Abstract
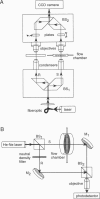
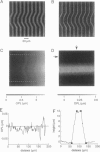
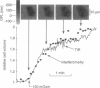
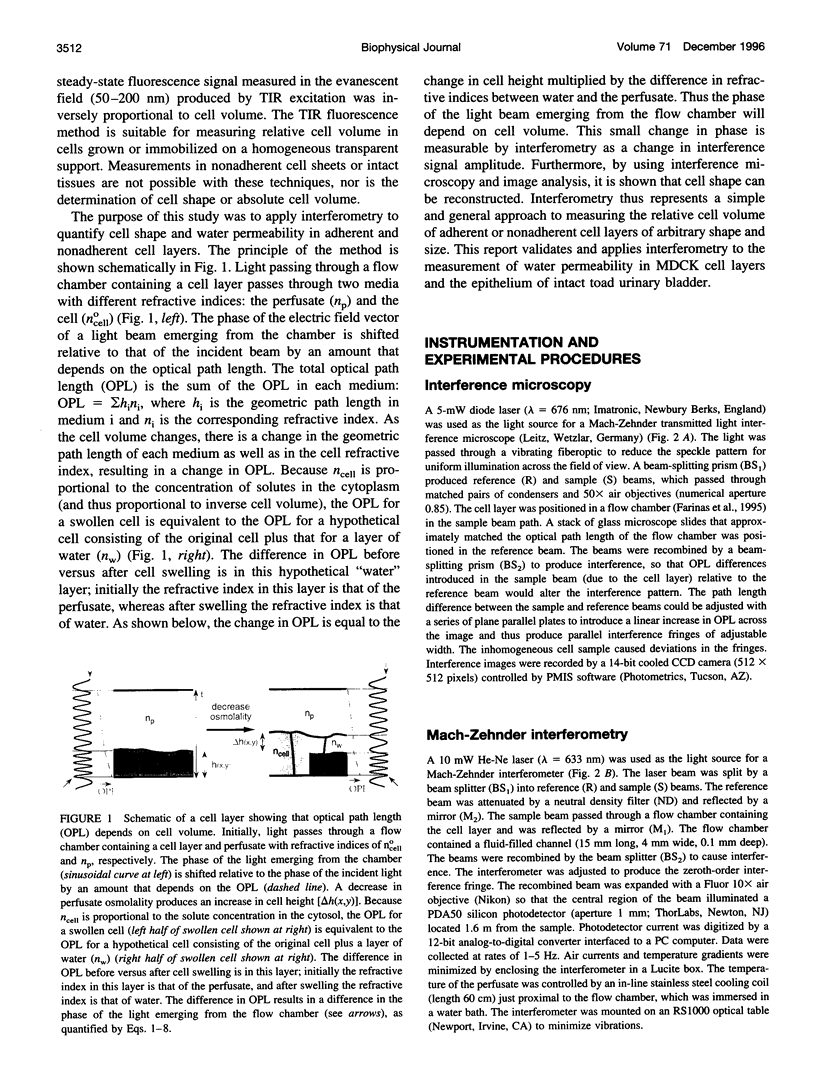
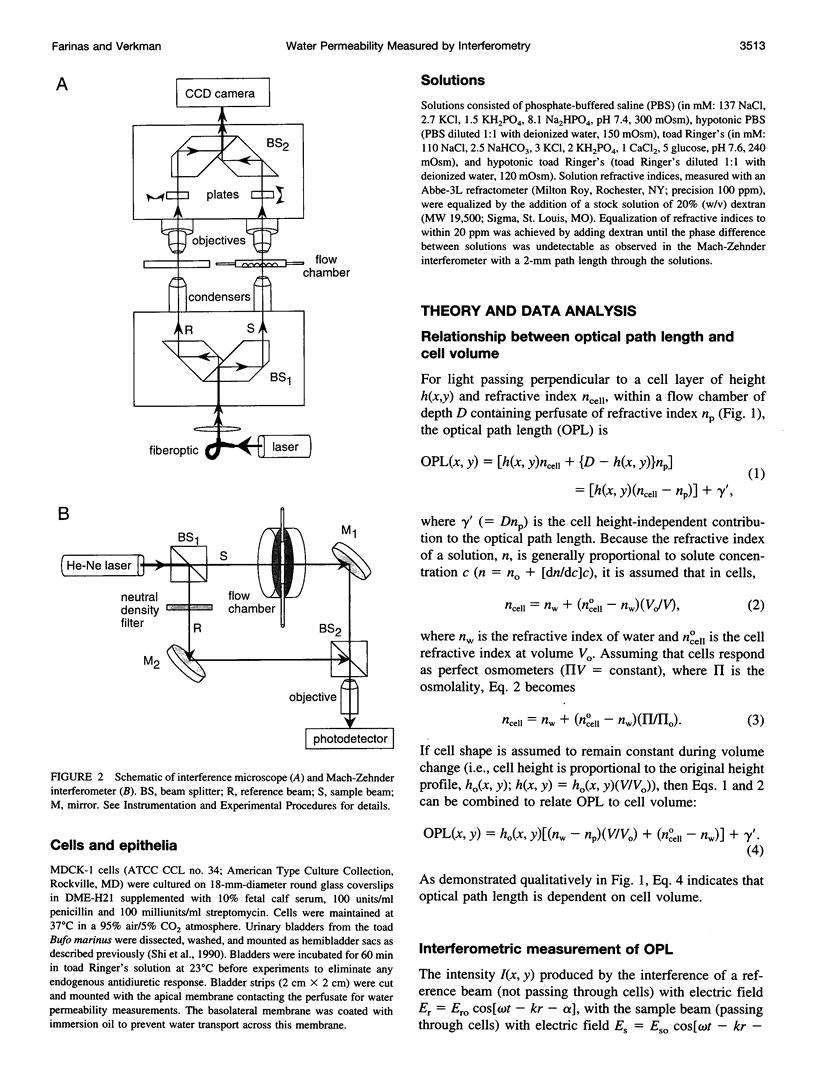
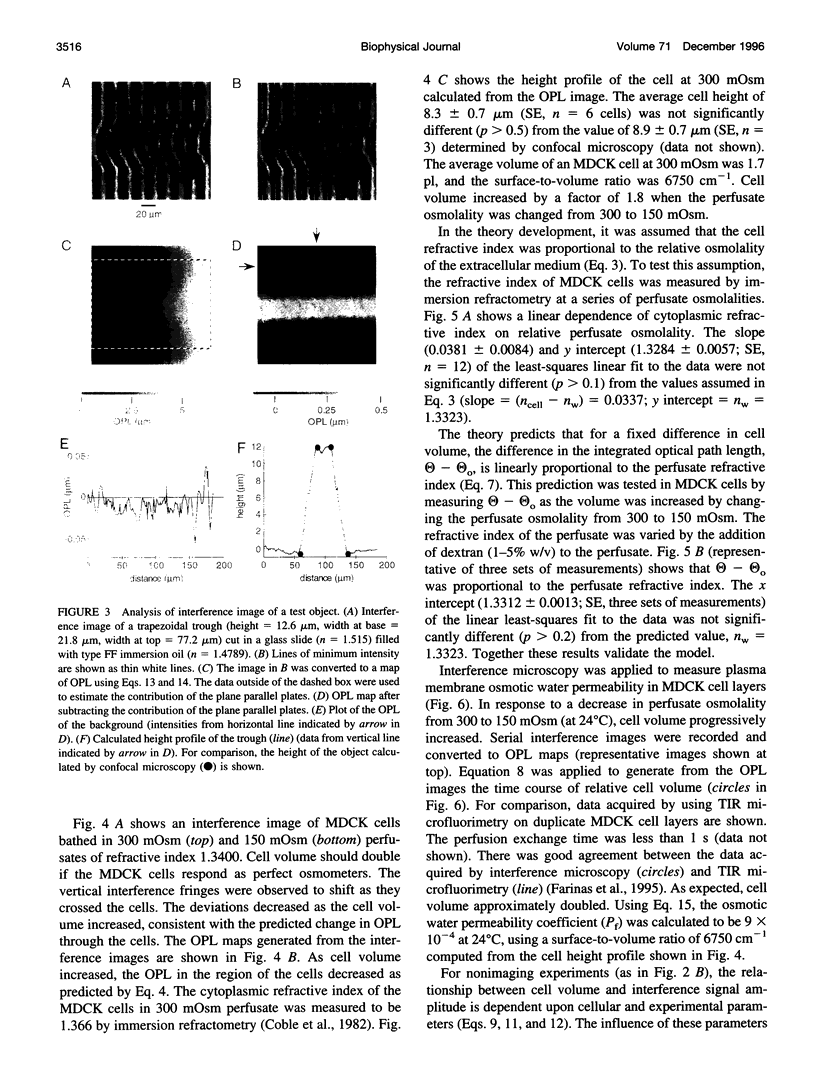
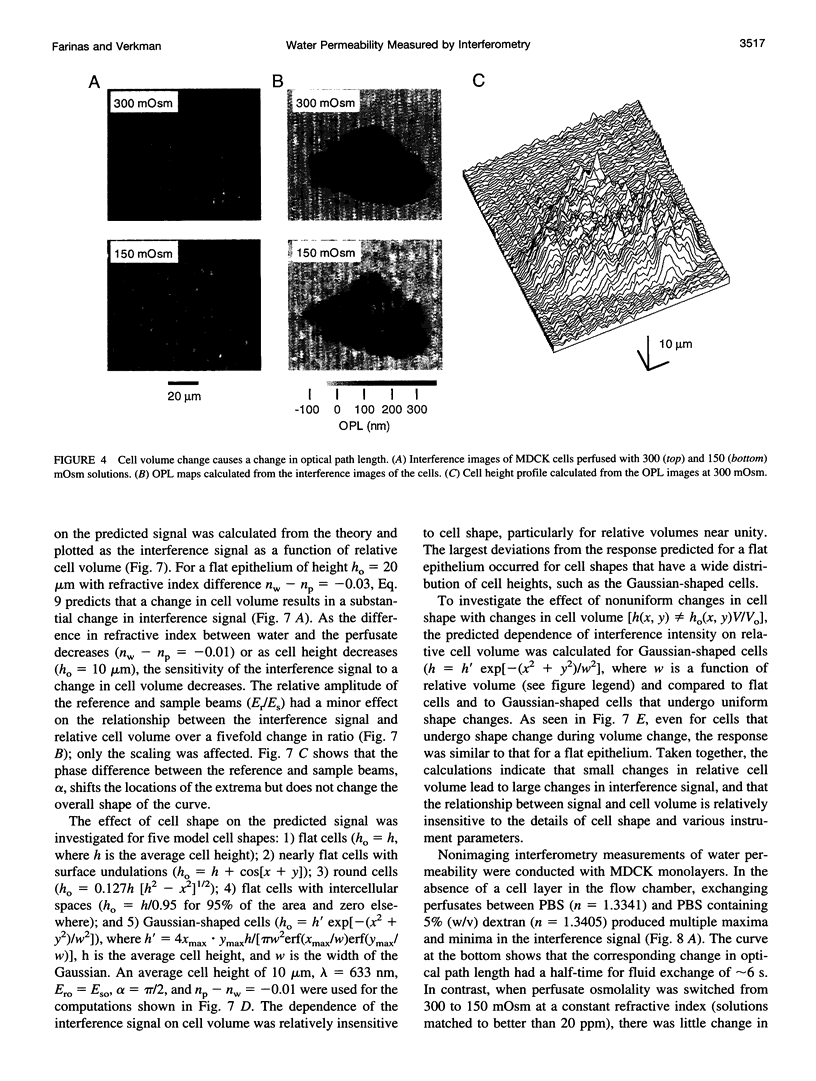
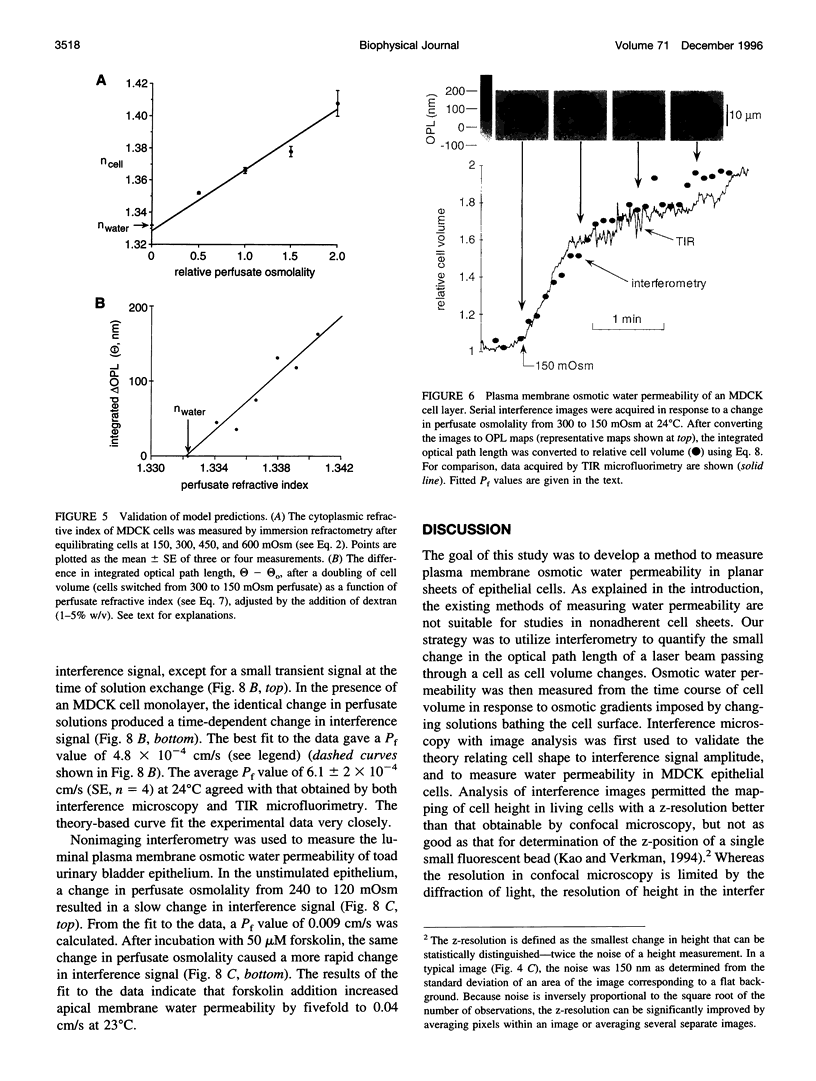
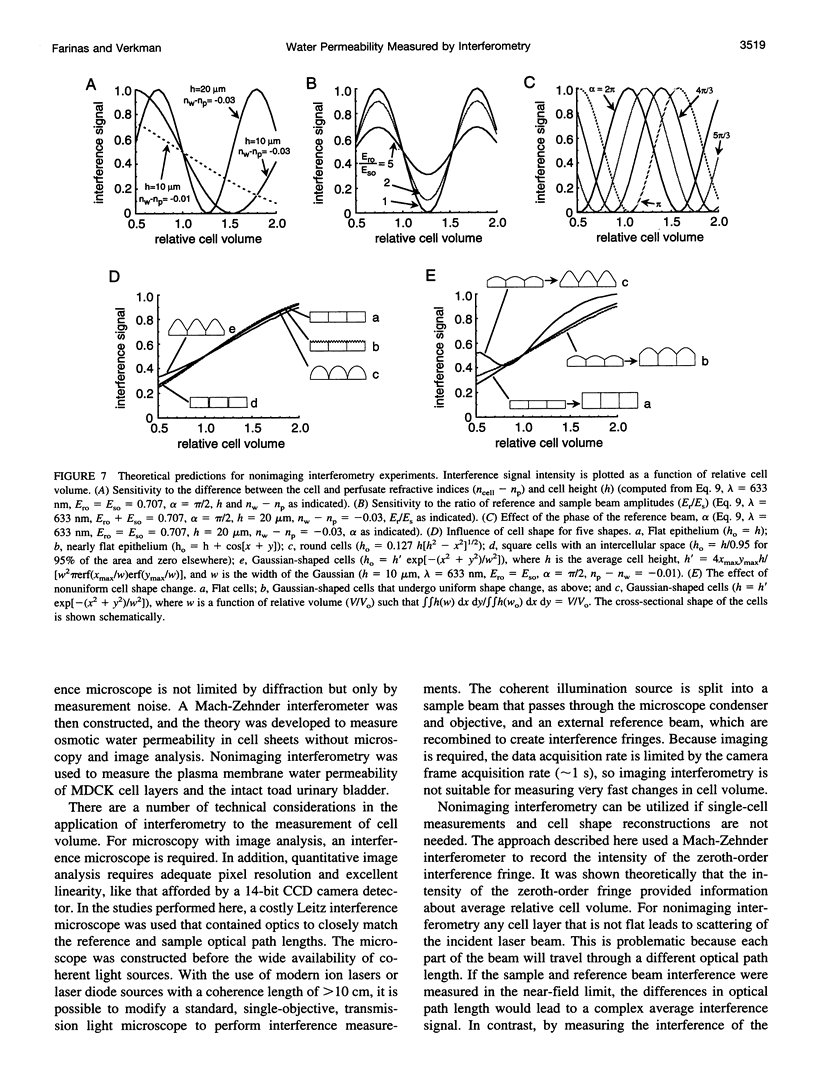
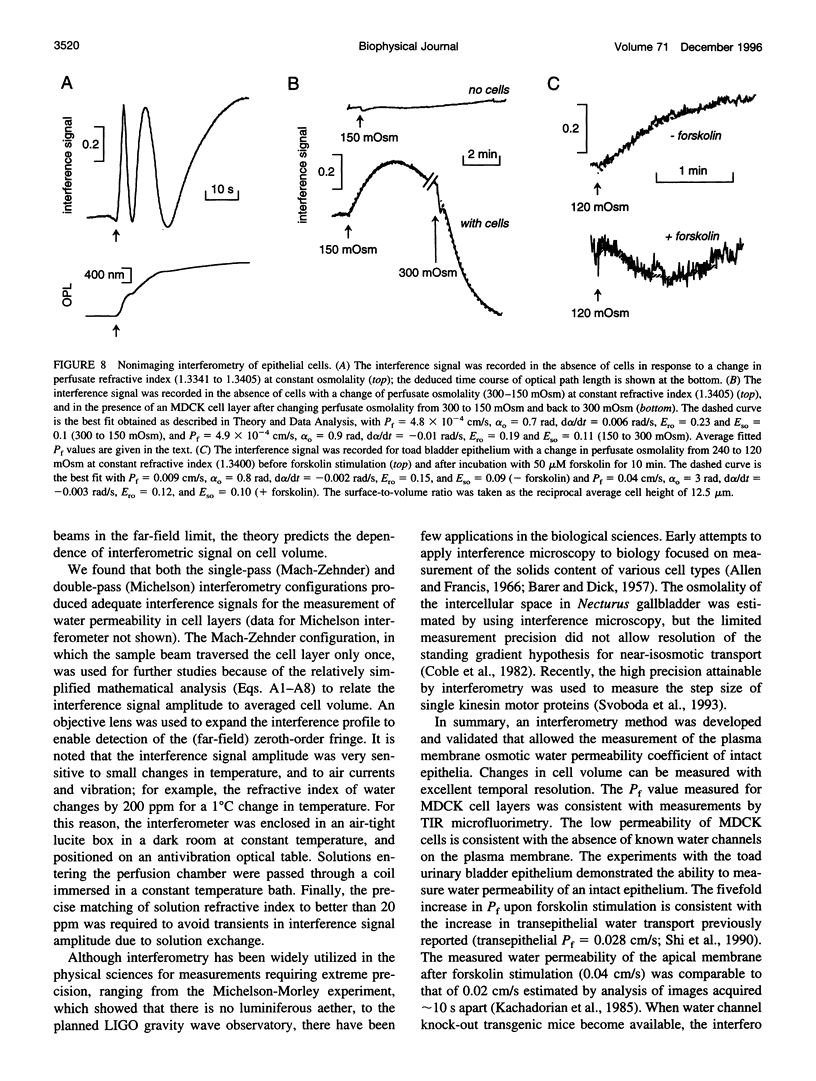
The development of strategies to measure plasma membrane osmotic water permeability (Pf) in epithelial cells has been motivated by the identification of a family of molecular water channels. A general approach utilizing interferometry to measure cell shape and volume was developed and applied to measure Pf in cell layers. The method is based on the cell volume dependence of optical path length (OPL) for a light beam passing through the cell. The small changes in OPL were measured by interferometry. A mathematical model was developed to relate the interference signal to cell volume changes for cells of arbitrary shape and size. To validate the model, a Mach-Zehnder interference microscope was used to image OPL in an Madin Darby Canine Kidney (MDCK) cell layer and to reconstruct the three-dimensional cell shape (OPL resolution < lambda/25). As predicted by the model, a doubling of cell volume resulted in a change in OPL that was proportional to the difference in refractive indices between water and the extracellular medium. The time course of relative cell volume in response to an osmotic gradient was computed from serial interference images. To measure cell volume without microscopy and image analysis, a Mach-Zehnder interferometer was constructed in which one of two interfering laser beams passed through a flow chamber containing the cell layer. The interference signal in response to an osmotic gradient was analyzed to quantify the time course of relative cell volume. The calculated MDCK cell plasma membrane Pf of 6.1 x 10(-4) cm/s at 24 degrees C agreed with that obtained by interference microscopy and by a total internal reflection fluorescence method. Interferometry was also applied to measure the apical plasma membrane water permeability of intact toad urinary bladder; Pf increased fivefold after forskolin stimulation to 0.04 cm/s at 23 degrees C. These results establish and validate the application of interferometry to quantify cell volume and osmotic water permeability in cell layers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARER R., DICK D. A. Interferometry and refractometry of cells in tissue culture. Exp Cell Res. 1957;13(Suppl 4):103–135. [PubMed] [Google Scholar]
- Crowe W. E., Altamirano J., Huerto L., Alvarez-Leefmans F. J. Volume changes in single N1E-115 neuroblastoma cells measured with a fluorescent probe. Neuroscience. 1995 Nov;69(1):283–296. doi: 10.1016/0306-4522(95)00219-9. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Echevarria M., Verkman A. S. Optical measurement of osmotic water transport in cultured cells. Role of glucose transporters. J Gen Physiol. 1992 Apr;99(4):573–589. doi: 10.1085/jgp.99.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas J., Simanek V., Verkman A. S. Cell volume measured by total internal reflection microfluorimetry: application to water and solute transport in cells transfected with water channel homologs. Biophys J. 1995 Apr;68(4):1613–1620. doi: 10.1016/S0006-3495(95)80335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Li J., Kuang K., Echevarría M., Iserovich P. Determination of volume and water permeability of plated cells from measurements of light scattering. Am J Physiol. 1993 Nov;265(5 Pt 1):C1412–C1423. doi: 10.1152/ajpcell.1993.265.5.C1412. [DOI] [PubMed] [Google Scholar]
- Folkesson H. G., Matthay M. A., Frigeri A., Verkman A. S. Transepithelial water permeability in microperfused distal airways. Evidence for channel-mediated water transport. J Clin Invest. 1996 Feb 1;97(3):664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachadorian W. A., Coleman R. A., Wade J. B. Water permeability and particle aggregates in ADH-, cAMP-, and forskolin-treated toad bladder. Am J Physiol. 1987 Jul;253(1 Pt 2):F120–F125. doi: 10.1152/ajprenal.1987.253.1.F120. [DOI] [PubMed] [Google Scholar]
- Kao H. P., Verkman A. S. Tracking of single fluorescent particles in three dimensions: use of cylindrical optics to encode particle position. Biophys J. 1994 Sep;67(3):1291–1300. doi: 10.1016/S0006-3495(94)80601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura T., Verbavatz J. M., Farinas J., Ma T., Ausiello D. A., Verkman A. S., Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7212–7216. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Zhang B. X., Loessberg P. A., Star R. A. Simultaneous recording of cell volume changes and intracellular pH or Ca2+ concentration in single osteosarcoma cells UMR-106-01. J Biol Chem. 1992 Sep 5;267(25):17658–17664. [PubMed] [Google Scholar]
- Nielsen S., Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995 Oct;48(4):1057–1068. doi: 10.1038/ki.1995.389. [DOI] [PubMed] [Google Scholar]
- Shi L. B., Wang Y. X., Verkman A. S. Regulation of the formation and water permeability of endosomes from toad bladder granular cells. J Gen Physiol. 1990 Oct;96(4):789–808. doi: 10.1085/jgp.96.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Optical methods to measure membrane transport processes. J Membr Biol. 1995 Nov;148(2):99–110. doi: 10.1007/BF00207267. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., van Hoek A. N., Ma T., Frigeri A., Skach W. R., Mitra A., Tamarappoo B. K., Farinas J. Water transport across mammalian cell membranes. Am J Physiol. 1996 Jan;270(1 Pt 1):C12–C30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]