Abstract
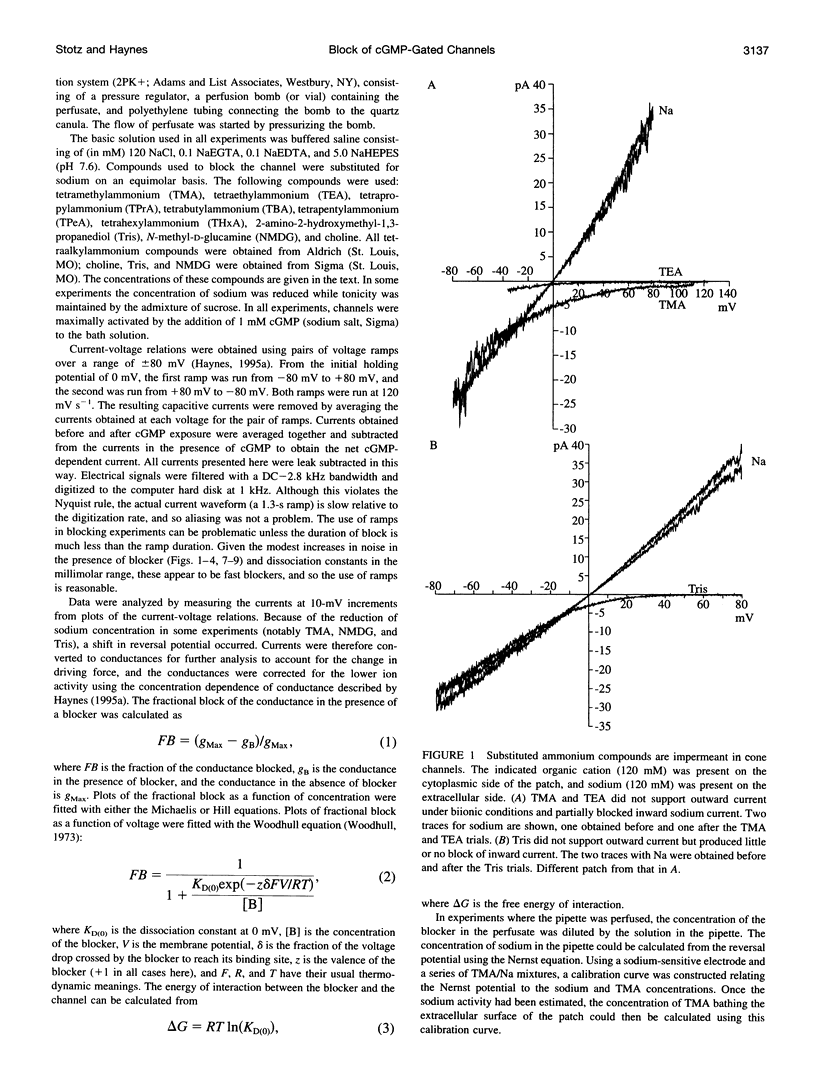
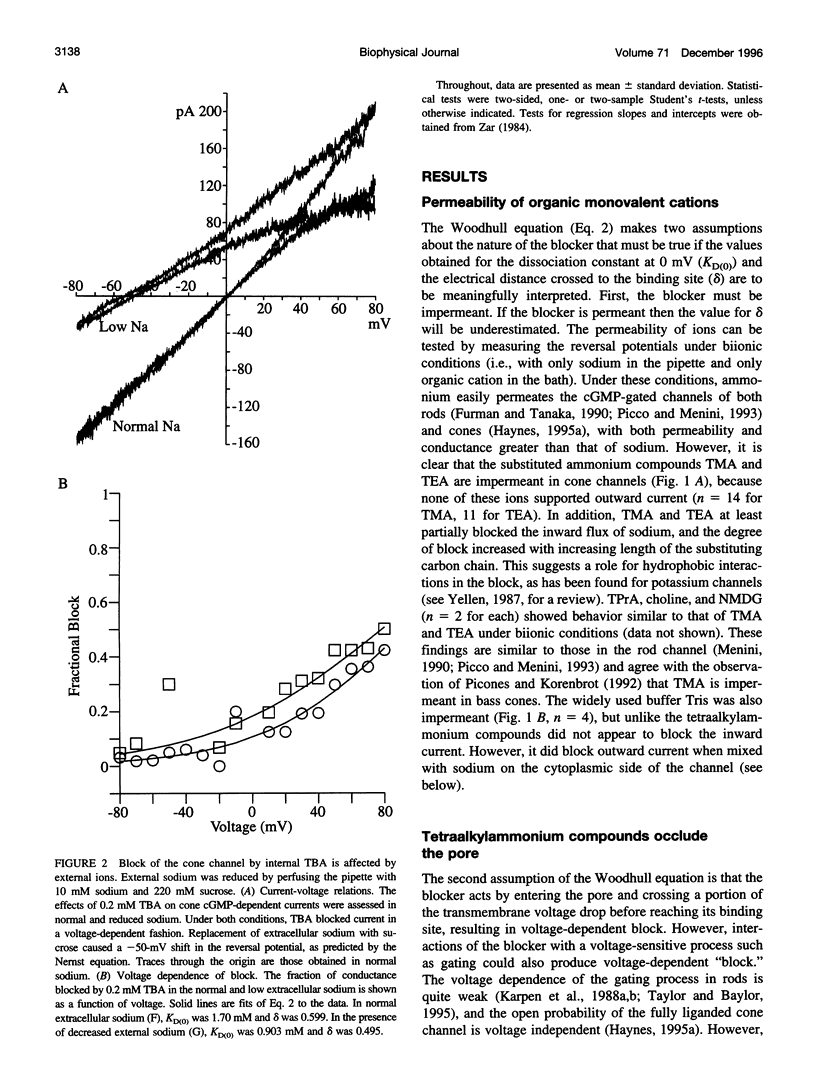
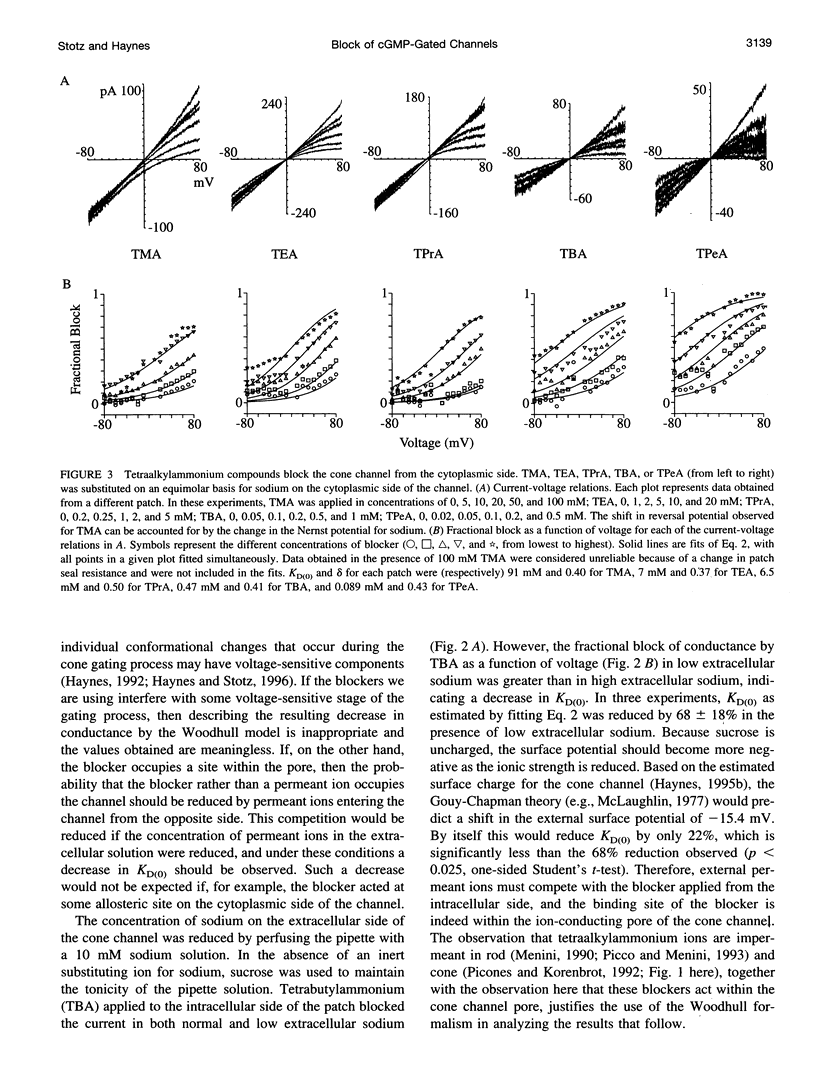
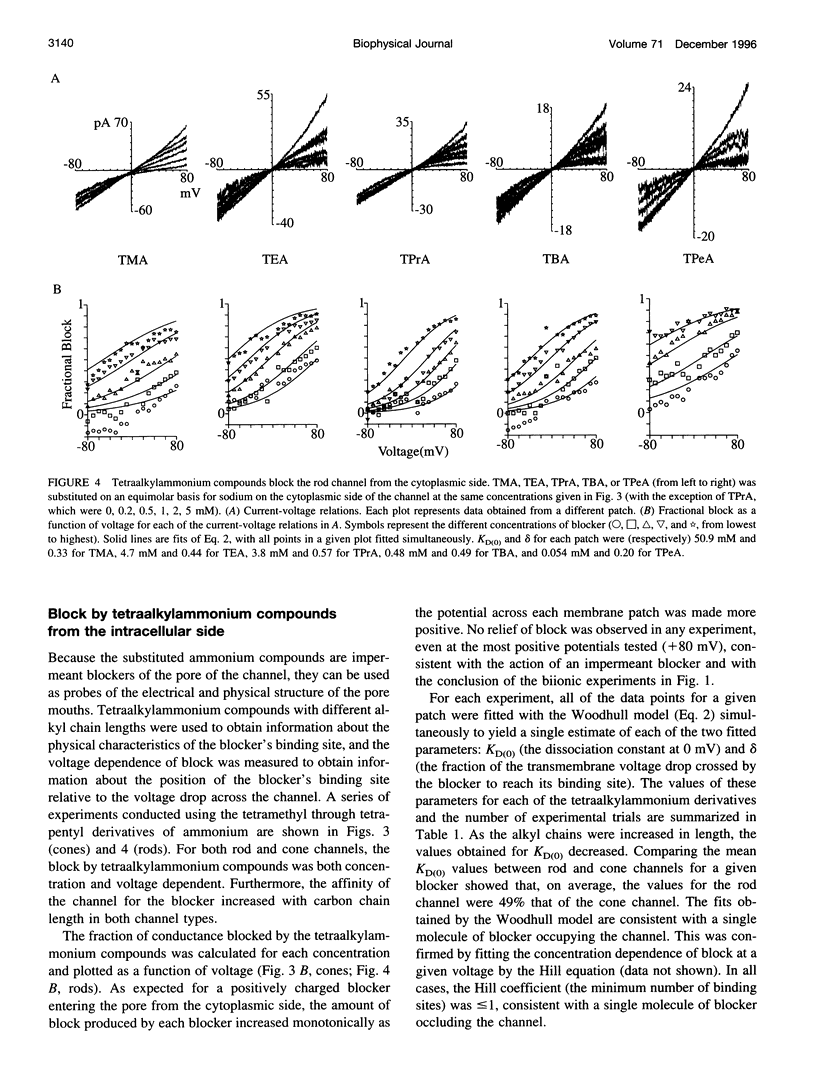
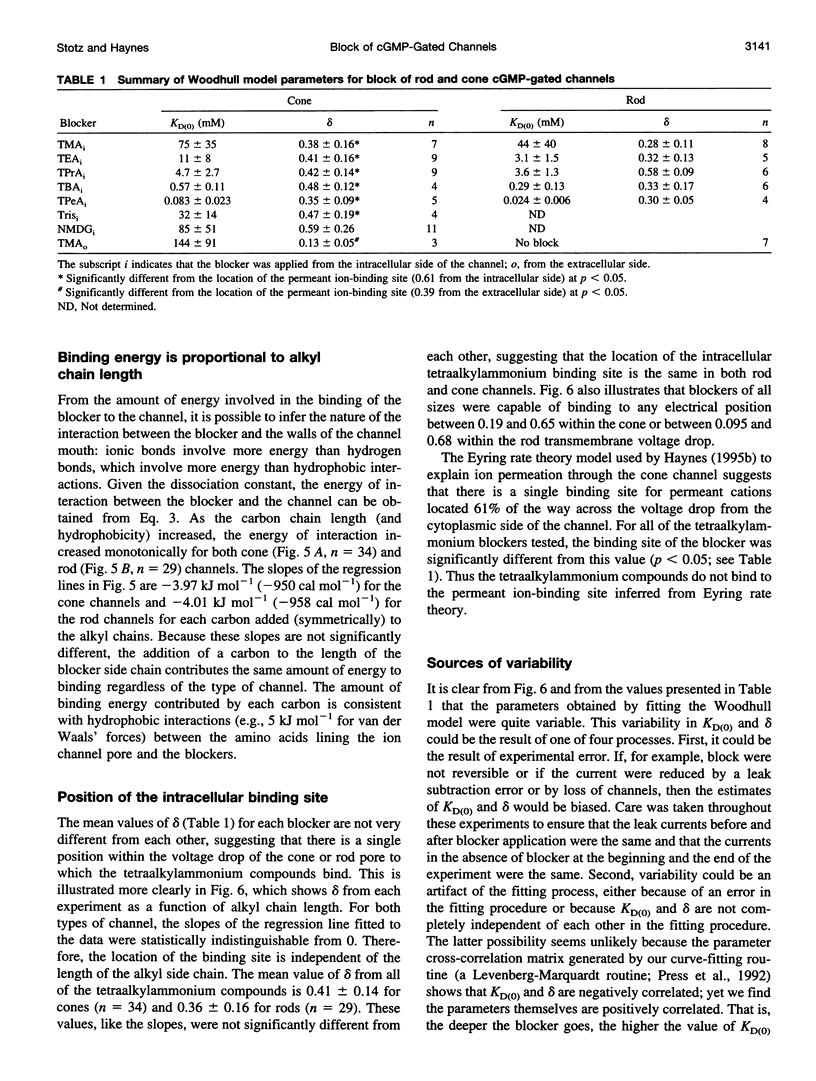
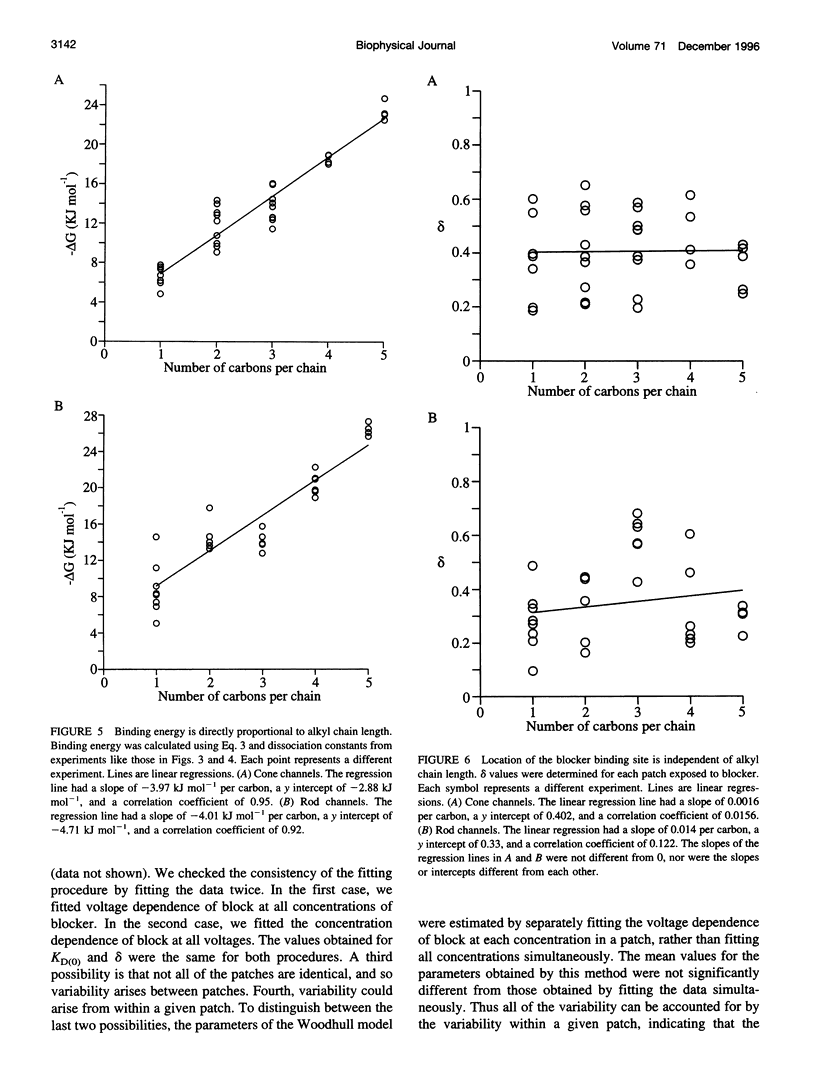
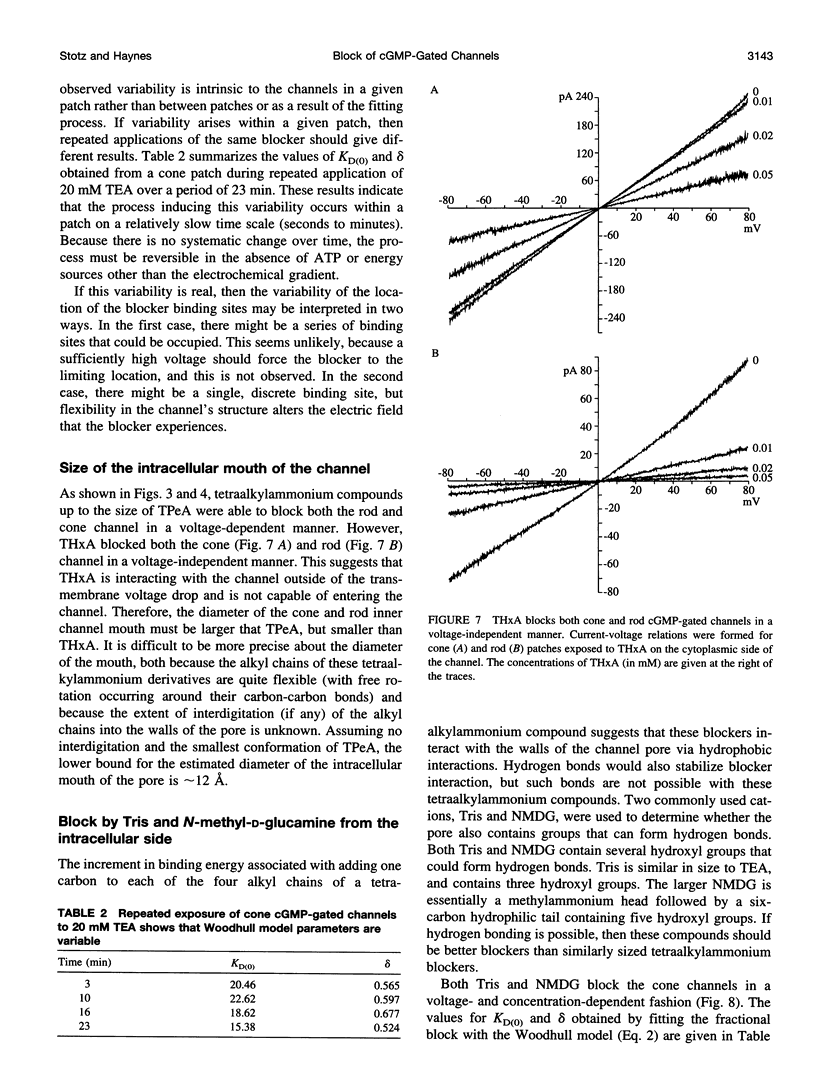
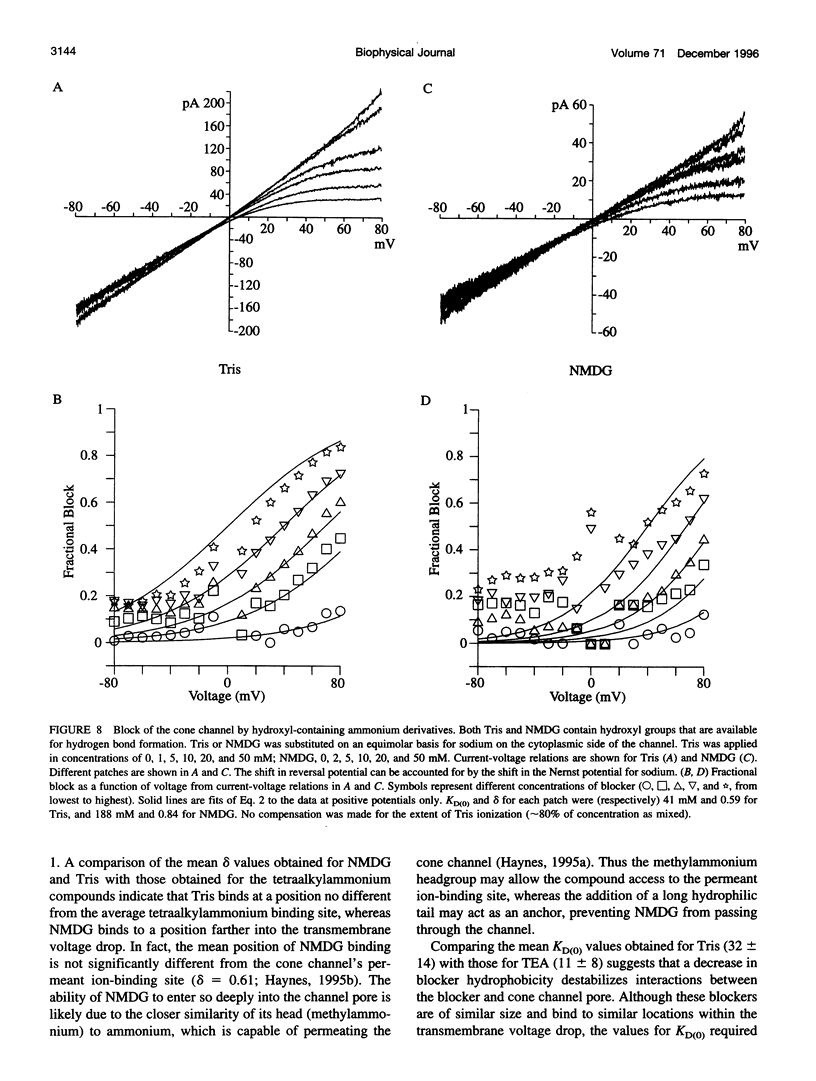
Tetraalkylammonium compounds and other organic cations were used to probe the structure of the internal and external mouths of the pore of cGMP-gated cation channels from rod and cone photoreceptors. Both rod and cone channels were blocked by tetramethyl- through tetrapentylammonium from the intracellular side in a voltage-dependent fashion at millimolar to micromolar concentrations. The dissociation constant at 0 mV (KD(O)) decreased monotonically with increasing carbon chain length from approximately 80 mM (TMA) to approximately 80 microM (TPeA), where the dissociation constant in rod channels is approximately 50% that of cone channels. N-Methyl-D-glucamine and the buffer Tris also blocked the cone channel in a voltage-dependent fashion at millimolar concentrations, but with lower affinity than similarly sized tetraalkylammonium blockers. Block by tetrahexylammonium (THxA) was voltage-independent, suggesting that the diameter of the intracellular mouth of these channels is less than the size of THxA but larger than TPeA. The location of the binding site for intracellular blockers was approximately 40% across the voltage-drop from the intracellular side. The addition of one carbon to each of the alkyl side chains increased the binding energy by approximately 4 kJ mol-1, consistent with hydrophobic interactions between the blocker and the pore. Cone, but not rod, channels were blocked by millimolar concentrations of extracellular TMA. The location of the extracellular binding site was approximately 13% of the voltage drop from the extracellular side. In cone channels, the two blocker binding sites flank the location of the cation binding site proposed previously.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönigk W., Altenhofen W., Müller F., Dose A., Illing M., Molday R. S., Kaupp U. B. Rod and cone photoreceptor cells express distinct genes for cGMP-gated channels. Neuron. 1993 May;10(5):865–877. doi: 10.1016/0896-6273(93)90202-3. [DOI] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Miller C. Conduction and block by organic cations in a K+-selective channel from sarcoplasmic reticulum incorporated into planar phospholipid bilayers. J Gen Physiol. 1982 Apr;79(4):529–547. doi: 10.1085/jgp.79.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J Gen Physiol. 1990 Jul;96(1):57–82. doi: 10.1085/jgp.96.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Haynes L. W. Block of the cyclic GMP-gated channel of vertebrate rod and cone photoreceptors by l-cis-diltiazem. J Gen Physiol. 1992 Nov;100(5):783–801. doi: 10.1085/jgp.100.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W. Permeation and block by internal and external divalent cations of the catfish cone photoreceptor cGMP-gated channel. J Gen Physiol. 1995 Sep;106(3):507–523. doi: 10.1085/jgp.106.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W. Permeation of internal and external monovalent cations through the catfish cone photoreceptor cGMP-gated channel. J Gen Physiol. 1995 Sep;106(3):485–505. doi: 10.1085/jgp.106.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Yau K. W. Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J Physiol. 1990 Oct;429:451–481. doi: 10.1113/jphysiol.1990.sp018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. A superfamily of ion channels. Nature. 1990 Jun 21;345(6277):672–672. doi: 10.1038/345672a0. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Molecular mechanics of the cyclic-GMP-activated channel of retinal rods. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):325–332. doi: 10.1101/sqb.1988.053.01.039. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Ludwig J., Margalit T., Eismann E., Lancet D., Kaupp U. B. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Lett. 1990 Sep 17;270(1-2):24–29. doi: 10.1016/0014-5793(90)81226-e. [DOI] [PubMed] [Google Scholar]
- Menini A. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol. 1990 May;424:167–185. doi: 10.1113/jphysiol.1990.sp018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picco C., Menini A. The permeability of the cGMP-activated channel to organic cations in retinal rods of the tiger salamander. J Physiol. 1993 Jan;460:741–758. doi: 10.1113/jphysiol.1993.sp019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J. I. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J Gen Physiol. 1992 Oct;100(4):647–673. doi: 10.1085/jgp.100.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Baylor D. A. Conductance and kinetics of single cGMP-activated channels in salamander rod outer segments. J Physiol. 1995 Mar 15;483(Pt 3):567–582. doi: 10.1113/jphysiol.1995.sp020607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A., Alvarez O., Oberhauser A., Latorre R. Probing a Ca2+-activated K+ channel with quaternary ammonium ions. Pflugers Arch. 1988 Dec;413(2):118–126. doi: 10.1007/BF00582521. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Permeation in potassium channels: implications for channel structure. Annu Rev Biophys Biophys Chem. 1987;16:227–246. doi: 10.1146/annurev.bb.16.060187.001303. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]



