Abstract
Introduction
Tirbanibulin, a topical treatment for actinic keratosis (AK) with a novel antiproliferative mechanism of action, has demonstrated efficacy and a favorable tolerability profile in clinical trials and real-life studies. An insight into best-practice use of tirbanibulin in different clinical presentations of AK would be useful.
Objectives
The aim of this article was to deliver advice, via expert consensus, on the optimal use of tirbanibulin in the real-life management of AK.
Methods
A panel of 11 dermatologists with expertise in the treatment of AK convened to develop consensus statements about key aspects of tirbanibulin treatment in AK patients based on selected literature data and on their own clinical experience.
Results
Two areas were examined and discussed: clinical assessment/diagnostic procedures and key aspects of tirbanibulin therapy. A total of 19 statements concerning clinical evaluation of AK patients, routine and advanced diagnostic instruments, patient selection, treatment modalities, and key messages for patient communication were drafted and voted on.
Conclusion
Tirbanibulin should be considered as a first-line option for most patients with AK owing to its proven efficacy, good local and systemic tolerability, and a short treatment regimen of five days. All these factors encourage patient acceptability and treatment compliance. The favorable safety profile and tolerability of tirbanibulin also enable flexible therapeutic schedules, including repeated treatment cycles, if needed.
Keywords: Actinic keratosis, Tirbanibulin, Topical treatment, Expert opinion
Introduction
Actinic keratosis (AK) is a chronic skin disease characterized by the abnormal proliferation of epidermal keratinocytes that typically presents as scaly erythematous papules or hyperkeratotic plaques in chronically sun-exposed areas [1,2]. AK lesions are considered either a precursor or an initial and superficial form of cutaneous squamous cell carcinoma (cSCC), although low rates of progression to invasive cSCC have been reported, ranging from 0% to 0.075% per lesion-year [1,3]. Mutations in oncogenic and tumor-suppressing genes (e.g., p53, CDKN2A and PTEN) induced by cumulative ultraviolet B (UVB) radiation are believed to be the main factor in the pathogenesis of both AK and cSCC [1]. Important risk factors for the development of AK include male sex, age >45 years, light Fitzpatrick phototypes, and the use of photosensitizing drugs [1,4]. The concept of field cancerization (FC), generally referred to as an area of subclinical changes surrounding AK lesions that displays genetic changes similar to those found in AK, has diagnostic and prognostic implications and is an important aspect in the management of AK [1,3,5]. A vast array of treatments for AK, either lesion- or field-directed, is currently available, including topical drugs, surgical procedures, cryotherapy, laser ablation, and photodynamic therapy. However, many therapies are associated with important local or systemic adverse reactions, and they often require a long duration of treatment, resulting in poor patient adherence and suboptimal outcomes [1,4]. The recently introduced tirbanibulin ointment, a promising topical treatment option that targets both AK lesions and surrounding FC, has rapidly gained favor among dermatologists mainly due to its good tolerability and ease of use, with a short-term course of therapy (once daily application for five days) [1,3,6,7]. A first-in-class microtubule inhibitor, tirbanibulin targets tubulin polymerization and Src tyrosine kinase signaling, two pathways that are upregulated in AK and cSCC [6,7]. Tirbanibulin has demonstrated efficacy in clearing AK lesions, with a good tolerability profile, in large randomized clinical trials as well as in real-world studies and is listed among recommended options for the treatment of AK in all major international guidelines [1,7–11].
Given the chronic, relapsing nature of AK, the growing body of knowledge about its pathophysiology, and the increasing availability of treatment options, clinicians face many challenges in the long-term management of this disease. The aim of this consensus document was to deliver real-world guidance on the optimal use of tirbanibulin in the treatment of AK throughout the patient journey.
Methods
Eleven dermatologists with extensive clinical experience in the treatment of AK convened to discuss best-practice patient management and optimal use of tirbanibulin based on the presentation of exemplary clinical cases and the review of relevant literature data. The panel then developed expert recommendations in the form of statements, subsequently voted on, related to two areas: 1) clinical assessment of patients and real-life diagnostic procedures; 2) key aspects of tirbanibulin therapy, including patient selection, treatment modalities, and patient education. Only statements with a consensus of 100% were included.
Clinical Assessment and Diagnostic Procedures
Initial Patient Evaluation
AK can have multiform clinical presentations (including distinct clinical variants such as pigmented, bowenoid, or lichenoid AK), with lesions of variable thickness and size [1,12–14]. Hyperkeratotic lesions are best examined after applying an ointment containing keratolytic agents, since the thick layer of scaly tissue may mask the real aspect of the lesion. AK lesions are most visible on the face and scalp but can also be found on the limbs and trunk. AK lesions located on the lower lip or ears are considered high-risk lesions that are often resistant to treatment [1,9]. In addition to assessing the number, aspect, and location of the lesions, it is important to accurately evaluate the photodamaged skin surrounding the lesions, since severe FC identifies a patient population at high risk for recurrence of AK and the development of multiple cSCCs that requires a prompt and aggressive therapeutic approach [4,15,16]. There is evidence from the literature that several UVB-induced mutations and gene expression alterations that are frequently observed in photodamaged skin (e.g., deregulation of tumor suppressor genes p53, CDKN2A and PTEN, disruption of the RAF-1/MER/ERK cell survival pathway) become increasingly more severe in AK and SCC, suggesting that photodamaged skin, AK, and SCC may be part of a disease continuum that is manifested as FC [17,18]. Despite the lack of a clear and consistent definition of FC, an expert consensus based on an extensive review proposed a clinical definition of FC as “the anatomical area with or adjacent to AK and visibly sun-damaged skin identified by at least two of the following signs: telangiectasia, atrophy, pigmentation disorders and sand paper-like texture” [17]. Even without diagnostic instruments, therefore, perilesional skin should be assessed for visible signs of inflammation and UV damage. The initial clinical evaluation should also always consider patient-specific factors such as demographics, Fitzpatrick type, comorbidities, current medications, past clinical history (especially regarding skin cancer), previous AK treatments, and sun-protection behavior. Particular attention should be paid to immunosuppression, as immunocompromised patients, mainly organ transplant recipients, have a greatly increased risk of developing more severe AK and SCC, with worse treatment outcomes compared to immunocompetent AK patients. In fact, immunocompromised patients represent a distinct population requiring a dedicated treatment algorithm [1,19].
The complexity of AK features cannot be captured by rigid classification systems. In the Olsen clinical classification scale, one of the most commonly used parameters for assessing AK severity, single lesions are graded based on their thickness [20]. The Olsen scale has been used in most clinical trials of AK treatments and is a valuable tool for the assessment of individual lesions, but it does not consider the severity of FC. For a more complete assessment of AK severity that includes FC, the Actinic Keratosis Field Assessment Scale (AK-FAS) is preferable [15]. This validated tool, a 4-grade scale which evaluates AK area, hyperkeratosis, and sun damage, provides objective and clinically relevant information and is easy to use in routine practice.
Instrumental Diagnostic Techniques and Histopathology
Dermatoscopy is an easy-to-use, readily available, non-invasive technique that should always be part of the clinical assessment of AK. Dermatoscopy enables clinicians to visualize typical clinical aspects of AK (e.g., erythematous pseudonetwork, ‘strawberry pattern’, large white follicles, and yellow or whitish scales), and thus it helps in the differential diagnosis of AK and superficial basal cell carcinoma, lentigo melanoma, irritated seborrheic keratosis, or other inflammatory skin diseases [1,14,21]. Dermatoscopy also plays an important role in monitoring treatment response and identifying early signs of invasive SCC such the presence of peripheral radial lines or vessels in the context of typical AK signs [14,21]. Histopathological examination of biopsy specimens should be carried out when atypical lesions are present, especially if they show features that may suggest a diagnosis of cSCC or other types of skin cancer [1]. Although more advanced imaging techniques, such as reflectance confocal microscopy (RCM), optical coherence tomography (OCT), and line-field confocal OCT (LC-OCT), are not part of the routine clinical assessment, when available, they can provide useful information in specific cases. Overall, these non-invasive techniques help increase diagnostic accuracy and provide additional information on the microenvironment of AK lesions, enabling detection of subclinical changes [1,14,22,23]. They also play an important role in monitoring treatment response, thus optimizing patient management [1,14,24,25] (Figure 1).
Figure 1.
Reflectance confocal microscopy image of a small area of field cancerization on the right temporal area of a 63-year-old male with multiple AK lesions on the face and scalp and a history of chronic myelofibrosis and resected melanomas: (A) before treatment, showing atypical honeycomb pattern together with small bright round inflammatory cells; (B) at 29 days after starting treatment with tirbanibulin 1% ointment, showing partial restoration of the typical honeycomb pattern with no atypical features. (Courtesy of Dr. F. Venturi)
Expert consensus statements with recommendations on clinical assessment and diagnosis of AK are reported in Table 1(A).
Table 1.
Expert Consensus Statements on Optimal Use of Tirbanibulin in the Treatment of Actinic Keratosis.
| A) Clinical assessment/ diagnostic procedures |
|
| B) Treatment |
|
AK: actinic keratosis; FC: field cancerization; LC-OCT: line-field confocal optical coherence tomography; NMSC: non-melanoma skin cancer; OCT: optical coherence tomography; RCM: reflectance confocal microscopy; SCC: squamous cell carcinoma.
Treatment of AK with Tirbanibulin
AK is a chronic disease that requires regular surveillance and long-term, personalized management based on prevention (i.e., photoprotection) and therapy of both visible lesions and FC; compliance is crucial to treatment success. In the natural history of the disease, the evolution of AK lesions (e.g., persistence, resolution, or progression to cSCC) is unpredictable, and disease recurrence is the norm owing to the continuous presence of mutagenic disease drivers induced by UV radiation [2,4,13,26]. The main objective of AK therapy is still a matter of debate. Although prevention of progression to invasive cSCC is still generally considered the key reason for treatment, there is no actual evidence that treating of AK and FC decreases the risk of developing cSCC in immunocompetent patients without a previous skin cancer history [13,27]. Few studies have specifically investigated the incidence of cSCC over time in patients treated for AK. A secondary analysis of a randomized clinical trial found that the 4-year risk of developing cSCC in a previously treated AK area was 3.7%, increasing to 20.9% in patients with Olsen grade III lesions and to 33.5% in those with severe AK needing retreatment, while a retrospective cohort study that followed 5700 patients for five years after treatment with 5-fluorouracil or imiquimod found no significant difference between the two treatments in the 2- or 5-year cumulative risk for a subsequent keratinocyte carcinoma [28,29]. However, since these studies did not include a control arm of untreated patients, the impact of AK treatment per se on the risk of developing cSCC remains unclear. Aside from the potential reduction in the risk of progression, the treatment goals of AK therapy are sustained total or partial clearance of visible lesions and improvement in FC. Field-directed therapies are currently the preferred option for most clinical cases of AK since, unlike lesion-directed treatments, they can target the subclinical changes underlying FC. However, many field therapies with proven efficacy (e.g., photodynamic therapy or 5-fluorouracil) are associated with adverse events such as intense local reactions or pain, which restrict their use and impact adherence [1,3–5]. Tirbanibulin, a topical ointment approved in the US (2020) and Europe (2021) for the treatment of AK on the face or scalp, has demonstrated significant clinical potential owing to its efficacy and good tolerability, supported by a novel mechanism of action, as well as to its short treatment course [1,3,6].
Evidence of Tirbanibulin Efficacy and Tolerability from Clinical Studies
In the phase III registration trials (two identical multicenter randomized double-blind placebo-controlled clinical studies enrolling a total of 702 patients with AK on the face or scalp), the percentage of patients with complete clearance (100% reduction in the number of lesions, as assessed at day 57 after completion of a 5-day regimen) was significantly higher for patients treated with tirbanibulin than for those who received control ointment (44% and 54% in trial 1 and 2, respectively, vs 5% and 13%, P<0.001 for both trails), while the percentages of patients with partial clearance (≥75% reduction in the number of lesions) were 68% and 76% vs 16% and 20%, respectively; P<0.001) [8]. At 1-year follow-up, the estimated percentage of tirbanibulin recipients with recurrent lesions, among those who had experienced complete clearance, was 47%. Tirbanibulin was well tolerated. Local reactions, including erythema, flaking/scaling, pruritus, and pain at the application site, were mostly graded as mild or moderate and resolved spontaneously. Unlike with most topical therapies, severe local reactions (e.g., vesicles/pustules, erosion/ulceration) were infrequent. As expected from the negligible absorption rate of tirbanibulin, systemic adverse events were uncommon. Notably, none of the patients discontinued treatment for tirbanibulin-related adverse events [8]. Post-hoc analyses of these phase 3 trials suggested that comorbidities and concomitant treatments (which were common in the elderly population enrolled in the studies) do not affect the efficacy and tolerability of tirbanibulin. Also, no correlation was found between the number of AK lesions at baseline and the severity of local reactions in tirbanibulin-treated patients [30]. The efficacy and favorable tolerability profile of tirbanibulin as well as the high rates of treatment compliance have been confirmed in several real-life studies, including case series and prospective or retrospective observational studies [9–11,30,31]. Despite their limitations (e.g., lack of a control group, small number of cases in some reports), the findings of real-life studies offer an insight into the effects of tirbanibulin in a much wider spectrum of patients and clinical presentations compared with the populations enrolled in randomized clinical trials. Although the European indication of tirbanibulin is for treatment of non-hyperkeratotic, non-hypertrophic AK (Olsen grade I) of the face or scalp, its use has been successfully extended to a range of clinical conditions, including AK patients with Olsen grade II or III lesions, AK lesions located on the trunk or limbs, or AK in organ transplant recipients [9,30,31]. High rates of patient satisfaction with tirbanibulin (as assessed by the Treatment Satisfaction Questionnaire for Medication) have also been reported, especially in domains related to treatment tolerability and convenience [10].
The efficacy and favorable tolerability profile of tirbanibulin are believed to be related to the novel mechanism of action of this molecule. Preclinical studies show that tirbanibulin, a potent antiproliferative agent, induces cell cycle arrest at G2/M phase and triggers programmed cell death of keratinocytes (via activation of intrinsic and extrinsic apoptotic pathways) by reversibly binding to tubulin, thus inhibiting tubulin polymerization, and by disrupting Src intracellular trafficking and Src-mediated signaling. Another possible antiproliferative mechanism is the induction of tumor suppressor p53 expression. Tirbanibulin antiproliferative effects have been demonstrated in several tumor cell lines, including SCC, suggesting that keratinocytes with an aberrant cell growth rate may be selectively targeted by tirbanibulin [32,33]. As for the mechanisms accounting for tolerability, tirbanibulin displayed an apoptotic effect without inducing overt toxicity in vitro owing to its fully reversible binding to tubulin [34]. The induction of apoptosis has also been demonstrated in a study using line-field confocal optical coherence tomography and confirmed by histopathology [35]. The severe adverse local reactions caused by many AK treatments are known to be triggered by the release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8). Notably, pro-inflammatory response assays show that tirbanibulin induces only a small increase in IL-8, unlike the significant increase in both TNF-α and IL-8 caused by 5-fluorouracil [32]. These data suggest a milder pro-inflammatory effect of this molecule compared with 5-fluorouracil, which could explain the favorable tolerability and safety profile observed in tirbanibulin-treated patients.
Patient Selection and Clinical Scenarios
As demonstrated in clinical trials and real-life studies, tirbanibulin is effective in achieving high clearance rates of AK lesions, with fewer severe local skin reactions compared with other commonly used topical treatments for AK. Importantly, tirbanibulin’s mechanism of action targets oncogenic pathways that are believed to be key factors in the progression from AK to SCC. The short duration of treatment is another aspect of tirbanibulin therapy which is relevant to real-life practice since, together with its favorable tolerability profile, it facilitates patient compliance, thus enhancing the chance of a successful outcome. Acceptance of therapy and treatment adherence are important considerations in real-world practice and are often underestimated when selecting AK therapies based on evidence derived solely from clinical trials [36]. In a systematic review investigating adherence and persistence associated with topical AK therapies, combined non-adherence and non-persistence rates were found to be as high as 88%, and key contributing factors were identified as duration of treatment, severity and persistence of local skin reactions, and patient confusion regarding treatment regimens [37]. Consistently with the data reported in clinical trials and real-life studies, this expert panel suggests that tirbanibulin should be considered as a first-line treatment for most AK patients, both naïve and previously treated, because of its efficacy, short treatment regimen, and favorable tolerability profile. As widely acknowledged, appropriate management of AK should consider patient preferences, clinical presentation (including localization, extension and severity of lesions and FC, duration and severity of symptoms) and patient characteristics (e.g., general health, comorbidities, risk factors for skin cancer) [4,12,27]. In the expert panel’s experience, tirbanibulin has been successfully used in a wide range of clinical scenarios, including isolated or multiple AK lesions of varying severity (Olsen grade I and II) located in different areas of the face and scalp, extensive FC, patients who had received multiple AK therapies, and those with a history of chronic sun damage. In particular, tirbanibulin therapy has been selected as the treatment of choice in subjects with comorbidities and those with a history of skin cancer owing to its tolerability and lack of systemic adverse events. The efficacy of tirbanibulin appears to be maintained in difficult-to-treat sites, including ears, lips, and limbs, and in areas with adnexa or pigmented lesions. Examples of tirbanibulin-treated clinical cases with various AK presentations are illustrated in Figures 2–5. Even in cases where total clearance of AK lesions was not achieved, a substantial clinical improvement in both lesions and FC was observed. According to the authors’ clinical experience, tirbanibulin therapy is readily accepted by patients, with a high degree of satisfaction and treatment compliance. Local reactions (mostly erythema and flaking/scaling) are generally mild, peak at approximately day 8 after treatment initiation (consistently with clinical study reports), and resolve spontaneously over an average of 10 days, without sequelae. Some patients experience a burning or itching sensation at the application site. The intensity of local reactions seems to be correlated to the extent of inflammation and photodamage in the treated field (Figure 3). However, severe local reactions such as swelling or erosion/ulceration are rarely observed. In addition to clearing or reducing AK signs, tirbanibulin treatment was also found to be associated with cosmetic benefits, such as improvement in skin quality and texture and a more uniform complexion (Figures 4 and 5). These emerging potential effects of tirbanibulin in targeting signs of skin aging, especially hyperpigmentation, have been investigated in recent preliminary studies [38,39]. In particular, tirbanibulin, unlike other AK therapies, appears to exert a skin-lightening effect on solar lentigines, hyperpigmented areas that are frequently observed in the context of AK and FC [39]. Although the mechanisms of action underlying these effects need to be further elucidated, the potential benefit of cosmetic improvement induced by tirbanibulin therapy should be mentioned to patients, as it may represent an additional motivation for treatment, further enhancing compliance.
Figure 2.
Images of AK lesions (Olsen grade I–II) in two small fields of cancerization on the scalp of a 75-year-old male with a long-standing history (>5 years) of AK on the scalp, previously treated with cryotherapy, topical diclofenac gel, and daylight photodynamic therapy: (A) before tirbanibulin treatment; (B) at the 2-month follow-up after treatment with tirbanibulin 1% ointment, showing complete clearance of occipital area and residual AK lesions (Olsen grade I) in the parietal field. The total area covered by tirbanibulin measured 26 cm2 (2x3 cm=6 cm2 for the occipital area and 4x5 cm=20 cm2 for the parietal area). (Courtesy of Dr. G. Nazzaro)
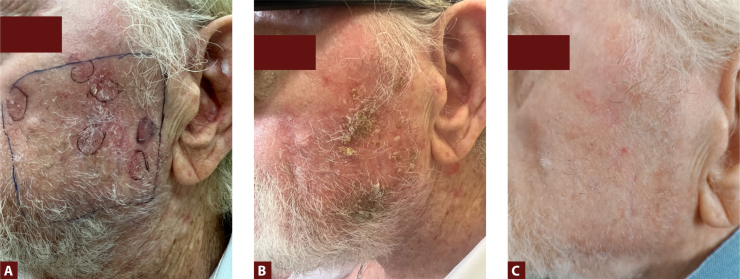
Figure 3.
Images of AK lesions (Olsen grade I–II) in a large field of cancerization on the left cheek of a 76-year-old male with a history of basal cell carcinomas on the trunk and limbs and with AK of the face and scalp (previously treated with physical therapies and topical drugs, with partial results): (A) before tirbanibulin treatment; (B) at 10 days after the end of treatment with tirbanibulin 1% ointment, showing erythema and scaling/flaking (the patient reported a burning sensation in the application area, but no intervention was required); (C) at 60 days after the end of treatment, showing complete clearance of the AK lesions (both Olsen grade I and II) and resolution of the local adverse reactions. (Courtesy of Dr. A. Villani)
Figure 4.
Images of multiple AK lesions (Olsen grade I–II), field of cancerization, and actinic cheilitis on the face of a 70-year-old treatment- naïve woman: A) before treatment, in the foreground square close-up; B) at four months after completion of 4 cycles of tirbanibulin 1% ointment (standard treatment of five days per cycle), showing efficacy on AK lesions, FC, and actinic cheilitis (with a close-up in the foreground square) as well as improvement in skin quality, with reduction in dyspigmentation and a more uniform complexion. A sequential treatment scheduled was adopted, with four cycles 10 to 30 days apart, as follows: cycle 1→right forehead and nose, cycle 2→left forehead and nose, cycle 3→right cheek, cycle 4→left cheek. (Courtesy of Dr. S. Guida)
Figure 5.
Images (with dermatoscopic view) of AK lesions (Olsen grade I–II) in the right frontotemporal region of an 83-year-old male, Fitzpatrick skin type I, with a history of chronic sun exposure and an extensive field of cancerization involving the head and neck which was treated with cycles of oral nicotinamide 250 mg four times daily. AK lesions had previously been treated with imiquimod 5% and piroxicam and sun filters for local use. The patient also had comorbid ischemic heart disease and depression (treated with calcium antagonists, clopidogrel, and sertraline) and had undergone surgery for a pigmented basal cell carcinoma on the forehead. (A) Before tirbanibulin treatment; (B) at 12 days after starting treatment with tirbanibulin 1% ointment, showing mild, well-tolerated local reactions (erythema and flaking); (C) at 58 days after treatment, showing complete clearance of the AK lesion. (Courtesy of Prof. E. Campione)
Treatment Modalities and Patient Education
The area of skin to be treated with tirbanibulin ointment depends on the distribution of AK lesions and FC on the face and scalp. In line with current indications, a thin layer of tirbanibulin 1% ointment should be applied to cover a 25 cm2 treatment field in each of four anatomical areas (scalp, forehead, left/right cheek with ear, nose, and chin). When treatment fields are not in close proximity, the total area to be covered by tirbanibulin can be divided into smaller sections, as shown in Figure 2. Addressing the needs of patients with large AK fields to be treated, recent studies have evaluated the pharmacokinetics and tolerability of tirbanibulin ointment when applied to fields larger than 25 cm2, supporting its use (as one 350 mg sachet applied daily) in fields measuring up to 100 cm2 [40,41]. The safety and tolerability profiles of tirbanibulin when used at approximately 100 cm2 fields were found to be consistent with those reported for smaller fields, and exploratory efficacy data also suggest similar benefits to those observed with the application of tirbanibulin over a more restricted area [41]. According to this expert panel’s experience, more than 25 cm2 can be treated by one sachet of tirbanibulin 1% ointment.
The standard period of treatment with tirbanibulin is five days. However, owing to the favorable tolerability profile of the drug, various protocols are possible. Forward planning of the treatment regimen (including repeated treatment of severely affected areas) is always advisable. When treating large fields (>25 cm2), sequential or simultaneous therapeutic schedules can be considered. Up to four consecutive treatment cycles one to two months apart can be planned if needed, based on disease and patient characteristics. Multiple treatment cycles can be performed in immunocompromised patients, those with a history of skin cancer, and those with advanced photodamage.
Good communication with the patient is essential in the long-term management of AK. Clinicians should understand the patient’s priorities and concerns, setting realistic treatment expectations [26]. Patient should be informed about the chronic nature of AK, the unpredictable evolution of AK lesions, and the high rates of recurrence, hence the need for repeated treatment courses over time. It is particularly important to inform patients about the occurrence and transient nature of local skin reactions associated with tirbanibulin treatment. Although skin reactions are usually mild, severe local adverse events may occur, and patients need reassurance that these will eventually resolve without sequelae. Correct treatment modalities are another important aspect of care that can affect the outcome of therapy. Patients should be given detailed instructions about tirbanibulin application at home.
Expert consensus statements with recommendations on tirbanibulin treatment are reported in Table 1(B).
Conclusion
With its novel mechanism of action, proven efficacy, good tolerability, and short duration of therapy, tirbanibulin is an important treatment for the management of AK and should be considered as a first-line option for most patients, including treatment-naïve and pre-treated subjects as well as those with difficult-to-treat lesions, comorbid conditions, or a history of previous skin cancer. Flexible therapeutic protocols with repeated treatment cycles are made possible by the favorable tolerability profile of this topical drug. In clinical studies as well as in routine clinical practice, tirbanibulin therapy is associated with high rates of patient acceptability and treatment compliance, thus increasing the chance of a successful outcome.
Footnotes
Key Message: Tirbanibulin use in the treatment of actinic keratosis is expanding owing to its proven efficacy, tolerability, and short treatment regimen. This expert consensus provides guidance on optimal use of tirbanibulin in real-life clinical practice.
Competing Interests: MA reports speaker fees from Almirall, La Roche-Posay, and Cantabria and advisory boards for Almirall. EC reports speaker fees from Almirall, Amgen, BMS, and UCB and advisory boards for Almirall, Amgen, BMS, Cantabria, and UCB. CL reports speaker fees from Almirall Hermal and advisory boards for Regeneron. GN reports speaker fees from Giuliani, Ideka, and Novartis and advisory boards for Almirall, L’Oreal, and Novartis. KP reports grants from Abbvie, Almirall, Lilly, Novartis, and Sanofi, consulting fees from Galderma, Leo Pharma, Lilly, and Sanofi, and participation in advisory boards for Abbvie, Almirall, Biogen, Galderma, Leo Pharma, Lilly, MSD, Pierre Fabre, Regeneron, Sun Pharma, Janssen, and Sanofi. AV reports speaker fees from Almirall. IZ reports speaker fees from Almirall Hermal, Sanofi, La Roche-Posay, Bioderma, Canova, MSD, Sanofi Genzyme, and FotoFinder and advisory boards for Philogen and Regeneron. SG reports speaker fees from L’Oreal and Almirall and consultation fees from Istituto Ganassini, Merz Aesthetics. GA, GM, and FV report no conflicts of interest.
Authorship: All authors have contributed significantly to this publication.
Funding: This article was sponsored by Almirall S.p.A. Assistance with coordination of the consensus process and medical writing was provided by Editamed and funded by Almirall S.p.A.
References
- 1.Kandolf L, Peris K, Malvehy J, et al. European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology and Union of Medical Specialists (Union Européenne des Médecins Spécialistes) European consensus-based interdisciplinary guideline for diagnosis, treatment and prevention of actinic keratoses, epithelial UV-induced dysplasia and field cancerization on behalf of European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology and Union of Medical Specialists (Union Européenne des Médecins Spécialistes) J Eur Acad Dermatol Venereol. 2024;38(6):1024–1047. doi: 10.1111/jdv.19897. [DOI] [PubMed] [Google Scholar]
- 2.Malvehy J, Stratigos AJ, Bagot M, Stockfleth E, Ezzedine K, Delarue A. Actinic keratosis: Current challenges and unanswered questions. J Eur Acad Dermatol Venereol. 2024;38(Suppl 5):3–11. doi: 10.1111/jdv.19559. [DOI] [PubMed] [Google Scholar]
- 3.Valenti M, Bianco M, Narcisi A, Costanzo A, Borroni R, Ardigò M. Topical Pharmacological Treatment of Actinic Keratoses: Focus on Tirbanibulin 1% Ointment. Dermatol Pract Concept. 2024;14(3 S1) doi: 10.5826/dpc.1403S1a145S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcuri D, Ramchatesingh B, Lagacé F, et al. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. Int J Mol Sci. 2023;24(5):4989. doi: 10.3390/ijms24054989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal I, Puyana C, Chandan N, Jetter N, Tsoukas M. Field Cancerization Therapies for the Management of Actinic Keratosis: An Updated Review. Am J Clin Dermatol. 2024;25(3):391–405. doi: 10.1007/s40257-023-00839-8. [DOI] [PubMed] [Google Scholar]
- 6.Dao DD, Sahni VN, Sahni DR, Balogh EA, Grada A, Feldman SR. 1% Tirbanibulin Ointment for the Treatment of Actinic Keratoses. Ann Pharmacother. 2022;56(4):494–500. doi: 10.1177/10600280211031329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen DB, Dellavalle RP, Frazer-Green L, Schlesinger TE, Shive M, Wu PA. Focused update: Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2022;87(2):373–374.e5. doi: 10.1016/j.jaad.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Kempers S, Lain E, et al. Phase 3 Tirbanibulin for Actinic Keratosis Group. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N Engl J Med. 2021;384(6):512–520. doi: 10.1056/NEJMoa2024040. [DOI] [PubMed] [Google Scholar]
- 9.Nazzaro G, Carugno A, Bortoluzzi P, et al. Efficacy and tolerability of tirbanibulin 1% ointment in the treatment of cancerization field: a real-life Italian multicenter observational study of 250 patients. Int J Dermatol. 2024;63(11):1566–1574. doi: 10.1111/ijd.17168. [DOI] [PubMed] [Google Scholar]
- 10.Campione E, Rivieccio A, Gaeta Shumak R, et al. Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals (Basel) 2023;16(12):1686. doi: 10.3390/ph16121686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchberger MC, Gfesser M, Erdmann M, Schliep S, Berking C, Heppt MV. Tirbanibulin 1% Ointment Significantly Reduces the Actinic Keratosis Area and Severity Index in Patients with Actinic Keratosis: Results from a Real-World Study. J Clin Med. 2023;12(14):4837. doi: 10.3390/jcm12144837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209–e233. doi: 10.1016/j.jaad.2021.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair R, Baker C, Spelman L, Supranowicz M, MacMahon B. A review of actinic keratosis, skin field cancerisation and the efficacy of topical therapies. Australas J Dermatol. 2021;62(2):119–123. doi: 10.1111/ajd.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conforti C, Ambrosio L, Retrosi C, et al. Clinical and Dermoscopic Diagnosis of Actinic Keratosis. Dermatol Pract Concept. 2024;14(3 S1):e2024147S. doi: 10.5826/dpc.1403S1a147S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dréno B, Cerio R, Dirschka T, et al. A Novel Actinic Keratosis Field Assessment Scale for GradingActinic Keratosis Disease Severity. Acta Derm Venereol. 2017;97(9):1108–1113. doi: 10.2340/00015555-2710. [DOI] [PubMed] [Google Scholar]
- 16.Willenbrink TJ, Ruiz ES, Cornejo CM, Schmults CD, Arron ST, Jambusaria-Pahlajani A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83(3):709–717. doi: 10.1016/j.jaad.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 17.Figueras Nart I, Cerio R, Dirschka T, et al. Progressing Evidence in AK (PEAK) Working Group. Defining the actinic keratosis field: a literature review and discussion. J Eur Acad Dermatol Venereol. 2018;32(4):544–563. doi: 10.1111/jdv.14652. [DOI] [PubMed] [Google Scholar]
- 18.Tan JM, Lambie D, Sinnya S, et al. Histopathology and reflectance confocal microscopy features of photodamaged skin and actinic keratosis. J Eur Acad Dermatol Venereol. 2016;30(11):1901–1911. doi: 10.1111/jdv.13699. [DOI] [PubMed] [Google Scholar]
- 19.Szeimies RM, Ulrich C, Ferrándiz-Pulido C, et al. The “Personalising Actinic Keratosis Treatment for Immunocompromised Patients” (IM-PAKT) Project: An Expert Panel Opinion. Dermatol Ther (Heidelb) 2024;14(7):1739–1753. doi: 10.1007/s13555-024-01215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen EA, Abernethy ML, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24(5 Pt 1):738–43. doi: 10.1016/0190-9622(91)70113-g. [DOI] [PubMed] [Google Scholar]
- 21.Zalaudek I, Giacomel J, Schmid K, et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: a progression model. J Am Acad Dermatol. 2012;66(4):589–97. doi: 10.1016/j.jaad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Cinotti E, Tognetti L, Cartocci A, et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: a descriptive study. Clin Exp Dermatol. 2021;46(8):1530–1541. doi: 10.1111/ced.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruini C, Schuh S, Gust C, et al. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers (Basel) 2021;13(12):2856. doi: 10.3390/cancers13122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacarrubba F, Verzì AE, Polita M, Aleo A, Micali G. Line-field confocal optical coherence tomography in the treatment monitoring of actinic keratosis with tirbanibulin: A pilot study. J Eur Acad Dermatol Venereol. 2023;37(9):e1131–e1133. doi: 10.1111/jdv.19147. [DOI] [PubMed] [Google Scholar]
- 25.Venturi F, Veronesi G, Baraldi C, Dika E. Reflectance confocal microscopy as noninvasive tool for monitoring tirbanibulin efficacy in actinic keratosis. Photodiagnosis Photodyn Ther. 2024;48:104235. doi: 10.1016/j.pdpdt.2024.104235. [DOI] [PubMed] [Google Scholar]
- 26.Morton C, Baharlou S, Basset-Seguin N, et al. Expert Recommendations on Facilitating Personalized Approaches to Longterm Management of Actinic Keratosis: The Personalizing Actinic Keratosis Treatment (PAKT) Project. Acta Derm Venereol. 2023;103:adv6229. doi: 10.2340/actadv.v103.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Berker D, McGregor JM, Mohd Mustapa MF, Exton LS, Hughes BR. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- 28.Ahmady S, Jansen MHE, Nelemans PJ, et al. Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022;158(6):634–640. doi: 10.1001/jamadermatol.2022.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: A cohort study. J Am Acad Dermatol. 2019;80(4):998–1005. doi: 10.1016/j.jaad.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellacani G, Schlesinger T, Bhatia N, et al. Efficacy and safety of tirbanibulin 1% ointment in actinic keratoses: Data from two phase-III trials and the real-life clinical practice presented at the European Academy of Dermatology and Venereology Congress 2022. J Eur Acad Dermatol Venereol. 2024;38(Suppl 1):3–15. doi: 10.1111/jdv.19636. [DOI] [PubMed] [Google Scholar]
- 31.Iglesias-Puzas Á, Conde-Taboada A, Campos-Muñoz L, Sirgado-Martínez A, López-Bran E. 1% Tirbanibulin Ointment for Actinic Keratoses on Upper Extremities: A Retrospective Case Review Study. Acta Derm Venereol. 2023;103:adv15296. doi: 10.2340/actadv.v103.15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlesinger T. Commentary: Impact of Prior Treatment in the Efficacy and Tolerability of Tirbanibulin Ointment 1% for Actinic Keratosis: Pooled Results from Two Phase III Studies. J Clin Aesthet Dermatol. 2022;15(10 Suppl 1):S11–S12. [PMC free article] [PubMed] [Google Scholar]
- 33.Smolinski MP, Bu Y, Clements J, et al. Discovery of Novel Dual Mechanism of Action Src Signaling and Tubulin Polymerization Inhibitors (KX2-391 and KX2-361) J Med Chem. 2018;61(11):4704–4719. doi: 10.1021/acs.jmedchem.8b00164. [DOI] [PubMed] [Google Scholar]
- 34.Niu L, Yang J, Yan W, et al. Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1’s low clinical toxicity. J Biol Chem. 2019;294(48):18099–18108. doi: 10.1074/jbc.RA119.010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacarrubba F, Verzì AE, Broggi G, Micali G. Line-field confocal optical coherence tomography shows in vivo apoptosis during a novel treatment for actinic keratosis: A histopathologic controlled study. J Eur Acad Dermatol Venereol. 2025;39(1):e60–e62. doi: 10.1111/jdv.20106. [DOI] [PubMed] [Google Scholar]
- 36.Pariser DM. Approaches to Field Therapy for Actinic Keratoses: Relating Clinical Trial Results to Real-world Practice-A Commentary. J Clin Aesthet Dermatol. 2022;15(4):40–43. [PMC free article] [PubMed] [Google Scholar]
- 37.Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: A literature review. J Dermatolog Treat. 2016;27(6):538–545. doi: 10.1080/09546634.2016.1178372. [DOI] [PubMed] [Google Scholar]
- 38.Li Pomi F, Peterle L, d’Aloja A, Di Tano A, Vaccaro M, Borgia F. Anti-aging Effects of Tirbanibulin 1% Ointment: A Real-Life Experience. Dermatol Ther (Heidelb) 2024;14(6):1683–1696. doi: 10.1007/s13555-024-01178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Pomi F, d’Aloja A, Rottura M, Vaccaro M, Borgia F. The Skin-Lightening Power of Tirbanibulin 1% Ointment. Dermatol Ther (Heidelb) 2025;15(1):95–110. doi: 10.1007/s13555-024-01310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuBois J, Jones TM, Lee MS, et al. Pharmacokinetics, Safety, and Tolerability of a Single 5-Day Treatment of Tirbanibulin Ointment 1% in 100 cm2: A Phase 1 Maximal-Use Trial in Patients with Actinic Keratosis. Clin Pharmacol Drug Dev. 2024;13(2):208–218. doi: 10.1002/cpdd.1368. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia N, Lain E, Jarell A, et al. Safety and tolerability of tirbanibulin ointment 1% treatment on 100 cm2 of the face or scalp in patients with actinic keratosis: A phase 3 study. JAAD Int. 2024;17:6–14. doi: 10.1016/j.jdin.2024.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]