Visual Abstract
Key Words: bicuspid aortic valve, congenital aortic valve stenosis, congenital heart disease, GATA5, NOTCH1, valve development
Highlights
-
•
Notch1;Gata5 compound mutant mice are a new model of highly penetrant congenital aortic valve disease without early lethality.
-
•
Notch1+/−;Gata5−/− mutant mice exhibit congenital aortic valve disease phenotypes including bicuspid aortic valve and progressive aortic valve stenosis, which are clinically relevant to human aortic valve disease.
-
•
Our findings demonstrate a novel genetic interaction between Notch1 and Gata5 in mice that is critical for proper aortic valve development.
-
•
Transcriptomic analysis reveals downregulation of smooth muscle genes in neonatal aortic valves in Notch1;Gata5 compound mutant mice consistent with an immature valve phenotype.
Summary
Here, we describe Notch1;Gata5 compound mutant mice as a novel mouse model of highly penetrant congenital aortic valve disease displaying bicuspid aortic valve and progressive aortic valve stenosis. Further, we find downregulation of smooth muscle genes in the neonatal aortic valves in Notch1;Gata5 compound mice consistent with an immature valve phenotype. Our findings demonstrate a novel genetic interaction between Notch1 and Gata5 in mice that is critical for proper aortic valve development. This novel model is an important tool to define dysregulated signaling pathways for congenital aortic valve stenosis and stenotic disease progression that can be investigated as therapeutic targets.
Congenital aortic valve disease (AVD), including bicuspid aortic valve (BAV), is among the most prevalent types of congenital heart diseases (CHDs) affecting 1% to 2% of the population.1 Congenital aortic valve stenosis (AVS) is a common valvular condition that occurs in 3.8 to 4.9 per 10,000 live births and accounts for 3% to 6% of all CHD cases.2, 3, 4 AVS is characterized by an obstruction of the aortic valve orifice, resulting from various aberrant aortic valve morphologies such as BAV, unicuspid aortic valve, or tricuspid aortic valve with dysplastic cusps.5 Without treatment, congenital AVS is progressive and leads to ventricular hypertrophy and heart failure with associated morbidity and mortality.6 Individuals with AVS or BAV are at elevated risk of developing aortopathy, aortic dissection, and calcific aortic valve disease (CAVD).7 Currently, the primary treatment for congenital AVS is balloon valvuloplasty or surgical aortic valve repair/replacement. Pharmacological therapies remain limited because of incomplete understanding of the molecular mechanisms underlying the disease.8
The normal aortic valve structure consists of 3 layers of extracellular matrix (ECM) that are oriented relative to blood flow: fibrosa composed of collagen, proteoglycan-rich spongiosa, and elastin fiber-containing ventricularis.9 Minor ECM components include fibronectin and lamin in the aortic valve cusps.10 In addition to the matrix, the aortic valve is composed of valvular interstitial cells (VICs) and valvular endothelial cells (VECs). VICs are located within the interior of each cusp and represent a fibroblast-like cell population.11,12 VICs are important for ECM synthesis and homeostasis, whereas VECs form a protective cellular layer surrounding each cusp. Proper organization of the ECM components is important for the structure and function of mature heart valves. In congenitally malformed and diseased valves, VICs become activated and overproduce ECM proteins, leading to valve stenosis or myxomatous degeneration, which is characterized by leaflet thickening, diffuse accumulation of proteoglycans, and fragmentation of collagen and elastin fibers.13 Interestingly, activated VICs, which express alpha smooth muscle actin (SMA), are also present in fetal valves during remodeling where they mediate extracellular remodeling.14,15
Despite significant advances in our understanding of the genetic basis of CHD, the molecular genetics of congenital AVD remains largely unknown and few genes have been associated with congenital AVS.5,16,17 We were the first to identify NOTCH1 pathogenic variants in 2 families with AVD, which included the phenotypes of AVS, BAV, and CAVD.18 Subsequently, NOTCH1 pathogenic variants have been reported to be associated with other left-sided CHD, including BAV, AVS, coarctation of the aorta, and hypoplastic left heart syndrome, as well as other types of CHD such as tetralogy of Fallot.19, 20, 21, 22, 23 NOTCH1 encodes a single-pass transmembrane receptor that functions in the highly conserved Notch signaling pathway, which is critical for cell fate determination during the development of multiple organs, including the heart.24 Notch1 is expressed in the developing cardiac outflow tract and required for proper semilunar valve development.18,25 Mice heterozygous for Notch1 (Notch1+/−) demonstrate minimal AVD with a low incidence of BAV and mild CAVD.26,27 We previously reported a highly penetrant mouse model of BAV with associated AVS and aortopathy by generating Notch1 haploinsufficient mice in the setting of complete deletion of endothelial nitric oxide synthase (Nos3) (Notch1+/−;Nos3−/−).28,29 Further, we demonstrated that endothelial-specific Notch1 haploinsufficiency in a Nos3-null background recapitulates the cardiac phenotype of BAV and abnormal aortic valve remodeling.30 However, Notch1+/−;Nos3−/− mice display significant perinatal lethality (≈65%) due to a spectrum of congenital cardiac outflow tract defects, limiting the ability to study congenital AVD development and progression.30
The GATA family of zinc-finger transcription factors, GATA4, GATA5, and GATA6, also play essential roles in cardiac development. Particularly, pathogenic variation in GATA5 has been well characterized in human BAV.31, 32, 33 GATA5 variants have also been associated with a spectrum of CHD, including septal defects and cardiac outflow tract defects.34, 35, 36 Importantly, genetic deletion of Gata5 in mice (Gata5−/−) displays partially penetrant BAV (incidence of 25%) with associated AVS.37 Endothelial cell-specific deletion of Gata5 also recapitulated the partially penetrant BAV phenotype, similar to Notch1.37 Together, these previous genetic murine models of congenital AVD have limitations with regard to high perinatal lethality or low penetrance. Interestingly, downregulation of the Notch signaling pathway was found in hearts of Gata5-deficient mice.37 These findings led us to hypothesize that Notch1 and Gata5 display a genetic interaction critical for proper aortic valve morphogenesis.
Here, we generate a new murine model of congenital AVD by intercrossing Notch1 and Gata5 heterozygous mice. We demonstrate that Notch1;Gata5 compound mutant mice display highly penetrant and clinically relevant congenital AVD without neonatal lethality. In addition, these compound mutant mice display partially penetrant BAV and aortopathy. Transcriptomic profiling of aortic valves from postnatal day (P) 10 mice suggests an immature, myxomatous valve. This work uncovers an in vivo genetic interaction between Notch1 and Gata5 important for aortic valve development and remodeling and describes a clinically relevant animal model for congenital myxomatous AVD.
Methods
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children’s Hospital (Protocol AR09-00057) and was conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Experimental mouse models
Notch1 mutant mice (Notch1+/−) were generated and genotyped as previously described and are publicly available (Jackson Laboratory, #002797) and have been maintained in a C57BL/6J strain.38 Gata5tm1b(KOMP)Wtsi targeted embryonic stem (ES) cells were obtained from the Knockout Mouse Project (KOMP) Repository (University of California at Davis).39 Targeted ES cell clones were injected into host blastocysts to generate chimeras. Resulting chimeras were mated to C57BL/6N mice to generate mice heterozygous for the Gata5-null allele (Gata5+/−) and then maintained in C57BL/6J background. Gata5+/− mice were interbred to generate homozygous Gata5-null mice (Gata5−/−). Notch1+/− male mice and Gata5+/− female mice were bred to obtain Notch1+/;Gata5+/− mice. Notch1+/;Gata5+/− male mice were bred with Gata5+/− female mice to obtain the following genotypes: Notch1+/−, Gata5+/−, Gata5−/−, Notch1+/;Gata5+/−, Notch1+/;Gata5−/−, and wild-type littermates. All mice were kept in a C57BL/6J background. These mice were maintained on a 12-hour light/dark cycle, with noon of the day of vaginal plug observation defined as embryonic day (E) 0.5.
Echocardiography
Transthoracic echocardiography was performed using a Vevo 2100 Imaging System equipped with 18- to 38-MHz linear-array transducer with a digital ultrasound system (VisualSonics Inc), as previously described.40,41 Mice were sedated with 2% isoflurane in 100% oxygen at a flow rate of 1.5 L/min and body temperature was monitored throughout the procedure. Pulse-wave Doppler analysis of 3 consecutive cardiac cycles across the aortic valve was performed and averaged along the parasternal long axis to obtain maximal transvalvular velocity. Additional measurements of the aorta and ventricular function were obtained. All echocardiographic experiments and analyses were performed by an experienced investigator in a genotype blinded fashion.
Histology and valve morphology
Experimental animals were euthanized and hearts were harvested for embryonic and neonatal time points or hearts were perfused with 4% paraformaldehyde in phosphate-buffered saline for adult time points before collection. Tissues were fixed in 4% paraformaldehyde at 4°C overnight. Subsequently, heart tissues were embedded in paraffin and serial sectioning was performed at a thickness of 6 μm. For gross histological analysis, staining was performed using hematoxylin and eosin (Sigma-Aldrich) and Russell-Movat's Pentachrome stain (American MasterTech, #KTRMP), following the manufacturer's protocols. All images were visualized using an Olympus BX51 microscope attached with an Olympus DP71 camera or a Keyence BZ-X800 Fluorescence microscope. Valve leaflet area was quantified using ImageJ software (National Institutes of Health) on at least 3 sections per aortic valve.
For gross valve morphology, the aortic valve was dissected out from the hearts of P10 and 16-week-old mice by removing both atria and ventricles, aortic arch, and pulmonary artery and grossly examined under the microscope to inspect the aortic valve morphology. Images were taken using an Olympus BX51 microscope attached to an Olympus DP71 camera.
Western blot
To prepare protein samples from heart ventricles harvested from adult mice, tissues were placed into RIPA buffer (Cell Signaling Technology, #9806) on ice, combined with chrome steel beads (BioSpec Products, #11079132c), and homogenized using a TissueLyser II (Qiagen, #69989) on ice for 3 minutes at a frequency of 30 Hz. Following homogenization, tissue samples were briefly sonicated (Fisher Scientific, #FB705). On lysis completion, tissue lysate was centrifuged at 13,000 rpm for 15 minutes at 4°C. The supernatants were collected, and BCA Protein Assay Kit (Thermo Fisher Scientific, #23227) was used to estimate protein concentration. Tissue lysates were mixed with 6X Laemmli SDS-Sample Buffer (Boston BioProducts, #BP-111R) containing β-mercaptoethanol and boiled for 5 minutes. Protein samples were separated in 4% to 20% Mini-PROTEAN TGX Precast Gels (Bio-Rad, #4561094), transferred into Immobilon-FL polyvinylidene difluoride membrane (Millipore, #IPFL00010), and blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20. Western blot was performed using standard protocols described for LI-COR Biosciences method of detection using infrared dye–conjugated secondary antibodies. After incubation with primary and secondary antibodies, membranes were scanned using the Odyssey CLx imaging system (LI-COR Biosciences). The following primary antibodies were used: rabbit anti-GATA5 (1:300; Proteintech, #55433-1-AP) and mouse anti-GAPDH (1:1,000; Novus Biologicals, #NB300-221). The following secondary antibodies were used: donkey anti-mouse IRDye 680RD (1:2,000; LI-COR Biosciences, #926-68072) and donkey anti-rabbit IRDye 800CW (1:2,000; LI-COR Biosciences, #926-32213).
Total RNA isolation for bulk RNA sequencing
Aortic valve tissue samples were collected in an RNase-free setting from wild-type, Notch1+/−;Gata5+/− and Notch1+/;Gata5−/− mice at P10. Aortic valve tissues were harvested in Trizol (Life Technologies, 15596018), snap frozen, and homogenized using TissueLyser II (Qiagen). RNA was isolated using a Total RNA Purification kit (Norgen Biotek Corp, 17200). DNase treatment (Zymo Research, R1013) was performed post RNA purification. Tissues from 3 aortic valves were pooled to form 1 biological replicate per each group. Bulk RNA sequencing was performed on 12 groups (generated from 36 aortic valve tissues) of total RNA samples from P10 mouse aortic valve tissues (n = 4 biological replicates of each genotype) in the Institute for Genomic Medicine Genomic Services Laboratory, Abigail Wexner Research Institute. RNA quantification was performed with Qubit (Life Sciences). RNA-sequencing libraries were generated using 100 ng to 1 μg of DNase-treated RNA input by ribodepletion with NEBNext Human/Mouse/Rat rRNA Depletion kit followed by library construction using the NEBNext Ultra II Directional RNA-Seq protocol (New England Biolabs). Illumina 2 × 150 paired end reads were generated on the NovaSeq 6000 sequencing platforms (Illumina).
RNA-sequencing data analysis and pathway analysis
DataPrudence (a Carboctet brand) performed RNA-sequencing data analysis. FASTQ files were trimmed using Trim Galore! to remove Illumina adapter sequences and low-quality reads. High-quality trimmed reads were aligned with STAR (v 2.7.10b)42 to mouse reference genome version GRCm39, GENCODE110. We observed more than 80% of the reads could be uniquely mapped to the genome using STAR. Gene expression was quantified using RESM (v 1.3.1),43 and differential expression analysis was performed with DESeq2 (v 1.34.0).44 Volcano and PCA plots were created using the ggplot2 package (v 3.5.1), and heat maps were generated using the pheatmap (v 1.0.12) package. Genes with an adjusted P < 0.10 were considered differentially expressed. Pathway analysis was performed using clusterProfiler (v 4.10.1) to calculate enrichment scores and the enrichplot package (v 1.22.0) to create plots, and background genes were imported from the Gene Set Enrichment Analysis (GSEA) database (M5, ontology gene sets). The bar plot and chord plot for gene ontology (GO terms) were generated with enrichplot and SRplot. Pathways with an adjusted P < 0.10 (Benjamini-Hochberg procedure was used to control the false discovery rate at 0.1) were considered significant.
Data availability
RNA-sequencing data are available in the Gene Expression Omnibus (GEO) under accession number GEO285189.
Statistics
All the experiments were performed at least in triplicate and results are presented as mean ± SD. Genotype frequencies are presented as expected probabilities and compared using the chi-square test. Logistic regression models with Dunnett’s post hoc test for multiple comparisons were used to compare disease incidence in Notch1+/−;Gata5−/− mice with other genotypes. For leaflet area comparisons, analysis of variance (ANOVA) followed by pairwise comparison controlling for multiple testing using Tukey’s post hoc test was used. To assess disease progression, we used a linear model with the outcome as the change in aortic velocity controlling for the baseline aortic velocity. Comparison of groups to wild-type was done with Dunnett’s post hoc test for multiple comparisons to a control group. The assumption of normality of the residuals was checked with residual plots. The nonparametric Kruskal-Wallace test was used as a sensitivity analysis for all ANOVA analyses. P < 0.05 was considered statistically significant. All analyses were performed using R version 4.4.0 (R Foundation for Statistical Computing).
Results
Generation of Notch1;Gata5 mutant mice
To determine if Notch1 and Gata5 exhibit an in vivo genetic interaction in the process of aortic valve development, we generated Notch1;Gata5 compound mutant mice. First, we generated Gata5-heterozygous (Gata5+/−) mice using ES cells imported from KOMP and bred them to homozygosity, as described in the Methods and the targeted allele is shown (Figure 1A). We confirmed the presence of the wild-type (262 base pairs) and mutant (661 base pairs) alleles by polymerase chain reaction analysis, the latter is the result of the lacZ cassette insertion (data not shown). GATA5 protein expression was undetectable by western blot in whole heart samples harvested from adult Gata5−/− mice, when compared with wild-type and Gata5+/− littermates (Figure 1B).
Figure 1.
Generation of Notch1+/−;Gata5−/− Compound Mutant Mice
(A) Gata5 locus and targeting strategy to generate a Gata5 null allele. Cre-mediated excision removes exons 1 to 6, leaving 1 loxP site. Coding exons are in light blue. (B) Western blotting demonstrates that protein levels of Gata5 are undetectable in Gata5−/− hearts (from 3 different mice) as compared with wild-type and Gata5+/− hearts (from 3 different mice per group). GAPDH is used as the loading control for whole hearts, showing equal protein loading. (C) Breeding scheme to generate Notch1+/−;Gata5+/− mutant mice. Notch1+/− male and Gata5+/− female mice were bred to generate Notch1+/−;Gata5+/− mice with normal Mendelian ratios noted at 16 weeks (chi-square P = 0.5). ∗One mouse died before 16 weeks. #Two mice died before 16 weeks. (D) Breeding scheme to generate Notch1+/−;Gata5−/− mutant mice. Notch1+/−;Gata5+/− male and Gata5+/− female mice were bred to generate Notch1+/−;Gata5−/− mice with normal Mendelian ratios are noted at 16 weeks (chi-square P = 0.41). N = number of mice per genotype.
Next, we bred Notch1+/− male mice with Gata5+/− female mice to generate mice heterozygous for both Notch1 and Gata5 (Notch1+/−;Gata5+/−). Offspring were born at normal Mendelian ratios and no postnatal lethality was observed up to 16 weeks (P = 0.5) (Figure 1C). In addition, we generated mice haploinsufficient for Notch1 in the context of complete deletion of Gata5 (Notch1+/−;Gata5−/−) by intercrossing Notch1+/−;Gata5+/− male mice with Gata5+/− female mice. Similar to the compound heterozygous mice, the Notch1+/−;Gata5−/− offspring were of expected Mendelian ratio and viable without any observed postnatal lethality (P = 0.41) (Figure 1D).
Aortic valve stenosis in Notch1+/−;Gata5+/− and Notch1+/−;Gata5−/− mice
To determine if Notch1+/−;Gata5+/− compound heterozygous mice had AVD, we performed transthoracic echocardiography on 16-week-old mice derived from the interbreeding of Notch1+/− and Gata5+/− mice to screen for cardiac structural and functional abnormalities. We found a 60% incidence of AVS, defined as an aortic velocity greater than 1,500 mm/s, in Notch1+/−;Gata5+/− mice (6 of 10), compared with wild-type (0 of 11), Notch1+/− (0/7), and Gata5+/− (2 of 14) littermates (Supplemental Figures 1A, top and 1B). Half (3 of 6) of the 1-year-old Notch1+/−;Gata5+/− mice had an increased aortic valve velocity based on echocardiographic analysis, and significantly thickened and dysmorphic aortic valve leaflets by histology consistent with AVS (Supplemental Figures 1A, bottom, 1C, and 1D). The velocity across the pulmonary valve, left ventricular systolic function (as assessed by ejection fraction), chamber size, and wall thickness in Notch1+/−;Gata5+/− mice were not significantly different from wild-type, Notch1+/−, and Gata5+/− mice at 16 weeks and 1 year (Supplemental Figure 2A).
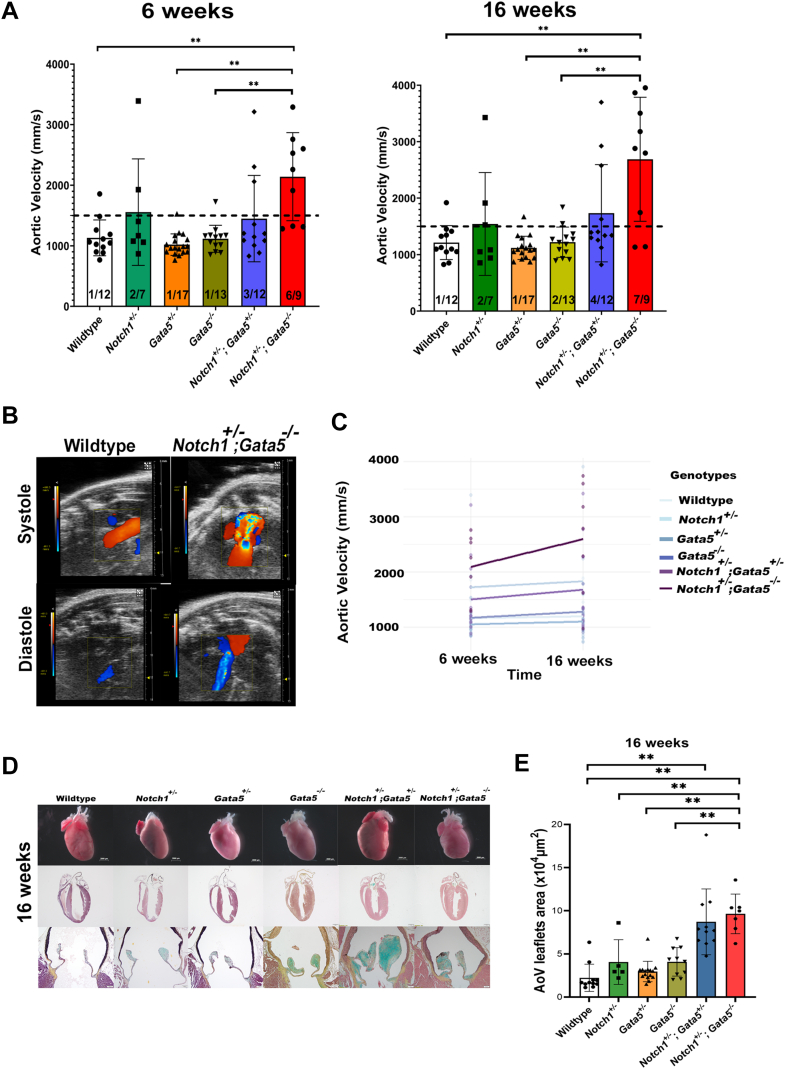
To determine if AVD severity increased with loss of the remaining Gata5 allele, we performed echocardiography on 6-week-old and 16-week-old offspring, derived from the interbreeding of Notch1+/−;Gata5+/− and Gata5+/− mice, which included Notch1+/−;Gata5−/− mice. Echocardiographic analysis demonstrated that most Notch1+/−;Gata5−/− mutant mice displayed AVS compared with wild-type, Notch1+/−, Gata5+/−, and Gata5−/− mice at 6 weeks (P < 0.01) and 16 weeks when compared with wild-type, Gata5+/−, and Gata5−/− mice (P < 0.01) (Figures 2A and 2B). Remarkably, significant AVS was displayed in 67% of Notch1+/−;Gata5−/− mice at 6 weeks (P = 0.05) and 78% at 16 weeks (P = 0.02) (Figure 2A). Aortic valve regurgitation was infrequently noted by 16 weeks in Notch1+/− mice (2 of 7), Notch1+/−;Gata5+/− mice (1 of 12), and Notch1+/−;Gata5−/− mutant mice (3 of 9) when compared with wild-type (0 of 12). Only the aortic valve was affected in Notch1;Gata5 compound mutant mice, as no difference was noted across the pulmonary valve when compared with other genotypes (Supplemental Figure 2B). There were no significant differences in left ventricular systolic function; however, a subset of 6- and 16-week-old Notch1+/−;Gata5−/− mice demonstrated left ventricular dilation and hypertrophy, although not statistically significant (Supplemental Figure 2B). Notably, the severity of AVS progressed between 6 and 16 weeks, as noted by the increase in aortic velocity from 6 to 16 weeks in Notch1+/−;Gata5/− mutant mice by echocardiography (by an average change of 502 mm/s) (Figure 2C). However, there was a high variability in these measures, and this was not statistically different from the average change in mean aortic velocity in the wild-type group (34 mm/s) (P = 0.10). Collectively, these echocardiographic studies showed highly penetrant and progressive AVS in Notch1+/−;Gata5−/− mutant mice.
Figure 2.
Characterization of Notch1+/−;Gata5−/− Mutant Mice Exhibits Progressive AVS
(A) Echocardiography demonstrates a significantly increased velocity across the aortic valve at 6 and 16 weeks in Notch1+/−;Gata5−/− mice as compared with other genotypes (Tukey’s post hoc test, ∗∗ indicates P < 0.01). Notch1+/−;Gata5+/− mice exhibit 33% incidence of aortic valve stenosis (AVS) (defined as aortic velocity >1,500 mm/s) by 16 weeks. Notch1+/−;Gata5−/− mice display ∼67% incidence of AVS by 6 weeks (Dunnett’s post hoc test P = 0.05) and ∼78% 16 weeks (Dunnett’s post hoc test P = 0.02). (B) Doppler images show AVS and regurgitation in Notch1+/−;Gata5−/− mice at 16 weeks. (C) Progression of AVS, as measured by increase in mean aortic velocity, is found in Notch1+/−;Gata5−/− mice (Dunnett’s post hoc test P = 0.10). (D) Gross examination of hearts of Notch1;Gata5 compound mutant mice demonstrates enlarged and hypertrophied hearts. Histological sections stained with Russell-Movat pentachrome show thickened, dysmorphic leaflets with proteoglycan deposition and left ventricular hypertrophy in Notch1;Gata5 mutant mice at 16 weeks. (E) Quantification of aortic valve (AoV) area shows increased size in Notch1;Gata5 mutant mice at 16 weeks (Tukey’s post hoc test, ∗∗P < 0.01).
Notch1;Gata5 mutant mice exhibit abnormal aortic valves
To further define the morphologic defects in the heart and aortic valve of Notch1+/−;Gata5−/− mutant mice, gross and histologic examinations were performed. Gross examination demonstrated grossly enlarged hearts in a subset of 16-week-old Notch1;Gata5 compound mutants (Figure 2D). Morphologic inspection of the aortic valve demonstrated that 3 of 12 (25%) Gata5−/− mice displayed BAV, consistent with previous reports (Figure 3A).37 Further, we found a significantly increased BAV incidence in 6 of 15 (40%) Notch1+/−;Gata5+/− and 3 of 6 (50%) Notch1+/−;Gata5−/− mice when compared with wild-type, Notch1+/−, and Gata5+/− littermates that all had normal tricuspid aortic valves (P = 0.60 and P = 0.05, respectively) (Figure 3A). A subset of 16-week-old Notch1;Gata5 compound mutant mice displayed left ventricular hypertrophy by histological examination (Figure 2D, Supplemental Figure 3A).
Figure 3.
Notch1+/−;Gata5−/− Mutant Mice Exhibit BAV and Partially Penetrant Aortopathy
(A) Top: Representative images of aortic valves at 16 weeks. Bottom: Bicuspid aortic valve (BAV) incidence is shown in Notch1+/−;Gata5+/− mice (40%) and Notch1+/−;Gata5−/− mice (50%). Data presented as count of BAV incidence/total mice in the group. (B) Aortic diameters of the aortic valve, aortic sinus, and sinotubular junction were assessed by echocardiography. (C) Partially penetrant aortic sinus dilation is found in Notch1+/−;Gata5−/− mice in comparison with wild-type littermates by 16 weeks.
Histological examination with Russell-Movat pentachrome staining of the aortic valve demonstrated significantly larger aortic valve leaflets in both Notch1+/−;Gata5+/− and Notch1+/−;Gata5−/− mice when compared with wild-type littermates (P = 1e-6). In addition, Notch1+/−;Gata5−/− mice showed significantly larger aortic valve leaflets when compared with Notch1+/−, Gata5+/−, and Gata5−/− mice (P ≤ 0.001) (Figure 2E). There was a notable gross increase in blue cytochemical staining in Notch1;Gata5 compound mutant aortic valves at 16 weeks, which indicated abnormal levels of proteoglycan abundance, consistent with a myxomatous aortic valve (Figure 2D). These histological studies showed highly penetrant proteoglycan abundant myxomatous aortic valves that were thickened and dysmorphic in Notch1+/−;Gata5−/− mutant mice.
Notch1;Gata5 mutant mice exhibit partially penetrant-associated aortopathy
Next, to determine if our mouse model of partially penetrant BAV and AVS also had associated aortopathy, the diameters of the aortic valve, aortic sinus, and sinotubular junction, defined as the distinct border between the aortic sinus and proximal ascending aorta, were assessed by echocardiography (Figures 3B and 3C). By 16 weeks, Notch1+/−;Gata5−/− mutant mice demonstrated partially penetrant aortic sinus dilation (3 of 9) (aortic sinus diameter measuring >2 mm), compared with wild-type littermates (0 of 12) (Figure 3C). It is unknown if the subset of mice with aortic dilation also had BAV, as BAV phenotyping was not performed.
Notch1;Gata5 mutant mice display congenital aortic valve malformations
To determine whether the aortic valve stenosis was congenital, we examined the aortic valves at E18.5 and P10. At both time points, the expected Mendelian ratios of Notch1+/−;Gata5−/− mice were observed. Histological examination using Russell-Movat pentachrome staining revealed that the aortic valve leaflets in Notch1+/−;Gata5−/− mutant embryos were dysmorphic and significantly larger with significant differences (adjusted P < 0.01) as compared with wild-type, Notch1+/−, Gata5+/−, and Gata5−/− E18.5 embryos (Figures 4A and 4B). At P10, based on histological examination with hematoxylin and eosin and Russell-Movat pentachrome staining, hearts of Notch1+/−;Gata5−/− mice displayed significantly thickened aortic valve leaflets (adjusted P < 0.001) due to markedly increased proteoglycan accumulation as compared with wild-type, Notch1+/−, Gata5+/−, and Gata5−/− genotypes (Figures 4C and 4D). Further, Notch1+/−;Gata5+/− displayed significantly larger aortic valves when compared with wild-type littermates at both E18.5 and P10 (Figures 4A and 4B).
Figure 4.
Notch1;Gata5 Mutant Mice Display Congenital Aortic Valve Malformations
(A) Histological sections stained with Russell-Movat pentachrome show thickened and dysmorphic leaflets in Notch1;Gata5 mutants at embryonic day (E) 18.5. (B) Quantification of aortic valve (AoV) area shows significantly increased size in Notch1;Gata5 mutants compared with other genotypes at E18.5 (Tukey’s post hoc test, ∗∗P < 0.01). (C) Histological examination of postnatal day (P)10 Notch1;Gata5 mutant mouse hearts displays enlarged heart, left ventricular hypertrophy, and significantly thickened and malformed aortic valve leaflets with markedly increased proteoglycan accumulation. (D) Quantification of AoV area demonstrates significantly increased size in Notch1;Gata5 mutants at P10 (Tukey’s post hoc test, ∗∗P < 0.001).
In a subset of mice, we performed transthoracic echocardiography on Notch1+/−;Gata5+/−, Notch1+/−;Gata5−/−, and wild-type littermates at P10 to determine if the aortic valve thickening was physiologically significant. Notch1;Gata5 compound mutant mice (Notch1+/−;Gata5+/− and Notch1+/−;Gata5−/−) demonstrated significantly increased aortic velocity compared with wild-type, consistent with hemodynamically significant AVS (Supplemental Figure 4). Further, we noted left ventricular hypertrophy in a subset of these P10 animals (Supplemental Figure 3B). Taken together, these findings suggest that Notch1;Gata5 compound mutant mice display a phenotype consistent with congenital AVS with dysmorphic aortic valves containing disorganized collagen and proteoglycan.
Notch1+/−;Gata5−/− mutant mice display dysregulation of genes involved in aortic valve remodeling
To determine the transcriptional profile of the dysmorphic aortic valve in Notch1;Gata5 compound mutant mice, we performed bulk RNA sequencing using total RNA isolated from P10 aortic valves from Notch1+/−;Gata5−/−, Notch1+/−;Gata5+/−, and wild-type littermate mice (Figure 5A).
Figure 5.
RNA Sequencing Reveals Gene Expression Changes in Notch1+/−;Gata5−/− Mutant Aortic Valves
(A) Workflow of the bulk RNA sequencing. Representative images of postnatal day (P) 10 aortic valves demonstrate the cardiac regions microdissected. At least 4 independent aortic valves per genotype were pooled and used for RNA-sequencing analysis. (B) Volcano plot representing differentially expressed genes (DEGs) between Notch1+/−;Gata5−/− vs wild-type aortic valves. Black points represent genes significantly differentially expressed. Red points represent 11 upregulated genes with Log2 fold change >0.58 and blue points represent 22 downregulated genes with Log2 fold change <−0.58. (C) Heatmap demonstrating differential gene expression of the top 10 upregulated and top 10 downregulated genes identified with Log2 fold change ± 1.5. (D) Top 10 gene ontology (GO) terms enriched among the significantly DEGs between the Notch1+/−;Gata5−/− vs wild-type aortic valves. Terms were identified through analysis with clusterprofiler R package. (E) Chord graph representing 19 DEGs between Notch1+/−;Gata5−/− vs wild-type aortic valves and the association of these genes to the corresponding top 10 GO terms. Genes are color coded to represent Log2 fold change, with blue corresponding to downregulation and red corresponding to upregulation.
Differential gene expression analysis revealed 33 significantly differentially expressed genes (DEGs) (false discovery rate adjusted P < 0.10) between Notch1+/−;Gata5−/− and wild-type P10 valves (11 upregulated genes with Log2 fold change >0.58 and 22 downregulated genes with Log2 fold change <−0.58) (Figures 5B and 5C). Among the downregulated DEGs, there was a preponderance of genes expressed in smooth muscle cells including Itih4, Synpo2, Cnn1, Tagln, Mylk, and Myh11.45, 46, 47 As expected, we found that Gata5 transcripts were the most downregulated in Notch1+/−;Gata5−/− valves based on DEG analysis. DEG analysis also revealed upregulation of Robo2, which is implicated in aortic valve development.48 Of note, using the same analysis threshold, no significantly differentially expressed genes were identified by comparing Notch1+/−;Gata5+/− and wild-type P10 valves likely due to reduced disease penetrance with this genotype; thus subsequent analysis focused on the Notch1+/−;Gata5−/− valves (data not shown).
GO analysis was performed to identify affected biological pathways in Notch1+/−;Gata5−/− P10 mice compared with wild-type. Pathway analysis was performed on DEGs with adjusted P < 0.10 and Log2 fold change >0.5 or <−0.5, and adjusted P < 0.10 of significantly enriched pathways. The top 3 enriched pathways were actomyosin, actin filament bundle, and actin cytoskeleton; other enriched pathways included smooth muscle tissue development, collagen containing extracellular matrix, and contractile fiber (Figure 5D). Among other DEGs, those involved in the cellular components of actin filament bundle and actomyosin (Fblim1, Myh11, Vcl, Mylk, Acta2, Synpo2), actin cytoskeleton (Cnn1, Fblim1, Myh11, Ppp1r12a, Vcl, Mylk, Acta2, Synpo2), and smooth muscle tissue development (Mylk, Itga8, Osr1) were the most significant (Figure 5E). To further explore gene enrichment among the top enriched GO pathways, we performed GSEA. We found significant enrichment (family-wise error rate P = 0.001) in wild-type samples for the Reactome, smooth muscle cell contraction, suggesting that genes involved in this pathway are downregulated in Notch1+/−;Gata5−/− P10 mice (Supplemental Figure 5A). PANTHER pathway analysis identified integrin, cytoskeletal regulation by Rho GTPase, and inflammation by chemokine and cytokine signaling pathways, all of which are reported to be associated with ECM disorganization in myxomatous heart valve disease (Supplemental Figure 5B).49, 50, 51, 52
Discussion
The genetic and molecular mechanisms underlying congenital AVD are not well understood because of a lack of suitable animal models. In this study, we describe a new murine model that is based on 2 genes implicated in the etiology of human BAV and AVS and was developed by intercrossing Notch1 and Gata5 heterozygous mice. We show that Notch1+/−;Gata5−/− mutant mice display highly penetrant congenital AVS and BAV by echocardiographic and histological analyses. Importantly, the valve disease in Notch1+/−;Gata5−/− mutant mice does not result in early lethality, allowing for the opportunity to study stenotic valve disease progression, which is highly clinically relevant for human AVS. Further, transcriptomic analysis of the mutant valves demonstrates downregulation of genes expressed in smooth muscle that are important for interactions with the actin cytoskeleton and accordingly are important mediators of the ECM, which is consistent with the abnormal myxomatous valve histologic phenotype. This novel model is an important tool to define dysregulated signaling pathways for congenital myxomatous aortic valve stenosis and stenotic disease progression that can be investigated as therapeutic targets.
The mouse model described here is unique, as it is the first to demonstrate congenital AVS with associated disease progression due to lack of lethality, which is clinically relevant to the phenotype found in infants and children. Although other mouse models of congenital AVD have been developed, they have limitations with regard to a low penetrance or embryonic or early neonatal lethality. Notch1+/−;Nos3−/− mice display congenital AVD, in the form of BAV and/or AVS at a penetrance of ∼90%, but this penetrance is found when examining only the adult survivors, as approximately two-thirds of mice with this genotype suffer early postnatal lethality due to severe cardiac outflow tract defects.28,30 Telomere shortening in Notch1 haploinsufficient mice elicit age-dependent AVS and aortic valve calcification at reduced penetrance or 70% early lethality with further telomere shortening.53 Mice with complete Gata5 genetic deletion or Gata6 haploinsufficiency develop BAV without an AVS phenotype with partial penetrance (25% and 27% to 56%, respectively).37,54 Gata5 and Gata6 have been shown to genetically interact in vivo and are critical for heart development.55 Further, genetic interaction between Gata6 and Mib1, a member of the Notch signaling pathway, has been reported where compound mutation of these genes impacts BAV penetrance consistent with importance of interactions between Notch and GATA factors for aortic valve development.56 Heterozygous Nkx2.5 knockout mice display a variety of cardiac phenotypes, including BAV with AVS at a low penetrance of 8.2%.57 Compared with these previous mice, our mouse model has unique features of surviving to adulthood while recapitulating the human congenital AVS phenotype with a high penetrance (nearly 80%). These characteristics allow for discovery of the underlying mechanisms of disease initiation and progression and the testing of novel therapeutics.
In addition to AVS, Notch1+/−;Gata5−/− compound mice display BAV at a penetrance of 50% and partially penetrant-associated aortopathy. We previously demonstrated that Notch1 haploinsufficiency causes ascending aortic aneurysms independent of BAV in mice,58 whereas Gata5-null mice display BAV with no associated aortopathy.37 BAV can alter hemodynamics in the ascending aorta by changing patterns of blood flow and shear stress, resulting in aortopathy characterized by aortic dilation.59 In addition to BAV, AVS with thickening of aortic valve cusps can be a contributor to aortopathy, as the increased blood velocity can contribute to post-stenotic dilation.60 It is unclear in our current study which is contributing, as we did not noninvasively diagnose BAV. Although technically challenging, this has been reported and will be a focus of future studies.61
Transcriptomic profiling of the aortic valve of P10 Notch1+/−;Gata5−/− mice demonstrated a consistent downregulation of genes that are well known to be expressed in smooth muscle (eg, Cnn1, Tagln, Mylk, Myh11). Smooth muscle gene expression is consistent with the myofibroblast VIC phenotype of activated VICs in the developing valve, where alpha smooth muscle actin (SMA/Acta2) and smooth muscle myosin heavy chain (Smemb/Myh10) are expressed.14 Reduced expression of these smooth muscle genes in the mutant myxomatous aortic valves was an intriguing observation, as increased SMA/Acta2 expression has been associated with increased cellular stiffness,62 and may represent an important developmental process for aortic valve remodeling after birth with increasing systemic blood pressure. SMA/ACTA2 transcript expression has been shown to be significantly increased in the mouse aortic valve at 4 months when compared with postnatal day 2.63 Additional analysis of this published transcriptomic dataset from neonatal and adult aortic valves shows several of these genes are upregulated with age (Supplemental Figure 6).63 Our bioinformatic analyses also showed that these gene expression differences impacted the actin cytoskeleton and actin filament organization. These alterations in the cellular architecture of VICs may affect the biomechanical properties of these cells during the transition from the fetal to neonatal to adult hemodynamics.64 Further, these changes in actin organization and dynamics are important mediators of the ECM,65 and accordingly may be consistent with the abundant proteoglycans found in the myxomatous aortic valves of Notch1+/−;Gata5−/− mutant mice. Alternatively, the decreased expression of smooth muscle genes may also suggest there are a reduced number of VICs with a myofibroblast-phenotype, as these cells have been proposed to promote normal physiologic valve remodeling.15 Surprisingly, no differences in collagen gene expression were noted in our RNA-sequencing analysis as has been described with diseased pediatric aortic valves in humans.66 In addition, the smooth muscle gene expression differences in our study were detected at the transcript level; thus, it is unknown if these differences will be found at the protein level. Accordingly, proteomic profiling is necessary to validate these differences in smooth muscle and collagen gene expression in this model.
Organization of ECM components, which include collagens, proteoglycans, and elastin, is important for the structure and function of mature heart valves. Valve ECM is secreted and maintained by VICs that reside within each cusp, and molecular communication between VICs and VECs via paracrine signaling is necessary to preserve ECM homeostasis and prevent valve disease.28,67,68 In congenitally malformed valves, VICs become activated and overproduce ECM proteins, leading to valve stenosis or myxomatous degeneration, which is characterized by leaflet thickening, diffuse accumulation of proteoglycans, and fragmentation of collagen and elastin fibers.13 AVD is observed in patients with genetic variants in ECM genes and mouse models with underlying connective tissue disorders, such as Marfan syndrome and osteogenesis imperfecta.69 Specifically, inhibiting transforming growth factor-β signaling or preventing infiltration of circulatory monocyte-derived macrophages can be potential therapeutic targets to prevent myxomatous valve disease in Marfan syndrome mice.70, 71, 72, 73 Recent studies have shown that Prox1 regulates VEC specialization critical for aortic valve development and ECM stratification of aortic valves in mice.74 Another recent study demonstrated that epicardial deletion of Sox9 leads to myxomatous valve degeneration in mice and identified Cd109 as a novel gene associated with valve development important for ECM organization.75 Future studies are warranted to explore novel therapeutics targeting ECM to maintain the trilaminar aortic valve cusp structure and prevent congenital AVS by utilizing the Notch1+/−;Gata5−/− mutant mouse model.
Notch1+/−;Gata5−/− mutant mice did not develop aortic valve calcification nor was there evidence of apparent inflammation in the thickened aortic valves (data not shown). Chronic inflammation is associated with CAVD and plays a key role in the degeneration of BAV with severe AVS.76,77 However, the role of inflammation remains unclear as a contributor to congenital AVD.
This study has clinical and pathologic significance for patients with congenital AVD. Exhibiting a highly penetrant and clinically relevant AVD phenotype, this mouse model can be broadly used to investigate the disease mechanisms for the pathogenesis and treatment of AVD. Further, this new mouse model allows for preclinical testing of new therapeutic targets of human AVD. However, this study has limitations, as the molecular basis for congenital AVD requires additional study. Although dysregulation of smooth muscle gene transcripts is found in the neonatal diseased aortic valves of this mouse model and was suggestive of an immature valve phenotype, this requires validation including investigation of the molecular pathways underlying the ECM abnormalities found with congenital myxomatous valves.
Conclusions
The Notch1+/−;Gata5−/− mutant mouse is a novel mouse model for congenital AVS. Characterization of these mice not only shows the physiologic changes that occur with congenital AVS, but histology and transcriptomic profiling identified ECM dysregulation at the mRNA and protein levels in neonatal aortic valves of the Notch1+/−;Gata5−/− mutant mice. By using 2 human AVS disease-causing genes (NOTCH1 and GATA5), we have created a highly clinically relevant genetic model of congenital AVS that has potential to be used for therapeutic discovery and preclinical testing.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Congenital AVD is one of the most common types of CHD, and an important contributor to infant mortality and morbidity from CHD. The current standard treatment option is balloon valvuloplasty in the catheterization laboratory or surgical aortic valve repair/replacement. No pharmacological treatments exist because of limited understanding of molecular genetic mechanisms and the lack of suitable model systems that recapitulate disease. The new mouse model described here allows investigation of disease mechanisms for the pathogenesis and treatment of early and later stages of AVS, and furthermore, provides a preclinical animal model for the testing of new therapeutic targets of congenital AVD.
TRANSLATIONAL OUTLOOK: Although this new mouse model demonstrates a highly penetrant and clinically relevant AVD phenotype to humans, the molecular mechanisms underlying the pathogenesis of congenital AVD remain elusive. Dysregulation of smooth muscle genes are identified in the neonatal diseased aortic valves, but multiple signaling pathways might lead to the improperly remodeled valve. Further studies are needed to identify the molecular pathways underlying abnormal aortic valve development and disease progression that result in the ECM disturbances found in the congenital myxomatous valve. Our new murine model provides the opportunity to gain these insights and to open the door for novel therapeutic discovery that may impact patients suffering from congenital AVD.
Funding Support and Author Disclosures
This work was supported by funding from the National Institutes of Health (T32HL134616 to Ms Aljuhani, T32HL166149 to Dr Rao, and R01HL121797 and R01HL175924 to Dr Garg). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Sara Adamczak and Holly Girard for their expert animal care and technical assistance; members of the Morphology Core at Nationwide Children’s Hospital for histology support; Aiman Q. Khan and Tong Zhou for RNA-sequencing analyses; Heather Costello, Saranga Wijeratne, and Benjamin J. Kelly in the Institute for Genomic Medicine Genomic Services Laboratory for providing RNA-sequencing library preparation and sequencing services; Dr Dennis Lewandowski for manuscript editing; and Dr Brenda Lilly for helpful comments and critical review of the manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman J.I., Kaplan S., Liberthson R.R. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kitchiner D.J., Jackson M., Walsh K., Peart I., Arnold R. Incidence and prognosis of congenital aortic valve stenosis in Liverpool (1960-1990) Br Heart J. 1993;69:71–79. doi: 10.1136/hrt.69.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuhara J., Schultz K., Bigelow A.M., Garg V. Congenital aortic valve stenosis: from pathophysiology to molecular genetics and the need for novel therapeutics. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1142707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keane J.F., Driscoll D.J., Gersony W.M., et al. Second natural history study of congenital heart defects. Results of treatment of patients with aortic valvar stenosis. Circulation. 1993;87:I16–I27. [PubMed] [Google Scholar]
- 7.Prakash S.K., Bossé Y., Muehlschlegel J.D., et al. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the International BAVCon (Bicuspid Aortic Valve Consortium) J Am Coll Cardiol. 2014;64:832–839. doi: 10.1016/j.jacc.2014.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 9.Koenig S.N., Lincoln J., Garg V. Genetic basis of aortic valvular disease. Curr Opin Cardiol. 2017;32:239–245. doi: 10.1097/HCO.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latif N., Sarathchandra P., Taylor P.M., Antoniw J., Brand N., Yacoub M.H. Characterization of molecules mediating cell-cell communication in human cardiac valve interstitial cells. Cell Biochem Biophys. 2006;45:255–264. doi: 10.1385/CBB:45:3:255. [DOI] [PubMed] [Google Scholar]
- 11.Filip D.A., Radu A., Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59(3):310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 12.Liu A.C., Joag V.R., Gotlieb A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim A.J., Xu N., Yutzey K.E. Macrophage lineages in heart valve development and disease. Cardiovasc Res. 2021;117:663–673. doi: 10.1093/cvr/cvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabkin-Aikawa E., Farber M., Aikawa M., Schoen F.J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13(5):841–847. [PubMed] [Google Scholar]
- 15.Horne T.E., VandeKopple M., Sauls K., et al. Dynamic heterogeneity of the heart valve interstitial cell population in mitral valve health and disease. J Cardiovasc Dev Dis. 2015;2(3):214–232. doi: 10.3390/jcdd2030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuhara J., Garg V. Genetics of congenital heart disease: a narrative review of recent advances and clinical implications. Transl Pediatr. 2021;10:2366–2386. doi: 10.21037/tp-21-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackah R.L., Yasuhara J., Garg V. Genetics of aortic valve disease. Curr Opin Cardiol. 2023;38:169–178. doi: 10.1097/HCO.0000000000001028. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg V., Muth A.N., Ransom J.F., et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed S.A., Aherrahrou Z., Liptau H., et al. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem Biophys Res Commun. 2006;345:1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 20.McKellar S.H., Tester D.J., Yagubyan M., Majumdar R., Ackerman M.J., Sundt T.M., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134:290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 21.McBride K.L., Riley M.F., Zender G.A., et al. NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum Mol Genet. 2008;17:2886–2893. doi: 10.1093/hmg/ddn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerstjens-Frederikse W.S., van de Laar I.M., Vos Y.J., et al. Cardiovascular malformations caused by NOTCH1 mutations do not keep left: data on 428 probands with left-sided CHD and their families. Genet Med. 2016;18:914–923. doi: 10.1038/gim.2015.193. [DOI] [PubMed] [Google Scholar]
- 23.Page D.J., Miossec M.J., Williams S.G., et al. Whole exome sequencing reveals the major genetic contributors to nonsyndromic tetralogy of Fallot. Circ Res. 2019;124:553–563. doi: 10.1161/CIRCRESAHA.118.313250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Pompa J.L., Epstein J.A. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell. 2012;22(2):244–254. doi: 10.1016/j.devcel.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain R., Engleka K.A., Rentschler S.L., et al. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nigam V., Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47:828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nus M., MacGrogan D., Martinez-Poveda B., et al. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 28.Bosse K., Hans C.P., Zhao N., et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol. 2013;60:27–35. doi: 10.1016/j.yjmcc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenig S.N., Bosse K.M., Nadorlik H.A., Lilly B., Garg V. Evidence of aortopathy in mice with haploinsufficiency of Notch1 in Nos3-null background. Cardiovasc Dev Dis. 2015;2:17–30. doi: 10.3390/jcdd2010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenig S.N., Bosse K., Majumdar U., Bonachea E.M., Radtke F., Garg V. Endothelial Notch1 is required for proper development of the semilunar valves and cardiac outflow tract. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padang R., Bagnall R.D., Richmond D.R., Bannon P.G., Semsarian C. Rare non-synonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J Mol Cell Cardiol. 2012;53:277–281. doi: 10.1016/j.yjmcc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bonachea E.M., Chang S.W., Zender G., et al. Rare GATA5 sequence variants identified in individuals with bicuspid aortic valve. Pediatr Res. 2014;76:211–216. doi: 10.1038/pr.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L.M., Tao J.W., Qiu X.B., et al. GATA5 loss-of-function mutations associated with congenital bicuspid aortic valve. Int J Mol Med. 2014;33:1219–1226. doi: 10.3892/ijmm.2014.1700. [DOI] [PubMed] [Google Scholar]
- 34.Jiang J.Q., Li R.G., Wang J., et al. Prevalence and spectrum of GATA5 mutations associated with congenital heart disease. Int J Cardiol. 2013;165:570–573. doi: 10.1016/j.ijcard.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Kassab K., Hariri H., Gharibeh L., et al. GATA5 mutation homozygosity linked to a double outlet right ventricle phenotype in a Lebanese patient. Mol Genet Genomic Med. 2016;4:160–171. doi: 10.1002/mgg3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei D., Bao H., Liu X.Y., et al. GATA5 loss-of-function mutations underlie tetralogy of Fallot. Int J Med Sci. 2013;10:34–42. doi: 10.7150/ijms.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laforest B., Andelfinger G., Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. J Clin Invest. 2011;121:2876–2887. doi: 10.1172/JCI44555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conlon R.A., Reaume A.G., Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 39.Valenzuela D.M., Murphy A.J., Frendewey D., et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 40.LaHaye S., Majumdar U., Yasuhara J., et al. Developmental origins for semilunar valve stenosis identified in mice harboring congenital heart disease-associated GATA4 mutation. Dis Model Mech. 2019;12 doi: 10.1242/dmm.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majumdar U., Manivannan S., Basu M., et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobin A., Davis C.A., Schlesinger F., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravindran A., Holappa L., Niskanen H., et al. Translatome profiling reveals Itih4 as a novel smooth muscle cell-specific gene in atherosclerosis. Cardiovasc Res. 2024;120(8):869–882. doi: 10.1093/cvr/cvae028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohanadan K., Assent M., Linnemann A., et al. Synaptopodin-2 isoforms have specific binding partners and display distinct, muscle cell type-specific expression patterns. Cells. 2023;13(1):85. doi: 10.3390/cells13010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin C.H., Lilly B. Notch signaling governs phenotypic modulation of smooth muscle cells. Vascul Pharmacol. 2014;63(2):88–96. doi: 10.1016/j.vph.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Mommersteeg M.T.M., Yeh M.L., Parnavelas J.G., Andrews W.D. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc Res. 2015;106:55–66. doi: 10.1093/cvr/cvv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu C.C., Liu M.M., Clinton M., Culshaw G., Argyle D.J., Corcoran B.M. Developmental pathways and endothelial to mesenchymal transition in canine myxomatous mitral valve disease. Vet J. 2015;206:377–384. doi: 10.1016/j.tvjl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Xu N., Alfieri C.M., Yu Y., Guo M., Yutzey K.E. Wnt signaling inhibition prevents postnatal inflammation and disease progression in mouse congenital myxomatous valve disease. Arterioscler Thromb Vasc Biol. 2024;44:1540–1554. doi: 10.1161/ATVBAHA.123.320388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagnozzi L.A., Butcher J.T. Mechanotransduction mechanisms in mitral valve physiology and disease pathogenesis. Front Cardiovasc Med. 2017;4:83. doi: 10.3389/fcvm.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majumdar U., Choudhury T.Z., Manivannan S., Ueyama Y., Basu M., Garg V. Single-cell RNA-sequencing analysis of aortic valve interstitial cells demonstrates the regulation of integrin signaling by nitric oxide. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.742850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theodoris C.V., Mourkioti F., Huang Y., et al. Long telomeres protect against age-dependent cardiac disease caused by NOTCH1 haploinsufficiency. J Clin Invest. 2017;127:1683–1688. doi: 10.1172/JCI90338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gharibeh L., Komati H., Bossé Y., et al. GATA6 regulates aortic valve remodeling, and its haploinsufficiency leads to right-left type bicuspid aortic valve. Circulation. 2018;138:1025–1038. doi: 10.1161/CIRCULATIONAHA.117.029506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laforest B., Nemer M. GATA5 interacts with GATA4 and GATA6 in outflow tract development. Dev Biol. 2011;358(2):368–378. doi: 10.1016/j.ydbio.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 56.Piñeiro-Sabarís R., MacGrogan D., de la Pompa J.L. Intricate MIB1-NOTCH-GATA6 interactions in cardiac valvular and septal development. J Cardiovasc Dev Dis. 2024;11(7):223. doi: 10.3390/jcdd11070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biben C., Weber R., Kesteven S., et al. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circ Res. 2000;87:888–895. doi: 10.1161/01.res.87.10.888. [DOI] [PubMed] [Google Scholar]
- 58.Koenig S.N., LaHaye S., Feller J.D., et al. Notch1 haploinsufficiency causes ascending aortic aneurysms in mice. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bäck M., Gasser T.C., Michel J.B., Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res. 2013;99:232–241. doi: 10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilton E., Jahangiri M. Post-stenotic aortic dilatation. J Cardiothorac Surg. 2006;1:7. doi: 10.1186/1749-8090-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guruji V., Zhou Y.Q., Tang M., et al. Identification of congenital aortic valve malformations in juvenile natriuretic peptide receptor 2-deficient mice using high-frequency ultrasound. Am J Physiol Heart Circ Physiol. 2024;327(1):H56–H66. doi: 10.1152/ajpheart.00769.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merryman W.D., Youn I., Lukoff H.D., et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290(1):H224–H231. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 63.Nordquist E., LaHaye S., Nagel C., Lincoln J. Postnatal and adult aortic heart valves have distinctive transcriptional profiles associated with valve tissue growth and maintenance respectively. Front Cardiovasc Med. 2018;5:30. doi: 10.3389/fcvm.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam N.T., Muldoon T.J., Quinn K.P., Rajaram N., Balachandran K. Valve interstitial cellcontractile strength and metabolic state are dependent on its shape. Integr Biol (Camb) 2016;8(10):1079–1089. doi: 10.1039/c6ib00120c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachir A.I., Horwitz A.R., Nelson W.J., Bianchini J.M. Actin-based adhesion modules mediate cell interactions with the extracellular matrix and neighboring cells. Cold Spring Harb Perspect Biol. 2017;9(7) doi: 10.1101/cshperspect.a023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clift C.L., Su Y.R., Bichell D., et al. Collagen fiber regulation in human pediatric aortic valve development and disease. Sci Rep. 2021;11(1):9751. doi: 10.1038/s41598-021-89164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hinton R.B., Jr., Lincoln J., Deutsch G.H., et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 68.Anstine L.J., Bobba C., Ghadiali S., Lincoln J. Growth and maturation of heart valves leads to changes in endothelial cell distribution, impaired function, decreased metabolism and reduced cell proliferation. J Mol Cell Cardiol. 2016;100:72–82. doi: 10.1016/j.yjmcc.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thatcher K., Mattern C.R., Chaparro D., et al. Temporal progression of aortic valve pathogenesis in a mouse model of osteogenesis imperfecta. J Cardiovasc Dev Dis. 2023;10:355. doi: 10.3390/jcdd10080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ng C.M., Cheng A., Myers L.A., et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Habashi J.P., Judge D.P., Holm T.M., et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim A.J., Xu N., Umeyama K., et al. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation. 2020;141:132–146. doi: 10.1161/CIRCULATIONAHA.119.042391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu N., Yutzey K.E. Therapeutic CCR2 blockade prevents inflammation and alleviates myxomatous valve disease in Marfan syndrome. JACC Basic Transl Sci. 2022;7:1143–1157. doi: 10.1016/j.jacbts.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Donnell A., Gonzalez B.A., Mukherjee S., et al. Localized Prox1 regulates aortic valve endothelial cell diversity and extracellular matrix stratification in mice. Arterioscler Thromb Vasc Biol. 2023;43:1478–1493. doi: 10.1161/ATVBAHA.123.319424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey A.B., Wolters R.A., Deepe R.N., et al. Epicardial deletion of Sox9 leads to myxomatous valve degeneration and identifies Cd109 as a novel gene associated with valve development. J Mol Cell Cardiol. 2024;186:16–30. doi: 10.1016/j.yjmcc.2023.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coté N., Mahmut A., Bosse Y., et al. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. 2013;36:573–581. doi: 10.1007/s10753-012-9579-6. [DOI] [PubMed] [Google Scholar]
- 77.Manno G., Bentivegna R., Morreale P., et al. Chronic inflammation: a key role in degeneration of bicuspid aortic valve. J Mol Cell Cardiol. 2019;130:59–64. doi: 10.1016/j.yjmcc.2019.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-sequencing data are available in the Gene Expression Omnibus (GEO) under accession number GEO285189.