Abstract
Punch biopsy specimens from Mycobacterium ulcerans disease lesions were used to compare the sensitivities and specificities of direct smear, culture, PCR, and histopathology in making a diagnosis of M. ulcerans disease in a field setting. PCR for the insertion element IS2404 was modified to include uracil-N-glycosylase and deoxyuridine triphosphate instead of deoxythymidine triphosphate to reduce the risk of cross contamination. The “gold standard” for confirmation of clinically diagnosed Buruli ulcer was a definite histological diagnosis, a positive culture for M. ulcerans, or a smear positive for acid-fast bacilli (AFB), together with a possible histological diagnosis. For 70 clinically diagnosed cases of M. ulcerans disease, the modified PCR was 98% sensitive and gave a rapid result. The sensitivities of microscopy, culture, and histology were 42%, 49%, and 82%, respectively. The use of a 4-mm punch biopsy specimen was preferred to a 6-mm punch biopsy specimen since the wound was less likely to bleed and to need stitching. Given adequate technical expertise and the use of controls, the PCR was viable in a teaching hospital setting in Ghana; and in routine practice, we would recommend the use of Ziehl-Neelsen staining of biopsy specimens to detect AFB, followed by PCR, in AFB-negative cases only, in order to minimize costs. Histology and culture remain important as quality control tests, particularly in studies of treatment efficacy.
Mycobacterium ulcerans disease (Buruli ulcer) manifests as a nodule, papule, plaque, or edematous lesion prior to ulceration (5). The disease has been reported in more than 31 countries, mostly countries with a tropical climate (2). The recommended treatment has been surgical excision, but areas of endemicity in tropical countries are often rural, with unmade roads and relatively poor health care facilities (5).
The clinical diagnosis of Buruli ulcer is relatively easy when a child from an area of endemicity presents looking well with a painless ulcer eroding into subcutaneous fat and the ulcer has undermined edges (2); but the disease can affect people of any age, and other ulcers can be mistaken for Buruli ulcer, particularly when they are around the ankles, for example, venous ulcer, cutaneous leishmaniasis, neurogenic ulcer, yaws, tropical ulcer, fungal lesions, and squamous cell carcinoma (7). Diagnosis is more difficult in the case of nodules, and the sensitivity of clinical diagnosis in experienced hands was 48% to 52% in one study (5). Confirmation of the clinical diagnosis of Buruli ulcer is becoming increasingly important now that there is growing evidence of a beneficial effect from treatment with antibiotics (4), which is beginning to replace excision surgery as the standard treatment. In the case of an ulcer, the diagnostic technique most often available in areas of endemicity is Ziehl-Neelsen (ZN) staining for acid-fast bacilli (AFB) of a smear from a swab taken from below the undermined edge of the ulcer base, but its sensitivity is low (40%) (16) and the technique cannot be used for nonulcerated lesions. Culture for M. ulcerans has a sensitivity (35 to 50%) similar to that of ZN staining for AFB (13, 16), but it is not widely available and takes up to 6 weeks for a result. Histology, which is more sensitive (63 to 90%) (7, 18) and has been performed on excised lesions, but it is not readily available in most regions where Buruli ulcer is prevalent.
Simple rapid tools for confirmation of the diagnosis without having to excise lesions need to be developed. In the present study we report on the sensitivity, specificity, and predictive values for diagnosis when punch biopsy specimens taken from 70 clinically diagnosed M. ulcerans lesions were examined by staining for acid-fast bacilli, culture, histology, and a modified PCR for the IS2404 repeat sequence, which is unique to M. ulcerans DNA (22) and which occurs 249 times in the genome of M. ulcerans (20).
MATERIALS AND METHODS
Clinical specimens.
From September 2003 to June 2004 skin biopsy specimens were obtained from subjects at St. Martin's Hospital, Agroyesum, Nkawie Hospital, and Tepa Hospital in districts in Ghana where Buruli ulcer is endemic. Punch biopsy specimens were obtained from subjects with a strong clinical suspicion of Mycobacterium ulcerans disease prior to treatment of the lesion by excision. Lesions were grouped as nonulcerative (nodules, plaques, or edematous lesions) or ulcers. A nodule was a localized subcutaneous swelling attached to the skin that was painless and firm (usually less than 10 cm in diameter). Plaques were firm, slightly raised lesions with irregular edges and were more than 10 cm in diameter. Edematous disease was characterized by painless, nonpitting swelling that spread rapidly from a central nodule or and which ulcerated and enlarged over a period of days to weeks. Ulcers were painless and had the characteristic appearances, including undermined edges (2).
By using 4- or 6-mm punch biopsy needles (Stiefel Laboratories, United Kingdom), biopsy specimens were taken within 1 to 2 cm of the center of a nodule or from the edge of the viable tissue when there was ulceration. Biopsy specimens were taken after the patient had been anesthetized, immediately before complete excision, which was being carried out as definitive treatment. Informed consent was obtained before the operation. Tissue was placed in sterile 2-ml skirted tubes with an “O” ring (Sarstedt, United Kingdom); and one biopsy specimen was used for microscopy and culture, one was used for histology, and one was used for PCR. Each sample for microscopy and culture was processed within 24 h; samples for histology were placed in 10% formal saline for later analysis; samples for PCR were stored at −20°C. Microscopy, culture, and PCR were performed at the Komfo Anokye Teaching Hospital in Ghana, while histological examination was undertaken at St. Thomas's Hospital in London. All laboratory tests were performed in a blinded fashion.
Microscopy and culture.
Biopsy specimens were cut into pieces with a scalpel blade and homogenized with a sterile mortar and pestle in 2 ml of normal saline. ZN-stained smears of supernatants were examined under a microscope. The supernatant was removed, and the tissue homogenate was decontaminated by the sodium hydroxide (modified Petroff) method (21). Briefly, an equal volume of 1 M NaOH was added to 1 ml of specimen, and the mixture was left to stand for 10 min with occasional shaking and was then neutralized with 1 ml of 1 M HCl. The mixture was centrifuged at 3,000 × g for 20 min, and the supernatant was removed. A total of 0.1 ml of the sediment was inoculated onto each of two Löwenstein-Jensen (LJ) slopes. The LJ slopes were incubated at 31°C, and the cultures were examined weekly until visible growth occurred. The slopes were discarded after 6 months of incubation. Cultures positive for M. ulcerans were subcultured on LJ slopes. ZN staining was done to confirm the presence of AFB in positive cultures, and the specificity was confirmed by PCR for the insertion sequence IS2404 of M. ulcerans, as described below.
DNA preparation.
DNA extraction was done by the guanidinium thiocyanate (GuSCN)-diatoms method, as described elsewhere (3, 8, 14). Briefly, 20 to 40 mg of tissue was minced with a sterile scalpel blade, and a solution containing 0.1 mg proteinase K in digestion buffer was added to the tissue to give final concentrations of 0.5% Triton X-100, 20 mM Tris-HCl, pH 8.3, and 1 mM EDTA. The suspension was incubated at 50 to 55°C for 18 h. To 100 μl of sample was added 900 μl lysis buffer L6 (5 M GuSCN in 50 mM Tris HCl, pH 6.4, 20 mM EDTA, 0.1% Triton X-100) in a 1.5-ml screw-cap vial. Twenty microliters of diatom suspension (10 g high-purity analytical grade Celite [Sigma-Aldrich, United Kingdom] in 50 ml H2O, 500 μl 32% [wt/vol] HCl) was added, and the mixture was vortexed and shaken on a gyratory shaker at 100 rpm for 10 min. The mixture was vortexed again and centrifuged in an Eppendorf microcentrifuge at 12,000 × g for 15 s, and the supernatant was removed. The pellet was washed twice with 1 ml L2 washing buffer (120 g GuSCN in 100 ml of 0.1 M Tris-HCl, pH 6.4), twice with 1 ml 70% ethanol, and once with 1 ml acetone. For each wash, the mixture was vortexed and centrifuged for 15 s before the supernatant was removed. The open vials were dried in a dry block heater at 56°C for 10 min. A total of 100 μl TE elution buffer (1 mM EDTA in 10 mM Tris HCl, pH 8.0) was added to the pellet, and the mixture was vortexed and incubated for 10 min at 56°C. The suspension was centrifuged at 12,000 × g for 30 s, and about 80 μl of the supernatant was carefully transferred into a new vial. A total of 10 μl of this supernatant was used in the PCR.
PCR for M. ulcerans.
The composition of the PCR mixture was similar to that described by Kox et al. (9), with final reaction concentrations of 10 mM Tris, 50 mM KCl, 0.01% gelatin, 1.5 mM MgCl2, 0.1% Triton X-100, 0.2 mM (each) deoxynucleoside diphosphate (dATP, dCTP, dGTP, and dUTP) (Roche Diagnostics GmbH, Germany), 0.2 μM each of forward primer PU4F (5′-GCGCAGATCAACTTCGCGGT 3′) and reverse primer PU7Rbio (5′-GCCCGATTGGTGCTCGGTCA-3′) (gene positions 548 to 567 bp and 702 to 683 bp, respectively), 1 unit of Taq DNA polymerase (HT Biotechnology, United Kingdom), and 0.1 U of uracil DNA glycosylase (UDG; Invitrogen, United Kingdom) per 50-μl reaction volume. The expected size of the PCR product was 154 bp. Portions of 40 μl were placed in 0.2-ml PCR vials. Ten microliters of the extracted DNA was added to each 40-μl PCR mixture in duplicate. PCR was performed in a heating block thermocycler (Hybaid, United Kingdom). The PCR incubations were as follows: 10 min at 40°C to allow the UDG to break down the amplicons, followed by 35 cycles of 1.5 min of denaturation at 94°C, 2 min of annealing at 65°C, and 3 min of primer extension at 72°C. Newly synthesized dU-containing PCR products were maintained at 72°C for a minimum of 1 h to prevent them from being broken down by any residual UDG activity. Electrophoresis of the PCR product was performed on a 2% agarose gel stained with ethidium bromide and visualized with UV light.
The quality of the PCR mixture in each reaction was tested by amplification of a series of dilutions with 100 fg, 10 fg, and 1 fg (that is, approximately 50 copies) of M. ulcerans DNA as in-house positive controls, with the assumption that one mycobacterial genome is equivalent to 4 to 5 fg of DNA. Each patient's sample was run in triplicate, two undiluted samples and one sample spiked with 5 fg of M. ulcerans DNA, to assess the sample for inhibitory activity. Cross contamination was checked by including TE-negative control vials with each PCR run. PCR mixtures, preparation of the samples to be added to the PCR mixture, and analysis of the PCR products were separated by using dedicated rooms, instruments, and laboratory coats to reduce the risk of contamination. Chemical decontamination of surfaces and equipment was done every other day with 0.5% sodium hypochlorite (bleach). After incubation for 5 to 10 min, the hypochlorite solution was removed with warm water. The PCR result was considered to be positive if all three PCR samples for each patient were positive, the positive controls were positive, and the negative controls with TE were negative. For PCR-negative specimens, positive controls were positive and negative controls with TE were negative.
PCR optimization.
The detection limit of the PCR was tested by using a series of PCRs with 100 fg, 10 fg, or 1 fg of M. ulcerans DNA and also with tissue spiked with 5 μl of 103 bacteria/ml (5 bacteria [absolute amount] per 50 mg tissue) or 5 μl of 104 bacteria/ml (50 bacteria [absolute amount] per 50 mg tissue). The M. ulcerans suspension used to spike the tissue was prepared as described elsewhere (9). To demonstrate the ability of UNG to break down the dUTP-containing PCR products without changing the efficiency of the PCR, a 154-bp dUTP-containing PCR product of primer pair PU4F and PU7Rbio was used. This PCR product, prepared with 10 pg M. ulcerans DNA, was successively diluted 10-fold with TE. Ten microliters of DNA diluted 105, 106, or 107 times was used as the PCR template with or without UNG.
Histology.
Each formalin-fixed specimen was embedded in its entirety and serially sectioned. Each of the paraffin sections was stained with hematoxylin-eosin and ZN stain by standard methods.
Every section was assessed in a blinded manner for the presence or the absence of epidermal ulceration, Buruli ulcer-type coagulative dermal and subcuticular necrosis, panniculitis, neuritis, venulitis, calcification, and scarring. A semiquantitative assessment of acute inflammation (neutrophils), chronic inflammation (lymphocytes, plasma cells, eosinophils), and granulomas was made for each section.
The histological case definition of “definite Buruli ulcer” required the presence of characteristic Buruli-type coagulative necrosis of the dermis or subcuticular tissue, with or without panniculitis, with or without granulomas, and with or without AFB. Because of the irregular distribution of clumps of AFB in Buruli ulcer lesions, not all histological sections may contain them.
The histological case definition of “possible Buruli ulcer,” whereby the histological appearances would be in keeping with Buruli ulcer if they were supported by other positive laboratory tests for the detection of Mycobacterium ulcerans, was absent Buruli-type coagulative dermal or subcuticular necrosis but the presence of panniculitis, with or without granulomas and with or without AFB.
Appearances of inflammation with or without granulomas and fibrosis, without coagulative dermal or subcuticular tissue necrosis, and without AFB were considered nonspecific, implying that, in the absence of other positive laboratory tests, the lesion was unlikely to be caused by M. ulcerans infection.
“Gold standard” for diagnosis.
A case diagnosed by clinical criteria was confirmed as Buruli ulcer when there was (i) a definite histological diagnosis, (ii) a positive culture result for M. ulcerans, or (iii) a positive smear result for AFB together with a possible histological diagnosis.
Cases which did not meet the gold standard were further analyzed by the histological criteria described above and by clinical assessment. The clinician judged all cases as definite or possible Buruli ulcer before knowing the results of any laboratory tests.
RESULTS
PCR optimization.
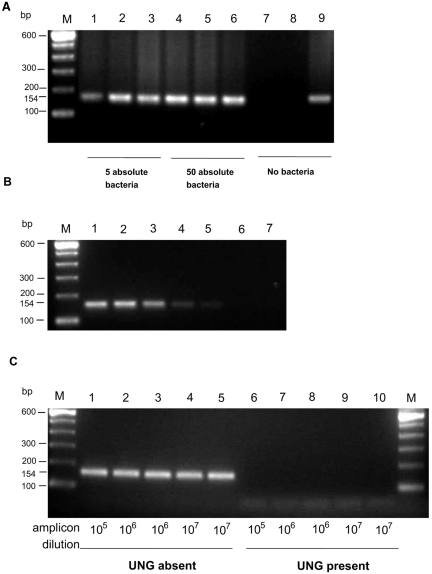
Figure 1A shows the results of initial experiments carried out to determine the detection limit of the DNA extraction and PCR technique by spiking skin biopsy samples with 5 μl of 103 bacteria/ml (5 bacteria [absolute amount] per 50 mg tissue), 5 μl of 104 bacteria/ml (50 bacteria [absolute amount] per 50 mg tissue), or no bacteria before the DNA extraction procedure. PCR was positive when tissue was spiked with the equivalent of 50 and 5 mycobacteria. No inhibition of the PCR was encountered (Fig. 1B). PCR with 100-fg, 10-fg, and 1-fg equivalents of M. ulcerans DNA always gave a positive result; so this was considered a reliable in-house positive control system. Figure 1C demonstrates the ability of UNG to break down dUTP-containing PCR products, without changing the efficiency of PCR, by using a 154-bp, dUTP-containing PCR product of primer pair PU4F and PU7Rbio. This PCR product, prepared with 10 pg M. ulcerans DNA, was successively diluted 10-fold with TE. Ten microliters of DNA diluted 105, 106, or 107 times was used as the PCR template with or without UNG. When UNG was included in vials with the PCR product as the template, no product was detected by PCR; but product was detected when UNG was excluded, showing that the amplicons previously made with dUTP had undergone hydrolytic cleavage by UNG. However, amplified DNA of M. ulcerans was detected in vials with M. ulcerans DNA as the template.
FIG. 1.
(A) The sensitivity of PCR for M. ulcerans was demonstrated by spiking normal skin tissue with 5 μl of a solution containing 103 bacteria/ml (5 bacteria; lanes 1 to 3), 5 μl of 104 bacteria/ml (50 bacteria; lanes 4 to 6), or no bacteria (lanes 7 to 9). Lanes 3, 6, and 9 were also spiked with 5 fg of M. ulcerans DNA before PCR to control for inhibition. Lane M, a 100-bp molecular ladder size marker. (B) Lane 1, 100 fg of M. ulcerans DNA (approximately 5,000 copies, based on 249 copies of the IS2404 insertion element per genome of M. ulcerans); lanes 2 and 3, 10 fg of M. ulcerans DNA (approximately 500 copies); lanes 4 and 5, 1 fg of M. ulcerans DNA (approximately 50 copies); lanes 6 and 7, TE control; lane M, a 100-bp molecular ladder size marker. (C) UNG in the reaction mixture breaks down dUTP-containing amplified PCR products, preventing false-positive results, as demonstrated with a dUTP-containing amplified product prepared with 10 pg M. ulcerans DNA solution and primer pair PU4F and PU7Rbio, which resulted in a 154-bp product. This amplified product was diluted 105, 106, and 107 times with TE and was further amplified in the absence of UNG (lanes 1 to 5) or in the presence of 0.01 unit UNG as the template in the PCR (lanes 6 to 10). Lane M, a 100-bp molecular ladder size marker.
Evaluation of diagnostic techniques.
Fifty patients fulfilled the gold standard criteria for a definite diagnosis, and all 50 had positive PCR results for M. ulcerans. Eight cases which did not meet the gold standard for conventional laboratory diag nosis had a histological diagnosis of possible Buruli ulcer with panniculitis, and seven of these had a positive PCR result. These seven cases have been diagnosed as probable Buruli ulcer (Table 1). The PCR-negative case was a 70-year-old woman with a nodular lesion on the foot; and the clinician had some doubt that it was due to M. ulcerans disease, so it is more likely that this patient had a different condition. This case has been diagnosed as possible Buruli ulcer.
TABLE 1.
Summary of final diagnosis based on laboratory and clinical features
| Final Buruli ulcer diagnosis | No. of patients |
No. of patients with the following histology result: |
No. of patients PCR positive | |||||
|---|---|---|---|---|---|---|---|---|
| Total | AFB positive | Culture positive | Definite | Possible | Nonspecific or alternative diagnosis | Normal skin | ||
| Definitea | 50 | 23 | 27 | 49 | 1 | 0 | 0 | 50 |
| Probable | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 7 |
| Possible | 3 | 0 | 0 | 0 | 1b | 1c | 1d | 2 |
| Not Buruli ulcer | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 |
Definite Buruli ulcer diagnosed on the basis of clinical and gold standard criteria.
A 70-year-old woman with a nodular lesion on the foot which was PCR negative.
A 18-year-old girl with a nodular lesion on the right upper limb close to the scar of a previous Buruli ulcer (the lesion sampled 4 months earlier was also PCR positive).
A 4-year-old boy with a nodular lesion on the left upper limb close to the scar of a previous Buruli ulcer (the lesion sampled 6 months earlier was also PCR positive).
Among the remaining 12 cases, histology suggested an alternative diagnosis in 3 cases, which were all PCR negative (Table 2), and nonspecific inflammation in 8, of which 7 were PCR negative and 1 was PCR positive. There was no histological abnormality in the other case, but the PCR result was positive. In both PCR-positive cases there was a strong clinical suspicion of M. ulcerans disease, so the nonspecific or normal histology was probably due to sampling error. These two PCR-positive cases have been diagnosed as possible Buruli ulcer (Table 1).
TABLE 2.
Histological findings for 11 biopsy specimens with negative PCR results for M. ulcerans
| Histological finding | No. of specimens |
|---|---|
| Alternative diagnosis | |
| Squamous cell carcinoma | 1 |
| Abscess | 1 |
| Reactive lymph node | 1 |
| Possible histological diagnosis | 1 |
| Nonspecific diagnosis | 7 |
| Total | 11 |
If all cases with a final diagnosis of definite, probable, or possible Buruli ulcer were true M. ulcerans disease, Table 3 shows the performance of the other laboratory tests compared to that of PCR. PCR was 98% sensitive, but if all three possible Buruli ulcer cases were not due to M. ulcerans disease, there was a false-positive PCR rate of 2.9%. However, if the clinician's initial judgment is taken into account, it is likely that all PCR-positive cases were due to M. ulcerans disease and that there were no false-negative results.
TABLE 3.
Comparison of results of microscopy for AFB, culture, histology, and PCR for 70 patients with a final diagnosis of M. ulcerans diseasea
| Test and result | No. with final diagnosis |
Sensitivity (% [95% CIb]) | Specificity (% [95% CI]) | Positive predictive value (% [95% CI]) | Negative predictive value (% [95% CI]) | |
|---|---|---|---|---|---|---|
| True positive | True negative | |||||
| Microscopyc | ||||||
| Positive | 23 | 0 | 42 (29-56) | 100 (69-100) | 100 (85-100) | 24 (12-39) |
| Negative | 32 | 10 | ||||
| Culturec | ||||||
| Positive | 27 | 0 | 49 (35-63) | 100 (69-100) | 100 (87-100) | 26 (13-43) |
| Negative | 28 | 10 | ||||
| Histology | ||||||
| Positive | 49 | 0 | 82 (70-90) | 100 (69-100) | 100 (93-100) | 48 (26-70) |
| Negative | 11 | 10 | ||||
| PCR | ||||||
| Positive | 59 | 0 | 98 (91-100) | 100 (69-100) | 100 (94-100) | 91 (59-100) |
| Negative | 1 | 10 | ||||
All cases with a final diagnosis of definite, probable, or possible Buruli ulcer (Table 1).
CI, confidence interval.
Microscopy and culture were not performed for five cases, and the data for these cases were excluded from this analysis but had definite histological diagnosis and a positive PCR result.
Results of microscopy for AFB and PCR for IS2404 were available within 24 h, whereas culture became positive after 6 to 8 weeks of incubation. Histology, which was 82% sensitive, was not available in Kumasi at the time of this study. The sensitivity of microscopy for AFB was 42%, and that of culture for M. ulcerans was 49%; the negative predictive values were 24% (95% confidence interval, 12 to 39%) for microscopy and 26% (95% confidence interval, 13 to 43%) for culture.
Table 4 shows the characteristics of the patients divided into groups according to the type of lesion and whether the clinical diagnosis was confirmed in the laboratory (group A) or not (group B). The mean age of the patients in group A was lower than that of the patients in group B for both preulcerative and ulcerative disease. The duration of disease before presentation was similar in groups A and B for preulcerative disease but was longer in group B for ulcerative disease. All the upper-limb lesions were shown to be due to M. ulcerans infection, whereas in 7 of 24 (29%) cases with lesions on the lower limb and 3 of 12 (25%) cases with lesions on the trunk, the clinical diagnosis was not confirmed in the laboratory.
TABLE 4.
Characteristics of 70 suspected Buruli ulcer patientsa
| Characteristic | Preulcerative disease |
Ulcerative disease |
||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| No. of patients | 26 | 6 | 33b | 5 |
| Mean age (yr) ± SE | 11.8 ± 2.2 | 28.1 ± 8.2 | 11.3 ± 1.2 | 35.8 ± 6.8 |
| M:F ratioc | 12:14 | 3:3 | 20:13 | 4:1 |
| Mean duration of skin lesion (mo) ± SE (range)d | 1.9 ± 0.3 (0.3-6.0) | 1.7 ± 1.1 (0.3-7.0) | 3.8 ± 0.7 (0.5-18.0) | 13.6 ± 6.2 (2.0-36.0) |
| Site of lesion (no. of patients) | ||||
| Upper limb | 17 | 0 | 15 | 0 |
| Lower limb | 1 | 3 | 16 | 4 |
| Trunk | 8 | 2 | 1 | 1 |
| Head or neck | 0 | 1 | 1 | 0 |
Group A, diagnosis confirmed by PCR; group B, diagnosis not confirmed by PCR.
Six patients had some associated edema.
M, male; F, female.
Patients' estimate of time since lesion first appeared. SE, standard error of the mean.
DISCUSSION
This study was carried out in a field setting and showed that punch biopsy specimens can be used to confirm M. ulcerans disease by microscopy, culture, histology, and PCR. It demonstrates the central role of PCR in diagnosis, especially in ZN staining- and culture-negative cases when histopathology is not diagnostic. PCR for the repeat sequence IS2404 of M. ulcerans (19, 21) was modified by using uracil DNA glycosylase and dUTP instead of dTTP to reduce the risk of false-positive results due to amplicon contamination (10, 12). Prior to thermal cycling, uracil-substituted PCR products served as the substrate for UDG, which catalyzes the hydrolysis of the N-glycosidic bonds in nucleic acids. This modification did not compromise the sensitivity of the test, and no problem was encountered with inhibition of PCR. Appropriate positive and negative controls were put in place to monitor the whole procedure, as suggested in several publications (6, 9).
Histopathology was suggestive of M. ulcerans disease in all but two cases for which the PCR result was positive. Of the exceptions, one biopsy specimen showed entirely normal skin, even though there was a nodular lesion on the upper limb that was strongly suggestive of M. ulcerans disease and that was close to the scar of a previous proven Buruli ulcer, so it is likely that the area of the lesion sampled was not representative. The other exception was an 18-year-old girl with a typical nodule that was, again, close to the scar of a previous proven Buruli ulcer. Both of these lesions probably represent recurrent disease, so it is unlikely there were any false-positive results in this series. However, we have not studied biopsy specimens from a large group of patients with a wide spectrum of diseases to assess the overall false-positive rate.
Among the PCR-negative cases, histology suggested an alternative diagnosis in three cases and a nonspecific diagnosis in seven cases. However, the histology for one PCR-negative case showed possible Buruli ulcer, so this would give a false-negative rate of 1.7%. This was a nodular lesion on the lower limb of a 70-year-old woman which the clinician classified as possibly due to M. ulcerans infection; in view of her age and the clinical uncertainty, it is more likely that this was a different condition. Thus, this PCR method was sensitive and specific as well as rapid, and it was particularly useful in cases with negative AFB and culture results in which histology did not yield a definitive diagnosis. This is in contrast to the IS2404-based nested PCR used in a previous study, in which the false-positive rate was 9.7% (7).
Microscopy for AFB with ZN staining was positive for 42% of the 54 cases diagnosed as having Buruli ulcer from whom a sample was taken, and it had the advantage of high specificity (100%). Other lesions containing AFB, such as those of tuberculosis, leprosy, or nontuberculous mycobacterial infection, are unlikely to be confused clinically with M. ulcerans disease. ZN staining was the quickest, cheapest, and simplest of the four techniques used in this study; and it would be the clinician's first diagnostic option. The sensitivity of culture for M. ulcerans was 49%; but it took 6 to 8 weeks for a positive culture result, so it could not be used to make a treatment decision; a positive culture result can take up to 6 months (15, 17). The sensitivity of culture for M. ulcerans in the present study was in the higher part of the range of 20 to 60% quoted for swab specimens taken from ulcers (18), probably because culture was set up in a local hospital on the day of the operation. The diagnostic yield from culture was not significantly greater than that from ZN staining.
Histology had a higher sensitivity (82%) if the five cases that did not have culture and smear are included. However, histopathology laboratories with rapid turnaround times are not available in countries where Buruli ulcer is endemic. An additional role of histology is to exclude other conditions, such as squamous cell carcinoma and reactive lymph node in this study, and as a quality control tool for other tests. Filarial nodules, keratin cysts, fungal lesions, actinomycosis, zygomycosis, leishmaniasis, and yaws can all cause lesions which could be confused with M. ulcerans disease (2, 7).
In this study multiple 4- or 6-mm punch biopsy specimens were taken immediately before the lesion was excised. Sometimes there was bleeding and the wound would have required stitching after a 6-mm biopsy specimen was obtained if the lesion were not being excised, so we would recommend the use of 4-mm biopsy specimens. In this study several biopsy specimens were taken, but it may not have been possible to obtain these specimens if excision was not planned. A single biopsy specimen could be used to stain for AFB and culture and another could be used for PCR; but a further specimen would be needed for histology, and the optimal size for this is 4 mm in diameter or larger. We have previously found that 2-mm biopsy specimens are inadequate for histology, as they show only nonspecific inflammation (unpublished observations). The depth of the biopsy specimen is important, since both the typical necrosis of M. ulcerans disease and AFB are seen in deep dermis and subcutaneous fat. Punch biopsy specimens have been used to diagnose several dermatological conditions, including carcinoma, dermatofibroma, sarcoidosis (11), and cutaneous tuberculosis (1). When Todd et al. (23) evaluated 2-mm punch biopsy specimens from patients with dermatological diseases, the diagnostic accuracy compared favorably with that obtained with the standard ellipse biopsy specimen, with an overall concordance rate of 94%, but this diameter has been found to be too small for M. ulcerans lesions.
The clinical diagnosis of M. ulcerans disease was more likely to be confirmed in the laboratory in a younger patient with a lesion on the upper limb (Table 4). Lesions on the lower limb were more difficult to be used for diagnosis on clinical grounds, especially in older patients. Not surprisingly, ulcers were of longer duration than nonulcerative disease, but the patients' estimates of duration must be treated with caution since they had kept no record of the time of onset of the lesion.
In conclusion, this field study has shown that a modified PCR for IS2404 carried out with punch biopsy specimens is the most sensitive of the diagnostic tests for M. ulcerans disease and that it has high specificity. PCR and ZN staining are the quickest diagnostic tools for making a treatment decision. The modified PCR used in this study is viable when adequate controls are put in place. For routine practice we would recommend ZN staining of biopsy specimens to detect AFB, followed by PCR for AFB-negative cases only, in order to minimize costs. PCR of AFB-negative cases would also reduce the risk of cross contamination. Histology and culture remain important as quality control tests, particularly in studies of treatment efficacy.
Acknowledgments
This study was supported by a grant from the Wellcome Trust. R. Phillips has a Wellcome Trust Training Research Fellowship Award for Research into Infectious Diseases for Scientists from Tropical and Developing Countries.
We thank Edwin Ampadu, Ghana National Buruli Ulcer Programme; Kofi Asare, Ashanti regional director of health services; Nsiah Asare, chief executive Komfo Anokye Teaching Hospital; Beatrice Appau, district director (Atwima); and Elizabeth Adentwe, district director (Tepa), for their assistance with this study.
REFERENCES
- 1.Arora, S. K., B. Kumar, and S. Sehgal. 2000. Development of a polymerase chain reaction dot-blotting system for detecting cutaneous tuberculosis. Br. J. Dermatol. 142:72-76. [DOI] [PubMed] [Google Scholar]
- 2.Asiedu, K., R. Scherpbier, and M. Raviglione. 2000. Buruli ulcer: Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/gtb-buruli/publications/index.html.
- 3.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etuaful, S., B. Carbonnelle, J. Grosset, S. Lucas, C. Horsefield, R. Phillips, M. Evans, D. Ofori-Adjei, E. Klustse, J. Owusu-Boateng, G. K. Amedofu, P. Awuah, E. Ampadu, G. Amofah, K. Asiedu, and M. Wansbrough-Jones. 2003. Bactericidal activity of rifampicin and streptomycin treatment for early human M. ulcerans lesions, p. 10. In Report of the 6th WHO Advisory Group Meeting on Buruli ulcer meeting. [Online.] http://www.who.int/gtb-buruli/activities/meetings.htm.
- 5.Evans, M. R., R. Phillips, S. N. Etuaful, G. Amofah, J. Adomako, O. Adjei, J. Dennis-Antwi, S. B. Lucas, and M. H. Wansbrough-Jones. 2003. An outreach education and treatment project in Ghana for the early stage of Mycobacterium ulcerans disease. Trans. R. Soc. Trop. Med. Hyg. 97:159-160. [DOI] [PubMed] [Google Scholar]
- 6.Grosset, J., and Y. Mouton. 1995. Is PCR a useful tool for the diagnosis of tuberculosis in 1995? Tuber. Lung Dis. 76:183-184. [DOI] [PubMed] [Google Scholar]
- 7.Guarner, J., J. Bartlett, E. A. Whitney, P. L. Raghunathan, Y. Stienstra, K. Asamoa, S. Etuaful, E. Klutse, E. Quarshie, T. S. van der Werf, W. T. van der Graaf, C. H. King, and D. A. Ashford. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 9:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolk, A. H., L. F. Kox, S. Kuijper, and C. Richter. 1994. Detection of Mycobacterium tuberculosis in peripheral blood. Lancet 344:694. [DOI] [PubMed] [Google Scholar]
- 9.Kox, L. F., D. Rhienthong, A. M. Miranda, N. Udomsantisuk, K. Ellis, J. van Leeuwen, S. van Heusden, S. Kuijper, and A. H. Kolk. 1994. A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J. Clin. Microbiol. 32:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok, S., and R. Higuchi. 1989. Avoiding false positives with PCR. Nature 339:237-238. [DOI] [PubMed] [Google Scholar]
- 11.Li, N., A. Bajoghli, A. Kubba, and J. Bhawan. 1999. Identification of mycobacterial DNA in cutaneous lesions of sarcoidosis. J. Cutan. Pathol. 26:271-278. [DOI] [PubMed] [Google Scholar]
- 12.Longo, M. C., M. S. Berninger, and J. L. Hartley. 1990. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 93:125-128. [DOI] [PubMed] [Google Scholar]
- 13.Meyers, W. M., W. M. Shelly, D. H. Connor, and E. K. Meyers. 1974. Human Mycobacterium ulcerans infections developing at sites of trauma to skin. Am. J. Trop. Med. Hyg. 23:919-923. [DOI] [PubMed] [Google Scholar]
- 14.Noordhoek, G. T., J. A. Kaan, S. Mulder, H. Wilke, and A. H. Kolk. 1995. Routine application of the polymerase chain reaction for detection of Mycobacterium tuberculosis in clinical samples. J. Clin. Pathol. 48:810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettit, J. H., N. J. Marchette, and R. J. Rees. 1966. Mycobacterium ulcerans infection. Clinical and bacteriological study of the first cases recognized in South East Asia. Br. J. Dermatol. 78:187-197. [DOI] [PubMed] [Google Scholar]
- 16.Portaels, F., J. Agular, K. Fissette, P. A. Fonteyne, H. De Beenhouwer, P. de Rijk, A. Guedenon, R. Lemans, C. Steunou, C. Zinsou, J. M. Dumonceau, and W. M. Meyers. 1997. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J. Clin. Microbiol. 35:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portaels, F., P. A. Fonteyene, H. de Beenhouwer, P. de Rijk, A. Guedenon, J. Hayman, and M. W. Meyers. 1996. Variability in 3′ end of 16S rRNA sequence of Mycobacterium ulcerans is related to geographic origin of isolates. J. Clin. Microbiol. 34:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portaels, F., P. Johnson, and W. Meyers. 2001. Buruli ulcer: diagnosis of Mycobacterium ulcerans disease. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/gtb-buruli/publications/index.html.
- 19.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and P. D. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinear, T. 2005. Mycobacterium ulcerans genome. In Report of the WHO Global Buruli Ulcer Initiative Meeting. [Online.] http://www.who.int/gtb-buruli/activities/meetings.htm.
- 21.Stinear, T., J. K. Davies, G. A. Jenkin, F. Portaels, B. C. Ross, F. Oppedisano, M. Purcell, J. A. Hayman, and P. D. Johnson. 2000. A simple PCR method for rapid genotype analysis of Mycobacterium ulcerans. J. Clin. Microbiol. 38:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stinear, T., B. C. Ross, J. K. Davies, L. Marino, R. M. Robins-Browne, F. Oppedisano, A. Sievers, and P. D. Johnson. 1999. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol. 37:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todd, P., J. J. Garioch, S. Humphreys, M. Seywright, J. Thomson, and A. W. du Vivier. 1996. Evaluation of the 2-mm punch biopsy in dermatological diagnosis. Clin. Exp. Dermatol. 21:11-13. [PubMed] [Google Scholar]