Abstract
From a 0.72-kb fragment universally generated in Paracoccidioides brasiliensis strains, primers were designed and tested on genomic DNA of this and other pathogenic fungi. They were specific and highly sensitive for P. brasiliensis DNA. Positive results were obtained when these were tested in clinical samples.
Paracoccidioidomycosis (PCM), caused by Paracoccidioides brasiliensis, is a chronic granulomatous systemic mycosis prevalent in rural areas of Latin America (16). PCM is routinely diagnosed by culture observation and microscopic detection of yeast cells in clinical specimens (10) and by serological tests, particularly with gp43, a reference P. brasiliensis antigen (17, 18).
Molecular methods (2, 6, 11, 19) allow the identification of many fungi without the need of culturing. They are rapid, highly specific and sensitive (3). The molecular identification of P. brasiliensis with primers of diverse origin has been reported (4, 5, 7, 9, 13, 14). Two specific DNA fragments (0.72 and 0.83 kb) common to and specific for all P. brasiliensis samples are generated when the arbitrary primer OPG18 (Operon Biotechnology) is used (5). We report specific primers designed on the 0.72-kb fragment, capable of identifying P. brasiliensis DNA from sputum and cerebrospinal fluid (CSF) of PCM patients.
P. brasiliensis DNA (strain Pb73; ATCC 32071) was prepared as described before (5). Other DNA samples were Histoplasma capsulatum (strains 7090, G222B, and G217B), Aspergillus terreus, Aspergillus fumigatus, Aspergillus nidulans, Blastomyces dermatitidis (strains 4.1 and 4.2), Candida albicans (strains B102A and 3), Candida krusei (strain W0701), Candida parapsilosis (strain 8992), Candida tropicalis (strain W0739), Candida guilliermondii (strain 6742), Candida pseudotropicalis (strain W0696), Candida dubliniensis, Cryptococcus neoformans (strain 90-2), Penicillium marneffei (strain 10742), Sporothrix schenckii (strain 5038), Trichophyton rubrum (strain A), Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium smegmatis.
Primers were designed on the 0.72-kb fragment generated by PCR with the arbitrary primer OPG18 (Operon Biotechnologies) (5). They were as follows: MG2(1)F, 5′-GGGATTCCCTAGGCAAACACTTGTGTGA-3′; MG2(1)R, 5′-GTGCAGTTATCCACAAGCCATATATTC-3′; MG2(2)F, 5′-GGAGATGATCTGACGTTAGTACGTGATG-3′; and MG2(2)R, 5′-ATGCTAATTTATGTCATTCCGCGTCTG-3′.
PCRs were carried out in 25-μl reaction mixtures with 2.5 μl 10× PCR buffer (200 mM Tris-HCl, pH 8.4, 500 mM KCl) containing 20 ng genomic DNA, 20 pmol of each primer, 0.75 μl 50 mM MgCl2, 1.25 μl 1 mM dNTP, and 0.5 U Taq DNA polymerase (Life Technologies). Amplifications were performed in a thermocycler (MJ Research, Inc.) with an initial cycle of 94°C (5 min), 30 cycles of 94°C (30 s), 65°C (30 s), and 72°C (1 min), and a final extension for 5 min at 72°C. Negative controls (water instead of fungal DNA) were included in all reactions. Sensitivity of the PCR assay was tested with P. brasiliensis genomic DNA at concentrations from 5 ng to 1 pg.
For diagnostic purposes, seven sputa and one CSF sample from PCM patients (Table 1) and three sputum samples from subjects without PCM were tested, undiluted and in 1:5 and 1:10 dilutions. An initial PCR with 5 μl DNA and the primer pair MG2(1)F-MG2(1)R was carried out, followed by reamplification (2 μl) under the same conditions. Southern hybridization with the specific 0.72-kb probe (5) was carried out according to the methods in reference 15.
TABLE 1.
Molecular detection of P. brasiliensis in samples from patients with various clinical forms of the disease
| Patient | Sexb(age in yrs) | Clinical and/or serological diagnosis (premolecular test) | Molecular PCM diagnosisc | Agreement between clinical and/or serological diagnosis with the molecular testd |
|---|---|---|---|---|
| 1 | M (39) | Chronic, multifocal PCM, 6-mo duration of symptoms | ++ | Sample from patient with a positive clinical and laboratorial diagnosis of PCM |
| 2 | M (55) | Chronic, multifocal PCM, 3-yr duration of symptoms | + (smear) | Sample from patient with a positive clinical and laboratorial diagnosis of PCM. This sample was exceedingly thick; it was subjected to three purification steps in a fruitless attempt to eliminate smear |
| 3 | F (38) | Chronic unifocal (pulmonary) PCM, 4-mo duration of symptoms | ++ | Sample from patient with a positive clinical and laboratorial diagnosis of PCM |
| 4 | M (34) | Chronic multifocal PCM, 4-mo duration of symptoms | +++ | Sample from patient with a positive clinical and laboratorial diagnosis of PCM |
| 5 | M (57) | Chronic unifocal (pulmonary) PCM; 14-mo duration of symptoms | +++ | Sample from patient with a positive clinical and laboratorial diagnosis of PCM |
| 6 | M (49) | Chronic, multifocal PCM diagnosed and treated in 2000. Probable relapse, unconfirmed at the time of the molecular test | +++ | Ten days after our positive report of PCM, clinical and serological confirmation was available (microscopic observation of P. brasiliensis multibudding yeast cells in sputum and positive serology) |
| 7 | F (47) | Chronic unifocal (pulmonary) PCM-diagnosed and treated in 1993. Probable relapse, unconfirmed at the time of the molecular test | − | Clinical and serological confirmation of no PCM relapse, 10 days after molecular results were available. Definitive negative mycological diagnosis, 1 month later: chronic bronchopulmonary obstructive emphysema and bilateral bronchiectasis after bacterial infection |
| 8a | M (38) | Chronic, multifocal PCM, with involvement of the central nervous system | ++ | CSF biochemistry revealed a discreet elevation in proteins. Direct microscopic examination, culture, and serology (DID) for P. brasiliensis were negative. CT scan with contrast showed the presence of multiple hypodense images with ring enhancement, surrounded by mild edema, localized mainly at posterior fossa and in lower numbers in basal ganglia and around periventricula |
Cerebrospinal fluid sample. All others were sputum.
M, male; F, female.
+, light band; ++, medium band; +++, intense band; −, no band.
CT, computed tomography; DID, double immunodiffusion.
To evaluate the method, control sputa were spiked with P. brasiliensis yeast cells (0, 10, 102, and 104 cells/ml). Samples were treated according to the methods in reference 8, although modifications were needed. (i) The lysis buffer consisted of 0.1 M Tris-HCl, pH 7.5; 5% sodium dodecyl sulfate; 30 mM EDTA; and both chitinase (from Streptomyces griseus, 1 mg/ml; Sigma, St. Louis, MO) and β-glucanase (yeast lytic enzyme, 2 mg/ml; ICN Biomedicals Inc., Aurora, OH) as lytic enzymes to soften the P. brasiliensis cell wall. It was added 1:1 (vol/vol) for 2 h at 37°C and a further 15 min at 100°C. (ii) Centrifugation conditions were modified to 12,000 rpm for 5 min after the potassium acetate step, 5,000 rpm at 4°C for 10 min after the phenol-chloroform extract, and 8,000 rpm at 4°C for 5 min following overnight incubation at −20°C.
Patients (Table 1) were farmers from rural areas nearby the Valencia Lake, in the north-central region of Venezuela (patients 1 through 7), or Lara State, the central-western region of the country (patient 8). Samples were treated in the same fashion as spiked samples. The project was submitted to and approved by the Bioethics Commission of the Venezuelan Institute for Scientific Research.
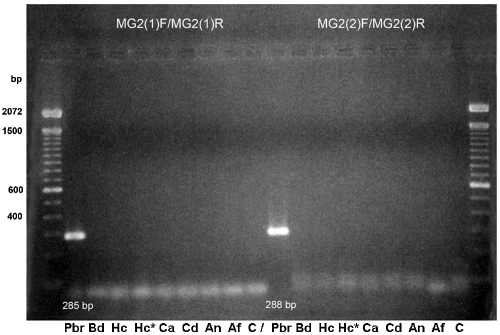
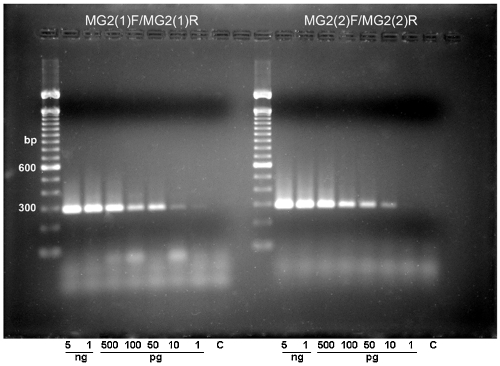
DNA from all microorganisms listed above were used for PCR assays with primer pairs MG2(1)F-MG2(1)R and MG2(2)F-MG2(2)R. Figure 1 shows results obtained with P. brasiliensis, B. dermatitidis, H. capsulatum, C. albicans, C. dubliniensis, A. nidulans, and A. fumigatus. All other fungal and mycobacterial DNAs and DNA from control samples were negative. Specific P. brasiliensis bands of 285 bp [MG2(1)F-MG2(1)R] and 288 bp [MG2(2)F-MG2(2)R] were generated. Southern hybridization (not shown) was positive for the P. brasiliensis amplicon only, confirming the specificities of the designed primers. They had a sensitivity limit of 10 pg (Fig. 2). The limit for spiked sputum samples was 10 cells/ml. Thereafter, only primer pair MG2(1)F-MG2(1)R was used.
FIG. 1.
PCR amplification with primers MG2(1)F-MG2(1)R and MG2(2)F-MG2(2)R. Lane 1, 100-bp ladder, Gibco Life Technologies. Genomic DNA from Paracoccidioides brasiliensis (Pbr), Blastomyces dermatitidis (Bd), Histoplasma capsulatum (Hc; Hc*, 1:10 dilution), Candida albicans (Ca), Candida dubliniensis (Cd), Aspergillus nidulans (An), and Aspergillus fumigatus (Af). Negative control, C.
FIG. 2.
Different amounts of genomic DNA were amplified with primers MG2(1)F-MG2(1)R (left) or MG2(2)F-MG2(2)R (right). Amplicons were detected by 2% agarose gel electrophoresis and ethidium bromide staining.
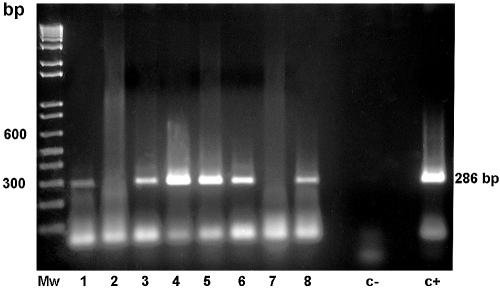
Experiments with serum and blood samples (not shown) were unsuccessful. With the exception of that of patient 2, samples listed in Table 1 generated a well-defined band (Fig. 3) visible even at a 1:10 dilution (not shown). Two sputum samples (those of patients 6 and 7) corresponded to previously treated PCM patients who returned to the hospital for presumptive PCM relapse. In both cases, molecular tests were done before clinical and laboratorial results were available. Sputum from patient 6 was positive for P. brasiliensis, while that from patient 7 was not. Clinical and mycological confirmations of both molecular diagnoses were reached at a later date.
FIG. 3.
Identification of P. brasiliensis DNA by reamplification with primers MG2(1)F-MG2(1)R of clinical samples from paracoccidioidomycosis patients (undiluted samples only; 1:5 and 1:10 dilutions not shown). Mw, standard; 1 to 7, sputum samples; 8, CSF sample, as referred to in Table 1; c−, negative control; c+, P. brasiliensis DNA.
So far, P. brasiliensis DNA sequences of potential diagnostic use include some derived from the rat β-actin gene (7), the 5.8S rRNA gene and its flanking internal transcribed spacer regions (9, 11, 13), or the gp43 gene (4, 8). The primers reported here produced positive identification bands in those patients with a confirmed diagnosis of chronic PCM, reflecting the frequent pulmonary involvement in such cases (12). Interestingly, in two cases of suspected relapses (patients 6 and 7), our molecular test produced results that preceded, by one or more weeks, information obtained by clinical, serological, or mycological tests. One of these patients (patient 6) turned out to have a PCM relapse, while the other one (patient 7) did not, as correctly predicted by the molecular test.
As for patient 8, he suffered from chronic multifocal PCM, and developed neurological symptoms of impairment, suggestive of an involvement of the central nervous system (CNS). Treatment with amphotericin B diminished mucosal lesions but not the CNS impairment. Our molecular test was able to detect P. brasiliensis in a CSF sample from this patient, although antibody detection and microscopic observation were negative for the presence of the fungus in this sample, as usually reported for CNS PCM (1). A positive molecular test could avoid a stereotaxic biopsy of the brain for diagnosis and could be useful to follow the treatment of patients with paracoccidioidal CNS involvement.
Acknowledgments
We thank Bruno Maresca (International Institute of Genetics and Biophysics, CNR, Naples, Italy) for kindly sequencing the P. brasiliensis 789-bp fragment, George Kobayashi (Washington University, Division of Infectious Diseases, St. Louis, MO) for providing DNA samples of fungi other than P. brasiliensis, Mireya Mendoza for mycelial preparation of H. capsulatum, and Howard Takiff (IVIC, Caracas, Venezuela) for mycobacterial DNAs. We thank Carmen Elena Kannee and María José De Armas (Hospital Vargas, Caracas, Venezuela) for the kind gift of a CSF sample from patient 8.
This work was supported by grant no. 2000-6, Instituto Venezolano de Investigaciones Científicas (IVIC). F.H.-B. was the recipient of a United Nations University Fellowship Training at Instituto Venezolano de Investigaciones Científicas (August-December, 2002).
REFERENCES
- 1.Almeida, S. M., F. Queiroz-Telles, H. A. G. Teive, C. E. L. Ribeiro, and L. C. Werneck. 2004. Central nervous system paracoccidioidomycosis: clinical features and laboratorial findings. J. Infect. 48:193-198. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, F. H., T. Imai, R. Tanaka, Y. Mikami, H. Taguchi, N. F. Nishimura, K. Nishimura, M. Miyaji, A. Z. Schreiber, and M. L. M. Branchini. 1999. New PCR primer pairs specific for Cryptococcus neoformans serotype A or B prepared on the basis of random polymorphic DNA fingerprint pattern analyses. J. Clin. Microbiol. 37:315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins, S. D., and I. M. Clark. 2004. Fungal molecular diagnostics: a mini review. J. Appl. Genet. 45:3-15. [PubMed] [Google Scholar]
- 4.Bialek, R., A. Ibricevik, C. Aepinus, L. K. Najvar, A. W. Fothergill, J. Knobloch, and J. R. Graybill. 2000. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J. Clin. Microbiol. 38:2940-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcagno, A. M., G. Niño-Vega, F. San-Blas, and G. San-Blas. 1998. Geographic discrimination of Paracoccidioides brasiliensis strains by randomly amplified polymorphic DNA analysis. J. Clin. Microbiol. 36:1733-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rotherhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldani, L. Z., A. L. Maia, and A. M. Sugar. 1995. Cloning and nucleotide sequence of a specific DNA fragment from Paracoccidioides brasiliensis. J. Clin. Microbiol. 33:1652-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes, G. M., P. S. Cisalpino, C. P. Taborda, and Z. P. Camargo. 2000. PCR for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 38:3478-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai, T., A. Sano, Y. Mikami, K. Watanabe, F. H. Aoki, M. L. M. Branchini, R. Negroni, K. Nishimura, and M. Miyaji. 2000. A new PCR primer for the identification of Paracoccidioides brasiliensis based on rRNA sequences coding the internal transcribed spacers (ITS) and 5.8S regions. Med. Mycol. 38:323-326. [DOI] [PubMed] [Google Scholar]
- 10.Lacaz, C. S. 1994. Mycological diagnosis, p. 339-344. In M. Franco, C. S. Lacaz, A. Restrepo-Moreno, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Inc., Boca Raton, Fla.
- 11.Lindsley, M. D., S. F. Hurst, N. J. Iqbal, and C. J. Morrison. 2001. Rapid identification of dimorphic and yeast-like fungal pathogens using specific DNA probes. J. Clin. Microbiol. 39:3505-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montenegro, M. R., and M. Franco. 1994. Pathology, p. 131-150. In M. Franco, C. S. Lacaz, A. Restrepo-Moreno, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Inc., Boca Raton, Fla.
- 13.Motoyama, A. B., E. J. Venancio, G. O. Brandao, S. Petrofeza-Silva, I. S. Pereira, C. M. Soares, and M. S. S. Felipe. 2000. Molecular identification of Paracoccidioides brasiliensis by PCR amplification of ribosomal DNA. J. Clin. Microbiol. 38:3106-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motta, T. R., C. A. Moreira-Filho, R. P. Mendes, L. R. Souza, M. F. Sugizak, S. Baueb, V. L. G. Calich, and C. A. C. Vaz. 2002. Evaluation of DNA polymorphisms amplified by arbitrary primers (RAPD) as genetically associated elements to differentiate virulent and non-virulent Paracoccidioides brasiliensis isolates. FEMS Immunol. Med. Microbiol. 33:151-157. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.San-Blas, G., G. Niño-Vega, and T. Iturriaga. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40:225-242. [DOI] [PubMed] [Google Scholar]
- 17.Silva, S. H., F. Queiroz-Telles, A. L. Colombo, M. H. Blotta, J. D. Lopes, and Z. P. Camargo. 2004. Monitoring gp43 antigenemia in paracoccidioidomycosis patients during therapy. J. Clin. Microbiol. 42:2419-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza, M. C., J. L. Gesztesi, A. R. Souza, J. Z. Moraes, J. D. Lopes, and Z. P. Camargo. 1997. Differences in reactivity of paracoccidioidomycosis sera with gp43 isoforms. J. Med. Vet. Mycol. 35:13-18. [DOI] [PubMed] [Google Scholar]
- 19.Trost, A., B. Graf, J. Eucker, O. Sezer, K. Possinger, U. B. Göbel, and T. Adam. 2004. Identification of clinically relevant yeasts by PCR/RFLP. J. Microbiol. Methods 56:201-211. [DOI] [PubMed] [Google Scholar]