Abstract
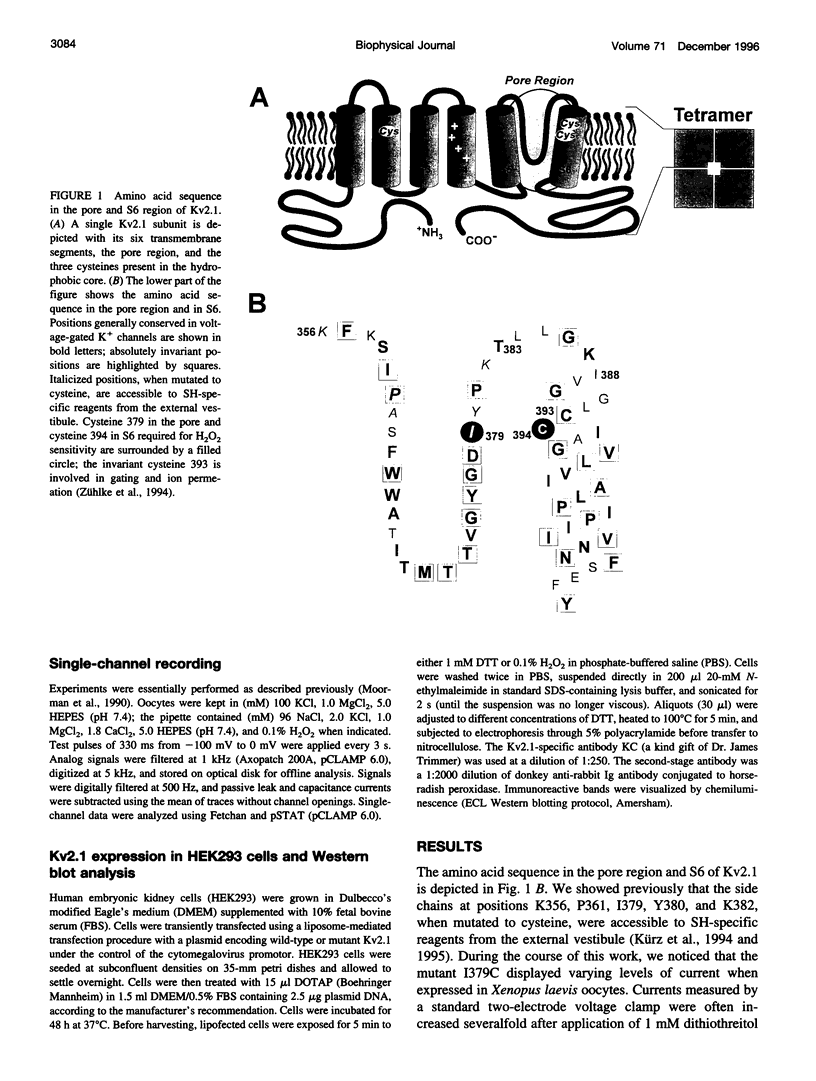
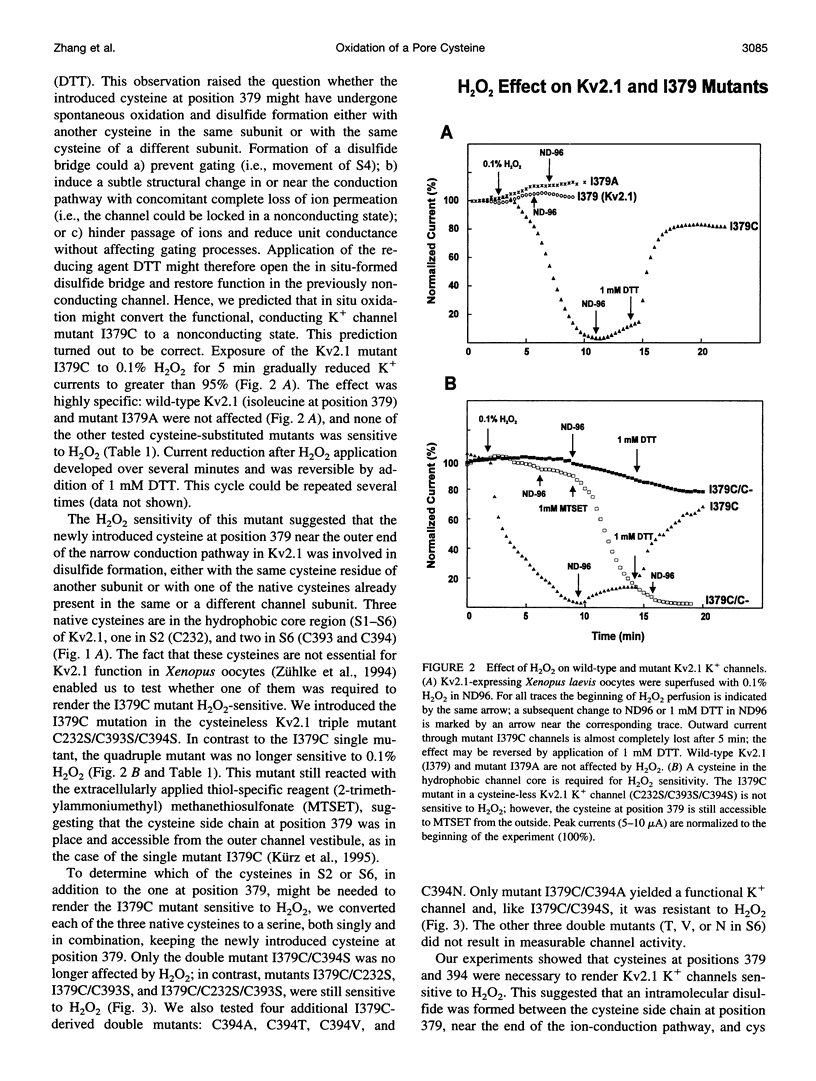
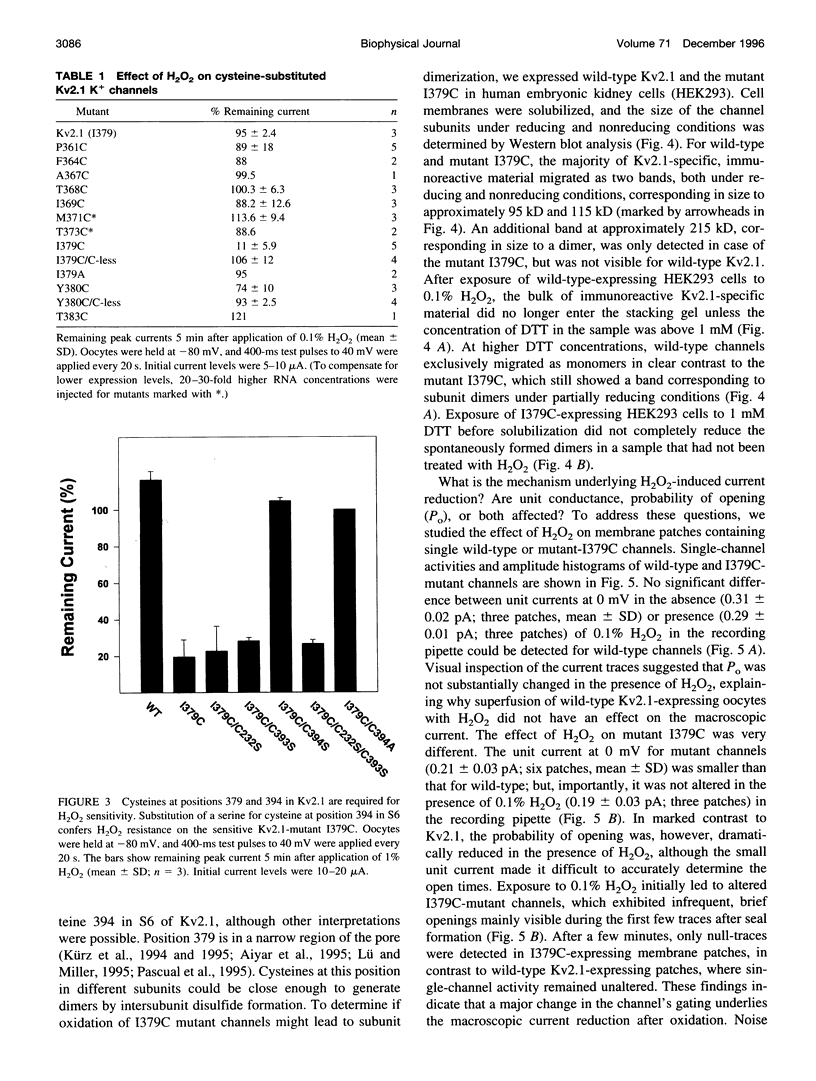
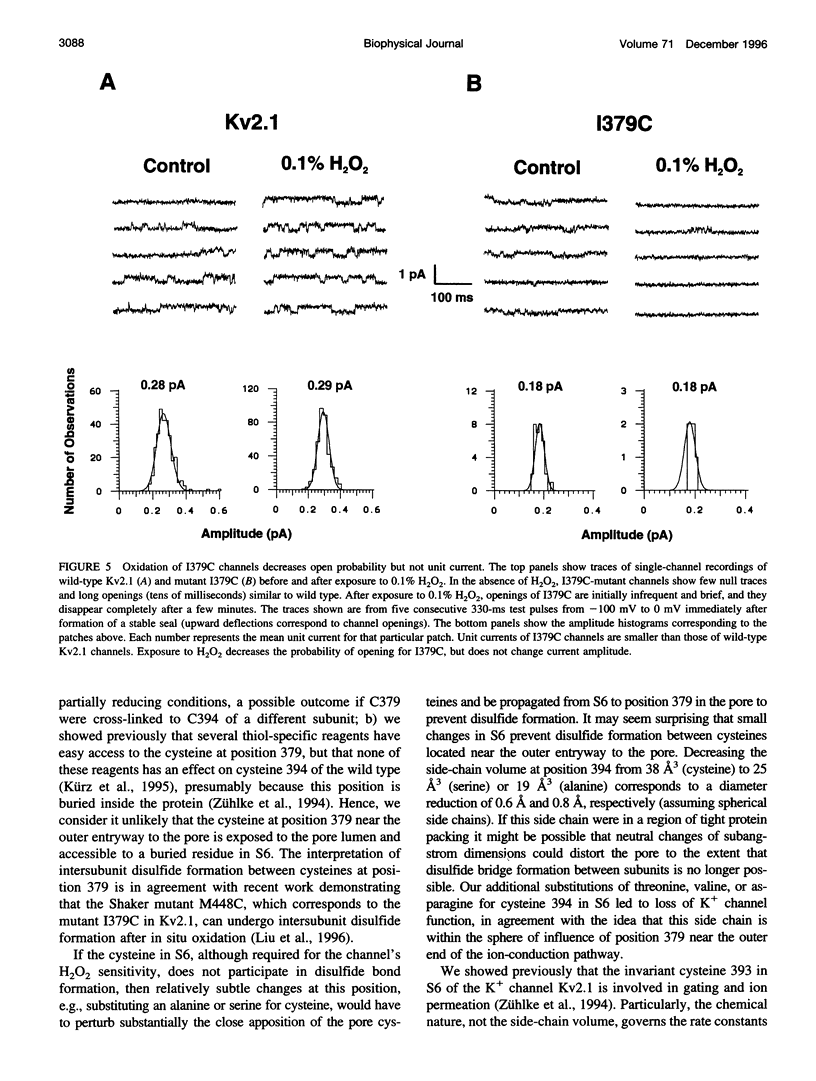
We report the use of cysteine-substituted mutants in conjunction with in situ oxidation to determine the physical proximity of a pair of engineered cysteines in the pore region of the voltage-gated K+ channel Kv2.1. We show that the newly introduced cysteine 1379C, located near the outer end of the narrow ion-conduction pathway, renders the K+ channel sensitive to oxidation by H2O2, but only if the native cysteine at position 394 in S6 remains in place. Conservative substitutions in S6 for cysteine 394 abolish H2O2 sensitivity in the Kv2.1 mutant 1379C. Comparative immunoblot analysis of wild-type and 1379C Kv2.1-expressing HEK293 cells demonstrates the presence of subunit dimers for 1379C, but not for wild-type Kv2.1. At the single-channel level, the probability of opening of 1379C channels, unlike wild-type, is reduced in the presence of H2O2; however, oxidation of 1379C does not alter unit current. These findings imply that cysteine 379, located near the outer end of the narrow ion-conduction pathway, participates in disulfide bridge formation, locking the channel in a nonconducting state from which it cannot undergo conformational transitions required for opening.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Withka J. M., Rizzi J. P., Singleton D. H., Andrews G. C., Lin W., Boyd J., Hanson D. C., Simon M., Dethlefs B. Topology of the pore-region of a K+ channel revealed by the NMR-derived structures of scorpion toxins. Neuron. 1995 Nov;15(5):1169–1181. doi: 10.1016/0896-6273(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Papazian D. M., Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991 Nov 1;254(5032):679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- Careaga C. L., Falke J. J. Structure and dynamics of Escherichia coli chemosensory receptors. Engineered sulfhydryl studies. Biophys J. 1992 Apr;62(1):209–219. doi: 10.1016/S0006-3495(92)81806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Kürz L. L., Zühlke R. D., Zhang H. J., Joho R. H. Side-chain accessibilities in the pore of a K+ channel probed by sulfhydryl-specific reagents after cysteine-scanning mutagenesis. Biophys J. 1995 Mar;68(3):900–905. doi: 10.1016/S0006-3495(95)80266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jurman M. E., Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996 Apr;16(4):859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Lopez G. A., Jan Y. N., Jan L. Y. Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 1994 Jan 13;367(6459):179–182. doi: 10.1038/367179a0. [DOI] [PubMed] [Google Scholar]
- Lynch B. A., Koshland D. E., Jr Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10402–10406. doi: 10.1073/pnas.88.23.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü Q., Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995 Apr 14;268(5208):304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., VanDongen A. M., Joho R. H., Brown A. M. Fast and slow gating of sodium channels encoded by a single mRNA. Neuron. 1990 Feb;4(2):243–252. doi: 10.1016/0896-6273(90)90099-2. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J. M., Shieh C. C., Kirsch G. E., Brown A. M. K+ pore structure revealed by reporter cysteines at inner and outer surfaces. Neuron. 1995 May;14(5):1055–1063. doi: 10.1016/0896-6273(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Jan Y. N., Jan L. Y. The S4-S5 loop contributes to the ion-selective pore of potassium channels. Neuron. 1993 Oct;11(4):739–749. doi: 10.1016/0896-6273(93)90083-4. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990 Oct;5(4):433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994 Apr;66(4):1068–1075. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Zühlke R. D., Zhang H. J., Joho R. H. Role of an invariant cysteine in gating and ion permeation of the voltage-sensitive K+ channel Kv2.1. Receptors Channels. 1994;2(3):237–248. [PubMed] [Google Scholar]





