ABSTRACT
During development, regulation of gene expression is key to cellular homeostasis. Gene expression regulation by non-coding RNAs involves the prevention of mRNA accumulation or the inhibition of translation of their target gene. In a forward-genetic screen to identify the microRNA involved in the growth and patterning of the Drosophila eye, we identified the highly conserved miR-137. Gain of function of miR-137 results in a reduced-eye phenotype by downregulating retinal determination and differentiation markers, and by upregulating negative regulators of eye development, such as Wingless (Wg) and Homothorax (Hth). Loss of function of miR-137 results in an enlarged-eye phenotype. Using bioinformatics and genetic approaches, we identified the oncogene Myc as the target of miR-137. Gain of function of Myc can rescue the reduced-eye phenotype of miR-137 gain of function, and vice versa. We tested the role of miR-137 in regulating Myc levels in the RasV12;scribRNAi, a tumor model of oncogenic cooperation that results in neoplastic tumors. Gain of function of miR-137 in the RasV12;scribRNAi background significantly reduced tumor phenotype as well as Myc levels in the eye. Our studies highlight miR-137 as a post-transcriptional regulator of Myc and a promising therapeutic target for diseases associated with Myc accumulation.
Keywords: Drosophila, Eye development, Retinal determination, Retina, miRNA, miR-137, Myc, Cell death, Cell proliferation
Summary: Identification and characterization of miR-137 as a post-transcriptional regulator of Myc that controls growth, offering insights into diseases such as neurodegenerative disorders and cancer, which are linked to Myc accumulation.
INTRODUCTION
During development, complex cell biological processes such as cell fate specification, cell proliferation, patterning, growth and cell death are strictly regulated by precise spatial and temporal regulation of the expression of genes. Regulation of gene expression occurs through multiple mechanisms, like regulation of transcription mediated by transcription factors, transcriptional pausing mechanisms or post-transcriptional mechanisms involving microRNAs (miRNAs) (Ben-Hamo and Efroni, 2015; Chimata et al., 2023; Li and Gilmour, 2011; Shang et al., 2023). Among these mechanisms, the developmental roles of miRNAs are beginning to emerge. miRNAs were initially discovered in Caenorhabditis elegans as a new mechanism of temporal regulation of genes and cell lineages (Lee et al., 1993). miRNAs are a class of small non-coding RNAs averaging 22 nucleotides in length that are encoded in the genome, transcribed into primary miRNAs, processed into precursors in the nucleus, and finally mature into miRNA in the cytoplasm (Bartel, 2004). miRNAs interact with 3′UTR of the target gene mRNAs to generate double-stranded RNA to induce target mRNA degradation, translational repression, mRNA deadenylation and decapping (Shang et al., 2023; Vishnoi and Rani, 2023). miRNAs confer specificity to the RNA-induced silencing complex (RISC) through partial sequence complementarity with specific mRNA targets (Ben-Hamo and Efroni, 2015; Shang et al., 2023). Recruitment of miRNA and the RISC complex mostly results in repression of the target mRNA by an increase in turnover and/or translational inhibition. Thus, miRNAs function in fine-tuning the activity of the target gene(s), thereby enhancing the robustness of gene regulatory networks (Ebert and Sharp, 2012). Recent findings indicate that miRNAs play a crucial role in development, differentiation, the modulation of multiple signaling pathways, cell cycle regulation, the stress response, behavior, hormone action and longevity (Ambros and Ruvkun, 2018; Ben-Hamo and Efroni, 2015; Frédérick and Simard, 2022). In humans, aberrant expression of miRNA(s) is implicated in cancer, and in cardiovascular and metabolic disorders; it is also used as a molecular marker for these conditions (Deshpande et al., 2024; Kariuki et al., 2023; Vaghf et al., 2022).
To study miRNA functions, Drosophila has proved to be an effective model system with its array of genetic tools and high degree of gene conservation (Bier, 2005; Perry et al., 2017; Singh and Irvine, 2012). The Drosophila eye model can be a suitable model for identifying and characterizing miRNA functions, as it has been extensively used to investigate cell fate specification, growth, cell-cell communication, cell survival and cell death mechanisms during organogenesis (Singh and Choi, 2003; Singh and Irvine, 2012; Singh et al., 2005b; Verghese et al., 2012; Warren and Kumar, 2023; Wolff and Ready, 1993). The power of the Drosophila eye as a model lies in the conservation of genetic machinery at the structural as well as the functional level. Furthermore, the roles of miRNAs in different cell types of the developing retina are conserved across species (Reh and Hindges, 2018).
Drosophila eye develops from a monolayer epithelium housed inside the Drosophila larva, called an eye imaginal disc. The larval eye imaginal disc gives rise to the adult compound eye, antenna and head cuticle (Cohen, 1993; Gogia et al., 2020a; Mishra and Sprecher, 2020; Tare et al., 2013). During the late second instar stage, the process of retinal differentiation begins as a synchronous wave, called the morphogenetic furrow (MF), at the posterior margin of the eye imaginal disc and moves toward the anterior end (Kumar, 2020; Ready et al., 1976). The cells posterior to the MF differentiate into photoreceptor neurons (Kumar, 2020; Warren and Kumar, 2023), controlled by the highly conserved retinal determination and differentiation (RD) gene network. The RD gene network comprises PAX-6 homolog eyeless (ey), twin of eyeless (toy), eye gone (eyg), twin of eyegone (toe), eyes absent (eya), sine oculis (so) and dachshund (dac), which are necessary and sufficient for normal eye development (Bui et al., 2000; Gogia et al., 2020a; Halder et al., 1998; Jang et al., 2003; Kango-Singh et al., 2003). In the first instar larva, ey, the master regulator of eye fate, is expressed in the entire eye disc. However, when differentiation begins, ey expression is downregulated in the cells posterior to the MF, and expression of downstream genes eya, so and dac is activated to promote retinal differentiation (Bessa et al., 2002; Kumar, 2010; Singh et al., 2002). At the MF, Eya, Dac and So activate atonal (ato), which is required for the initiation of R8 differentiation (Jarman et al., 1994; Tanaka-Matakatsu and Du, 2008). The uniform spacing between photoreceptor clusters is maintained by the fibrinogen-related secreted protein Scabrous (Sca) (Baker et al., 1990). Prospero (Pros) is a marker for R7 photoreceptor fate and cone cells in the eye (Hayashi et al., 2008).
The differentiation along the MF is also guided by signaling molecules, such as Hedgehog (Hh) and Decapentaplegic (Dpp) (Domínguez and Hafen, 1997; Kumar, 2020; Sarkar et al., 2018). The dpp expression moves dynamically with the MF, thus serving as an excellent marker for MF progression (Kumar, 2020; Sarkar et al., 2018; Warren and Kumar, 2023). Dpp promotes MF progression by downregulating wingless (wg), a ligand for the evolutionarily conserved Wg/WNT signaling pathway. In the developing eye, wg is expressed along the antero-lateral margins of the third-instar eye imaginal disc. Wg blocks MF progression by negatively regulating photoreceptor differentiation and thereby determines the eye versus head fate (Ma and Moses, 1995; Puli et al., 2024; Tare et al., 2013; Treisman and Rubin, 1995; Won et al., 2015). Furthermore, Wg regulates the expression of homothorax (hth), which encodes a TALE (three amino-acid loop extension) type homeodomain protein, to suppress eye fate. During larval eye development, Hth expression evolves and retracts anteriorly, ahead of the MF, in the late second instar. In the third instar larval eye-antennal imaginal disc, Hth expression is in the anterior region of the eye disc (Bessa et al., 2002; Pai et al., 1998; Singh et al., 2004, 2011, 2012). Along with the control of differentiation, the overall growth of the eye is also tightly regulated by growth regulatory pathways such as Hippo and PI3K/TOR. These pathways control overall organismal growth by controlling cell proliferation, cell size and cell death (Brennecke et al., 2003; Verghese et al., 2012; Wittkorn et al., 2015). Thus, eye differentiation is orchestrated by the interactions of the RD genes and signaling pathways.
Ectopic Wg can suppress eye fate through activation of caspase-dependent cell death (Singh et al., 2006). The initiator caspase Dronc is activated by upstream genes reaper (rpr), head involution defective (hid) and grim (also known as RHG) (Chen et al., 1996; Grether et al., 1995; White et al., 1994). Caspases are essential for maintaining cellular homeostasis by facilitating a programmed cell death process called apoptosis. It involves the formation of the apoptosome, which is a complex of adaptor protein(s) that triggers the activation of the initiator caspase leading to apoptosis. Another mode of cellular homeostasis, autophagy, represents a unique mechanism of cell death that involves the removal of damaged or surplus cytoplasmic components through a catabolic process mediated by lysosomal degradation. Autophagy is genetically regulated by evolutionarily conserved genes involved in autophagolysosomal degradation. In Drosophila, there are 20 highly conserved autophagy-related genes (Atg) identified. Among these, the Atg8 gene family (Atg8a and Atg8b) has been extensively studied, with its members serving as reliable biomarkers for autophagy (Chimata et al., 2023; Larkin et al., 2021; Shpilka et al., 2011).
We performed a forward-genetic screen to uncover miRNAs involved in eye development. Specifically, we screened for changes in eye size upon overexpression of the miRNAs in the developing eye using the well-established GAL4-UAS system (Brand and Perrimon, 1993; Deshpande et al., 2024). Here, we report the identification of miR-137 in a forward-genetic screen for modifiers of eye size. Gain of function of miR-137 results in reduced-eye phenotype by affecting the RD gene network and activating the expression of negative regulators such as Wg and Hth. The gain-of-function phenotype of miR-137 has no domain constraint. Loss of function of miR-137 results in an eye-enlargement phenotype. We identified Myc (also known as dMyc and cMyc), a Drosophila homolog of the vertebrate Myc oncogene (Gallant et al., 1996; Johnston et al., 1999), as the target of miR-137 in the eye, where it regulates cell homeostasis to determine the size of the eye field. Myc is a transcription factor and a proto-oncogene that plays a dual role during development. Myc expression is closely linked to cell-cycle progression in normal tissues, whereas uncontrolled Myc expression is a hallmark of hyperproliferation observed in many cancers. Interestingly, despite its role in promoting cell proliferation, Myc also serves as a potent inducer of apoptosis. Thus, Myc regulates growth via proliferation, cell death and metabolism during development. We employed the RasV12; scribRNAi tumor model for oncogenic cooperation to demonstrate that the gain of function of miR-137 can significantly rescue the neoplastic overgrowth phenotype(s) by downregulating Myc levels. Here, we present a previously unreported function for highly conserved miR-137 in regulating tissue homeostasis and growth by targeting Myc during eye development.
RESULTS
Gain of function of miR-137 results in reduced-eye phenotype
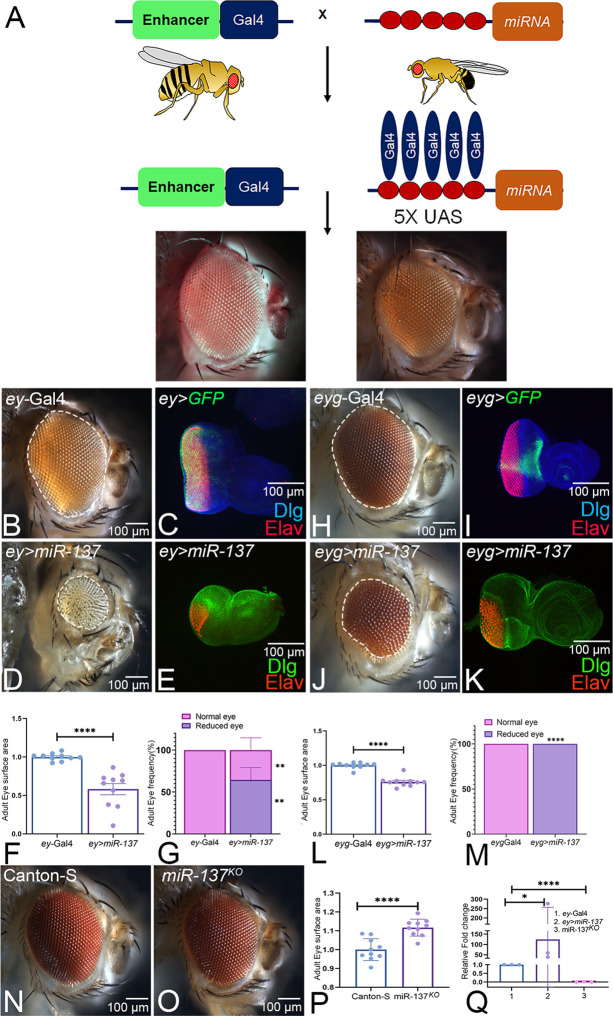
We performed a forward genetic screen in Drosophila to look for miRNAs involved in eye development, where we individually express miRNA transgene(s) in the developing eye (Fig. 1A). We used the ey-Gal4, which drives the expression of GFP reporter (ey-GAL4>UAS-GFP, ey>GFP) in the entire eye field (Fig. 1C), and assayed its effect on the eye phenotype in the F1 progeny (Fig. 1A) (Singh et al., 2005a). Among various miRNA(s) misexpressed in the developing eye field (Fig. 1C), we found that, compared to the control ey>GFP (Fig. 1B,C), the gain of function of miR-137 reduces the eye size significantly both in the adult and the larval eye imaginal disc (Fig. 1D,E). Furthermore, statistical analysis of the eye-surface area of ey>miR-137 flies showed a significant reduction (42%) compared to the adult eyes of ey-Gal4 control (Fig. 1F). In addition, the frequency of reduced-eye phenotype of adult eye in ey>miR-137 was significantly higher at 64.5% (n=387/600) when compared to the ey-Gal4 control, where 100% of flies showed normal wild-type adult eye (Fig. 1G). We used eyegone (eyg)-GAL4, another Gal4, to confirm the phenotypic defects in the eye caused by the gain of function of miR-137. eyg-Gal4 drives the GFP reporter expression (eyg-Gal4>UAS-GFP, eyg>GFP) in an antero-dorsal stripe in the mid-late third-instar larval eye disc (Fig. 1I), a pattern that closely mimics eyg gene expression (Jang et al., 2003). The control eyg-Gal4 exhibits a wild-type eye phenotype in the imaginal discs and adults (Fig. 1H,I). Targeted expression of miR-137 by eyg-Gal4 (eyg>miR137) resulted in a reduced-eye phenotype, as seen in the adult as well as in the eye imaginal disc (Fig. 1J,K). Furthermore, statistical analysis of the eye-surface area and the frequency of reduced-eye phenotype of eyg>miR-137 adult eye showed a significant reduction (24%) of eye-surface area and a significant increase in reduced-eye frequency, when compared to the eyg-Gal4 control (Fig. 1L,M). These results suggest that gain of function of miR-137 results in reduced-eye phenotype.
Fig. 1.
Screening for miRNA involved in eye development results in the identification of miR-137. (A) The screening strategy to identify miRNA(s) involved in eye development. In the developing eye, individual miRNA(s) are misexpressed using the Gal4/UAS Target system, and their effect on eye phenotype is assayed. (B) The adult eye of an ey-Gal4 control, to which misexpressed miRNA(s) phenotypes were compared. (C) The domain of expression (UAS GFP, green) of ey-GAL4 in the third-instar larval eye-antennal imaginal disc stained for Discs large (Dlg, blue), to mark cellular outlines, and Elav (red), to mark photoreceptor neurons. (D,E) Gain of function of miR-137 in the developing eye using ey-Gal4 driver (ey>miR-137) results in the reduced-eye phenotype, as seen in (D) adults and (E) larval eye imaginal disc. (F,L,P) Quantification of adult eye surface area (μm2) using Fiji/ImageJ software with statistical analysis of normalized adult eye surface area (150 pixels/inch) carried out using an unpaired Student's t-test. The analyzed area is indicated with white dotted lines for denoted genotypes (B,D,H,J) (n=10). (G,M) Adult eye phenotype frequency of indicated genotypes (n=200, three replicates). Analysis was carried out using a two-way ANOVA with Sidak's multiple comparison test. (H) Adult eye of the eyg-Gal4 driver serves as a control. (I) The third-instar larval eye-antennal imaginal disc showing eyg-Gal4 expression (UAS-GFP, green). (J,K) eyg>miR-137 exhibits the reduced-eye phenotype in (J) adult and (K) larval eye imaginal disc. (N,O) Adult eyes of Canton-S control (N) and miR-137KO homozygous (O) flies, which exhibit an enlarged eye phenotype. (Q) The log2 fold-change values of miR-137 normalized to 2S-rRNA in ey-Gal4, ey>miR-137 and miR-137KO. Pairwise comparisons were made with an unpaired t-test. miR-137 is expressed in the developing eye. All graphs were plotted using GraphPad Prism 8.3.1. Data are mean±s.e.m. Statistical significance is indicated in each graph: ****P<0.0001; **P<0.01; *P<0.05; ns, non-significant. Orientation of all the imaginal discs is posterior to the left and dorsal upwards. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification, unless specified otherwise. Scale bars: 100 µm.
To understand the role of miR-137 in eye development, we assayed the miR-137 loss-of-function phenotype using three different alleles, i.e. miR-137KO (Fig. 1O), miR-137CR and ey>miR-137 sponge (Fig. S1). We found a significant increase in the eye size of miR-137KO (Fig. 1O,P) when compared to Canton-S (Fig. 1N,P). In addition, homozygous miR-137CR and ey>miR-137 sponge exhibits increased eye size in comparison to Canton-S and ey-Gal4 (Fig. S1). Additionally, to validate the expression levels of miR-137 during eye development, we performed qRT-PCR and found that miR-137 is expressed in the control larval eye disc (Fig. 1Q). These miR-137 levels increased significantly in the gain-of-function background of ey>miR-137 and were non-detectable in miR-137KO (Fig. 1Q). Our results show that miR-137 is expressed in the developing eye field, and its loss of function leads to the enlarged eye phenotype. It is important to determine the effect of the reduced-eye phenotype of miR-137 misexpression on the retinal determination and differentiation gene cascade.
miR-137 suppresses retinal determination and differentiation
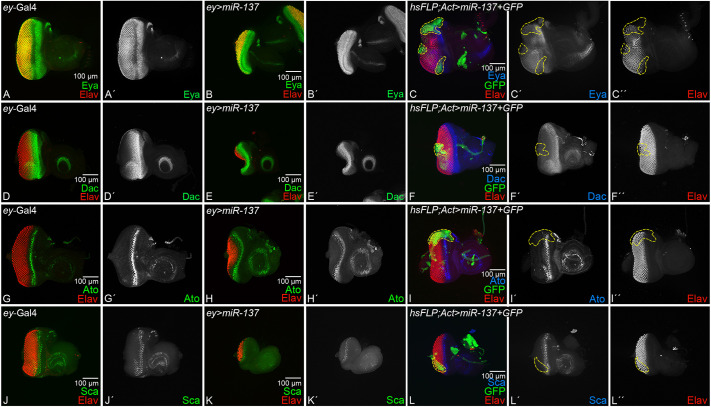
We examined the retinal determination (RD) gene expression in the gain-of-function background of miR-137 using ey-Gal4 driver (ey>miR-137) and random FLP-out clones using heat-shock Flippase (hsFLP; Actin>y+>GAL4/UAS-miR-137) in the developing eye imaginal discs. There is a significant reduction in the size of the eye field in ey>miR-137 background. We tested the expression of the two core RD genes, eyes absent (eya) and dacshund (dac) in developing eye imaginal discs. The eya is expressed in a stripe of retinal precursor cells ahead of the advancing morphogenetic furrow (MF), as well as in the differentiating photoreceptor neurons, cones and pigment cells of the neural retina behind the MF (Bonini et al., 1993), (Fig. 2A,A′). Furthermore, eya, along with sine oculis (so), activates expression of the downstream RD gene dac in two different domains that span anteriorly and posteriorly to the MF (Chen et al., 1997), (Fig. 2D,D′). Gain of function of miR-137 in the developing eye (ey>miR-137), showed that Eya (Fig. 2B,B′) and Dac (Fig. 2E,E′) expression domains are significantly reduced posteriorly to the MF, as compared to the control (Fig. 2A,A′,D,D′). To address the issue of domain constraint, we generated heat-shock Flippase-mediated random FLP-out clones of miR-137 . We found downregulation of expression of the retinal fate marker Elav as well as Eya and Dac expression downregulation close to the clonal boundary (Fig. 2C,C′,F,F′). Additionally, the arrangement of photoreceptors was disrupted and there was an increase in the distance between photoreceptors (Fig. 2C″,F″).
Fig. 2.
Gain of function of miR-137 downregulates retinal determination and retinal differentiation genes. (A-F″) Expression of the retinal determination genes (A-C″) Eya (green) and (D-F″) Dac in eye-antennal imaginal disc of (A,A′,D,D′) ey-Gal4, (B,B′,E,E′) ey>miR-137 and (C-C″,F-F″) hsFLP-out clones of miR-137. (A′,B′,C′,D′,E′,F′) Single channel images for (A′,B′,C′) Eya and (D′,E′,F′) Dac. Elav (red) marks the retinal neuron. (B,B′) Eya and (E,E′) Dac expression is downregulated posterior to the MF in the ey>miR-137 background, which exhibits a reduced-eye phenotype. FLP-out clones showed abnormal spacing between neighboring photoreceptors and exhibited downregulation of (C,C′) Eya and (F,F′) Dac. (C″,F″) Elav indicates where retinal neurons are affected. (G-L″) Expression of a retinal differentiation genes (G-I″) Ato (green) and (J-L″) Sca (green) in eye-antennal imaginal disc of (G,G′,J,J′) ey-Gal4 control, (H,H′,K,K′) ey>miR-137 and (I,I′,I″,L,L′,L″) FLP-out clones of miR-137. (G′,H′,I′,J′,K′,L′) Single channel images for (G′,H′,I′) Ato and (J′,K′,L′) Sca. (H,H′) Ato and (K,K′) Sca expression is downregulated in MF in the ey>miR-137 background and a similar observation was seen in (I,I′,L,L′) hsFLP-out clones. All the imaginal discs are oriented posterior to the left and dorsal upwards. All eye-antennal imaginal discs were captured at 20× magnification, unless specified otherwise. Scale bars: 100 µm.
Furthermore, in the ey>miR-137 background, we examined the expression of retinal differentiation markers such as Atonal (Ato) (Fig. 2G,G′), Scabrous (Sca) (Fig. 2J,J′) and Prospero (Pros) (Fig. S2) (Baker et al., 1990; Jarman et al., 1994; Mlodzik et al., 1990). Compared to the ey-Gal4 control (Fig. 2G,G′,J,J′), gain of function of miR-137 in the developing eye (ey>miR-137) showed reduced expression of Ato (Fig. 2H,H′), Sca (Fig. 2K,K′) and Pros (Fig. S2). In FLP-out clones, the Ato and Sca expression domains were affected. Ato was downregulated in the clone near dorsal eye margin (Fig. 2I,I′) and Sca also exhibited downregulation in the clone (Fig. 2L,L′), and there was disruption of photoreceptor arrangement and spacing (Fig. 2I″,L″). These observations suggest that the gain of function of miR-137 downregulates the retinal determination and differentiation markers, and FLP-out clones show evidence of impaired photoreceptor arrangement. Our results show that the entire eye field is reduced (evident in Fig. 2B,E,H,K) compared to the controls (Fig. 2A,D,G,J). Taken together, the reduced-eye phenotype of miR-137 overexpression showed reduced retinal determination and differentiation markers.
miR-137 blocks MF progression by activation of negative regulators of the eye
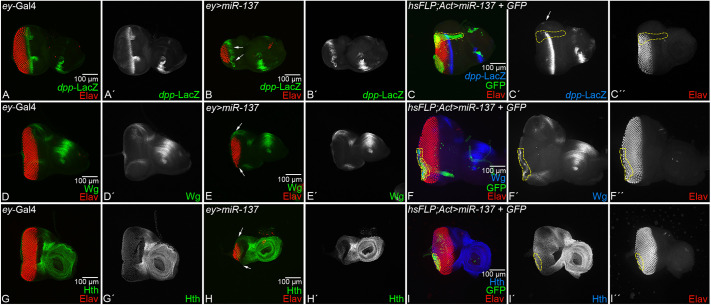
We investigated the effect of gain of function of miR-137 (ey>miR-137) on MF progression by using a transcriptional reporter of dpp as a MF marker (Blackman et al., 1991). In the control eye imaginal discs, dpp-lacZ marks a thin stripe that overlays the apical constrictions caused by the MF cells. Compared to the control, in which dpp-lacZ expression marks the MF (Fig. 3A,A′), dpp-lacZ expression in the ey>miR-137 background is significantly downregulated (Fig. 3B,B′, arrows). A similar downregulation of dpp-lacZ reporter was seen in random FLP-out clones of miR-137 near the dorsal eye margin in the developing eye (Fig. 3C-C″, arrow). All the random clone boundaries are marked by a yellow dotted line.
Fig. 3.
Gain of function of miR-137 blocks morphogenetic furrow progression and ectopically induces the negative regulators of eye fate. (A-C′) Eye-antennal imaginal discs stained for the dpp-LacZ reporter (green), which marks the morphogenetic furrow (MF) in the (A,A′) ey-Gal4 (control) and (B,B′) ey>miR-137 background, and in (C,C′) heat shock FLP-out clones of miR-137 in the developing eye imaginal disc. Elav (red) marks the retinal neuron. (A′,B′,C′) Single channel images for dpp-lacZ expression. dpp-lacZ is significantly downregulated in ey>miR-137 background (marked by arrow in C′) when compared to the control and in FLP-out clones. dpp-LacZ is downregulated in the border of the clone and does not extend to the dorsal margin (marked by arrows in B,D,H). (D-I″) Eye-antennal imaginal disc of third-instar larvae stained for (D-F″) Wg and (G-I″) Hth. (D-F″) Wg (green), a negative regulator of MF progression, is ectopically induced in (E,E′) the ey>miR-137 background and in (F,F′) the FLP-out clone of miR-137 (outlined in yellow) in comparison to the control (D,D′) ey-Gal4. (G-I″) Hth (green), a head fate marker and negative regulator of eye fate, is ectopically induced in (H,H′) the ey>miR-137 background and in (I-I″) the FLP-out clone of miR-137 (outlined in yellow) in comparison to the control (G,G′) ey-Gal4. (D′,E′,F′,G′,H′,I′) Single channel images for (D′,E′,F′) Wg and (G′,H′,I′) Hth. All the imaginal discs are oriented posterior to the left and dorsal upwards. All eye-antennal imaginal discs were captured at 20× magnification, unless specified otherwise. Scale bars: 100 µm.
In the developing eye, Wg serves as a negative regulator of eye fate and is known to block MF progression (Kumar, 2011; Ma and Moses, 1995; Swarup and Verheyen, 2012; Treisman and Rubin, 1995). Therefore, we tested if the gain of function of miR-137 (ey>miR-137) affects Wg expression. In the third-instar larva eye disc, Wg is expressed on the antero-lateral margins of the control eye imaginal disc (Fig. 3D,D′). In the ey>miR-137 background, which exhibits a reduced-eye phenotype, there is a strong induction of Wg on the margins (Fig. 3E,E′, arrows). Random FLP-out clones of miR-137 on the posterior margin of the developing eye disc exhibit loss of photoreceptor cells along with the ectopic induction of Wg expression (Fig. 3F-F″). Wg is known to activate Hth – a negative regulator of eye development (Bessa et al., 2002; Pai et al., 1998; Singh et al., 2002). In the wild-type eye disc, hth is expressed in the peripodial membrane cells, and in a band of retinal precursor cells that are anterior to the MF (Fig. 3G,G′). We found an ectopic induction of Hth in the ey>miR-137 reduced-eye field compared to the control (Fig. 3H,H′, arrows). We also tested Hth expression in random FLP-out clones of miR-137 where the clone boundary is marked by yellow dotted line. We found that Hth is induced on the posterior margin, accompanied by a loss of photoreceptor cells (Fig. 3I-I″). We also tested the expression of F-actin, which also marks MF in the eye (Benlali et al., 2000). We found that in gain of function of miR-137, F-actin levels were downregulated (Fig. S2). Overall, these data suggest that miR-137 plays a role in suppressing retinal fate by promoting the expression of negative regulators of eye, Wg and Hth, and by downregulating the MF marker dpp in the developing eye.
Gain of function of miR-137 triggers cell death
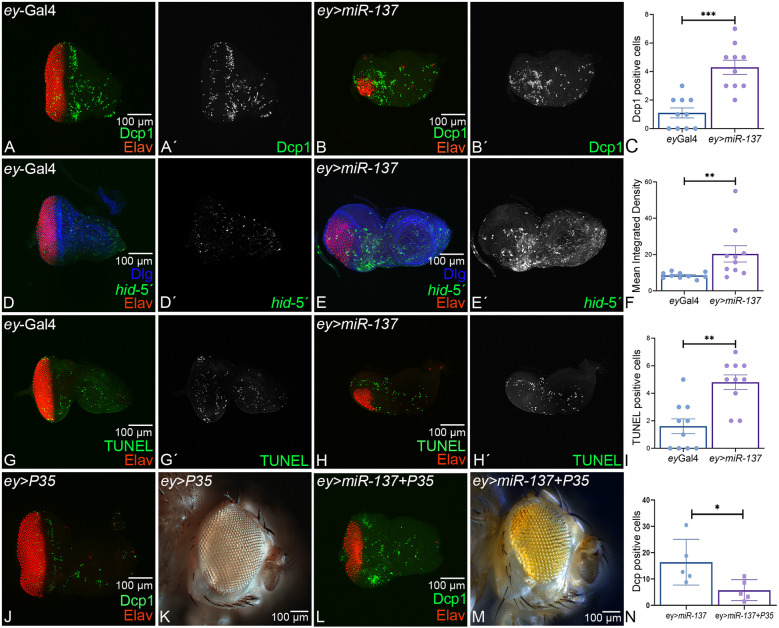
It is possible that the reduced-eye phenotype of ey>miR-137 is due to induction of cell death. Therefore, we tested a series of cell death markers in the eye-antennal disc of third-instar larvae. We used Death caspase-1 (Dcp-1) in Drosophila, a homolog of mammalian caspase 3, as an apoptotic cell marker to mark developmental cell death. Full-length Dcp-1 does not have proteolytic activity; however, when cleaved Dcp-1 is active, the cells undergo apoptosis (Mehta et al., 2021; Song et al., 1997). In comparison to the control, ey-Gal4, eye imaginal disc (Fig. 4A,A′), the ey>miR-137 background showed an increase in the number of retinal neurons undergoing cell death (Fig. 4B,B′). Statistical analysis of the number of Dcp1-positive cells between ey-Gal4 and ey>miR-137 confirmed a significant increase in Dcp-1 positive cells (Fig. 4C). We also employed a hid5′F-WT-GFP reporter for pro-apoptotic factor head involution defective (hid), which is involved in regulating apoptosis and programmed cell death to mark dying cells. The reporter is expressed in cells undergoing apoptosis (Tanaka-Matakatsu et al., 2009). In controls, a basal level of GFP (green) signal that marks hid5′F-WT-GFP reporter expression is generally seen in a few cells of the third-instar eye-antennal imaginal disc (Fig. 4D,D′), whereas the number of dying cells marked by the hid5′F-WT-GFP reporter was significantly higher in gain-of-function miR-137 (ey>miR-137) eye discs, even though the discs were smaller (Fig. 4E,E′). Statistical analysis of hid5′ GFP mean signal intensity exhibited a significant increase in the miR-137 gain-of-function background (ey>miR-137) when compared to the control (Fig. 4F).
Fig. 4.
Gain of function of miR-137 triggers cell death in the developing eye. (A-B′,D-E′,G-H′) Eye-antennal imaginal discs of third-instar larvae stained for Elav (red), (A-B′) Dcp1 (green, a marker for apoptotic cells), (D-E′) hid5′-WT-GFP (green, a proapoptotic gene, hid reporter) and (G-H′) TUNEL (green, a marker for dying cells nuclei) in (A,A′,D,D′,G,G′) ey-Gal4 and (B,B′,E,E′,H,H′) ey>miR-137 background. Single channel images for (A′,B′) Dcp-1, (D′,E′) hid5′-WT-GFP and (G′,H′) TUNEL alone. (C,F,I) Statistical analysis showed that ectopic expression of miR-137 (ey>miR-137) exhibits a significant increase in (C) Dcp-1, (F) hid5′-WT-GFP and (I) TUNEL-positive nuclei as compared to the ey-Gal4 controls as shown in the graphs. The signal intensity was calculated in three regions of interest per disc within a sample of n=5 for each genotype. (J-M) Ectopic expression of baculovirus P35, which blocks cell death, in (J,K) ey-Gal4 (ey>P35, control) and (L,M) ey>miR-137 (ey>miR-137+P35) background. P35 rescues the ey>miR-137 reduced-eye phenotype, as seen in (L) eye imaginal disc and (M) adult eye. (N) Statistical analysis showed a decrease in Dcp1-positive cells. Dcp1-positive cells were calculated in a sample of n=5 for each genotype. (C,F,I,N) The statistical analysis was carried out using an unpaired Student's t-test. Data are mean±s.e.m. Statistical significance is indicated in each graph: ***P<0.001; **P<0.01; *P<0.05. All graphs were plotted using GraphPad Prism 8.3.1. All the imaginal discs are oriented posterior to the left and dorsal upwards. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification, unless specified otherwise. Scale bars: 100 µm.
A TUNEL assay detects fragmented DNA ends and marks the nuclei of the dying cells (White et al., 1994). Using a TUNEL assay, we quantitate the cell death in ey>miR-137 eye disc. In comparison to the ey-Gal4 control eye discs (Fig. 4G,G′), there was a significant increase in the number of dying cells in the ey>miR-137 eye disc (Fig. 4H,H′). Statistical analysis of TUNEL-positive nuclei mean signal intensity confirmed a significant increase in the miR-137 gain-of-function background (ey>miR-137) when compared to the control (Fig. 4I). To test if the observed reduction in eye size was due to caspase-dependent cell death, we misexpressed P35, which is a pan-caspase inhibitor (Hay et al., 1994). In comparison to controls (ey>P35) (Fig. 4J,K), co-expression of miR-137 and P35 (ey>miR-137+ P35) in developing eye significantly rescued the ey>miR-137 phenotype, as seen in the eye imaginal disc and the adult eye (Fig. 4L,M). Statistical analysis of Dcp-1-positive cells in ey>P35 and ey>miR-137+P35 eye imaginal discs revealed a significant reduction in the number of Dcp-1-positive cells in experimental conditions (ey>miR-137+P35) with respect to the control (Fig. 4N). Thus, blocking cell death using P35 can rescue the ey>miR-137 reduced-eye phenotype. However, it is not a complete rescue. Therefore, the reduced-eye phenotype of ey>miR-137 may not be totally dependent on caspase-dependent cell death mechanisms.
We also tested autophagy, which is another mode of homeostasis involving a cell elimination pathway (Das et al., 2012; Shpilka et al., 2011), using the well-established Atg8a-mCherry reporter line (Shukla et al., 2019). We checked the levels of Atg8a as an indicator of the entire process of autophagy in the gain-of-function background of miR-137. However, we found no significant difference in the levels of atg8a-mCherry between ey>GFP control and ey>miR-137 discs (Fig. S3). We also tested if downregulating autophagy using Atg8a1 mutant can rescue the miR-137-mediated reduced-eye phenotype. However, reducing Atg8a function using the heterozygous mutant of Atg8a1 in miR-137 gain-of-function background (ey>miR-137+Atg8a1) did not affect the reduced-eye phenotype (Fig. S3). These data suggest that miR-137 might increase cell death through apoptosis but not through autophagy. It will be important to investigate if miR-137 affects other developing fields.
Gain of function of miR-137 suppresses the size of other developing fields
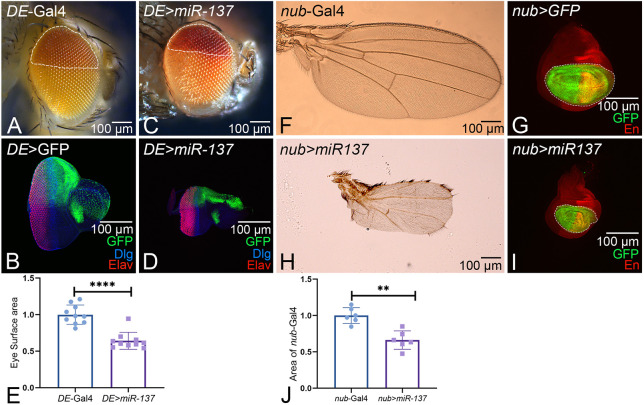
We assayed the effect of the gain of function of miR-137 in other developing fields to address whether there is any domain-specific constraint in the gain-of-function phenotype of miR-137 with reference to the compartments within the eye and other developing fields such as the wing. In comparison to the control, in which DE-Gal4 drives expression of the mini-white reporter in the dorsal compartment of the adult eye (Fig. 5A, boundary of the dorsal compartment of the adult eye is marked by dotted white line) and expression of the GFP (green) reporter in the dorsal half of the eye imaginal disc (Fig. 5B), gain of function of miR-137 using DE-Gal4 (DE>miR-137) driver showed a significant reduction in the dorsal compartment of eye imaginal disc and the adult eye (Fig. 5C,D). Furthermore, statistical analysis of the surface area of the reduced-eye phenotype of DE>miR-137 adult eye showed a significant reduction (∼30%) compared to DE-Gal4 control (Fig. 5E). Additionally, the overall size of the eye imaginal disc was reduced because the generation of the DV boundary is crucial for eye development (Singh et al., 2012). Furthermore, we also tested another developing field similar to wing imaginal disc. We employed nub-Gal4 driver to misexpress the gene of interest in the wing pouch region. As compared to the control nub-Gal4 adult wing and wing disc (Fig. 5F,G), gain of function of miR-137 using nub-Gal4 (nub>miR-137), which drives expression in the wing pouch region, marked by a GFP reporter in the larval wing imaginal disc (Fig. 5G), exhibits a significant reduction of the wing pouch region in the wing disc (Fig. 5I) as well as in the adult wing (Fig. 5H). The statistical analysis of the wing pouch area of nub>miR-137 wing imaginal disc showed a significant reduction (∼30%) compared to the nub-Gal4 control (Fig. 5J). We also tested the gain-of-function phenotype of miR-137 in the entire fly using Tub-Gal4 (Tub>miR-137), which caused embryonic or early larval lethality. We also employed a temperature-sensitive Gal80 (Gal80ts) approach to titrate the levels of miR-137 but failed to achieve survival of the adults (data not shown). Overall, our data suggest that miR-137 affects the size of the developing field.
Fig. 5.
The gain-of-function phenotype of miR-137 does not exhibit domain constraint. (A-D) Gain of function of miR-137 in the dorsal half of the adult eye (A,C, marked by white dotted lines) using the DE-Gal4 driver (domain marked by GFP reporter) marked by expression of mini-white reporter (red, white dotted line outlines the domain). (C,D) The gain of function of miR-137 in the background of DE-Gal4 (DE>miR-137) reduces the dorsal domain of the eye field in (C) the adult eye and (D) the eye-imaginal disc. (B,D) Eye-antennal imaginal disc of third-instar larvae stained for Elav (red), Dlg (blue) and GFP (green) reporter. (E) Dorsal half expression of DE>miR-137 (n=10) normalized to the control DE-Gal4 (n=10). (E) The bar graph shows the length of the dorsal half of eye in DE>miR-137 (n=10) normalized to the control, DE-Gal4 (n10). (F,H) Adult wings of (F) nub-Gal4 and (H) nub>miR-137. The wings of nub>miR-137 (H) are reduced when compared to the control (F) nub-Gal4. (G,I) Wing imaginal discs of (G) nub-Gal4 and (I) nub>miR-137 stained for Engrailed (En) (red, a posterior compartment fate marker) and GFP (green, reporter marks nub-Gal4 expression domain). (J) A nub-Gal4 expression domain in the wing disc (n=5) of nub>miR-137 normalized to control nub-Gal4 (n=5). The nub-Gal4 domain is significantly reduced in the nub>miR-137 background. Data are mean±s.e.m. Statistical significance is indicated in each graph: ****P<0.0001; **P<0.01. All graphs were plotted using GraphPad Prism 8.3.1. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification, unless specified otherwise. Scale bars: 100 µm.
miR-137 targets Myc in the developing eye
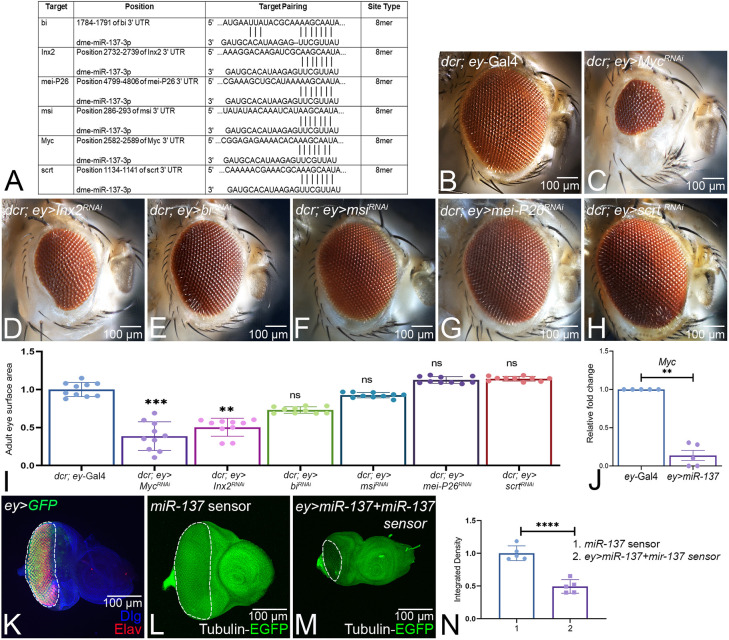
To understand the mechanism underlying the miR-137-mediated reduced-eye phenotype, we screened for miR-137 target genes using bioinformatics analysis tools such as TargetScanFly (http://www.targetscan.org/fly_72/) and DIANA (https://dianalab.e-ce.uth.gr/microt_webserver/#/interactions) to predict the potential targets of miR-137 (Maragkakis et al., 2009; Tastsoglou et al., 2023) (Fig. 6A). TargetScanFly predicts the targets of fly miRNA by matching the seed sequence (consensus sequence) of the 3′ untranslated region of the miRNA and their target mRNA. miRNAs bind the 3′UTR of their target mRNAs and thereby regulate gene expression. We found multiple putative targets predicted by bioinformatics analysis, including Myc, Innexin 2 (Inx2), bifid (bi), musashi (msi) and meiotic-P26 (mei-P26) (Fig. 6A). To identify the miR-137 target(s), we used genetic approaches. The rationale was that the loss-of-function phenotype of the target gene(s) in the developing eye should be similar to the gain of function of miR-137 (ey>miR-137). We used the ey-Gal4 driver to downregulate expression of the putative targets using RNAi lines to determine if they exhibit a reduced-eye phenotype (Fig. 6B-H). In these experiments, co-expression of dcr2 with a RNAi transgene results in a stronger phenotype by enhancing the RNA interference effect. In comparison to the control, dcr; ey-Gal4 (Fig. 6B), downregulation of Myc (dcr; ey>MycRNAi) (Fig. 6C) and Inx2 (dcr; ey>Inx2RNAi) (Fig. 6D) produces a reduced-eye phenotype. However, bi (dcr; ey>biRNAi) (Fig. 6E), msi (dcr; ey>msiRNAi) (Fig. 6F), mei-p26 (dcr; ey>mei-P26RNAi) (Fig. 6G) and scratch (scrt; dcr; ey>scrtRNAi) (Fig. 6H) did not show any reduction in eye size. The eye surface area of the miR-137 targets was quantified, and the statistical analyses revealed that Myc and Inx2 could be the targets, as their downregulation results in a reduced-eye phenotype similar to that in ey>miR-137 (Fig. 6I). However, the frequency of rescue of the reduced-eye phenotype with Inx2 was not as high as with Myc (data not shown). We used reverse transcription-quantitative PCR (qPCR) to determine the expression levels of Myc, the target of miR-137, in the eye imaginal disc. We found a significant reduction in Myc expression levels (∼70%) in the gain-of-function background of miR-137 (ey>miR-137) as compared to the control (Fig. 6J). Thus, we confirmed Myc as the target of miR-137, the absence of which in the developing eye affects the eye size.
Fig. 6.
Screening for miR-137 gene target(s) identified Myc. (A) The miR-137 binding site on its target(s) predicted by the bioinformatic tools TargetScan and DIANA. (B-H) Loss-of-function phenotypes in the adult eye of the putative targets identified using bioinformatics approaches. Adult eye phenotypes of (B) dcr2; ey-Gal4 (control), (C) dcr; ey>MycRNAi, (D) dcr; ey>Inx2RNAi, (E) dcr; ey>biRNAi, (F) dcr; ey>msiRNAi, (G) dcr; ey>mei-P26RNAi and (H) dcr; ey>scrtRNAi. In these experiments, dcr2 is co-expressed in the ey expression domain for a stronger RNA interference effect. (C) dcr; ey>MycRNAi and (D) dcr; ey>Inx2RNAi exhibit a strong reduction in eye size when compared to the control (B). (I) The normalized eye-surface area of miR-137 targets (n=10 per genotype) compared to the control dcr; ey-Gal4. The statistical analysis was performed using a one-way ANOVA with Dunnett's multiple comparison test. (J) Relative transcriptional (expression) levels of Myc (from triplicate sets) obtained using quantitative PCR (qPCR) in ey-Gal4 and ey>miR-137 backgrounds. Statistical analysis was carried out using an unpaired Student's t-test for independent samples. (K) Eye-antennal imaginal disc of third-instar larvae stained for Elav (red), Dlg (blue) a membrane-specific marker and GFP (green) reporter expressing in ey-Gal4 domain. (L,M) The Tubulin–GFP–miR-137 sensor shows ubiquitous expression of GFP under the control of tubulin promoter in the eye discs (L) and the ey>miR-137+miR-137-sensor (ey>miR-137+sensor) shows reduced GFP fluorescence in ey-producing cells the eye discs (M). (N) Quantification of GFP shows a significant reduction in GFP intensity in ey>miR-137+miR-137-sensor eye discs when compared to the control. The statistical analysis was carried out using an unpaired Student's t-test. Data are mean±s.e.m. Statistical significance is indicated in each graph: ****P<0.0001; ***P<0.001; **P<0.01. All graphs were plotted using GraphPad Prism 8.3.1. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification unless specified otherwise. Scale bars: 100 µm.
To further validate that miR-137 targets Myc in the developing eye and that the ey>miR-137-induced reduced-eye phenotype is due to downregulation of the target Myc, we generated the Tubulin–GFP–miR-137 sensor line (Brennecke et al., 2003). We modified the Tubulin-GFP-sensor toolbox with the 3′ UTR of Myc (which has a miR-137-binding site). In the control eye discs, GFP is ubiquitously expressed in all the cells (Fig. 6L). In the ey>miR-137 background, miR-137 mRNA will bind to its 3′ UTR binding site on Myc, and will degrade the mRNA with GFP and the miR-137-binding site. This will reduce the GFP levels in ey-Gal4 driver domain (Fig. 6K) where miR-137 is misexpressed. This reduction in GFP levels in the ey-Gal4 driver expression domain will serve as evidence that Myc is the target of miR-137. The larval eye discs in the Tubulin–GFP–miR-137 sensor flies expressing miR-137 exhibited reduced GFP levels in the ey-expressing cells (Fig. 6M, highlighted by a white dotted line) when compared to the Tubulin–GFP–miR-137 sensor controls (Fig. 6L). Quantification of GFP levels in the eye discs expressing Tubulin–GFP–miR-137 showed a significant reduction of GFP signal intensity levels in the Tubulin–GFP–miR-137 sensor larvae expressing miR-137 in the ey cells when compared to the control discs (Fig. 6L). Taken together, these findings strongly suggest that Myc is the target of miR-137.
Modulation of Myc levels affects the reduced-eye phenotype of miR-137 gain of function
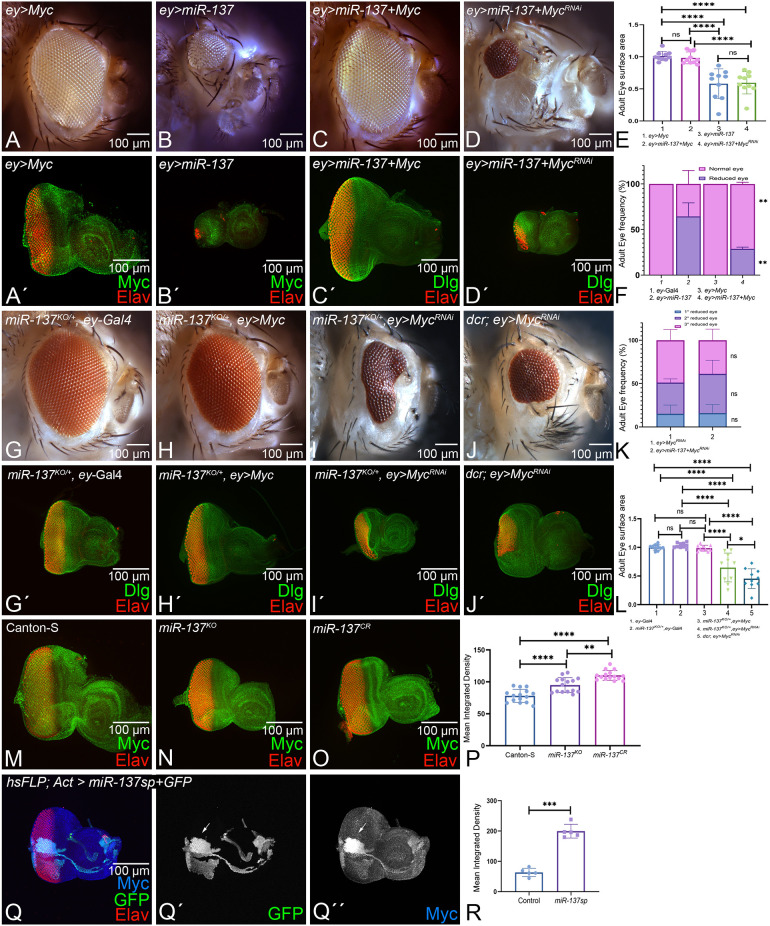
Myc is a regulator of growth during development (Gallant et al., 1996; Prober and Edgar, 2002). To determine if miR-137 has a role in growth regulation like its target Myc, we employed classical genetic approaches. The rationale was that if Myc is the downstream target of miR-137, then gain of function of Myc can rescue the reduced-eye phenotype of ey>miR-137. Conversely, loss of function of Myc will result in the reduced-eye phenotype (ey>MycRNAi). In comparison to the control, ey-Gal4 (Fig. 1B,D), gain of function of Myc (ey>Myc) exhibits near wild-type eye (Fig. 7A,A′), and gain of function of miR-137 (ey>miR-137), which served as control, shows the reduced-eye phenotype in the adult eye and eye imaginal disc (Fig. 7B,B′). In comparison to the controls, gain of function of miR-137 and Myc together (ey>miR-137+ Myc) results in rescue of the reduced-eye phenotype of ey>miR-137 alone, as seen in the adult eye and the eye imaginal disc (Fig. 7C,C′). Conversely, downregulation of Myc using a RNAi strategy in the ey>miR-137 background (ey>miR-137+MycRNAi) results in a reduced-eye phenotype (Fig. 7D,D′). The statistical analysis of adult eye-surface area analysis showed that gain of function of Myc in the ey>miR-137 background (ey>miR-137+ Myc) rescues the reduced-eye phenotype significantly (Fig. 7E). Furthermore, gain of function of Myc in the ey>miR-137 background (ey>miR-137+ Myc) significantly reduced the frequency of the reduced-eye phenotype of the gain of function of miR-137 (ey>miR-137, Fig. 7F). Additionally, gain of function of miR-137 along with downregulation of Myc RNAi (ey>miR-137+MycRNAi) exhibit the reduced-eye phenotype, as seen in controls such as ey>miR-137 and ey> MycRNAi alone. However, the frequency of the reduced-eye phenotype did not change significantly, suggesting that it is a threshold-dependent response (Fig. 7K).
Fig. 7.
miR-137 targets Myc during eye development. (A-B′) Gain of function of Myc (ey>Myc) (A,A′) and miR-137 (ey>miR-137) (B,B′) in (A,B) adult eye and (A′,B′) eye imaginal disc. (C′,D′) Eye-antennal imaginal discs stained for Elav, a pan-neuronal marker (red), and Dlg, a membrane-specific marker (green). (C-D′) Modulation of Myc levels in ey>miR-137 background using (C,C′) gain of function of Myc (ey>miR-137+Myc) and (D,D′) loss of function of Myc (ey>miR-137+MycRNAi), as seen in (C,D) adult eye and (C′,D′) eye imaginal disc. (E) The normalized eye-surface area of (A) ey>Myc, (B) ey>miR-137, (C) ey>miR-137+Myc and (D) ey>miR-137+MycRNAi showed a significant increase in eye size in ey>miR-137 +Myc compared to controls (ey>miR-137, n=10 per genotype). (F) Statistical analysis of adult eye phenotype frequency between (B) ey>miR-137 and (C) ey>miR-137+ Myc. There was significant rescue seen in ey>miR-137+ Myc when compared to ey>miR-137. (G-H′) Loss of function of miR-137 in (G,G′) ey-Gal4 (miR-137KO, ey-Gal4) and (H,H′) ey>Myc (miR-137KO, ey>Myc) background produces a wild-type phenotype in (G,H) adult eye and (G′,H′) eye imaginal disc. (I-J′) Loss of function of Myc in (I,I′) miR-137KO (ey>miR-137KO+MycRNAi) and (J,J′) ey-Gal4 (ey>MycRNAi) background, as seen in (I,J) adult eye and (I′,J′) eye imaginal disc, produces a reduced-eye phenotype. (K) Statistical analysis of adult eye phenotype frequency in (J) ey>MycRNAi and (D) ey>miR-137+MycRNAi. The reduced-eye phenotype is classified into: (1) 1° reduced eye, representing reduced eye in one or both of the eyes (blue); (2) 2° reduced eye, representing highly reduced to no eye in one of the eyes (purple); and (3) 3° reduced eye, representing highly reduced to no eye in both the eyes (pink). (L) The normalized eye-surface area of ey-Gal4 (Fig. 1B,C), miR-137KO/+, ey-Gal4 (G,G′), miR-137KO/+, ey>Myc (H,H′), miR-137KO/+, ey>MycRNAi (I,I′) and dcr; ey>MycRNAi (J,J′). (M-O) Eye-antennal imaginal disc of (M) Canton-S, (N) miR-137KO and (O) miR-137CR stained for Elav (red) and Myc (green). (P) Mean integrated density of Myc levels from Canton-S (M), miR-137KO (N) and miR-137CR (O). The signal intensity of Myc was calculated in five regions of interest per disc within a sample of n=5 for each genotype. Myc intensity was significantly increased in miR-137KO and miR-137CR. (Q-Q″) Eye-antennal imaginal disc of (Q) random flp out clones of miR-137sp (hsFLP; Act>miR-137sp+ GFP) stained for Elav (red) and Myc (blue). (Q′) Single channel image of GFP marking the clones of miR-137sp. (Q′′) Single channel image of Myc. Myc intensity was dramatically increased with flp out clones of miR-137sp. (R) Mean integrated density of Myc levels between control and flp out clones of miR-137sp. The signal intensity of Myc was calculated at the ROI (50×50) of flp out clones (n=5) and non-flp out (n=5) of the same disc. Myc intensity was significantly increased in hsFLP-out clones of miR-137sp. One-way ANOVA with Tukey's multiple comparison test was used for comparing more than two groups, and an unpaired Student's t-test was used to compare two groups. For adult frequency analysis, three replicates of 200 flies (200×3=600) were used to calculate the frequency of each genotype. Data are mean±s.e.m. Statistical significance is indicated in each graph: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification unless specified otherwise. Scale bars: 100 µm.
Conversely, we also modulated Myc levels in the heterozygous background of miR-137KO/+ (knockout, downregulated miR-137 levels due to heterozygosity) (Fig. 7G-I). Here, miR-137KO/+, which exhibits near-normal eyes, served as a control (Fig. 7G,G′). Gain of function of Myc in the miR-137KO/+ background (miR-137KO/+; ey>Myc) exhibits a normal eye (Fig. 7H,H′), whereas downregulation of Myc using a RNAi approach in the miR-137KO/+ background (miR-137KO/+; ey>MycRNAi) exhibits the reduced-eye phenotype (Fig. 7I,I′). The control ey>MycRNAi also exhibits a reduced-eye phenotype (Fig. 7J,J′), which is similar to gain of function of ey>miR-137 in the eye imaginal disc and adult eye (Fig. 7B,B′). The statistical analysis of adult eye surface areas of these different combinations revealed that there is no significant change in eye size when Myc levels are upregulated in miR-137KO/+ background. However, there is a significant reduction in eye size when Myc levels are downregulated in the miR-137KO/+ background (Fig. 7L). These results suggest that modulation of Myc expression levels can change the ey>miR-137 eye phenotypes. Thus, Myc is one of the downstream targets of miR-137 in the developing eye.
Additionally, we used Canton-S (Fig. 7M) to identify changes in Myc levels in miR-137KO (Fig. 7N) and miR-137CR (Fig. 7O). The statistical analysis of mean integrated density of Myc showed a significant increase in Myc levels in miR-137KO and miR-137CR backgrounds (Fig. 7P). Furthermore, random FLP-out clones of miR-137sponge (miR-137sp) were created using heat-shock Flippase in developing eye imaginal discs (Fig. 7Q), where GFP marks the clones of FLP-out miR-137sp (hsFLP; Actin>y+>GAL4/UAS-miR-137sp). These miR-137sp clones showed a robust increase in Myc levels (Fig. 7Q′,Q″, marked by white arrows). The statistical analysis of mean integrated density of Myc (Fig. 7R) showed a significant increase in Myc levels in random FLP-out clones of miR-137sp. These results demonstrate that Myc expression levels are significantly increased in miR-137KO and miR-137CR, and in random FLP-out clones of miR-137sp, emphasizing the role of miR-137 in targeting Myc to regulate its expression in developing eye.
miR-137 targets Myc expression for the growth regulation response
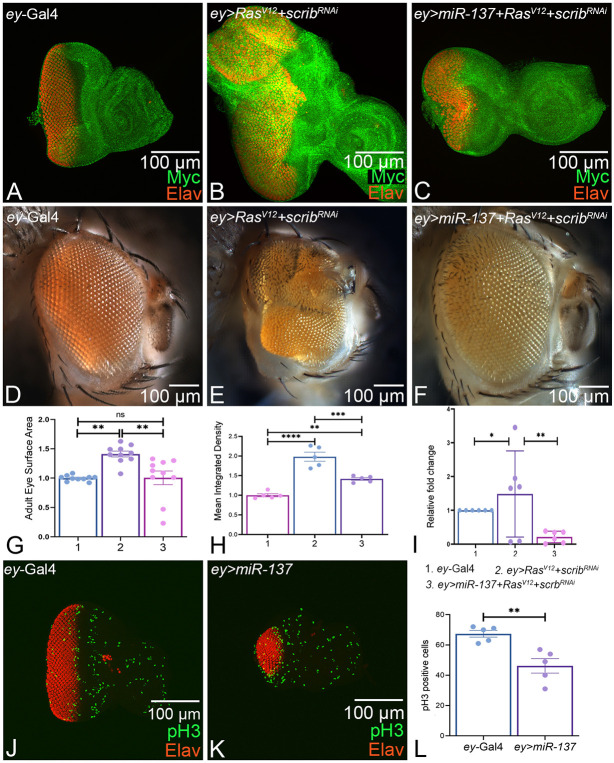
During tumorigenesis, Myc and Ras are the two most commonly activated oncogenes. Myc is upregulated in tumor models (Atkins et al., 2016; Prober and Edgar, 2002). Since Myc is one of the targets of miR-137, we tested the role of miR-137 in regulating Myc expression levels in a well-established oncogenic cooperation model of RasV12; scribRNAi. Gain of function of Ras along with downregulation of scrib using a RNAi approach (ey>RasV12+scribRNAi) result in neoplastic tumors (Brumby and Richardson, 2003; Pagliarini and Xu, 2003). In comparison to the ey-Gal4 control (Fig. 8A,D,G), there is a significant increase in the eye size in the ey>RasV12+scribRNAi (Fig. 8B,D,G) background, along with high levels of Myc signal intensity (Fig. 8H). Gain of function of miR-137 in the oncogenic cooperation model (ey>miR137+ RasV12+ scribRNAi) resulted in a significant reduction of the overgrowth phenotype of ey> RasV12+scribRNAi in eye imaginal disc and the adult eye (Fig. 8C,F), which was further validated by statistical analysis of surface area comparisons (Fig. 8G), by the Myc staining signal intensity (Fig. 8H) and by the adult eye phenotypes frequency (Fig. S4). Gain of function of miR-137 in the ey>RasV12+scrib RNAi background (ey>miR-137 +RasV12+scribRNAi) (Fig. 8C,F,G) results in a significant reduction in Myc levels (Fig. 8H). We also compared the relative transcriptional (expression) levels of the Myc mRNA in the developing eye using a qPCR approach. We found that Myc transcript levels were significantly increased in ey>RasV12+scribRNAi compared to ey-Gal4 and were decreased in the ey> RasV12+scribRNAi+miR-137 background compared to ey-Gal4 (Fig. 8I).
Fig. 8.
miR-137 exhibits growth regulation function in the developing eye. (A,D) Eye imaginal disc (A) and adult eye (D) of ey-Gal4 control. (B,E) ey>RasV12+ scribRNAi serves as a model for oncogenic cooperation resulting in neoplastic tumors and overgrowth. (C,F) Gain of function of miR-137: ey>miR-137+ RasV12+ scribRNAi significantly rescues the overgrowth phenotype of (B,E) ey> RasV12+ scribRNAi both at the eye imaginal disc level and in the adult eye. (A-C) All eye-imaginal discs are stained for Myc (green) and Elav (red). (G) Statistical analyses of normalized adult eye surface area (pixels/inch) were carried out using one-way ANOVA with Tukey's multiple comparison test for (A) ey-Gal4, (C) ey> RasV12+ scribRNAi and (E) ey> miR-137+RasV12+ scribRNAi (n=10 per genotype). The eye surface is reduced by gain of function of miR-137 in the ey> RasV12+ scribRNAi background. (H) Average intensity of Myc levels within the three backgrounds: (1) ey-Gal4, (2) ey> RasV12+ scribRNAi and (3) ey>miR-137+ RasV12+ scribRNAi. The signal intensity of Myc was calculated in three region of interest per disc within a sample of n=5 for each genotype. Statistical analysis was performed using an unpaired Student's t-test for independent samples. Myc intensity was significantly reduced in the ey>miR-137+ RasV12+ scribRNAi background. (I) Relative transcriptional (expression) levels of Myc using the quantitative real-time PCR (qRT-PCR) in ey-Gal4, ey>RasV12+scribRNAi and ey> RasV12+scribRNAi+miR-137 backgrounds. Statistical analysis was carried out in triplicate, using an unpaired Student's t-test for independent samples. Myc transcript levels were significantly increased in ey>RasV12+scribRNAi compared to ey-Gal4 and decreased in ey> RasV12+scribRNAi+miR-137 background compared to ey-Gal4. (J,K) Eye-antennal imaginal disc of (J) ey-Gal4 and (K) ey>miR-137 stained for phospho-histone 3 (pH3) (green, a marker for cell division) and Elav (red). (L) Statistical analysis of pH3 counts in the retinal field marked by Elav in comparable region of interest in ey-Gal4 (n=5) and ey>miR-137 (n=5) shows a significant difference in proliferating cells between control and ey>miR-137. The statistical analysis was carried out using an unpaired Student's t-test. Quantification of pH3 and eye-surface area was calculated using Fiji/ImageJ software to assay the differences. Data are mean±s.e.m. Statistical significance is indicated in each graph: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05. All graphs were plotted using GraphPad Prism 8.3.1. All the imaginal discs are oriented posterior to the left and dorsal upwards. All eye-antennal imaginal discs were captured at 20× magnification and adult eyes at 10× magnification unless specified otherwise. Scale bars: 100 µm.
To understand whether the reduced-eye phenotype of ey>miR-137 is due to decreased cell proliferation, we assessed expression of anti-phospho-histone H3 (pH3), which is a mitotic marker. In the histone H3 tail, phosphorylation of a highly conserved serine residue (Ser-10) marks the onset of mitosis and represents the completion of the cell cycle (Kim et al., 2017). The gain of function of miR-137 (ey>miR-137) exhibits reduced cell proliferation (Fig. 8K) as compared to the ey-Gal4 control (Fig. 8J). In the control eye disc, these cells can be seen as a band of cells at the MF and in a broad domain, anterior to the MF, in the head capsule region (Fig. 8J). These results suggest that the reduction in the eye field in the ey>miR-137 background may also be due to reduced cell proliferation rates. Statistical analysis of pH3 counts in the retinal field marked by Elav in a comparable region of interest in ey-Gal4 and ey>miR-137 revealed a significant difference in proliferating cells between control and ey>miR-137, suggesting that miR-137 also affects cell proliferation.
miR-137 targets Myc to regulate growth and patterning of the developing eye field
Based on all these data, we provide a model with which we demonstrate the growth regulation function of miR-137, a microRNA, which binds to 3′UTR of Myc mRNA to generate double-stranded RNA to induce Myc mRNA degradation (Fig. 9A,B). Consequently, Myc levels are downregulated, which results in a reduced-eye phenotype. Thus, miR-137 regulates the levels of its downstream target Myc, which in turn regulates both cell death and cell proliferation to maintain the size of the developing eye field (Fig. 9).
Fig. 9.
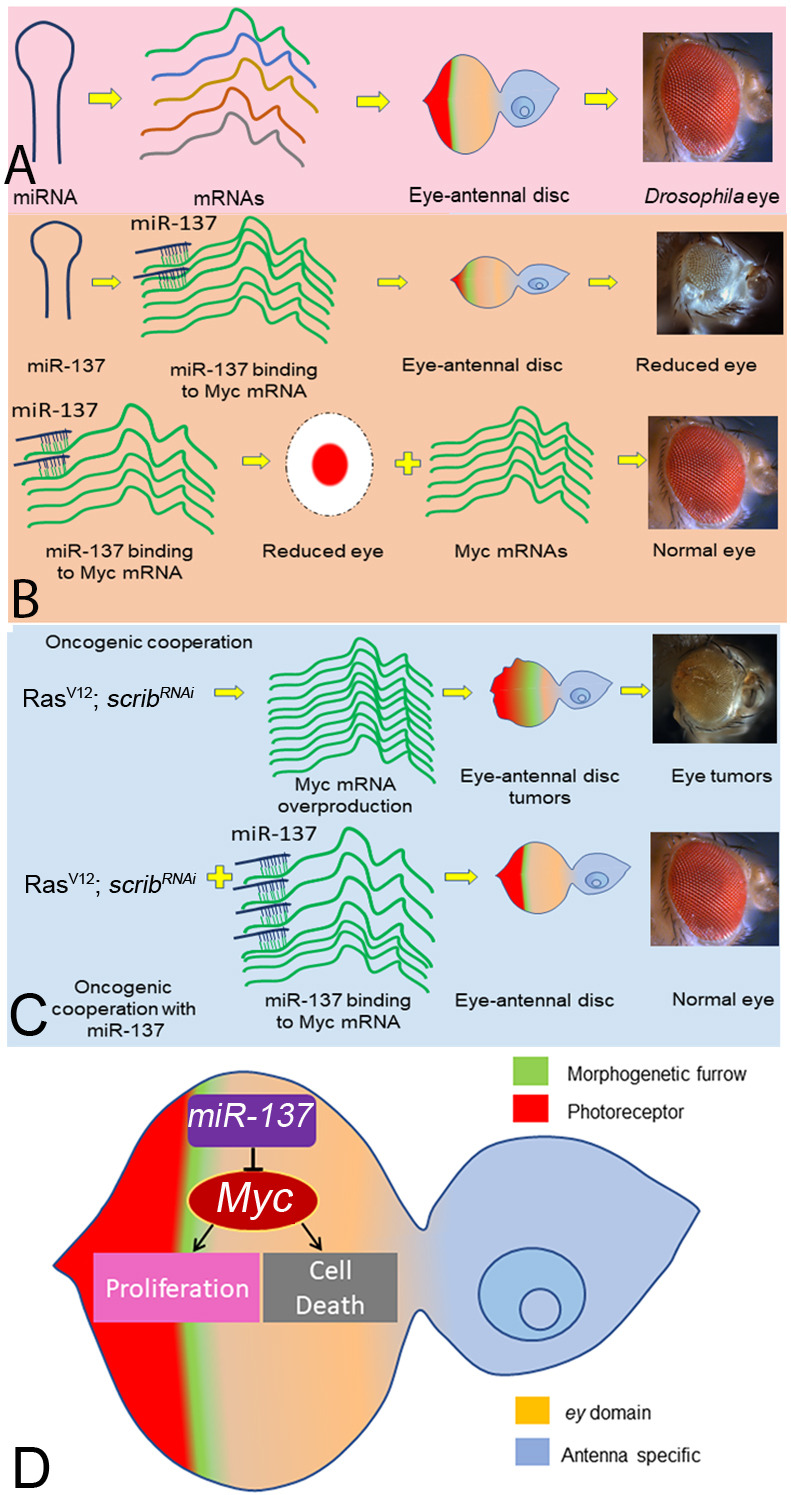
A model to demonstrate the role of miR-137 in growth regulation by targeting Myc during eye development. (A) When miRNAs are expressed at normal levels, a normal eye is produced. (B) Gain of function of miR-137 in the ey-Gal4 domain (ey>miR-137) results in a reduced-eye phenotype. Our results indicate that miR-137 targets Myc to reduce the size of eye. (C) miR-137 targets Myc to regulate growth during development. Gain of function of miR-137 can rescue overgrowth phenotypes observed in the tumor model of oncogenic cooperation in Drosophila. (D) Our results show that miR-137 targets Myc to regulate cell proliferation and cell death in the developing eye and in other tissues.
DISCUSSION
In eukaryotes, microRNAs (miRNAs) represent a class of small non-coding RNAs (∼22 nucleotides in length) that post-transcriptionally regulate protein-coding gene expression by translational repression or by RNA degradation of target mRNA. The human genome comprises a number of miRNA genes that account for nearly 1-5% of all predicted human genes that regulate ∼30% of all protein-coding genes (Shang et al., 2023; Vishnoi and Rani, 2023). It has been estimated that every miRNA can interact with an average of 200 mRNA transcripts (Colaianni and De Pittà, 2022). Consequently, miRNAs may play a fundamental role in biological processes such as development, metabolism, proliferation, growth regulation, apoptotic cell death and diseases. The structural and genetic similarities between the visual system of Drosophila and vertebrates, along with evolutionary conservation of miRNAs involved in development and regulation, underscore the value of the fruit fly as a model organism. These similarities suggest that Drosophila, with its strong repertoire of molecular genetic tools, can provide valuable insights into the molecular and neuronal circuits regulated by miRNAs in the specification and functioning of the visual system, which is relevant to so-called higher organisms, such as humans (Bier, 2005; Deshpande et al., 2024; Shang et al., 2023; Singh and Irvine, 2012). Drosophila has a genome-wide collection of miRNA overexpression and miRNA depletion transgenes (Fulga et al., 2015). We employed the Drosophila eye model for a forward genetic screen to look for the miRNAs involved in patterning and growth (Deshpande et al., 2024, 2023). We identified miR-137 as a negative regulator of the eye because the gain of function of miR-137 results in the reduced-eye phenotype due to activation of negative regulators of eye development, which results in MF progression. Our qPCR data, along with the high-throughput miRNA data (Leader et al., 2018), show that miR-137 is expressed during the development, particularly in early development. Interestingly, the miR-137 gain-of-function phenotype is similar in the wing and in another developing field, which suggest that miR-137 gain of function exhibits a phenotype of reduction in size of a developing field, as seen in the eye and wing. However, the gain-of-function phenotype of the entire fly larva could not be observed due to early lethality.
miR-137 employs caspase-dependent cell death
Gain of function of miR-137 can activate cell death, which results in the reduced-eye phenotype. However, when we block the caspase-dependent cell death pathway using the baculovirus P35 (Hay et al., 1994), there was rescue of the reduced-eye phenotype by miR-137 gain of function in a small population of flies. Since the rescue was not complete and the frequency of rescue was less, it suggests that other mechanisms might be involved. A basal level of cell death occurs in the developing eye to maintain cellular homeostasis. During development, both caspase-dependent cell death, called apoptosis, and a lysosomal-mediated degradation process, called autophagy, are used to regulate patterning and growth (Chimata et al., 2023). These data rule out the possibility of any significant role of autophagy in the reduced-eye phenotype of miR-137 gain of function (Fig. S3). However, in certain conditions, Myc promotes autophagy (Nagy et al., 2013). Therefore, miR-137-mediated reduced-eye phenotype employs caspase-dependent cell death.
miR-137 targets Myc to regulate growth
We screened for the target of miR-137 using bioinformatics, followed by molecular-genetic analysis, and found that loss of function of Myc and Inx2 using a RNAi strategy produced reduced-eyes phenotypes. However, the extent and frequency of the phenotype of Myc was highly consistent. Myc, an oncogene and a bHLH class transcription factor, is the master regulator, controlling diverse processes such as homeostasis. In Drosophila, Myc mutants have smaller eyes and body size (Johnston et al., 1999). Furthermore, complete loss of Myc (null mutant) results in larval lethality (Pierce et al., 2004), which is similar to the gain of function of miR-137 in the entire larva using the TubP-Gal4 driver. Since Myc is upregulated in the majority of cancers, it is plausible that our findings, where Myc is identified as a target of miR-137, the gain of function of which produces the reduced-eye phenotype, are significant. We further validated Myc as a target for miR-137 using a qPCR assay, which showed that Myc levels are significantly reduced in the gain-of-function background of miR-137 in the developing eye. Furthermore, the reduced-eye phenotype produced by the gain of function of miR-137 was rescued by the gain of function of Myc. Thus, Myc is a target of miR-137 in the developing eye.
Our studies suggest that optimum levels of miR-137 are crucial for development, as gain of function of miR-137 (ey>miR-137) produces a reduced-eye phenotype, whereas loss of function causes the converse phenotype of an enlarged eye. Interestingly, we found that gain of function of miR-137 ectopically induced Wg expression, whereas Myc levels are downregulated as Myc is its target. Interestingly, both Myc and wg, which is a secretory morphogen, regulate growth. Wg represses Myc levels in the zone of non-proliferating cells (ZNC) at the Drosophila wing margin. Blocking Wg signaling with dominant-negative TCF restores Myc expression (Gallant, 2013; Johnston et al., 1999). Our findings suggest miR-137 plays a role in this repressive interaction between Wg and Myc during development.
miR-137 regulates Myc in Drosophila tumor models
During eye development, Myc prevents ommatidium loss and eye size reduction (Huang et al., 2017), and its mutation leads to smaller eyes and body size (Johnston et al., 1999). We have shown a reduced-eye phenotype in both MycRNAi and ey>miR-137, where the phenotype of the latter emulates the former. The oncogenic cooperation between RasV12 and scribRNAi drives malignant tumors in the eye-antennal disc, although individually, these constructs can only drive benign tumors. Myc plays a central role in the oncogenic cooperation between RasV12 and scribRNAi (Atkins et al., 2016), where increased Myc levels promote hyperplasia in RasV12 (Prober and Edgar, 2002) and scribRNAi. We have shown that miR-137 reduces tumor growth in RasV12 and scribRNAi by targeting Myc, supporting a tumor-suppressor role for miR-137. Further studies will uncover the molecular mechanisms by which miR-137 regulates eye development, and the expression of Myc and other target genes (Fig. S4).
miR-137 is conserved and implicated in diseases
Based on the miRbase release 22 (https://mirbase.org/), D. melanogaster currently consists of 471 mature and 238 precursor miRNA(s), while Homo sapiens consists of 2693 mature and 1917 precursor miRNA(s) (Ambros et al., 2003; Kozomara and Griffiths-Jones, 2014). Most miRNA(s) belong to highly conserved non-coding sequences, where conservation of mature sequences is common (Price et al., 2011). Based on the conserved seed sequence, the Homo sapiens (human) ortholog of D. melanogaster miR-137 is hsa-miR-137. Furthermore, the Drosophila miR-137 shows an 87% similarity in the mature sequence to humans (Ibáñez-Ventoso et al., 2008) (Fig. S5). Recent genome-wide association studies (GWASs) have shown that single-nucleotide polymorphisms near miR-137 are statistically associated with neurodegenerative and psychiatric disorders (Pergola et al., 2024; Whalley et al., 2012). They regulate neural stem cell proliferation and differentiation during development (Sun et al., 2011), and their host gene, MIR-137HG, is associated with many psychiatric disorders (Whalley et al., 2012). Furthermore, Myc overexpression is linked to immune disorders such as myasthenia gravis, psoriasis and pemphigus vulgaris.
The Drosophila miR-137 human homolog hsa-miR-137, which is located on chromosome 1p22, acts as a tumor suppressor in colorectal cancer, squamous cell carcinoma and melanoma (Rupaimoole and Slack, 2017). In many cancers, miR-137 inhibits proliferation and differentiation (Althoff et al., 2013; Bi et al., 2018; Silber et al., 2008), which has led to its recognition as a therapeutic target in glioma (Wang et al., 2020). It has been reported that high levels of Myc expression in many cancers are associated with poor survival and disease progression. In many cancers, miR-137 can serve as tumor suppressor by targeting the mRNAs that encode oncoproteins and thereby inhibit proliferation and differentiation (Althoff et al., 2013; Bi et al., 2018; Silber et al., 2008). Around 70% of human cancers showed deregulation of Myc (Sorolla et al., 2020), and has been suggested as a target for molecular therapeutics (Duffy et al., 2021; Sorolla et al., 2020; Wang et al., 2021). Both miR-137 and Myc have been identified as possible therapeutic targets, but no studies have reported Myc as a direct target of miR-137 in humans and Drosophila. Interestingly, in a cohort of 706 patients with high KRAS expression and SCRIB loss, 395 patients (56%) exhibited low copy number expression (log2≤−0.12) of miR-137/5p, suggesting a significant heterozygous loss that may further affect the tumor-suppressive activity of miR-137 (Cerami et al., 2012; de Bruijn et al., 2023; Gao et al., 2013). These studies highlight the utility of miR-137 as a possible therapeutic target for cancers linked to Myc overexpression. Recently, another study reported that protein tyrosine phosphatase 61F (PTP61F), and ortholog of mammalian TC-PTP/PTP18, is one of the targets of miR-137, which is a highly conserved brain-enriched miRNA (Saedi et al., 2024). PTP61F is known to dephosphorylate the Insulin receptor InR (InR-P). These studies demonstrate that miR-137 regulates levels of PTP61F to maintain normal insulin signaling and energy homeostasis. In the future, it will be interesting to study molecular-genetic interactions between targets of miR-137, such as Myc and PTP61F, which are involved in growth regulation and tissue homeostasis.
Study limitations
microRNA characterization studies have some challenges regarding redundancy and conservation of expression amongst species, both of which may affect their targets and functions. This is because miRNA families often share common seed sequences, leading to functional redundancy. Findings from tissue-specific expression of miRNAs from one model can be extrapolated to another only after verification of expression.
MATERIALS AND METHODS
Fly stocks
The fly stocks used in this study are described in FlyBase (https://flybase.org/). We have used ey-Gal4 (Hazelett et al., 1998) and UAS-miR-137 (BL59881) to screen the eye phenotype described. Other stocks used include UAS-biRNAi (BL28341), UAS-E(z)RNAi (BL31617), UAS-Inx2RNAi (BL29306), UAS-mei-P26RNAi (BL36855), UAS-mitf RNAi (BL34835), UAS-msiRNAi (BL55152), UAS-MycRNAi (BL25783) and UAS-scrtRNAi (BL27025) to screen for the targets of miR-137. Additionally, miR-137KO (Chen et al., 2014), miR-137CR (Saedi et al., 2024), UAS-miR-137 sponge (miR-137sp, BL61395), UAS-Myc (BL9764) (Johnston et al., 1999), hid5′FWT-GFP (Tanaka-Matakatsu et al., 2009), UAS-P35 (Hay et al., 1994), UAS-mCherry-Atg8a and Atg8a mutant were used (Shukla et al., 2019). A stock UAS-RasV12; UAS-scribRNAi; was used to study the neoplastic tumors generated using UAS-scribRNAi (BL59080) and UASRasV12 (BL64196) (Snigdha et al., 2021). In this study, we have used ey-Gal4 driver (Hazelett et al., 1998), eyg-Gal4 (Jang et al., 2003), DE-Gal4 (Morrison and Halder, 2010), nubbin-Gal4 (Verghese et al., 2012), TubP (Tubulin)-Gal4 (BL5138) and TubP-Gal80ts; Tub-Gal4/TM6B (BL67065) for characterizing the role of miR-137 in eye, wing and whole fly development. All candidate RNAi lines were co-expressed with UAS-dicer2 (BL24650) by crossing them to the Gal4 driver(s) to increase the efficacy of RNA interference effects (Dietzl et al., 2007). All the stocks are maintained at 25°C on cornmeal, yeast and molasses food medium.
Generation of Tubulin-GFP-SV40 sensor for Myc
We have modified the original Tubulin–GFP–SV40 sensor, gifted by Eric Lai (Sloan-Kettering Institute, New York, USA), with the addition of tubulin promoter sites. We generated the miR-137 sensor line by inserting the miR-137 binding sites of 3′UTR of Myc into the tubulin-GFP-SV40 plasmid (gifted by Eric Lai, Sloan-Kettering Institute, New York, USA) between the NotI and XhoI restriction sites (GenScript). Transgenic Drosophila strains were generated by standard injection with the Δ2-3 helper transposase (GenetiVision) (Brennecke et al., 2003) using random P element insertion and balanced to perform experiments.
Genetic crosses
The Gal4/UAS system was used to misexpress the transgene(s) of interest in a spatiotemporal manner (Brand and Perrimon, 1993). All crosses were maintained at 25°C and 29°C, unless specified, to observe the changes in induction levels (Singh et al., 2005a).
Genetic mosaic analysis
To generate gain-of-function random flp out clones in the developing eye, we crossed y,w, hsFLP122; P(Act>y+>Gal4)25P (UAS-GFPS65T)/CyO (Struhl and Basler, 1993) flies to the UAS-miR-137 (gain of function) or UAS-miR137 sp (loss of function) flies. The progeny from these cultures were heat-shocked to generate flp out clones.
Immunohistochemistry
Wandering third-instar larvae were dissected for the eye-antennal imaginal disc in 1×phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde (fixative) in 1×PBS for 20 min and washed thrice in PBS-Triton-X100 (PBST). The tissues (eye-imaginal discs) were blocked using the serum and stained using a combination of antibodies following the standard protocol (Singh et al., 2002; Tare et al., 2020). Primary antibodies used were mouse anti-β-GAL (1:100; Developmental Studies Hybridoma Bank, DSHB), goat anti-Atonal (Ato; 1:250; Santa Cruz Biotechnology, sc-15699), goat anti-Homothorax (Hth; 1:250; Santa Cruz Biotechnology, sc-26187), mouse anti-Dachshund (Dac; 1:100; DSHB), rabbit anti-Death Caspase-1 (Dcp1; 1:250; Cell Signaling Technology, 9578), mouse anti-Disc-large (Dlg; 1:100; DSHB), rat anti- Embryonic Lethal Abnormal Vision (Elav; 1:100; DSHB), mouse anti-Eyes absent (Eya; 1:100; DSHB), rabbit anti-Myc (1:100; Santa Cruz Biotechnology, D2704), mouse anti-Phospho histone 3 (pH3; 1:200; Cell Signaling Technology, 9701), mouse anti-Scabrous (Sca; 1:100; DSHB), mouse anti-Prospero (Pros; 1:100; DSHB) and mouse anti-Wingless (Wg; 1:100; DSHB). The discs were washed thrice in PBST for 10 min. Secondary antibodies used were donkey anti-rat IgG conjugated to Cy5 (1:250; 712-175-153), donkey anti-rabbit IgG conjugated to Cy3 (1:300; 711-166-152) or goat anti-mouse IgG conjugated to Cy3 (1:200; 115-166-062) (all from Jackson ImmunoResearch). Discs were mounted in Vectashield and scanned on a Fluoview 3000 Laser Scanning Confocal Microscope (Steffensmeier et al., 2013; Tare et al., 2016). We captured the images at 20× magnification unless specified. We prepared and analyzed the figures using Adobe Photoshop CS6 software.
Detection of cell death
Cell death was detected by using a Terminal Transferase dUTP Nick End Labeling (TUNEL) kit from Roche Diagnostics. A standardized protocol was used for TUNEL staining in the eye antennal imaginal discs (Chimata et al., 2022; White et al., 1994). The TUNEL-positive cells were counted from five sets of imaginal discs per genotype. These numbers were used for statistical analysis the P-values were calculated using an unpaired Student's t-test (Deshpande et al., 2024, 2023). The graphs were plotted using GraphPad Prism 8. The error bars represent the s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Adult eye imaging
The adult flies were frozen at −20°C for ∼4 h before imaging their eyes. The flies were mounted onto the needles after dissecting the wings and legs, and imaged at 10× magnification. The needle with the fly is inserted in a clay putty on a glass slide to position it stably for the lateral view of the fly eye and head. Images were taken on a MrC5 color camera mounted on an Axioimager.Z1 Zeiss Apotome using a z-sectioning function of Axiovision software 4.6.3 (Cutler et al., 2015; Gogia et al., 2020b; Irwin et al., 2020; Mehta et al., 2021). The final z-projections were compiled using Adobe Photoshop CS6 software.
Frequency of eye phenotype
Three independent sets of 200 flies were screened (200×3=600) and the frequencies of eye phenotype(s) were calculated for each genetic cross (Raj et al., 2020; Singh et al., 2024). The eye phenotypes were categorized as reduced-eye or normal eye. The graphs were plotted using statistical methods described below in GraphPad Prism 8.
Quantitative analyses of eye surface area and eye imaginal discs
Image J software was used to quantify the area (pixels/inch) as described previously (Deshpande et al., 2021). We measured the eye surface area by drawing the region of interest (ROI) along the perimeter of the adult eye. The values were analyzed and plotted as mean±s.e.m. using the statistical methods described below. Each genotype was analyzed with at least ten images, unless specified otherwise. For measuring intensity in eye imaginal discs, three ROIs of 50×50 pixels were selected and normalized to controls (Deshpande et al., 2021). Each genotype was analyzed with at least five images unless specified otherwise.
Adult wing imaging
The adult flies were sequentially immersed in 50% and 70% of ethanol for 2 days, and 90% of ethanol for 4 days. Wings were then dissected in 70-100% ethanol and mounted using Canada balsam. Images were taken on a MrC5 color camera mounted on an Axioimager.Z1 Zeiss Apotome using a z-sectioning function of Axiovision software 4.6.3. The final z-projections were compiled using Adobe Photoshop CS6 software.
Real-time quantitative PCR
We used 60-80 eye-antennal imaginal discs for RNA isolation (Mehta and Singh, 2017; Mehta et al., 2019). The tissue was collected and homogenized in TRIzol reagent (Invitrogen, 15596026). We used RNA Clean & Concentrator (Zymo Research, R1013) columns after total RNA extraction and eluted in about 20 µl DNase/RNase-free water. The quality and quantity of RNA were assayed by a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific). The cDNA was synthesized from 500 ng of total mRNA through a reverse transcription reaction using a first-strand cDNA synthesis kit (GE Healthcare, 27926101). Real time-qPCR was performed using BioRad iQ SYBR Green Supermix (Bio-Rad, 1708860) according to the standard protocol (Chimata et al., 2023; Deshpande et al., 2024, 2023). For miRNA gene expression levels, miRNA isolation from 60-70 pairs of eye-antennal imaginal discs was executed using a miRNeasy kit (Qiagen, 217084). The reverse transcription was performed using a TaqMan MicroRNA Reverse Transcription Kit (ThermoFisher Scientific, 4366596) with miR-137 and 2S-rRNA probes (ThermoFisher Scientific, 4440886 and 4427975). Real time-qPCR was performed using a TaqMan Fast Advances Master Mix (ThermoFisher Scientific, 4444557) with probes (miR-137 and 2S-rRNA). Fold change was calculated using the comparative CT method (2−ΔΔCT method). The primers used were: GAPDH-Fwd, 5′-GCGGATAAAGTAAATGTGTGC-3′; GAPDH-Rev, 5′-AGCTCCTCGTAGACGAACAT-3′; Myc-Fwd, 5′-TGTCCTCGATGTGCTCAACC-3′; Myc-Rev, 5′-CACTATCAGAGCCGGTCGTC-3′; miR-137, 5′-UAUUGCUUGAGAAUACACGUAG-3′; 2S rRNA, 5′-TGCTTGGACTACATATGGTTGAGGGTTGTA-3′.
Statistics
We used GraphPad Prism 8 for all our statistical analysis: two-way ANOVA with Sidak multiple comparison tests and an unpaired Student's t-test for the eye frequency data; a non-parametric one-way ANOVA, Mann–Whitney test for mean integrated intensity quantification data (>2 groups). Data are plotted as mean±s.e.m. with the individual values indicated. Statistical significance is set at 95% CI and is shown by ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05 (Chimata et al., 2023).
Supplementary Material
Acknowledgements
We thank Bloomington Drosophila Stock Center (BDSC) for Drosophila strains and the Developmental Studies Hybridoma Bank (DSHB) for antibodies. Confocal microscopy was supported by the core facility at the University of Dayton. We thank Dr Susan Tsunoda of the University of Pennsylvania (miR-137CR) and Dr Eric Lai (tubulin-GFP-SV40 plasmid) for their generous gifts to our work. We also thank Aditi Singh for comments on the manuscript.
Footnotes
Author contributions
Conceptualization: M.K.-S., A. Singh; Data curation: R.P., A.V.C., A.R., A. Singh; Formal analysis: R.P., M.S., A. Sangeeth, M.K.-S.; Funding acquisition: M.K.-S., A. Singh; Investigation: R.P., A.V.C., A. Singh; Methodology: R.P., M.S., A.V.C., A.R., S.Y., A. Sangeeth, M.K.-S., A. Singh; Resources: A.R., S.Y., M.K.-S., A. Singh; Software: A.V.C.; Supervision: M.K.-S., A. Singh; Validation: A. Singh; Visualization: R.P.; Writing – original draft: R.P., A. Singh; Writing – review & editing: R.P., M.S., A.V.C., A.R., S.Y., A. Sangeeth, M.K.-S., A. Singh
Funding
R.P. is supported by the National Institutes of Health (1RO1EY032959-01 to A. Singh). M.S. is supported by the Knights Templar Eye Foundation (KTEF-411281). A. Singh is supported by the Brother Leonard A Mann Chair in Natural Sciences Endowment Fund from the University of Dayton. M.K.-S. is supported by the National Institutes of Health (1RO1EY032959-01) and the Schuellein Chair Endowment Fund from the University of Dayton. Open Access funding provided by the University of Dayton. Deposited in PMC for immediate release.
Data and resource availability
All relevant data and details of resources can be found within the article and its supplementary information.
References
- Althoff, K., Beckers, A., Odersky, A., Mestdagh, P., Köster, J., Bray, I. M., Bryan, K., Vandesompele, J., Speleman, F., Stallings, R. L.et al. (2013). MiR-137 functions as a tumor suppressor in neuroblastoma by downregulating KDM1A. Int. J. Cancer 133, 1064-1073. 10.1002/ijc.28091 [DOI] [PubMed] [Google Scholar]
- Ambros, V. and Ruvkun, G. (2018). Recent molecular genetic explorations of Caenorhabditis elegans microRNAs. Genetics 209, 651-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros, V., Bartel, B., Bartel, D. P., Burge, C. B., Carrington, J. C., Chen, X., Dreyfuss, G., Eddy, S. R., Griffiths-Jones, S., Marshall, M.et al. (2003). A uniform system for microRNA annotation. RNA 9, 277-279. 10.1261/rna.2183803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins, M., Potier, D., Romanelli, L., Jacobs, J., Mach, J., Hamaratoglu, F., Aerts, S. and Halder, G. (2016). An ectopic network of transcription factors regulated by Hippo signaling drives growth and invasion of a malignant tumor model. Curr. Biol. 26, 2101-2113. 10.1016/j.cub.2016.06.035 [DOI] [PubMed] [Google Scholar]
- Baker, N. E., Mlodzik, M. and Rubin, G. M. (1990). Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science 250, 1370-1377. 10.1126/science.2175046 [DOI] [PubMed] [Google Scholar]
- Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281-297. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Ben-Hamo, R. and Efroni, S. (2015). MicroRNA regulation of molecular pathways as a generic mechanism and as a core disease phenotype. Oncotarget 6, 1594-1604. 10.18632/oncotarget.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlali, A., Draskovic, I., Hazelett, D. J. and Treisman, J. E. (2000). act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell 101, 271-281. 10.1016/S0092-8674(00)80837-5 [DOI] [PubMed] [Google Scholar]
- Bessa, J., Gebelein, B., Pichaud, F., Casares, F. and Mann, R. S. (2002). Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev. 16, 2415-2427. 10.1101/gad.1009002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, W. P., Xia, M. and Wang, X. J. (2018). miR-137 suppresses proliferation, migration and invasion of colon cancer cell lines by targeting TCF4. Oncol. Lett. 15, 8744-8748. 10.3892/ol.2018.8364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier, E. (2005). Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 6, 9-23. 10.1038/nrg1503 [DOI] [PubMed] [Google Scholar]
- Blackman, R. K., Sanicola, M., Raftery, L. A., Gillevet, T. and Gelbart, W. M. (1991). An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development 111, 657-666. 10.1242/dev.111.3.657 [DOI] [PubMed] [Google Scholar]
- Bonini, N. M., Leiserson, W. M. and Benzer, S. (1993). The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72, 379-395. 10.1016/0092-8674(93)90115-7 [DOI] [PubMed] [Google Scholar]
- Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. 10.1242/dev.118.2.401 [DOI] [PubMed] [Google Scholar]
- Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. and Cohen, S. M. (2003). bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25-36. 10.1016/S0092-8674(03)00231-9 [DOI] [PubMed] [Google Scholar]
- Brumby, A. M. and Richardson, H. E. (2003). scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769-5779. 10.1093/emboj/cdg548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui, Q. T., Zimmerman, J. E., Liu, H. and Bonini, N. M. (2000). Molecular analysis of Drosophila eyes absent mutants reveals features of the conserved Eya domain. Genetics 155, 709-720. 10.1093/genetics/155.2.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., Jacobsen, A., Byrne, C. J., Heuer, M. L., Larsson, E.et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401-404. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., Nordstrom, W., Gish, B. and Abrams, J. M. (1996). grim, a novel cell death gene in Drosophila. Genes Dev. 10, 1773-1782. 10.1101/gad.10.14.1773 [DOI] [PubMed] [Google Scholar]
- Chen, R., Amoui, M., Zhang, Z. and Mardon, G. (1997). Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell 91, 893-903. 10.1016/S0092-8674(00)80481-X [DOI] [PubMed] [Google Scholar]
- Chen, Y.-W., Song, S., Weng, R., Verma, P., Kugler, J.-M., Buescher, M., Rouam, S. and Cohen, S. M. (2014). Systematic study of Drosophila microRNA functions using a collection of targeted knockout mutations. Dev. Cell 31, 784-800. 10.1016/j.devcel.2014.11.029 [DOI] [PubMed] [Google Scholar]
- Chimata, A. V., Deshpande, P., Mehta, A. S. and Singh, A. (2022). Protocol to study cell death using TUNEL assay in Drosophila imaginal discs. STAR Protoc. 3, 101140. 10.1016/j.xpro.2022.101140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimata, A. V., Darnell, H., Raj, A., Kango-Singh, M. and Singh, A. (2023). Transcriptional pausing factor M1BP regulates cellular homeostasis by suppressing autophagy and apoptosis in Drosophila eye. Autophagy Rep. 2, 2252307. 10.1080/27694127.2023.2252307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. M. (1993). Imaginal Disc development. In The Development of Drosophila melanogaster (ed. Bate M. and Martinez-Arias A.), Volume 2, Chapter 13. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Colaianni, D. and De Pittà, C. (2022). The role of microRNAs in the Drosophila melanogaster visual system. Front. Cell Dev. Biol. 10, 889677. 10.3389/fcell.2022.889677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, T., Sarkar, A., Moran, M., Steffensmeier, A., Puli, O. R., Mancini, G., Tare, M., Gogia, N. and Singh, A. (2015). Drosophila eye model to study neuroprotective role of CREB binding protein (CBP) in Alzheimer's disease. PLoS ONE 10, e0137691. 10.1371/journal.pone.0137691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, G., Shravage, B. V. and Baehrecke, E. H. (2012). Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4, a008813. 10.1101/cshperspect.a008813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn, I., Kundra, R., Mastrogiacomo, B., Tran, T. N., Sikina, L., Mazor, T., Li, X., Ochoa, A., Zhao, G., Lai, B.et al. (2023). Analysis and visualization of longitudinal genomic and clinical data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 83, 3861-3867. 10.1158/0008-5472.CAN-23-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, P., Gogia, N., Chimata, A. V. and Singh, A. (2021). Unbiased automated quantitation of ROS signals in live retinal neurons of Drosophila using Fiji/ImageJ. BioTechniques 71, 416-424. 10.2144/btn-2021-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, P., Chimata, A. V., Snider, E., Singh, A., Kango-Singh, M. and Singh, A. (2023). N-Acetyltransferase 9 ameliorates Abeta42-mediated neurodegeneration in the Drosophila eye. Cell Death Dis. 14, 478. 10.1038/s41419-023-05973-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, P., Chen, C.-Y., Chimata, A. V., Li, J.-C., Sarkar, A., Yeates, C., Chen, C.-H., Kango-Singh, M. and Singh, A. (2024). miR-277 targets the proapoptotic gene-hid to ameliorate Abeta42-mediated neurodegeneration in Alzheimer's model. Cell Death Dis. 15, 71. 10.1038/s41419-023-06361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl, G., Chen, D., Schnorrer, F., Su, K.-C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S.et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Domínguez, M. and Hafen, E. (1997). Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye. Genes Dev. 11, 3254-3264. 10.1101/gad.11.23.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, M. J., O'Grady, S., Tang, M. and Crown, J. (2021). MYC as a target for cancer treatment. Cancer Treat. Rev. 94, 102154. 10.1016/j.ctrv.2021.102154 [DOI] [PubMed] [Google Scholar]
- Ebert, M. S. and Sharp, P. A. (2012). Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515-524. 10.1016/j.cell.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frédérick, P. M. and Simard, M. J. (2022). Regulation and different functions of the animal microRNA-induced silencing complex. Wiley Interdiscip. Rev. RNA 13, e1701. 10.1002/wrna.1701 [DOI] [PubMed] [Google Scholar]
- Fulga, T. A., McNeill, E. M., Binari, R., Yelick, J., Blanche, A., Booker, M., Steinkraus, B. R., Schnall-Levin, M., Zhao, Y., DeLuca, T.et al. (2015). A transgenic resource for conditional competitive inhibition of conserved Drosophila microRNAs. Nat. Commun. 6, 7279. 10.1038/ncomms8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, P. (2013). Myc function in Drosophila. Cold Spring Harb. Perspect. Med. 3, a014324. 10.1101/cshperspect.a014324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, P., Shiio, Y., Cheng, P. F., Parkhurst, S. M. and Eisenman, R. N. (1996). Myc and Max homologs in Drosophila. Science 274, 1523-1527. 10.1126/science.274.5292.1523 [DOI] [PubMed] [Google Scholar]
- Gao, J., Aksoy, B. A., Dogrusoz, U., Dresdner, G., Gross, B., Sumer, S. O., Sun, Y., Jacobsen, A., Sinha, R., Larsson, E.et al. (2013). Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogia, N., Puli, O. R., Raj, A. and Singh, A. (2020a). Generation of third dimension: axial patterning in the developing Drosophila eye. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 53-95. Springer NewYork Heidelberg Dordrecht London: Springer International Publishing. [Google Scholar]
- Gogia, N., Sarkar, A., Mehta, A. S., Ramesh, N., Deshpande, P., Kango-Singh, M., Pandey, U. B. and Singh, A. (2020b). Inactivation of Hippo and cJun-N-terminal Kinase (JNK) signaling mitigate FUS mediated neurodegeneration in vivo. Neurobiol. Dis. 140, 104837. 10.1016/j.nbd.2020.104837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether, M. E., Abrams, J. M., Agapite, J., White, K. and Steller, H. (1995). The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 9, 1694-1708. 10.1101/gad.9.14.1694 [DOI] [PubMed] [Google Scholar]
- Halder, G., Callaerts, P., Flister, S., Walldorf, U., Kloter, U. and Gehring, W. J. (1998). Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development 125, 2181-2191. 10.1242/dev.125.12.2181 [DOI] [PubMed] [Google Scholar]
- Hay, B. A., Wolff, T. and Rubin, G. M. (1994). Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121-2129. 10.1242/dev.120.8.2121 [DOI] [PubMed] [Google Scholar]
- Hayashi, T., Xu, C. and Carthew, R. W. (2008). Cell-type-specific transcription of prospero is controlled by combinatorial signaling in the Drosophila eye. Development 135, 2787-2796. 10.1242/dev.006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelett, D. J., Bourouis, M., Walldorf, U. and Treisman, J. E. (1998). decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125, 3741-3751. 10.1242/dev.125.18.3741 [DOI] [PubMed] [Google Scholar]
- Huang, J., Feng, Y., Chen, X., Li, W. and Xue, L. (2017). Myc inhibits JNK-mediated cell death in vivo. Apoptosis 22, 479-490. 10.1007/s10495-016-1340-4 [DOI] [PubMed] [Google Scholar]
- Ibáñez-Ventoso, C., Vora, M. and Driscoll, M. (2008). Sequence relationships among C. elegans, D. melanogaster and human microRNAs highlight the extensive conservation of microRNAs in biology. PLoS ONE 3, e2818. 10.1371/journal.pone.0002818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin, M., Tare, M., Singh, A., Puli, O. R., Gogia, N., Riccetti, M., Deshpande, P., Kango-Singh, M. and Singh, A. (2020). A positive feedback loop of Hippo- and c-Jun-amino-terminal kinase signaling pathways regulates amyloid-beta-mediated neurodegeneration. Front. Cell Dev. Biol. 8, 117. 10.3389/fcell.2020.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, C.-C., Chao, J.-L., Jones, N., Yao, L.-C., Bessarab, D. A., Kuo, Y. M., Jun, S., Desplan, C., Beckendorf, S. K. and Sun, Y. H. (2003). Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development 130, 2939-2951. 10.1242/dev.00522 [DOI] [PubMed] [Google Scholar]
- Jarman, A. P., Grell, E. H., Ackerman, L., Jan, L. Y. and Jan, Y. N. (1994). Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398-400. 10.1038/369398a0 [DOI] [PubMed] [Google Scholar]
- Johnston, L. A., Prober, D. A., Edgar, B. A., Eisenman, R. N. and Gallant, P. (1999). Drosophila myc regulates cellular growth during development. Cell 98, 779-790. 10.1016/S0092-8674(00)81512-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kango-Singh, M., Singh, A. and Sun, Y. H. (2003). Eyeless collaborates with Hedgehog and Decapentaplegic signaling in Drosophila eye induction. Dev. Biol. 256, 48-60. 10.1016/S0012-1606(02)00123-9 [DOI] [PubMed] [Google Scholar]
- Kariuki, D., Asam, K., Aouizerat, B. E., Lewis, K. A., Florez, J. C. and Flowers, E. (2023). Review of databases for experimentally validated human microRNA-mRNA interactions. Database (Oxford) 2023, baad014. 10.1093/database/baad014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Y., Jeong, H. S., Chung, T., Kim, M., Lee, J. H., Jung, W. H. and Koo, J. S. (2017). The value of phosphohistone H3 as a proliferation marker for evaluating invasive breast cancers: a comparative study with Ki67. Oncotarget 8, 65064-65076. 10.18632/oncotarget.17775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara, A. and Griffiths-Jones, S. (2014). miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68-D73. 10.1093/nar/gkt1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, J. P. (2010). Retinal determination the beginning of eye development. Curr. Top. Dev. Biol. 93, 1-28. 10.1016/B978-0-12-385044-7.00001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, J. P. (2011). My what big eyes you have: How the Drosophila retina grows. Dev. Neurobiol. 71, 1133-1152. 10.1002/dneu.20921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, J. P. (2020). Catching the next wave: patterning of the Drosophila eye by the morphogenetic furrow. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 75-97. Springer NewYork Heidelberg Dordrecht London: Springer International Publishing. [Google Scholar]
- Larkin, A., Marygold, S. J., Antonazzo, G., Attrill, H., dos Santos, G., Garapati, P. V., Goodman, J. L., Gramates, L. S., Millburn, G., Strelets, V. B.et al. (2021). FlyBase: updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 49, D899-D907. 10.1093/nar/gkaa1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader, D. P., Krause, S. A., Pandit, A., Davies, S. A. and Dow, J. A. T. (2018). FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46, D809-D815. 10.1093/nar/gkx976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R. C., Feinbaum, R. L. and Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843-854. 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- Li, J. and Gilmour, D. S. (2011). Promoter proximal pausing and the control of gene expression. Curr. Opin. Genet. Dev. 21, 231-235. 10.1016/j.gde.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. and Moses, K. (1995). Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development 121, 2279-2289. 10.1242/dev.121.8.2279 [DOI] [PubMed] [Google Scholar]
- Maragkakis, M., Reczko, M., Simossis, V. A., Alexiou, P., Papadopoulos, G. L., Dalamagas, T., Giannopoulos, G., Goumas, G., Koukis, E., Kourtis, K.et al. (2009). DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Res. 37, W273-W276. 10.1093/nar/gkp292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, A. and Singh, A. (2017). Real time quantitative PCR to demonstrate gene expression in an undergraduate lab. Dros. Inf. Serv. 100, 5. [Google Scholar]
- Mehta, A. S., Luz-Madrigal, A., Li, J.-L., Tsonis, P. A. and Singh, A. (2019). Total RNA extraction from transgenic flies misexpressing foreign genes to perform Next generation RNA sequencing. 10.17504/protocols.io.5bng2me [DOI] [Google Scholar]
- Mehta, A. S., Deshpande, P., Chimata, A. V., Tsonis, P. A. and Singh, A. (2021). Newt regeneration genes regulate Wingless signaling to restore patterning in Drosophila eye. iScience 24, 103166. 10.1016/j.isci.2021.103166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, A. K. and Sprecher, S. G. (2020). Early eye development: specification and determination. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 1-52. Springer NewYork Heidelberg Dordrecht London: Springer. [Google Scholar]
- Mlodzik, M., Baker, N. E. and Rubin, G. M. (1990). Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 4, 1848-1861. 10.1101/gad.4.11.1848 [DOI] [PubMed] [Google Scholar]
- Morrison, C. M. and Halder, G. (2010). Characterization of a dorsal-eye Gal4 line in Drosophila. Genesis 48, 3-7. 10.1002/dvg.20571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, P., Varga, A., Pircs, K., Hegedűs, K. and Juhász, G. (2013). Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genet. 9, e1003664. 10.1371/journal.pgen.1003664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini, R. A. and Xu, T. (2003). A genetic screen in Drosophila for metastatic behavior. Science 302, 1227-1231. 10.1126/science.1088474 [DOI] [PubMed] [Google Scholar]
- Pai, C.-Y., Kuo, T.-S., Jaw, T. J., Kurant, E., Chen, C.-T., Bessarab, D. A., Salzberg, A. and Sun, Y. H. (1998). The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 12, 435-446. 10.1101/gad.12.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola, G., Rampino, A., Sportelli, L., Borcuk, C. J., Passiatore, R., Di Carlo, P., Marakhovskaia, A., Fazio, L., Amoroso, N., Castro, M. N.et al. (2024). A miR-137-related biological pathway of risk for schizophrenia is associated with human brain emotion processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 9, 356-366. 10.1016/j.bpsc.2023.11.001 [DOI] [PubMed] [Google Scholar]
- Perry, N., Volin, M. and Toledano, H. (2017). microRNAs in Drosophila regulate cell fate by repressing single mRNA targets. Int. J. Dev. Biol. 61, 165-170. 10.1387/ijdb.160271ht [DOI] [PubMed] [Google Scholar]
- Pierce, S. B., Yost, C., Britton, J. S., Loo, L. W. M., Flynn, E. M., Edgar, B. A. and Eisenman, R. N. (2004). dMyc is required for larval growth and endoreplication in Drosophila. Development 131, 2317-2327. 10.1242/dev.01108 [DOI] [PubMed] [Google Scholar]
- Price, N., Cartwright, R. A., Sabath, N., Graur, D. and Azevedo, R. B. R. (2011). Neutral evolution of robustness in Drosophila microRNA precursors. Mol. Biol. Evol. 28, 2115-2123. 10.1093/molbev/msr029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober, D. A. and Edgar, B. A. (2002). Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 16, 2286-2299. 10.1101/gad.991102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puli, O. R., Gogia, N., Chimata, A. V., Yorimitsu, T., Nakagoshi, H., Kango-Singh, M. and Singh, A. (2024). Genetic mechanism regulating diversity in the placement of eyes on the head of animals. Proc. Natl. Acad. Sci. USA 121, e2316244121. 10.1073/pnas.2316244121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, A., Chimata, A. V. and Singh, A. (2020). Motif 1 Binding Protein suppresses wingless to promote eye fate in Drosophila. Sci. Rep. 10, 17221. 10.1038/s41598-020-73891-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready, D. F., Hanson, T. E. and Benzer, S. (1976). Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53, 217-240. 10.1016/0012-1606(76)90225-6 [DOI] [PubMed] [Google Scholar]
- Reh, T. A. and Hindges, R. (2018). MicroRNAs in retinal development. Annu. Rev. Vis. Sci. 4, 25-44. 10.1146/annurev-vision-091517-034357 [DOI] [PubMed] [Google Scholar]
- Rupaimoole, R. and Slack, F. J. (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16, 203-222. 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- Saedi, H., Waro, G., Giacchetta, L. and Tsunoda, S. (2024). miR-137 regulates PTP61F, affecting insulin signaling, metabolic homeostasis, and starvation resistance in Drosophila. Proc. Natl. Acad. Sci. USA 121, e2319475121. 10.1073/pnas.2319475121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, A., Gogia, N., Farley, K., Payton, L. and Singh, A. (2018). Characterization of a morphogenetic furrow specific Gal4 driver in the developing Drosophila eye. PLoS ONE 13, e0196365. 10.1371/journal.pone.0196365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, R., Lee, S., Senavirathne, G. and Lai, E. C. (2023). microRNAs in action: biogenesis, function and regulation. Nat. Rev. Genet. 24, 816-833. 10.1038/s41576-023-00611-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka, T., Weidberg, H., Pietrokovski, S. and Elazar, Z. (2011). Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 12, 226. 10.1186/gb-2011-12-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, A. K., Spurrier, J., Kuzina, I. and Giniger, E. (2019). Hyperactive innate immunity causes degeneration of dopamine neurons upon altering activity of Cdk5. Cell Rep. 26, 131-144.e134. 10.1016/j.celrep.2018.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber, J., Lim, D. A., Petritsch, C., Persson, A. I., Maunakea, A. K., Yu, M., Vandenberg, S. R., Ginzinger, D. G., James, C. D., Costello, J. F.et al. (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 6, 14. 10.1186/1741-7015-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. and Choi, K.-W. (2003). Initial state of the Drosophila eye before dorsoventral specification is equivalent to ventral. Development 130, 6351. 10.1242/dev.00864 [DOI] [PubMed] [Google Scholar]
- Singh, A. and Irvine, K. D. (2012). Drosophila as a model for understanding development and disease. Dev. Dyn. 241, 1-2. 10.1002/dvdy.23712 [DOI] [PubMed] [Google Scholar]
- Singh, A., Kango-Singh, M. and Sun, Y. H. (2002). Eye suppression, a novel function of teashirt, requires Wingless signaling. Development 129, 4271-4280. 10.1242/dev.129.18.4271 [DOI] [PubMed] [Google Scholar]
- Singh, A., Kango-Singh, M., Choi, K.-W. and Sun, Y. H. (2004). Dorso-ventral asymmetric functions of teashirt in Drosophila eye development depend on spatial cues provided by early DV patterning genes. Mech. Dev. 121, 365-370. 10.1016/j.mod.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Singh, A., Chan, J., Chern, J. J. and Choi, K.-W. (2005a). Genetic interaction of Lobe with its modifiers in dorsoventral patterning and growth of the Drosophila eye. Genetics 171, 169-183. 10.1534/genetics.105.044180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A., Lim, J. and Choi, K.-W. (2005b). Dorso-ventral boundary is required for organizing growth and planar polarity in the Drosophila eye. In “Planar Cell Polarization during Development: Advances in Developmental Biology and Biochemistry” (ed. Mlodzik M.), pp. 59-91. Elsevier Science & Technology Books. [Google Scholar]
- Singh, A., Shi, X. and Choi, K.-W. (2006). Lobe and Serrate are required for cell survival during early eye development in Drosophila. Development 133, 4771-4781. 10.1242/dev.02686 [DOI] [PubMed] [Google Scholar]
- Singh, A., Tare, M., Kango-Singh, M., Son, W.-S., Cho, K.-O. and Choi, K.-W. (2011). Opposing interactions between homothorax and Lobe define the ventral eye margin of Drosophila eye. Dev. Biol. 359, 199-208. 10.1016/j.ydbio.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A., Tare, M., Puli, O. R. and Kango-Singh, M. (2012). A glimpse into dorso-ventral patterning of the Drosophila eye. Dev. Dyn. 241, 69-84. 10.1002/dvdy.22764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A., Chimata, A. V., Deshpande, P., Bajpai, S., Sangeeth, A., Rajput, M. and Singh, A. (2024). SARS-CoV2 Nsp3 protein triggers cell death and exacerbates amyloid beta42-mediated neurodegeneration. Neural Regen. Res. 19, 1385-1392. 10.4103/1673-5374.382989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha, K., Singh, A. and Kango-Singh, M. (2021). Yorkie-Cactus (IkappaBalpha)-JNK axis promotes tumor growth and progression in Drosophila. Oncogene 40, 4124-4136. 10.1038/s41388-021-01831-4 [DOI] [PubMed] [Google Scholar]
- Song, Z., McCall, K. and Steller, H. (1997). DCP-1, a Drosophila cell death protease essential for development. Science 275, 536-540. 10.1126/science.275.5299.536 [DOI] [PubMed] [Google Scholar]
- Sorolla, A., Wang, E., Golden, E., Duffy, C., Henriques, S. T., Redfern, A. D. and Blancafort, P. (2020). Precision medicine by designer interference peptides: applications in oncology and molecular therapeutics. Oncogene 39, 1167-1184. 10.1038/s41388-019-1056-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensmeier, A. M., Tare, M., Puli, O. R., Modi, R., Nainaparampil, J., Kango-Singh, M. and Singh, A. (2013). Novel neuroprotective function of apical-basal polarity gene crumbs in amyloid beta 42 (aβ42) mediated neurodegeneration. PLoS ONE 8, e78717. 10.1371/journal.pone.0078717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl, G. and Basler, K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527-540. 10.1016/0092-8674(93)90072-X [DOI] [PubMed] [Google Scholar]
- Sun, G. Q., Ye, P., Murai, K., Lang, M.-F., Li, S., Zhang, H., Li, W., Fu, C., Yin, J., Wang, A.et al. (2011). miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2, 529. 10.1038/ncomms1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, S. and Verheyen, E. M. (2012). Wnt/Wingless signaling in Drosophila. Cold Spring Harbor Perspect. Biol. 4, a007930. 10.1101/cshperspect.a007930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Matakatsu, M. and Du, W. (2008). Direct control of the proneural gene atonal by retinal determination factors during Drosophila eye development. Dev. Biol. 313, 787-801. 10.1016/j.ydbio.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Matakatsu, M., Xu, J., Cheng, L. and Du, W. (2009). Regulation of apoptosis of rbf mutant cells during Drosophila development. Dev. Biol. 326, 347-356. 10.1016/j.ydbio.2008.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare, M., Puli, O. R. and Singh, A. (2013). Molecular genetic mechanisms of axial patterning: mechanistic insights into generation of axes in the developing eye. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 37-75. Springer NewYork Heidelberg Dordrecht London: Springer. [Google Scholar]
- Tare, M., Sarkar, A., Bedi, S., Kango-Singh, M. and Singh, A. (2016). Cullin-4 regulates Wingless and JNK signaling-mediated cell death in the Drosophila eye. Cell Death Dis. 7, e2566. 10.1038/cddis.2016.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare, M., Chimata, A. V., Gogia, N., Narwal, S., Deshpande, P. and Singh, A. (2020). An E3 ubiquitin ligase, cullin-4 regulates retinal differentiation in Drosophila eye. Genesis 58, e23395. 10.1002/dvg.23395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastsoglou, S., Alexiou, A., Karagkouni, D., Skoufos, G., Zacharopoulou, E. and Hatzigeorgiou, A. G. (2023). DIANA-microT 2023: including predicted targets of virally encoded miRNAs. Nucleic Acids Res. 51, W148-W153. 10.1093/nar/gkad283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman, J. E. and Rubin, G. M. (1995). wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121, 3519-3527. 10.1242/dev.121.11.3519 [DOI] [PubMed] [Google Scholar]
- Vaghf, A., Khansarinejad, B., Ghaznavi-Rad, E. and Mondanizadeh, M. (2022). The role of microRNAs in diseases and related signaling pathways. Mol. Biol. Rep. 49, 6789-6801. 10.1007/s11033-021-06725-y [DOI] [PubMed] [Google Scholar]
- Verghese, S., Bedi, S. and Kango-Singh, M. (2012). Hippo signalling controls Dronc activity to regulate organ size in Drosophila. Cell Death Differ. 19, 1664-1676. 10.1038/cdd.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnoi, A. and Rani, S. (2023). miRNA biogenesis and regulation of diseases: an updated overview. Methods Mol. Biol. 2595, 1-12. 10.1007/978-1-0716-2823-2_1 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Chen, R., Zhou, X., Guo, R., Yin, J., Li, Y. and Ma, G. (2020). miR-137: a novel therapeutic target for human glioma. Mol. Ther. Nucleic Acids 21, 614-622. 10.1016/j.omtn.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Zhang, J., Yin, J., Gan, Y., Xu, S., Gu, Y. and Huang, W. (2021). Alternative approaches to target Myc for cancer treatment. Signal. Transduct. Target Ther. 6, 117. 10.1038/s41392-021-00500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, J. and Kumar, J. P. (2023). Patterning of the Drosophila retina by the morphogenetic furrow. Front. Cell Dev. Biol. 11, 1151348. 10.3389/fcell.2023.1151348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley, H. C., Papmeyer, M., Romaniuk, L., Sprooten, E., Johnstone, E. C., Hall, J., Lawrie, S. M., Evans, K. L., Blumberg, H. P., Sussmann, J. E.et al. (2012). Impact of a microRNA MIR137 susceptibility variant on brain function in people at high genetic risk of schizophrenia or bipolar disorder. Neuropsychopharmacology 37, 2720-2729. 10.1038/npp.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, K., Grether, M. E., Abrams, J. M., Young, L., Farrell, K. and Steller, H. (1994). Genetic control of programmed cell death in Drosophila. Science 264, 677-683. 10.1126/science.8171319 [DOI] [PubMed] [Google Scholar]
- Wittkorn, E., Sarkar, A., Garcia, K., Kango-Singh, M. and Singh, A. (2015). The Hippo pathway effector Yki downregulates Wg signaling to promote retinal differentiation in the Drosophila eye. Development 142, 2002-2013. 10.1242/dev.117358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, T. and Ready, D. F. (1993). Pattern formation in the Drosophila retina. In The Development of Drosophila melanogaster (ed. Bate M. and Martinez Arias A.), pp. 1277-1325. Cold Spring Harbor Laboratory Press [Google Scholar]
- Won, J.-H., Tsogtbaatar, O., Son, W., Singh, A., Choi, K.-W. and Cho, K.-O. (2015). Correction: cell type-specific responses to wingless, hedgehog and decapentaplegic are essential for patterning early eye-antenna disc in Drosophila. PLoS ONE 10, e0128169. 10.1371/journal.pone.0128169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.