Abstract
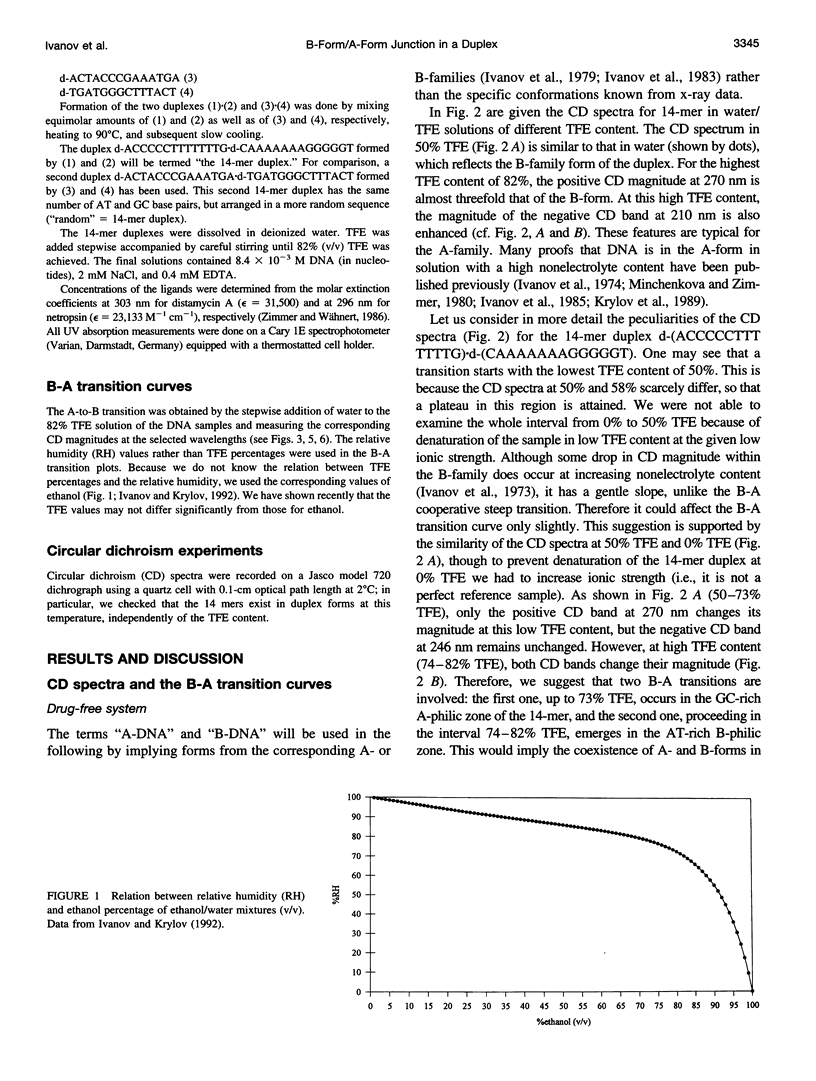
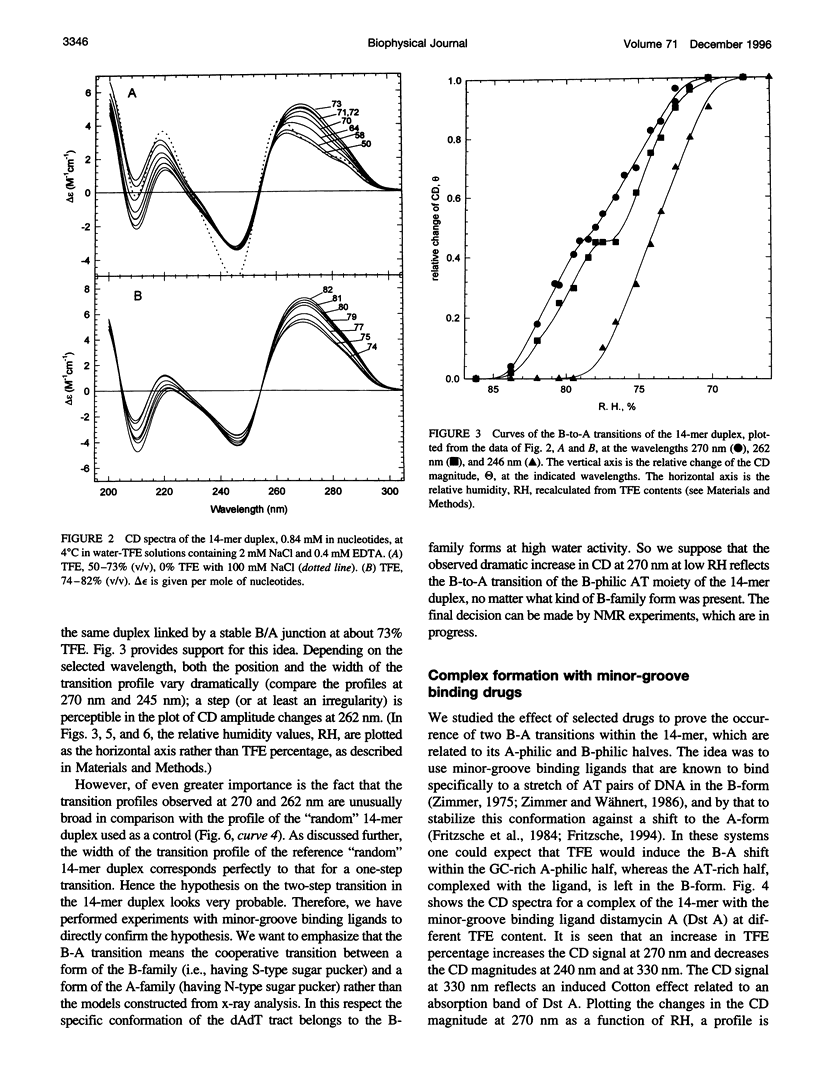
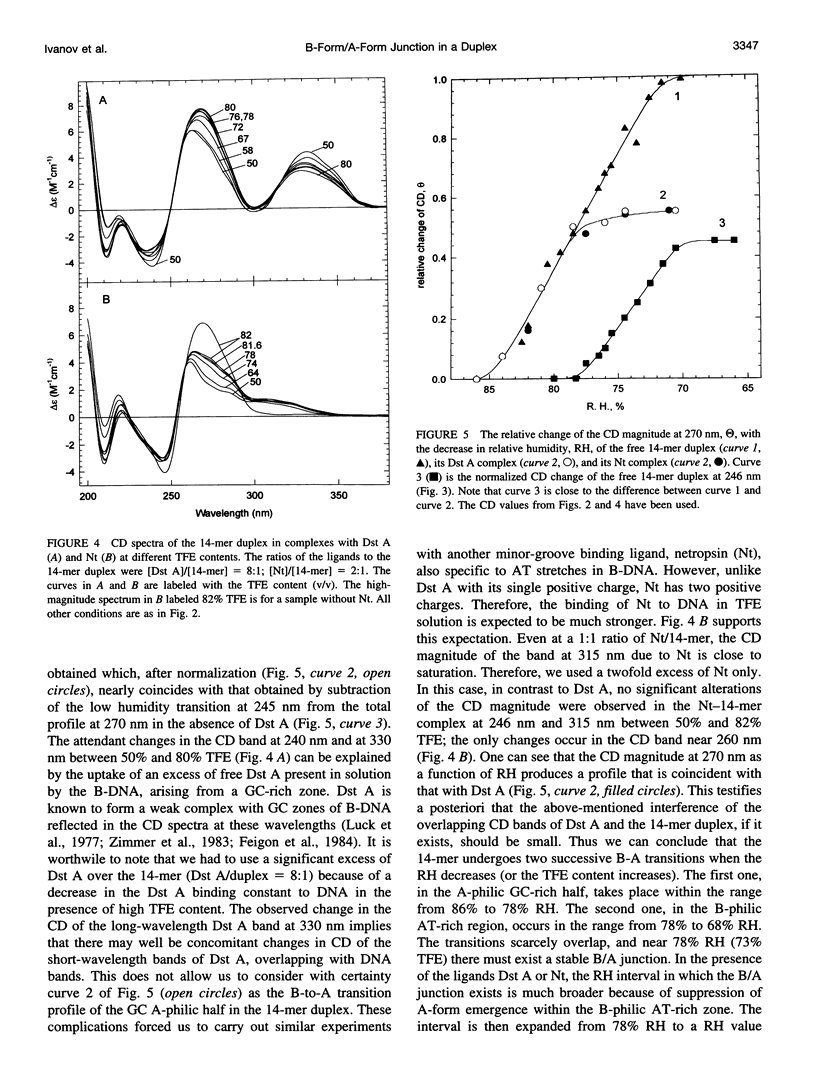
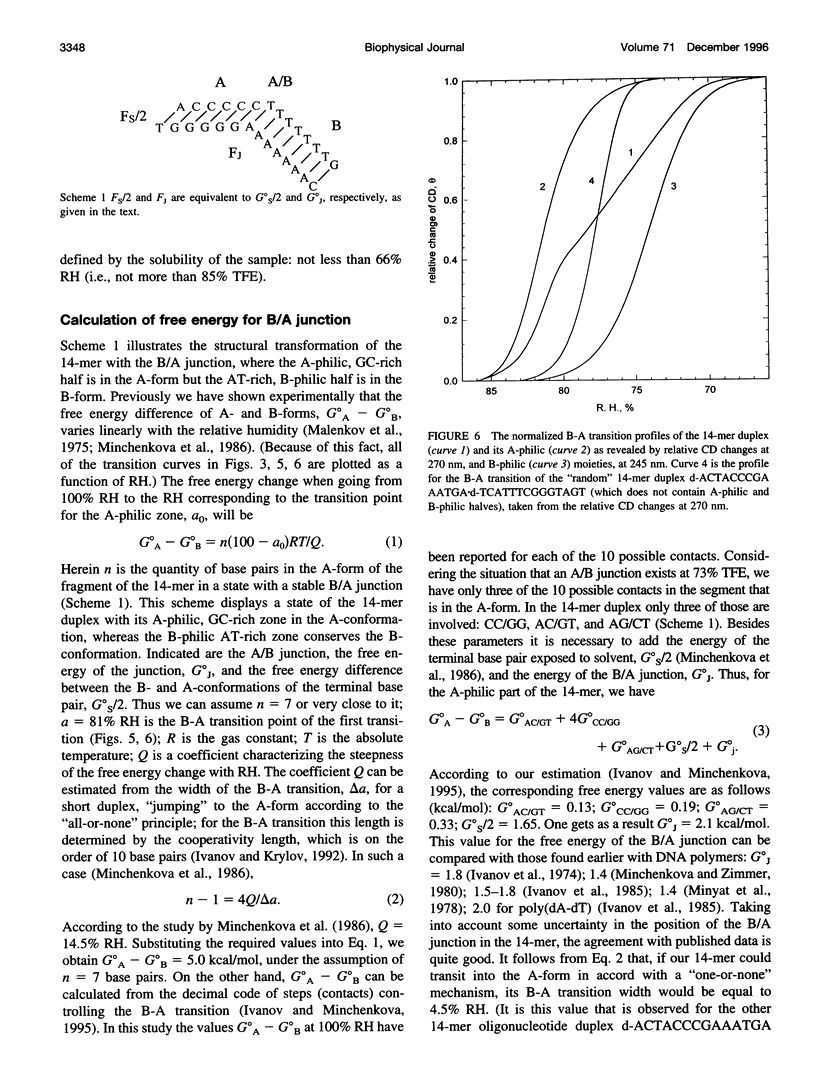
The transition of the 14-meric deoxyoligonucleotide duplex d-(ACCCCCTTTTTTTG).d-(CAAAAAAAGGGGGT) from the B- to the A-conformation in water/trifluorethanol (TFE) solution was studied with the use of circular dichroism. An increase in the fraction of TFE induces a two-step B-A transition. In the first step, up to 73% TFE, the A-form is generated from the GC-rich part; in the second step, 73-82% TFE, the AT-rich part shifts to the A-form. By this we suggest the existence of a B/A junction near 73% TFE. Emergence of the B/A junction has been directly confirmed with the use of distamycin A and netropsin, ligands known to selectively bind to AT stretches of B-DNA. It can be shown that both ligands suppress formation of the A-form in the B-philic part. The free energy value for the B/A junction was estimated to be 2.1 kcal/mol, which agrees well with known data for polymeric DNAs. The obtained results may have biological relevance in connection with recently published x-ray data about the occurrence of the B/A junction in the complex of DNA with reverse transcriptase of HIV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAHMS J., MOMMAERTS W. F. A STUDY OF CONFORMATION OF NUCLEIC ACIDS IN SOLUTION BY MEANS OF CIRCULAR DICHROISM. J Mol Biol. 1964 Oct;10:73–88. doi: 10.1016/s0022-2836(64)80029-2. [DOI] [PubMed] [Google Scholar]
- Basham B., Schroth G. P., Ho P. S. An A-DNA triplet code: thermodynamic rules for predicting A- and B-DNA. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6464–6468. doi: 10.1073/pnas.92.14.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigon J., Denny W. A., Leupin W., Kearns D. R. Interactions of antitumor drugs with natural DNA: 1H NMR study of binding mode and kinetics. J Med Chem. 1984 Apr;27(4):450–465. doi: 10.1021/jm00370a007. [DOI] [PubMed] [Google Scholar]
- Fritzsche H. Infrared linear dichroism studies of DNA-drug complexes: quantitative determination of the drug-induced restriction of the B-A transition. Nucleic Acids Res. 1994 Mar 11;22(5):787–791. doi: 10.1093/nar/22.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche H., Rupprecht A., Richter M. Infrared linear dichroism of oriented DNA-ligand complexes prepared with the wet-spinning method. Nucleic Acids Res. 1984 Dec 11;12(23):9165–9177. doi: 10.1093/nar/12.23.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. A. Sequence-dependent DNA structure. The role of base stacking interactions. J Mol Biol. 1993 Apr 5;230(3):1025–1054. doi: 10.1006/jmbi.1993.1217. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Krylov DYu A-DNA in solution as studied by diverse approaches. Methods Enzymol. 1992;211:111–127. doi: 10.1016/0076-6879(92)11008-7. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Krylov DYu, Minyat E. E. Three-state diagram for DNA. J Biomol Struct Dyn. 1985 Aug;3(1):43–55. doi: 10.1080/07391102.1985.10508397. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Schyolkina A. K. Cooperative transitions in DNA with no separation of strands. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):243–250. doi: 10.1101/sqb.1983.047.01.029. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Ding J., Nanni R. G., Clark A. D., Jr, Lu X., Tantillo C., Williams R. L., Kamer G., Ferris A. L., Clark P. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov DYu, Makarov V. L., Ivanov V. I. The B-A transition in superhelical DNA. Nucleic Acids Res. 1990 Feb 25;18(4):759–761. doi: 10.1093/nar/18.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazurkin Y. S., Frank-Kamenetskii M. D., Trifonov E. N. Melting of DNA: its study and application as a research method. Biopolymers. 1970 Nov;9(11):1253–1306. doi: 10.1002/bip.1970.360091102. [DOI] [PubMed] [Google Scholar]
- Luck G., Zimmer C., Reinert K. E., Arcamone F. Specific interactions of distamycin A and its analogs with (A-T) rich and (G-C) rich duplex regions of DNA and deoxypolynucleotides. Nucleic Acids Res. 1977 Aug;4(8):2655–2670. doi: 10.1093/nar/4.8.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenkov G., Minchenkova L., Minyat E., Schyolkina A., Ivanov V. The nature of the B-A transition of DNA in solution. FEBS Lett. 1975 Mar 1;51(1):38–42. doi: 10.1016/0014-5793(75)80850-7. [DOI] [PubMed] [Google Scholar]
- Minchenkova L. E., Schyolkina A. K., Chernov B. K., Ivanov V. I. CC/GG contacts facilitate the B to A transition of DNA in solution. J Biomol Struct Dyn. 1986 Dec;4(3):463–476. doi: 10.1080/07391102.1986.10506362. [DOI] [PubMed] [Google Scholar]
- Minyat E. E., Ivanov V. I., Kritzyn A. M., Minchenkova L. E., Schyolkina A. K. Spermine and spermidine-induced B to A transition of DNA in solution. J Mol Biol. 1979 Mar 5;128(3):397–409. doi: 10.1016/0022-2836(79)90094-9. [DOI] [PubMed] [Google Scholar]
- Pohle W., Zhurkin V. B., Fritzsche H. The DNA phosphate orientation. Infrared data and energetically favorable structures. Biopolymers. 1984 Nov;23(11 Pt 2):2603–2622. doi: 10.1002/bip.360231131. [DOI] [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Alden C. J., Arnott S. Bent DNA: visualization of a base-paired and stacked A-B conformational junction. J Biol Chem. 1979 Jun 25;254(12):5417–5422. [PubMed] [Google Scholar]
- Sheardy R. D., Suh D., Kurzinsky R., Doktycz M. J., Benight A. S., Chaires J. B. Sequence dependence of the free energy of B-Z junction formation in deoxyoligonucleotides. J Mol Biol. 1993 May 20;231(2):475–488. doi: 10.1006/jmbi.1993.1295. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Minchenkova L. E., Frank-Kamenetskii M. D., Ivanov V. I. On the flexibility of the boundaries between the A-form and B-form sections in DNA molecule. Nucleic Acids Res. 1978 Jul;5(7):2657–2663. doi: 10.1093/nar/5.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Birch-Hirschfeld E., Weiss R., Arcamone F., Guschlbauer W. Chain length-dependent association of distamycin-type oligopeptides with A X T and G X C pairs in polydeoxynucleotide duplexes. Biochim Biophys Acta. 1983 Oct 13;741(1):15–22. doi: 10.1016/0167-4781(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]


