Abstract
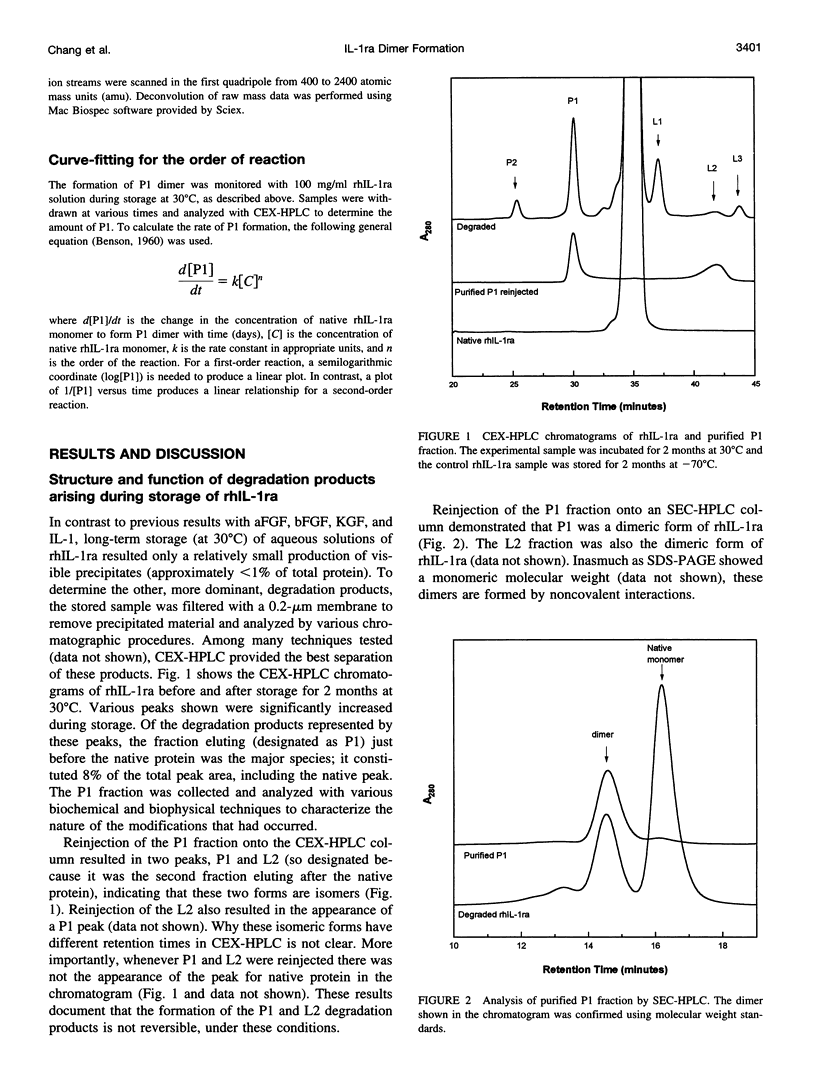
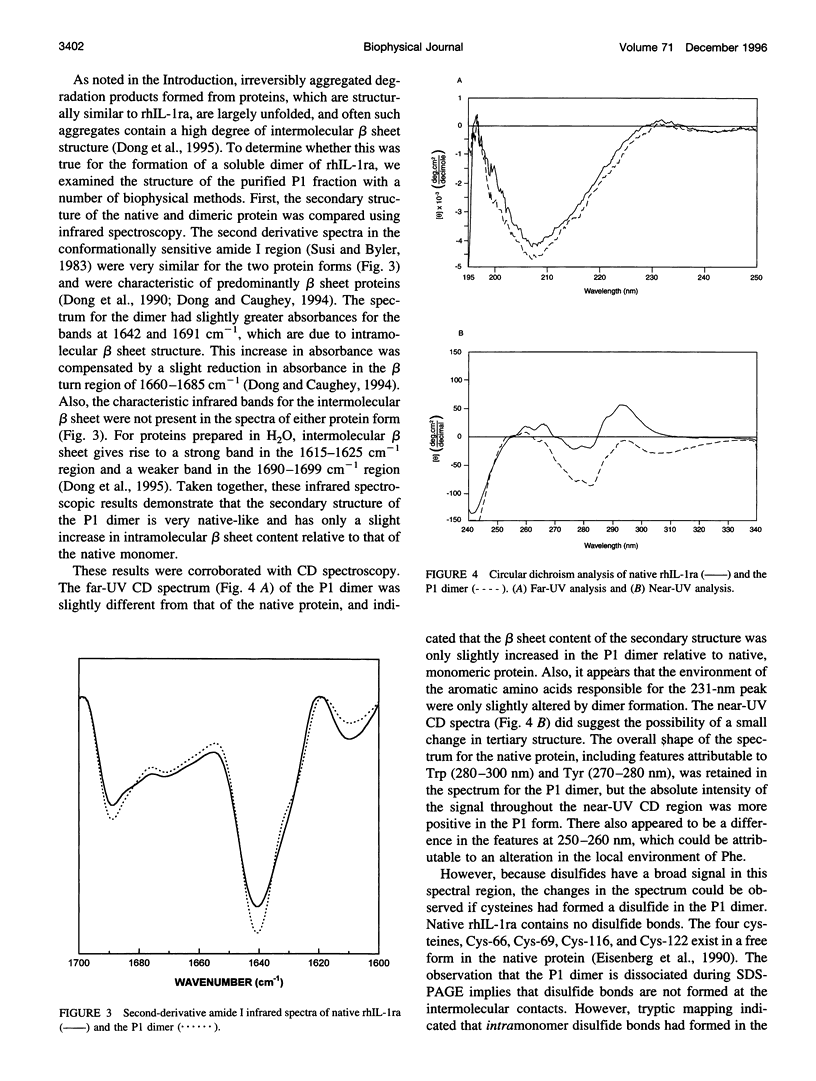
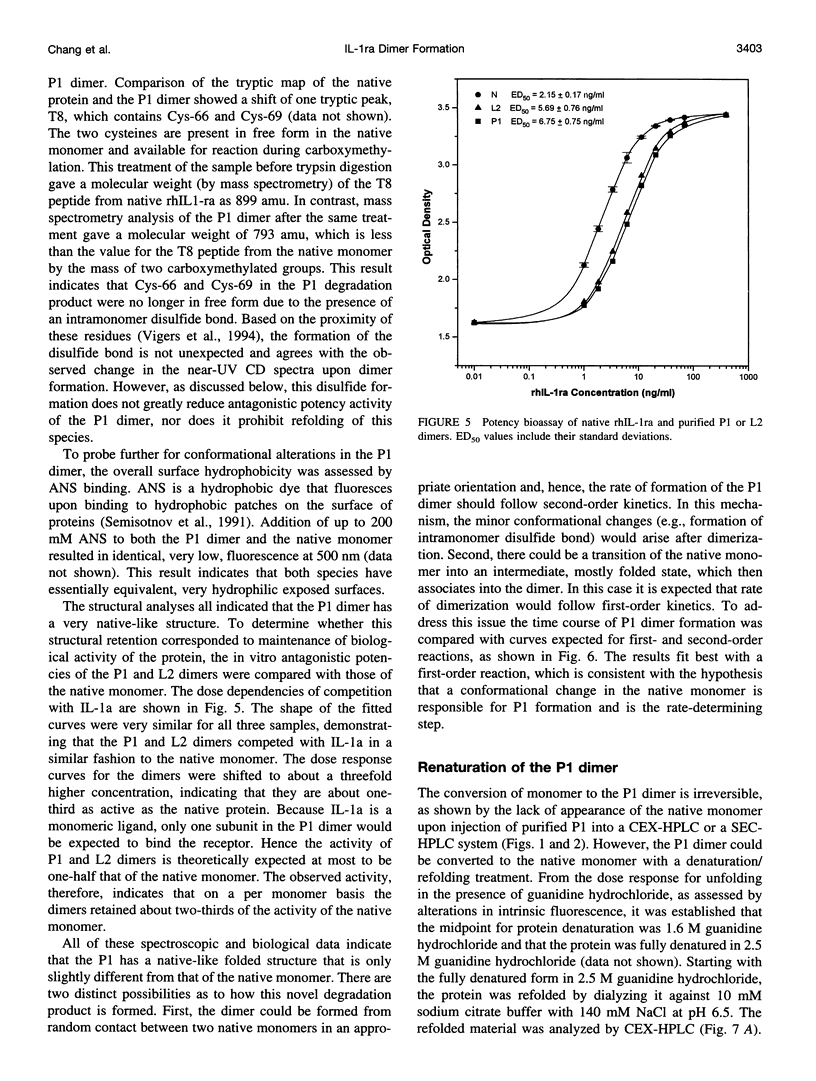
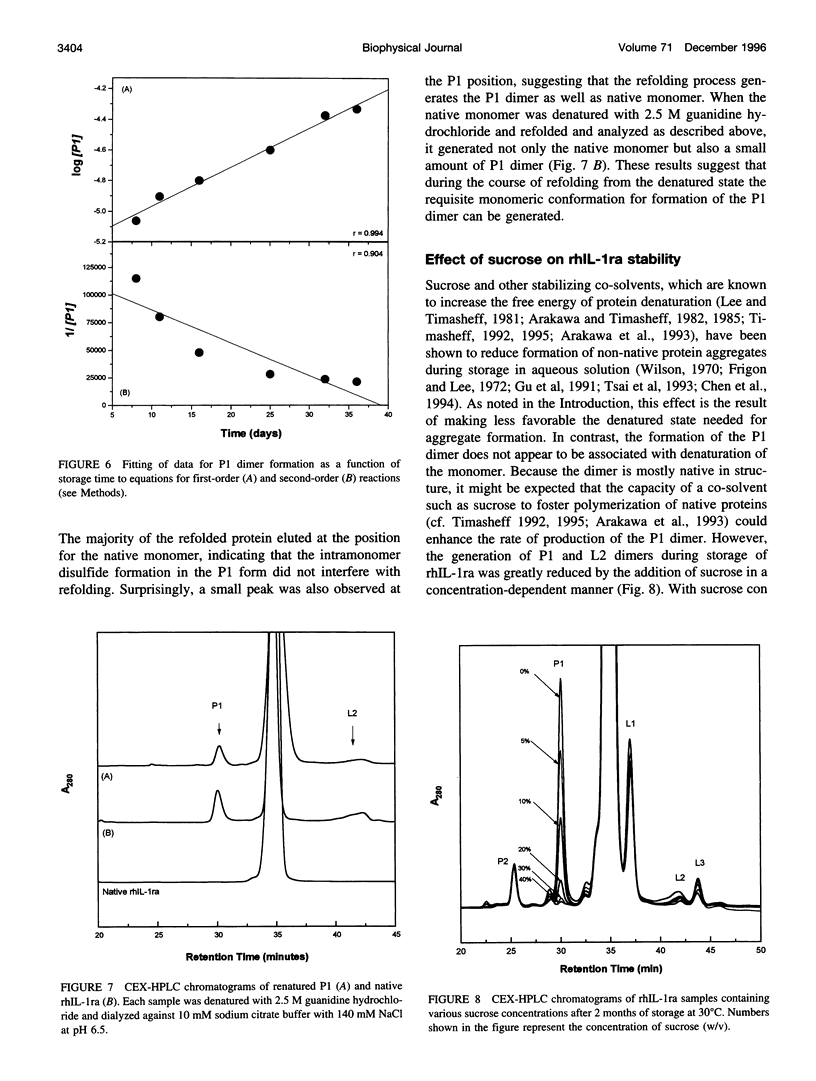
The degradation products of recombinant human interleukin-1 receptor antagonist (rhIL-1ra) formed during storage at 30 degrees C in aqueous solution were characterized. Cationic exchange chromatography of the stored sample showed two major, new peaks eluting before (P1) and after (L2) the native protein, which were interconvertible. Size-exclusion chromatography and electrophoresis documented that both the P1 and L2 fractions were irreversible dimers, formed by noncovalent interactions. A competition assay with interleukin-1 indicated that on a per monomer basis the P1 and L2 dimers retained about two-thirds of the activity of the native monomer. Infrared and far-UV circular dichroism spectroscopies showed that only minor alterations in secondary structure arose upon the formation of the P1 dimer. However, alteration in the near-UV circular dichroism spectrum suggested the presence of disulfide bonds in the P1 dimer, which are absent in the native protein. Mass spectroscopy and tryptic mapping, before and after carboxymethylation, demonstrated that the P1 dimer contained an intramolecular disulfide bond between Cys-66 and Cys-69. Although conversion of native protein to the P1 dimer was irreversible in buffer alone, the native monomer could be regained by denaturing the P1 dimer with guanidine hydrochloride and renaturing it by dialysis, suggesting that the intramolecular disulfide bond does not interfere with refolding. Analysis of the time course of P1 formation during storage at 30 degrees C indicated that the process followed first-order, and not second-order, kinetics, suggesting that the rate-limiting step was not dimerization. It is proposed that a conformational change in the monomer is the rate-limiting step in the formation of the P1 dimer degradation product. Sucrose stabilized the native monomer against this process. This result can be explained by the general stabilization mechanism for this additive, which is due to its preferential exclusion from the protein surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ago H., Kitagawa Y., Fujishima A., Matsuura Y., Katsube Y. Crystal structure of basic fibroblast growth factor at 1.6 A resolution. J Biochem. 1991 Sep;110(3):360–363. doi: 10.1093/oxfordjournals.jbchem.a123586. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. Stabilization of protein structure by sugars. Biochemistry. 1982 Dec 7;21(25):6536–6544. doi: 10.1021/bi00268a033. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. The stabilization of proteins by osmolytes. Biophys J. 1985 Mar;47(3):411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. L., Arakawa T., Morris C. F., Kenney W. C., Wells C. M., Pitt C. G. Aggregation pathway of recombinant human keratinocyte growth factor and its stabilization. Pharm Res. 1994 Nov;11(11):1581–1587. doi: 10.1023/a:1018905720139. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Wingfield P. T., Gronenborn A. M. High-resolution three-dimensional structure of interleukin 1 beta in solution by three- and four-dimensional nuclear magnetic resonance spectroscopy. Biochemistry. 1991 Mar 5;30(9):2315–2323. doi: 10.1021/bi00223a005. [DOI] [PubMed] [Google Scholar]
- Dong A., Caughey W. S. Infrared methods for study of hemoglobin reactions and structures. Methods Enzymol. 1994;232:139–175. doi: 10.1016/0076-6879(94)32047-0. [DOI] [PubMed] [Google Scholar]
- Dong A., Huang P., Caughey W. S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry. 1990 Apr 3;29(13):3303–3308. doi: 10.1021/bi00465a022. [DOI] [PubMed] [Google Scholar]
- Dong A., Prestrelski S. J., Allison S. D., Carpenter J. F. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995 Apr;84(4):415–424. doi: 10.1002/jps.2600840407. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Cousens L. S., Weaver L. H., Matthews B. W. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzel B. C., Clancy L. L., Holland D. R., Muchmore S. W., Watenpaugh K. D., Einspahr H. M. Crystal structure of recombinant human interleukin-1 beta at 2.0 A resolution. J Mol Biol. 1989 Oct 20;209(4):779–791. doi: 10.1016/0022-2836(89)90606-2. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Lee J. C. The stabilization of calf-brain microtubule protein by sucrose. Arch Biochem Biophys. 1972 Dec;153(2):587–589. doi: 10.1016/0003-9861(72)90376-1. [DOI] [PubMed] [Google Scholar]
- Gerlsma S. Y. Reversible denaturation of ribonuclease in aqueous solutions as influenced by polyhydric alcohols and some other additives. J Biol Chem. 1968 Mar 10;243(5):957–961. [PubMed] [Google Scholar]
- Gerlsma S. Y. The effects of polyhydric and monohydric alcohols on the heat induced reversible denaturation of chymotrypsinogen A. Eur J Biochem. 1970 May 1;14(1):150–153. doi: 10.1111/j.1432-1033.1970.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Hatada M. H., Hendrickson W. A., Miller J. K., Madison V. S., Satow Y. Structure of interleukin 1 alpha at 2.7-A resolution. Biochemistry. 1990 Mar 20;29(11):2679–2684. doi: 10.1021/bi00463a009. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Gu L. C., Erdös E. A., Chiang H. S., Calderwood T., Tsai K., Visor G. C., Duffy J., Hsu W. C., Foster L. C. Stability of interleukin 1 beta (IL-1 beta) in aqueous solution: analytical methods, kinetics, products, and solution formulation implications. Pharm Res. 1991 Apr;8(4):485–490. doi: 10.1023/a:1015851228163. [DOI] [PubMed] [Google Scholar]
- Kajio T., Kawahara K., Kato K. Stabilization of basic fibroblast growth factor with dextran sulfate. FEBS Lett. 1992 Jul 20;306(2-3):243–246. doi: 10.1016/0014-5793(92)81009-b. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The stabilization of proteins by sucrose. J Biol Chem. 1981 Jul 25;256(14):7193–7201. [PubMed] [Google Scholar]
- Liu Y., Bolen D. W. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry. 1995 Oct 3;34(39):12884–12891. doi: 10.1021/bi00039a051. [DOI] [PubMed] [Google Scholar]
- Priestle J. P., Schär H. P., Grütter M. G. Crystallographic refinement of interleukin 1 beta at 2.0 A resolution. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9667–9671. doi: 10.1073/pnas.86.24.9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Rodgers G. H., Hatfield S. M., Glasebrook A. L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991 Sep 13;142(2):257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- Semisotnov G. V., Rodionova N. A., Razgulyaev O. I., Uversky V. N., Gripas' A. F., Gilmanshin R. I. Study of the "molten globule" intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers. 1991 Jan;31(1):119–128. doi: 10.1002/bip.360310111. [DOI] [PubMed] [Google Scholar]
- Shaanan B., Gronenborn A. M., Cohen G. H., Gilliland G. L., Veerapandian B., Davies D. R., Clore G. M. Combining experimental information from crystal and solution studies: joint X-ray and NMR refinement. Science. 1992 Aug 14;257(5072):961–964. doi: 10.1126/science.1502561. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Protein structure by Fourier transform infrared spectroscopy: second derivative spectra. Biochem Biophys Res Commun. 1983 Aug 30;115(1):391–397. doi: 10.1016/0006-291x(83)91016-1. [DOI] [PubMed] [Google Scholar]
- Tsai P. K., Volkin D. B., Dabora J. M., Thompson K. C., Bruner M. W., Gress J. O., Matuszewska B., Keogan M., Bondi J. V., Middaugh C. R. Formulation design of acidic fibroblast growth factor. Pharm Res. 1993 May;10(5):649–659. doi: 10.1023/a:1018939228201. [DOI] [PubMed] [Google Scholar]
- Vigers G. P., Caffes P., Evans R. J., Thompson R. C., Eisenberg S. P., Brandhuber B. J. X-ray structure of interleukin-1 receptor antagonist at 2.0-A resolution. J Biol Chem. 1994 Apr 29;269(17):12874–12879. [PubMed] [Google Scholar]
- Volkin D. B., Tsai P. K., Dabora J. M., Gress J. O., Burke C. J., Linhardt R. J., Middaugh C. R. Physical stabilization of acidic fibroblast growth factor by polyanions. Arch Biochem Biophys. 1993 Jan;300(1):30–41. doi: 10.1006/abbi.1993.1005. [DOI] [PubMed] [Google Scholar]
- Vrkljan M., Foster T. M., Powers M. E., Henkin J., Porter W. R., Staack H., Carpenter J. F., Manning M. C. Thermal stability of low molecular weight urokinase during heat treatment. II. Effect of polymeric additives. Pharm Res. 1994 Jul;11(7):1004–1008. doi: 10.1023/a:1018935420680. [DOI] [PubMed] [Google Scholar]
- Wen J., Hsu E., Kenney W. C., Philo J. S., Morris C. F., Arakawa T. Characterization of keratinocyte growth factor binding to heparin and dextran sulfate. Arch Biochem Biophys. 1996 Aug 1;332(1):41–46. doi: 10.1006/abbi.1996.0314. [DOI] [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]
- Zhang J. D., Cousens L. S., Barr P. J., Sprang S. R. Three-dimensional structure of human basic fibroblast growth factor, a structural homolog of interleukin 1 beta. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3446–3450. doi: 10.1073/pnas.88.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Komiya H., Chirino A., Faham S., Fox G. M., Arakawa T., Hsu B. T., Rees D. C. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991 Jan 4;251(4989):90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]


