Abstract
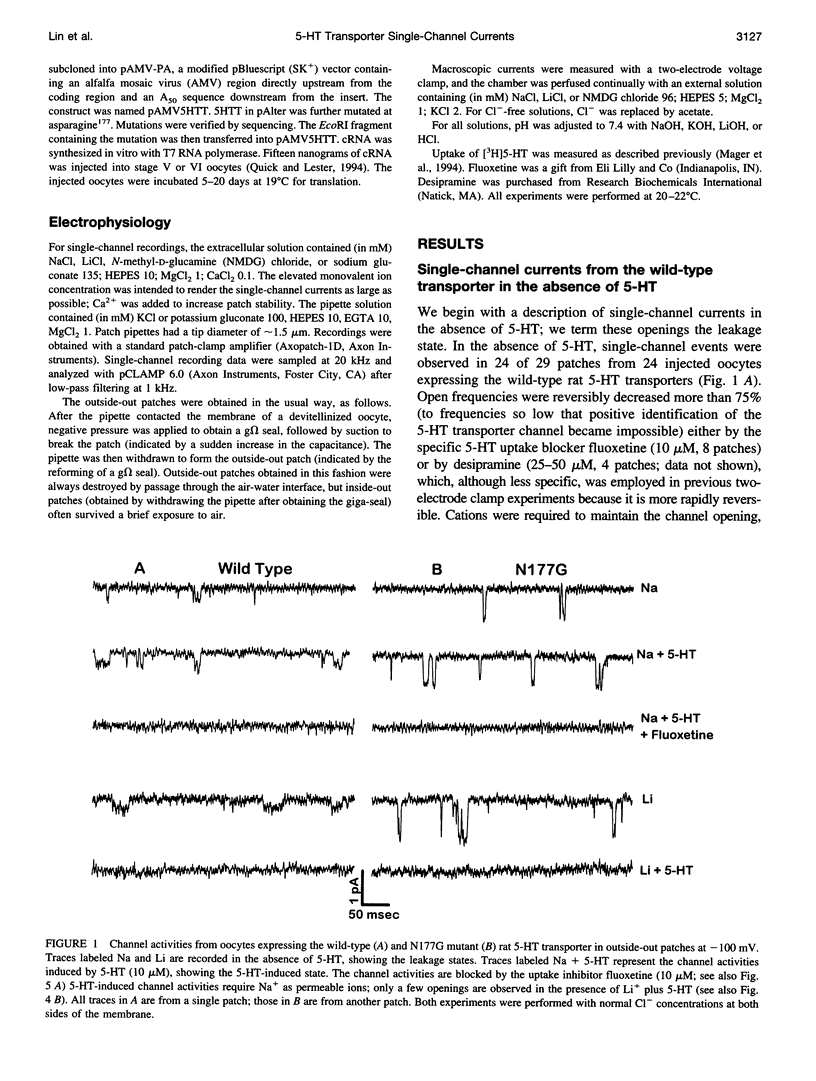
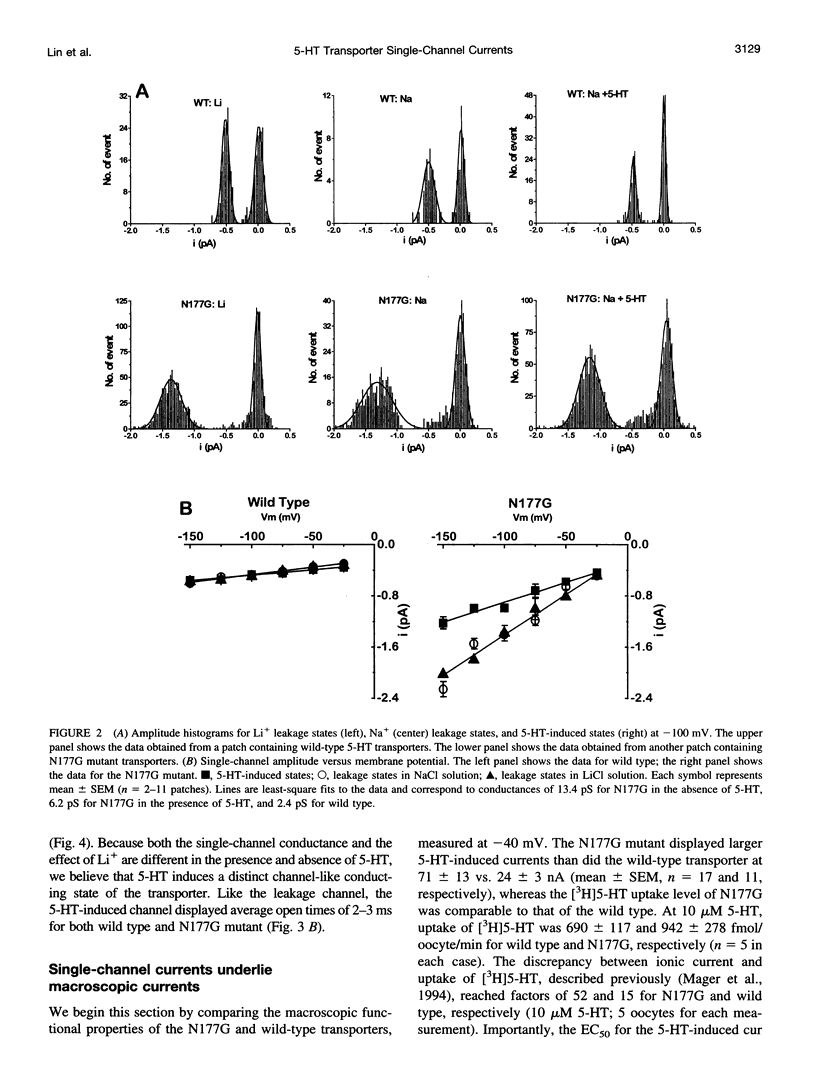
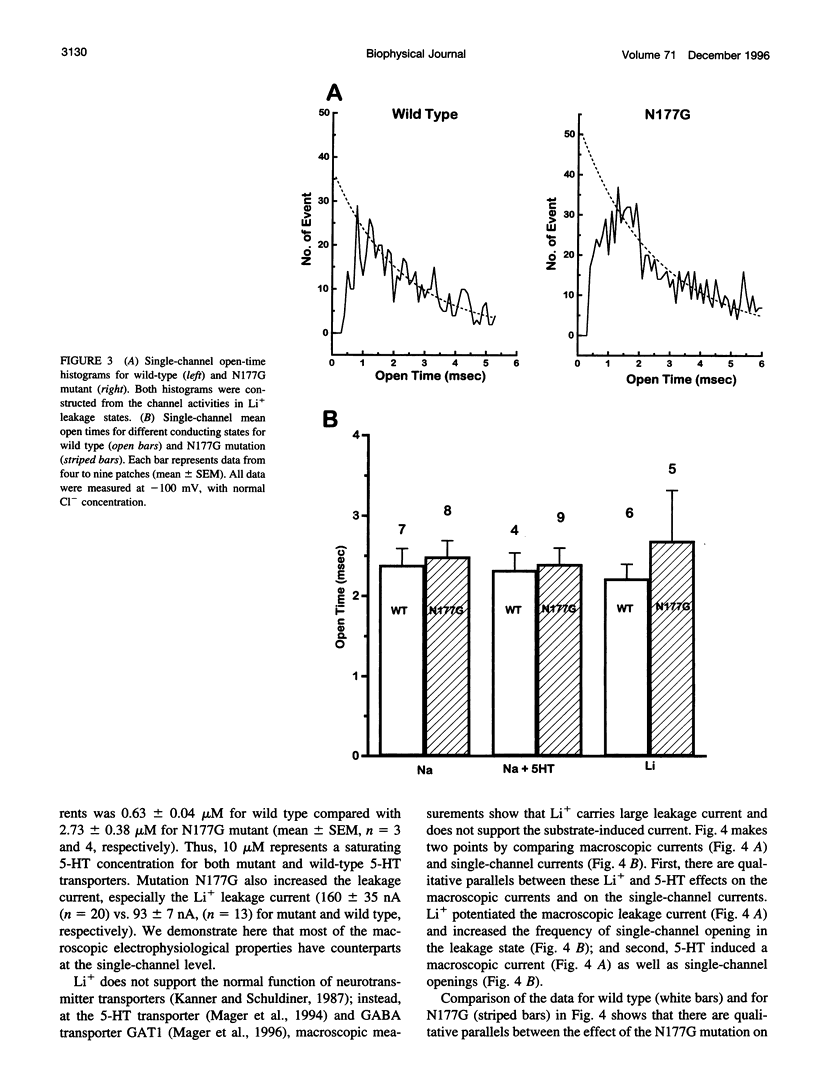
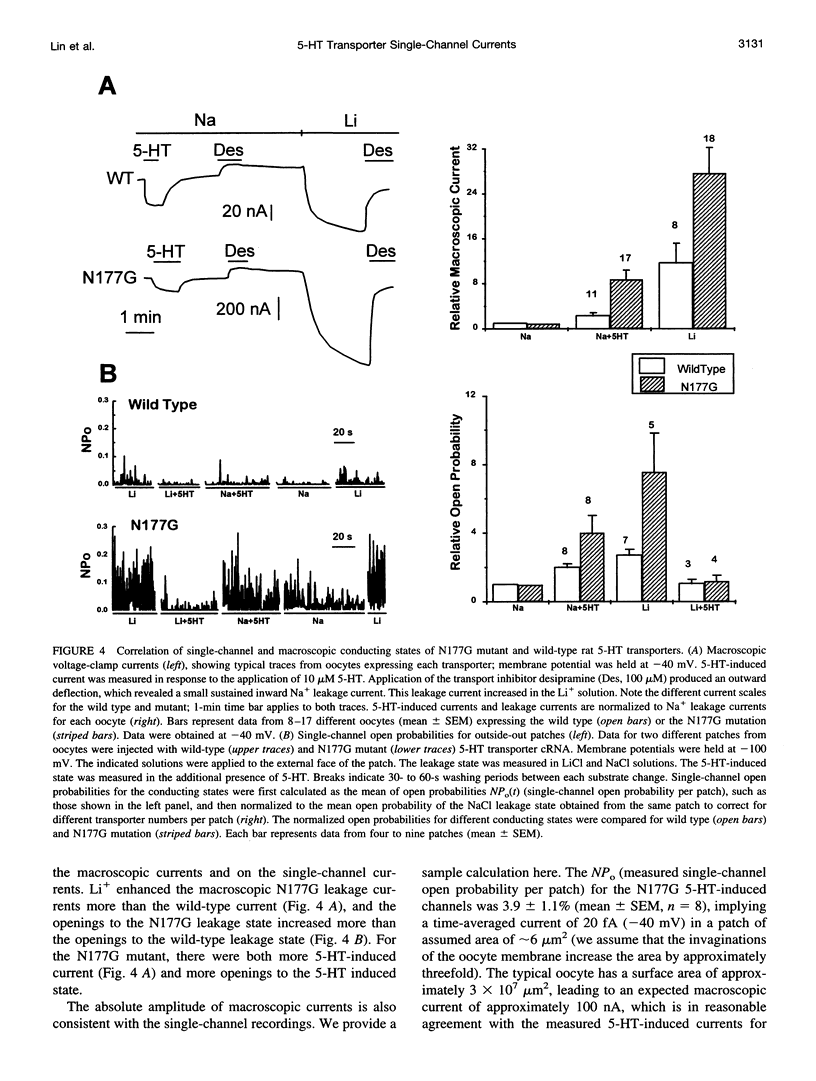
Single-channel activities were observed in outside-out patches excised from oocytes expressing a mammalian 5-hydroxytryptamine (5-HT) transporter. Channel conductance was larger for a mutant in which asparagine177 of the third putative transmembrane domain was replaced by glycine, suggesting that this residue lies within or near the permeation pathway. The N177G mutant enables quantitative single-channel measurements; it displays two conducting states. One state, with conductance of approximately 6 pS, is induced by 5-HT and is permeable to Na+. The other state (conductance of approximately 13 pS) is associated with substrate-independent leakage current and is permeable to both Na+ and Li+. Cl- is not a major current carrier. Channel lifetimes under all conditions measured are approximately 2.5 ms. The single-channel phenomena account for previously observed macroscopic electrophysiological phenomena, including 5-HT-induced transport-associated currents and substrate-independent leakage currents. The channel openings occur several orders of magnitude less frequently than would be expected if one such opening occurred for each transport cycle and therefore do not represent an obligatory step in transport. Nevertheless, single-channel events produced by neurotransmitter transporters indicate the functional and structural similarities between transporters and ion channels and provide a new tool, at single-molecule resolution, for detailed structure-function studies of transporters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Kuhar M. J. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Berson H. E., Fremeau R. T., Jr, Caron M. G., Peek M. M., Prince H. K., Bradley C. C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991 Nov 7;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Schwartz E. A. Channel behavior in a gamma-aminobutyrate transporter. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice L. J., Blakely R. D. Pore models for transporters? Biophys J. 1996 Feb;70(2):579–580. doi: 10.1016/S0006-3495(96)79604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman W. A., Vandenberg R. J., Arriza J. L., Kavanaugh M. P., Amara S. G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995 Jun 15;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Wong D. T. Serotonin uptake and serotonin uptake inhibition. Ann N Y Acad Sci. 1990;600:68–80. doi: 10.1111/j.1749-6632.1990.tb16873.x. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Rakowski R. F., De Weer P. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 1993 Apr 2;260(5104):100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Galli A., DeFelice L. J., Duke B. J., Moore K. R., Blakely R. D. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol. 1995 Oct;198(Pt 10):2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 1994 Mar 11;263(5152):1429–1432. doi: 10.1126/science.8128223. [DOI] [PubMed] [Google Scholar]
- Hoffman B. J., Mezey E., Brownstein M. J. Cloning of a serotonin transporter affected by antidepressants. Science. 1991 Oct 25;254(5031):579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Larsson H. P., Picaud S. A., Werblin F. S., Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996 Feb;70(2):733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Mager S., Quick M. W., Corey J. L. Permeation properties of neurotransmitter transporters. Annu Rev Pharmacol Toxicol. 1994;34:219–249. doi: 10.1146/annurev.pa.34.040194.001251. [DOI] [PubMed] [Google Scholar]
- Mager S., Min C., Henry D. J., Chavkin C., Hoffman B. J., Davidson N., Lester H. A. Conducting states of a mammalian serotonin transporter. Neuron. 1994 Apr;12(4):845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Nelson P. J., Rudnick G. The role of chloride ion in platelet serotonin transport. J Biol Chem. 1982 Jun 10;257(11):6151–6155. [PubMed] [Google Scholar]
- O'Reilly C. A., Reith M. E. Uptake of [3H]serotonin into plasma membrane vesicles from mouse cerebral cortex. J Biol Chem. 1988 May 5;263(13):6115–6121. [PubMed] [Google Scholar]
- Picaud S. A., Larsson H. P., Grant G. B., Lecar H., Werblin F. S. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995 Oct;74(4):1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. J Biol Chem. 1977 Apr 10;252(7):2170–2174. [PubMed] [Google Scholar]
- Rudnick G., Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993 Oct 4;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Talvenheimo J., Fishkes H., Nelson P. J., Rudnick G. The serotonin transporter-imipramine "receptor". J Biol Chem. 1983 May 25;258(10):6115–6119. [PubMed] [Google Scholar]
- Talvenheimo J., Nelson P. J., Rudnick G. Mechanism of imipramine inhibition of platelet 5-hydroxytryptamine transport. J Biol Chem. 1979 Jun 10;254(11):4631–4635. [PubMed] [Google Scholar]
- Wadiche J. I., Amara S. G., Kavanaugh M. P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995 Sep;15(3):721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Wood M. D. Examination of the relationship between the uptake site for 5-hydroxytryptamine and the high affinity binding site for [3H]imipramine. II. The role of sodium ions. Neuropharmacology. 1987 Aug;26(8):1081–1085. doi: 10.1016/0028-3908(87)90251-6. [DOI] [PubMed] [Google Scholar]


