Abstract
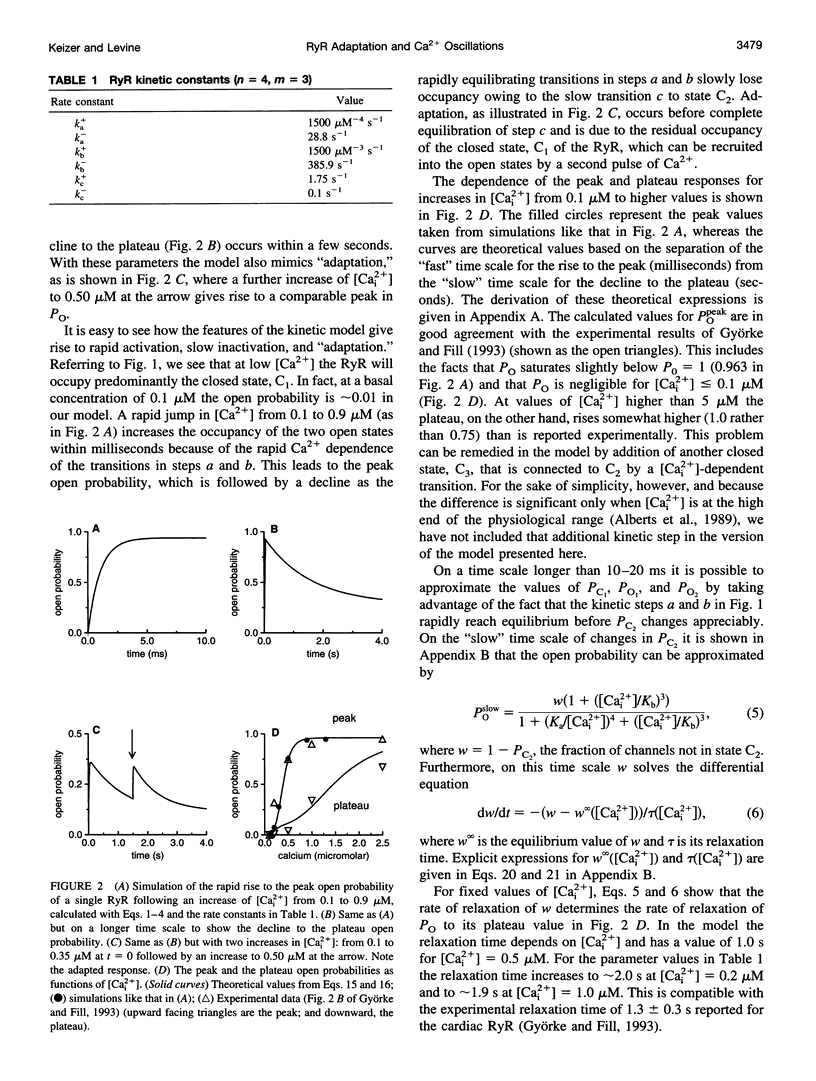
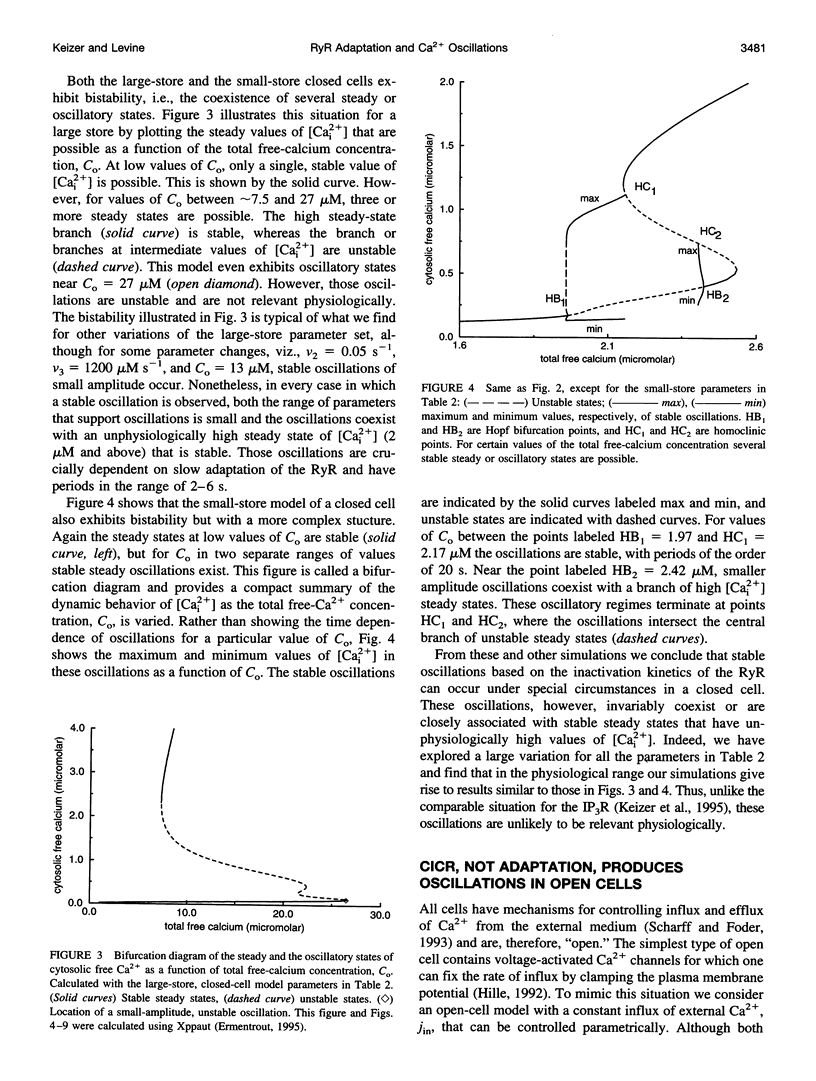
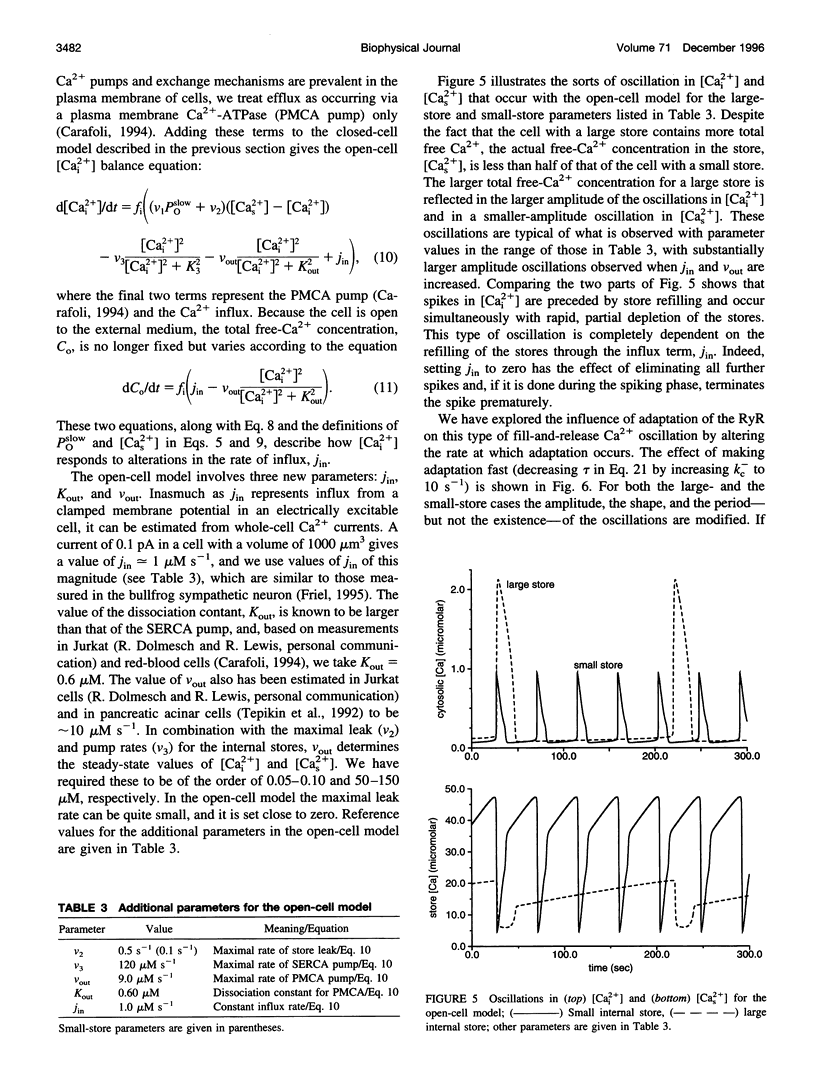
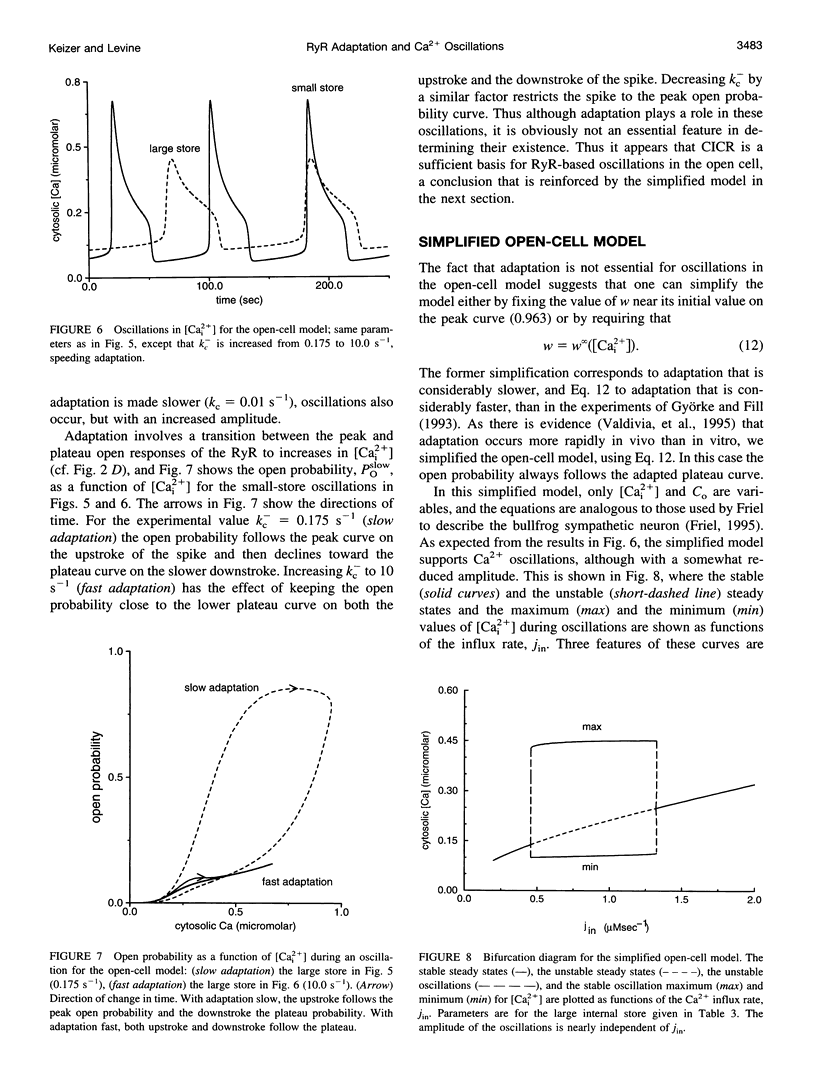
A simplified mechanism that mimics "adaptation" of the ryanodine receptor (RyR) has been developed and its significance for Ca2+(-)induced Ca2+ release and Ca2+ oscillations investigated. For parameters that reproduce experimental data for the RyR from cardiac cells, adaptation of the RyR in combination with sarco/endoplasmic reticulum Ca2+ ATPase Ca2+ pumps in the internal stores can give rise to either low [Cai2+] steady states or Ca2+ oscillations coexisting with unphysiologically high [Cai2+] steady states. In this closed-cell-type model rapid, adaptation-dependent Ca2+ oscillations occur only in limited ranges of parameters. In the presence of Ca2+ influx and efflux from outside the cell (open-cell model) Ca2+ oscillations occur for a wide range of physiological parameter values and have a period that is determined by the rate of Ca2+ refilling of the stores. Although the rate of adaptation of the RyR has a role in determining the shape and the period of the Ca2+ spike, it is not essential for their existence. This is in marked contrast with what is observed for the inositol 1,4,5-trisphosphate receptor for which the biphasic activation and inhibition of its activity by Ca2+ are sufficient to produce oscillations. Results for this model are compared with those based on Ca2+(-)induced Ca2+ release alone in the bullfrog sympathetic neuron. This kinetic model should be suitable for analyzing phenomena associated with "Ca2+ sparks," including their merger into Ca2+ waves in cardiac myocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994 Oct;8(13):993–1002. [PubMed] [Google Scholar]
- Cheng H., Fill M., Valdivia H., Lederer W. J. Models of Ca2+ release channel adaptation. Science. 1995 Mar 31;267(5206):2009–2010. doi: 10.1126/science.7701326. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Chu A., Fill M., Stefani E., Entman M. L. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca(2+)-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol. 1993 Jul;135(1):49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Phase-dependent contributions from Ca2+ entry and Ca2+ release to caffeine-induced [Ca2+]i oscillations in bullfrog sympathetic neurons. Neuron. 1992 Jun;8(6):1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- Friel D. D. [Ca2+]i oscillations in sympathetic neurons: an experimental test of a theoretical model. Biophys J. 1995 May;68(5):1752–1766. doi: 10.1016/S0006-3495(95)80352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Response. Science. 1994 Feb 18;263(5149):987–988. doi: 10.1126/science.263.5149.987. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Vélez P., Suárez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J. 1994 Jun;66(6):1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Direct measurement of free Ca in organelles of gastric epithelial cells. Am J Physiol. 1994 Sep;267(3 Pt 1):G442–G451. doi: 10.1152/ajpgi.1994.267.3.G442. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Tsukioka M. Feedback control of inositol trisphosphate signalling bycalcium. Mol Cell Endocrinol. 1994 Jan;98(2):141–146. doi: 10.1016/0303-7207(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Jafri M. S., Keizer J. Diffusion of inositol 1,4,5-trisphosphate but not Ca2+ is necessary for a class of inositol 1,4,5-trisphosphate-induced Ca2+ waves. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9485–9489. doi: 10.1073/pnas.91.20.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M. S., Keizer J. On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys J. 1995 Nov;69(5):2139–2153. doi: 10.1016/S0006-3495(95)80088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer J., Li Y. X., Stojilković S., Rinzel J. InsP3-induced Ca2+ excitability of the endoplasmic reticulum. Mol Biol Cell. 1995 Aug;6(8):945–951. doi: 10.1091/mbc.6.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R., Dulhunty A. F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995 Sep;147(1):7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Keizer J., Stojilković S. S., Rinzel J. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations. Am J Physiol. 1995 Nov;269(5 Pt 1):C1079–C1092. doi: 10.1152/ajpcell.1995.269.5.C1079. [DOI] [PubMed] [Google Scholar]
- Louis C. F. Caged calcium and the ryanodine receptor. Biophys J. 1994 Jun;66(6):1739–1740. doi: 10.1016/S0006-3495(94)80968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Crit Rev Biochem Mol Biol. 1994;29(4):229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Sachs F., Qin F., Palade P. Models of Ca2+ release channel adaptation. Science. 1995 Mar 31;267(5206):2010–2011. doi: 10.1126/science.7701327. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Regulation of cytosolic calcium in blood cells. Physiol Rev. 1993 Jul;73(3):547–582. doi: 10.1152/physrev.1993.73.3.547. [DOI] [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys J. 1994 Dec;67(6):2223–2235. doi: 10.1016/S0006-3495(94)80707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]


