptide than linear RGD or adsorbed Fn, 2) increased adhesion is due to a greater affinity between cyclic RGD and its receptor, and 3) the affinity of RGD peptides and adsorbed Fn for their receptors is increased after immobilization.
Full text
PDF



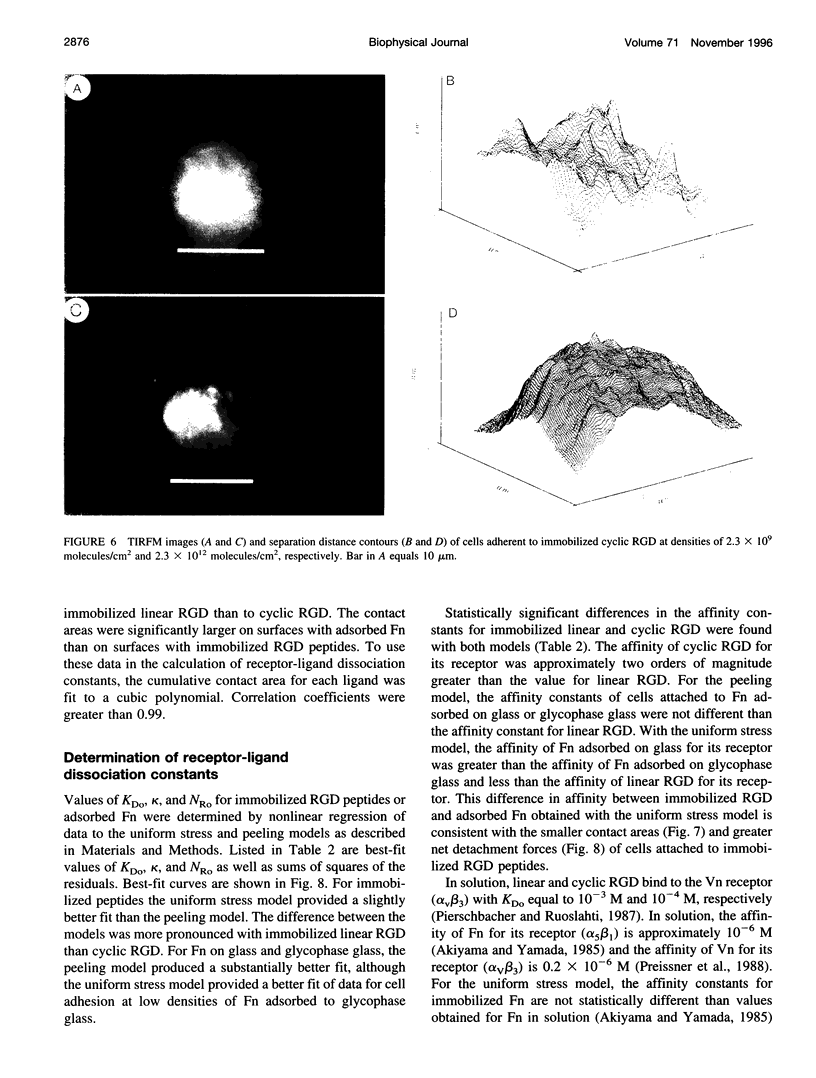
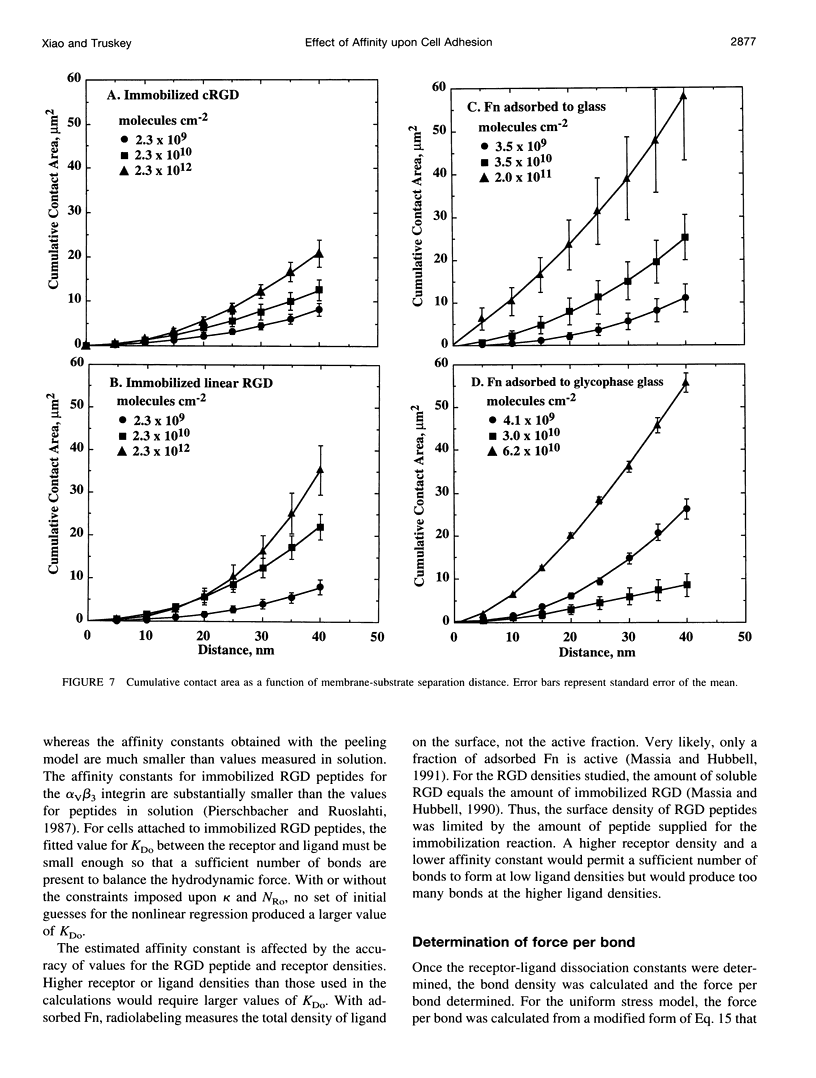

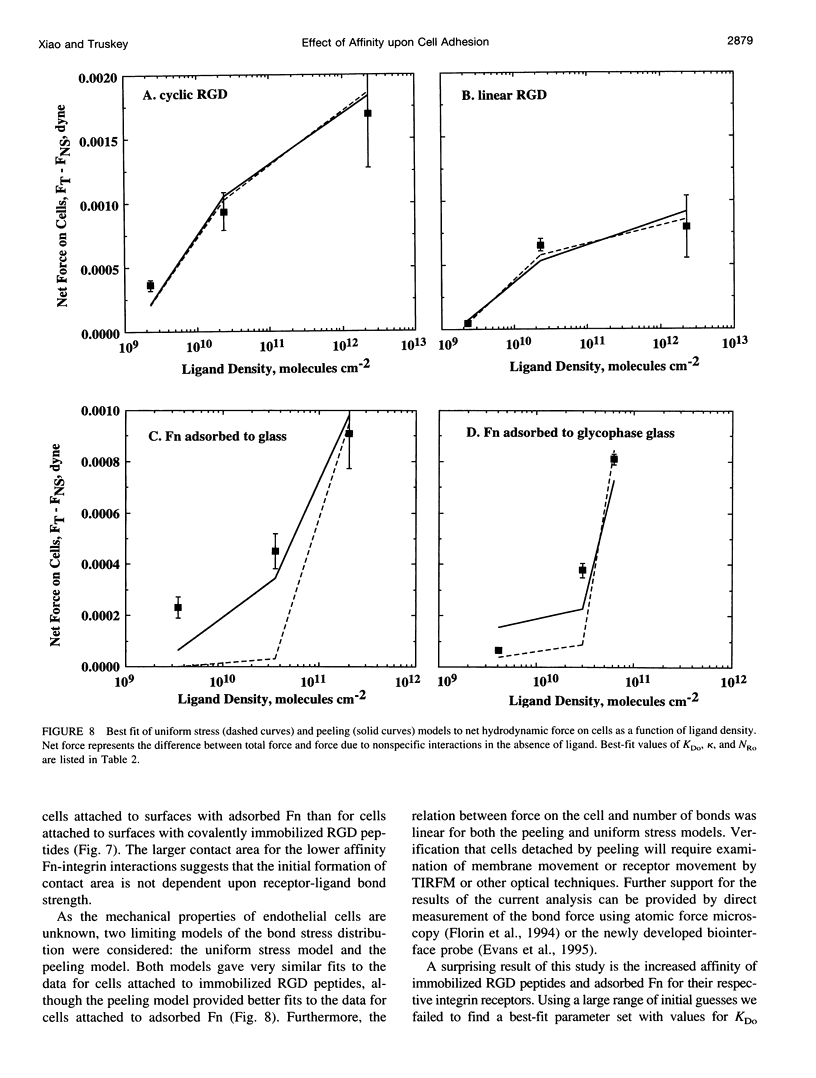

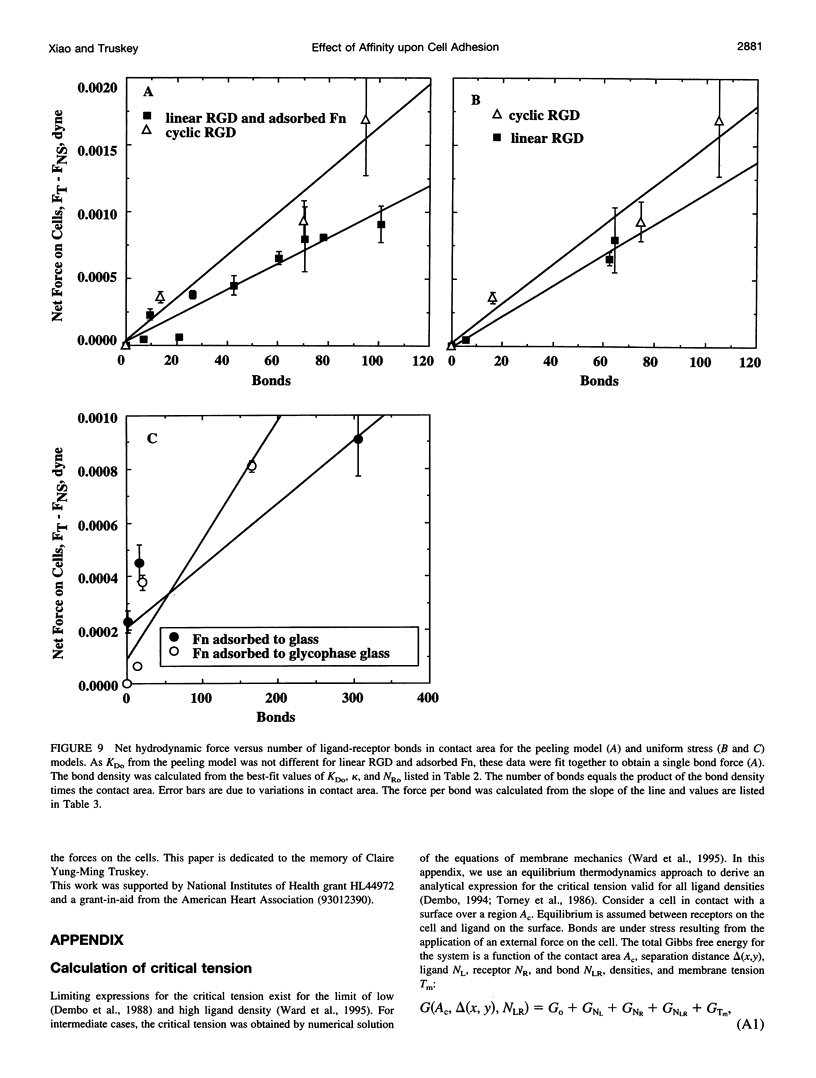
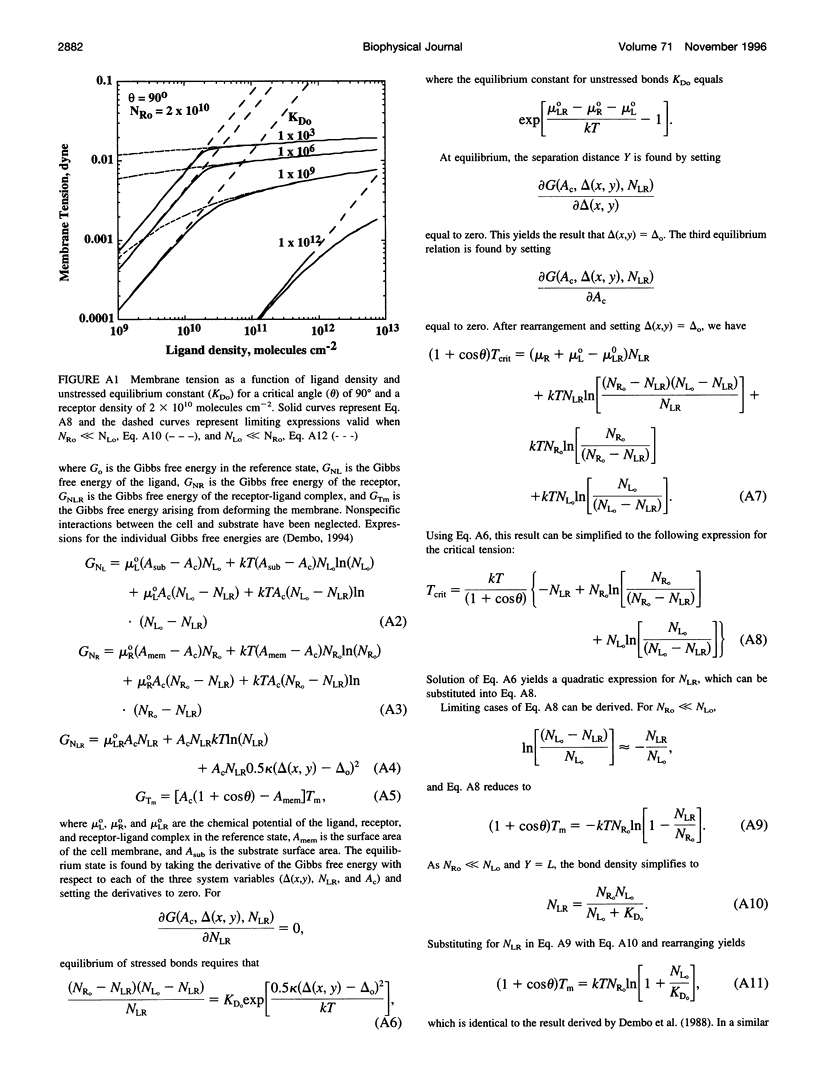


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Alon R., Hammer D. A., Springer T. A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995 Apr 6;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Burmeister J. S., Truskey G. A., Reichert W. M. Quantitative analysis of variable-angle total internal reflection fluorescence microscopy (VA-TIRFM) of cell/substrate contacts. J Microsc. 1994 Jan;173(Pt 1):39–51. doi: 10.1111/j.1365-2818.1994.tb03426.x. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti G., Zanetti A., Colella S., Abbadini M., Marchisio P. C., Pytela R., Giancotti F., Tarone G., Languino L. R., Dejana E. Interaction of fibronectin with cultured human endothelial cells: characterization of the specific receptor. Blood. 1989 May 1;73(6):1576–1585. [PubMed] [Google Scholar]
- Craig W. S., Cheng S., Mullen D. G., Blevitt J., Pierschbacher M. D. Concept and progress in the development of RGD-containing peptide pharmaceuticals. Biopolymers. 1995;37(2):157–175. doi: 10.1002/bip.360370209. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys J. 1985 Jul;48(1):175–183. doi: 10.1016/S0006-3495(85)83770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Ritchie K., Merkel R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys J. 1995 Jun;68(6):2580–2587. doi: 10.1016/S0006-3495(95)80441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Gingell D., Owens N. How do cells sense and respond to adhesive contacts? Diffusion-trapping of laterally mobile membrane proteins at maturing adhesions may initiate signals leading to local cytoskeletal assembly response and lamella formation. J Cell Sci. 1992 Feb;101(Pt 2):255–266. doi: 10.1242/jcs.101.2.255. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982 May 10;257(9):4888–4893. [PubMed] [Google Scholar]
- Grinnell F. Focal adhesion sites and the removal of substratum-bound fibronectin. J Cell Biol. 1986 Dec;103(6 Pt 2):2697–2706. doi: 10.1083/jcb.103.6.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Lauffenburger D. A. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987 Sep;52(3):475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman W. A. Shear flow over a protrusion from a plane wall: addendum. J Biomech. 1972 Nov;5(6):643–643. doi: 10.1016/0021-9290(72)90036-x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iuliano D. J., Saavedra S. S., Truskey G. A. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res. 1993 Aug;27(8):1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lauffenburger D. A. Relationship between receptor/ligand binding affinity and adhesion strength. Biophys J. 1993 Nov;65(5):2191–2200. doi: 10.1016/S0006-3495(93)81277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. M., Burdsal C. A., Erickson H. P., McClay D. R. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989 Oct;109(4 Pt 1):1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991 Sep;114(5):1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990 Jun;187(2):292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. Immobilized amines and basic amino acids as mimetic heparin-binding domains for cell surface proteoglycan-mediated adhesion. J Biol Chem. 1992 May 15;267(14):10133–10141. [PubMed] [Google Scholar]
- Narasimhan C., Lai C. S., Haas A., McCarthy J. One free sulfhydryl group of plasma fibronectin becomes titratable upon binding of the protein to solid substrates. Biochemistry. 1988 Jul 12;27(14):4970–4973. doi: 10.1021/bi00414a003. [DOI] [PubMed] [Google Scholar]
- Nicol A., Gowda D. C., Urry D. W. Cell adhesion and growth on synthetic elastomeric matrices containing Arg-Gly-Asp-Ser-3. J Biomed Mater Res. 1992 Mar;26(3):393–413. doi: 10.1002/jbm.820260309. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987 Dec 25;262(36):17294–17298. [PubMed] [Google Scholar]
- Plopper G., Ingber D. E. Rapid induction and isolation of focal adhesion complexes. Biochem Biophys Res Commun. 1993 Jun 15;193(2):571–578. doi: 10.1006/bbrc.1993.1662. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Anders E., Grulich-Henn J., Müller-Berghaus G. Attachment of cultured human endothelial cells is promoted by specific association with S protein (vitronectin) as well as with the ternary S protein-thrombin-antithrombin III complex. Blood. 1988 Jun;71(6):1581–1589. [PubMed] [Google Scholar]
- Reichert W. M., Truskey G. A. Total internal reflection fluorescence (TIRF) microscopy. I. Modelling cell contact region fluorescence. J Cell Sci. 1990 Jun;96(Pt 2):219–230. doi: 10.1242/jcs.96.2.219. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Rupnick M. A., Hubbard F. A., Pratt K., Jarrell B. E., Williams S. K. Endothelialization of vascular prosthetic surfaces after seeding or sodding with human microvascular endothelial cells. J Vasc Surg. 1989 Jun;9(6):788–795. doi: 10.1067/mva.1989.vs0090788. [DOI] [PubMed] [Google Scholar]
- Schmidt C. E., Horwitz A. F., Lauffenburger D. A., Sheetz M. P. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993 Nov;123(4):977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torney D. C., Dembo M., Bell G. I. Thermodynamics of cell adhesion. II. Freely mobile repellers. Biophys J. 1986 Feb;49(2):501–507. doi: 10.1016/S0006-3495(86)83660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truskey G. A., Burmeister J. S., Grapa E., Reichert W. M. Total internal reflection fluorescence microscopy (TIRFM). II. Topographical mapping of relative cell/substratum separation distances. J Cell Sci. 1992 Oct;103(Pt 2):491–499. doi: 10.1242/jcs.103.2.491. [DOI] [PubMed] [Google Scholar]
- Truskey G. A., Pirone J. S. The effect of fluid shear stress upon cell adhesion to fibronectin-treated surfaces. J Biomed Mater Res. 1990 Oct;24(10):1333–1353. doi: 10.1002/jbm.820241006. [DOI] [PubMed] [Google Scholar]
- Truskey G. A., Proulx T. L. Relationship between 3T3 cell spreading and the strength of adhesion on glass and silane surfaces. Biomaterials. 1993;14(4):243–254. doi: 10.1016/0142-9612(93)90114-h. [DOI] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Ward M. D., Dembo M., Hammer D. A. Kinetics of cell detachment: effect of ligand density. Ann Biomed Eng. 1995 May-Jun;23(3):322–331. doi: 10.1007/BF02584432. [DOI] [PubMed] [Google Scholar]
- Ward M. D., Hammer D. A. A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys J. 1993 Mar;64(3):936–959. doi: 10.1016/S0006-3495(93)81456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. K., Rose D. G., Jarrell B. E. Microvascular endothelial cell sodding of ePTFE vascular grafts: improved patency and stability of the cellular lining. J Biomed Mater Res. 1994 Feb;28(2):203–212. doi: 10.1002/jbm.820280210. [DOI] [PubMed] [Google Scholar]