Abstract
DNA fingerprinting of Candida dubliniensis isolates using the species-specific probe Cd25 previously showed that this species consists of two distinct groups, termed Cd25 group I and Cd25 group II. The present study investigated the population structure of 30 C. dubliniensis oral isolates from Saudi Arabia and Egypt using Cd25 fingerprinting and rRNA gene internal transcribed spacer region-based genotyping. Cd25 fingerprinting analysis of these isolates revealed two distinct populations, the first of which consisted of 10 closely related genotype 1 isolates (average similarity coefficient [SAB] value, 0.86). The second population of 20 isolates was much more heterogeneous (average SAB value, 0.35) and consisted of two distinct subpopulations, one of which consisted of genotype 3 isolates (n = 13) and the other of genotype 4 isolates (n = 7). A mixed dendrogram generated from the fingerprint data from the 30 Saudi Arabian and Egyptian isolates, 5 Israeli isolates, and 51 previously characterized international isolates (32 of Cd25 group I and 19 of Cd25 group II) revealed the presence of three distinct main clades. The first corresponded to the previously described Cd25 group I and contained all the Saudi Arabian, Egyptian, and Israeli genotype 1 isolates mixed with international isolates. The second clade corresponded to the previously described Cd25 group II and contained three Israeli isolates, one genotype 2 isolate, one genotype 3 isolate, and a genotype 4 variant isolate, which were mixed with international isolates. The third clade has not been described before and consisted solely of the 20 Saudi Arabian and Egyptian genotype 3 and 4 isolates identified in this study and a previously described genotype 4 Israeli isolate. All 20 Cd25 group III isolates exhibited high-level resistance to 5-flucytosine (MIC ≥ 128 μg/ml), whereas all Cd25 group I and Cd25 group II isolates tested (10 Saudi Arabian and Egyptian, 16 Israeli, and 24 international) were susceptible to 5-flucytosine (MIC ≤ 0.125 μg/ml). The results of this study show for the first time the presence of a novel 5-flucytosine-resistant clade of C. dubliniensis (Cd25 group III) that is predominant among isolates from Saudi Arabia and Egypt and absent from a previously characterized international collection of 98 isolates from 15 countries.
Candida dubliniensis was first described in 1995 and was initially associated with oral carriage and infection in Irish and Australian human immunodeficiency virus (HIV)-infected and AIDS patients (48). Since then, numerous reports have confirmed the presence of C. dubliniensis in the oral cavities of HIV-infected individuals from widespread geographic locations (11, 12, 37, 45, 46, 47). C. dubliniensis has also been found as an oral carriage organism in a small percentage of healthy individuals and more recently has been implicated as an agent of oral colonization and disease in other immunocompromised groups, such as diabetics and patients with cancer (4, 25, 27, 36, 42, 49, 52). In addition, C. dubliniensis has also been isolated from a range of samples from other body sites and specimens, including vaginas, blood and urine samples, sputa, and wounds, mainly from patients with severe underlying disease (7, 18, 19, 28, 36). Recently, the first reported case of C. dubliniensis endocarditis (with fatal outcome) was described for an Irish patient (10).
The development of the C. dubliniensis species-specific complex DNA fingerprinting probe Cd25 by Joly et al. (22) has allowed the genetic relatedness of individual isolates and the population structure of C. dubliniensis to be investigated in detail. Computer-assisted analysis of Cd25-generated fingerprint profiles of 57 independent C. dubliniensis isolates from 11 different countries by Joly et al. (22) revealed the existence of two distinct C. dubliniensis populations. The results of this study were subsequently confirmed by Gee et al. (18) with 98 C. dubliniensis isolates recovered from 94 separate individuals from 15 different countries. In the latter study, Cd25 fingerprint group I isolates were found to be associated predominantly with HIV-infected individuals (67.6%), whereas Cd25 fingerprint group II isolates were associated predominantly with non-HIV-infected individuals (70.4%). Cd25 group I isolates were found to consist of closely related isolates (average similarity coefficient [SAB] value, 0.8), whereas Cd25 group II isolates were found to be less closely related (average SAB value, 0.57) and included a number of distantly related subpopulations and outliers (18). The close relatedness of Cd25 group I isolates was further confirmed in a recent study by Moran et al. (29) that showed that 14/24 (58%) of Cd25 group I isolates tested from 11 different countries contained identical nonsense mutations within the CdCDR1 gene encoding the ABC transporter protein CdCdr1p, suggesting that many of these isolates were clonal in origin. None of 16 Cd25 group II isolates tested from five different countries contained the nonsense mutation. The study of Gee et al. (18) also revealed that Cd25 fingerprint group I isolates consisted of a single genotype (genotype 1) based on the nucleotide sequence of the internal transcribed spacer region (ITS) of the rRNA gene cluster, whereas Cd25 fingerprint group II isolates were found to consist of three genotypes (genotypes 2 to 4).
Most reported isolates of C. dubliniensis have been recovered from individuals in Europe, the United States, South America, and Australia (18, 45, 47), but there is relatively little information on the prevalence or population structure of this organism in the Middle East. There have been a few reports of the isolation of C. dubliniensis in Israel, Saudi Arabia, and Kuwait (1, 16-18, 24, 26, 36), but little is known about the relatedness of C. dubliniensis isolates from this area to isolates from the rest of the world. The purpose of the present study was to investigate the relatedness of C. dubliniensis isolates recovered from individuals in Saudi Arabia and Egypt by analysis of genotypes and Cd25-generated fingerprint profiles. The results of the study identified the presence of a novel flucytosine (5FC)-resistant C. dubliniensis clade predominant among Saudi Arabian and Egyptian isolates that, apart from a single related isolate originally recovered from an Arab individual in Israel, was completely absent from a previously characterized group of 98 C. dubliniensis isolates recovered from 94 separate individuals from 15 different countries.
MATERIALS AND METHODS
C. dubliniensis isolates.
The C. dubliniensis clinical isolates used in this study that were recovered from individual patients in Saudi Arabia, Egypt, and Israel are listed in Table 1. A total of 309 Saudi Arabian patients at the Riyadh Medical Center and the Jeddah Kidney Center, Saudi Arabia, and 125 Egyptian patients at the National Institute of Diabetes and the National Institute of Cancer, Cairo, Egypt, were tested for the presence of oral C. dubliniensis. Prior to sampling, the presence of oral clinical signs and symptoms indicative of oral candidiasis, including pseudomembranous candidiasis, erythematous candidiasis, median rhomboid glossitis, and angular cheilitis (12), were recorded. Swab specimens from the mid-dorsum of the tongue were taken and plated onto CHROMagar Candida medium (11, 45). After 48 h of incubation at 37°C, the number of colonies present on each plate and their colors and relative abundances were recorded. Single-colony isolates were presumptively identified as C. dubliniensis or Candida albicans on the basis of their dark green or light green coloration, respectively (11, 21, 23, 45), and subsequently by their colony morphology following growth on Staib agar and Pal's agar as described previously (2, 3, 44). In addition, 5 other C. dubliniensis clinical isolates (SA100 to SA104) previously recovered from various specimens from separate patients at the King Faisal and King Khalid Hospitals, Riyadh, Saudi Arabia, and 16 C. dubliniensis isolates recovered from various specimens from patients at the Hadassah Medical Center, Jerusalem, Israel, were included in the study (Table 1). Ten of the 16 Israeli C. dubliniensis isolates were recovered as pure cultures, 4 in mixed culture with non-C. albicans Candida species and 2 in mixed culture with Aspergillus species.
TABLE 1.
C. dubliniensis clinical isolates used in the studya
| C. dubliniensis isolateb | Country of originc | Yr of isolation | Underlying patient condition | Sample | 5FC MIC50 (μg/ml)d | Cd25 fingerprint groupe | Genotypef |
|---|---|---|---|---|---|---|---|
| Eg200* | Egypt | 2002 | Diabetes | Oral | 128 | III | 4 |
| Eg201 | Egypt | 2002 | Cancer | Oral | 128 | III | 4 |
| Eg202 | Egypt | 2002 | Cancer | Oral | 128 | III | 4 |
| Eg203 | Egypt | 2002 | Cancer | Oral | 0.125 | I | 1 |
| Eg204 | Egypt | 2002 | Cancer | Oral | 0.125 | I | 1 |
| Eg205 | Egypt | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| Eg206 | Egypt | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| Eg207 | Egypt | 2002 | Diabetes | Oral | 128 | III | 4A |
| SA100* | S. Arabia | 2002 | Leukemia | Oral | 128 | III | 3 |
| SA101* | S. Arabia | 2002 | Leukemia | Oral | 0.125 | I | 1 |
| SA102* | S. Arabia | 2002 | Leukemia | Blood | 0.125 | I | 1 |
| SA103 | S. Arabia | 2002 | Pneumonia | BAL | 128 | III | 3 |
| SA104 | S. Arabia | 2002 | Pneumonia | Oral | 128 | III | 4 |
| SA105* | S. Arabia | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| SA106 | S. Arabia | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| SA107* | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA108 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA109 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA110 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA111 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA112 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA113* | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 4 |
| SA114 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA115 | S. Arabia | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| SA116 | S. Arabia | 2002 | Diabetes | Oral | 0.125 | I | 1 |
| SA117 | S. Arabia | 2002 | Diabetes | Oral | 128 | III | 3 |
| SA118 | S. Arabia | 2002 | S/P renal Tx | Oral | 128 | III | 3 |
| SA119 | S. Arabia | 2002 | S/P renal Tx | Oral | 128 | III | 3 |
| SA120 | S. Arabia | 2002 | S/P renal Tx | Oral | 128 | III | 3 |
| SA121 | S. Arabia | 2002 | S/P renal Tx | Oral | 128 | III | 4 |
| Is34 | Israel | 2000 | Liver transplant | BAL | 0.125 | ND | 3 |
| Is35 | Israel | 2001 | Nephritis | Oral | 0.125 | II | 4B |
| Is36 | Israel | 2001 | CF | Sputum | 0.125 | II | 3 |
| Is38 | Israel | 2001 | SBE | Urine | 0.125 | ND | 1 |
| Is39 | Israel | 2001 | Stroke | Sputum | 0.125 | ND | 1 |
| Is40 | Israel | 2002 | Renal failure | Throat | 0.125 | ND | 1 |
| Is41 | Israel | 2002 | CF | Sputum | 0.125 | ND | 1 |
| Is42 | Israel | 2002 | Leukemia | Throat | 0.125 | ND | 1 |
| Is43 | Israel | 2002 | CF | Sputum | 0.125 | ND | 1 |
| Is44 | Israel | 2002 | CF | Sputum | 0.125 | ND | 1 |
| Is45 | Israel | 2002 | CF | Sputum | 0.125 | I | 1 |
| Is46 | Israel | 2002 | Trauma | BAL | 0.125 | ND | 1 |
| Is47 | Israel | 2002 | Ciliary dyskinesia | Sputum | 0.125 | I | 1 |
| Is48 | Israel | 2002 | CF | Sputum | 0.125 | ND | 1 |
| Is49 | Israel | 2003 | CF | Sputum | 0.125 | II | 2 |
| Is51 | Israel | 2003 | Postsurgery | ENT | 0.125 | ND | 1 |
The provenance of the isolates is detailed in Materials and Methods. Abbreviations: S. Arabia, Saudi Arabia; BAL, broncheoalveolar lavage; ENT, endotracheal aspirate; SBE, subacute bacterial endocarditis; S/P renal Tx, stage post-renal transplantation; CF, cystic fibrosis; ND, not done.
Isolates marked with an asterisk had their FCY1 and FUR1 genes amplified and sequenced.
Egyptian and Saudi Arabian isolates were all recovered from Arab individuals, whereas Israeli isolates were recovered from Jewish individuals, apart from Is35, Is40, Is43, and Is44, which were recovered from Arab individuals in Israel.
MIC50 values were determined according to NCCLS document M27-A2 (31).
Refers to major groups of isolates (Fig. 2) identified following computer-assisted analysis of Cd25-generated hybridization fingerprint profiles of EcoRI-digested genomic DNA using the DENDRON computer software package. Only 5 selected Israeli isolates were fingerprinted in the present study, although all 16 were genotyped.
Determined by PCR using the genotype-specific primers described by Gee et al. (18). The nucleotide sequences of the ITS regions of the variant genotypes 4A and 4B differed from the genotype 4 ITS consensus sequence at one and two nucleotide positions, respectively.
Definitive identification of all C. dubliniensis isolates was confirmed by the inability or ability of the isolates to grow at 45°C (35), by their substrate assimilation profiles determined with the API ID 32C yeast identification system (bioMérieux, Marcy l'Etoile, France) (9, 34), and by PCR analysis with C. dubliniensis-specific primers as described previously (14).
Isolates were routinely cultured on potato dextrose agar (PDA; Oxoid) medium, pH 5.6, at 37°C. For liquid culturing, isolates were grown overnight in yeast extract-peptone-dextrose (YPD) broth at 37°C in an orbital incubator (Gallenkamp, Leicester, United Kingdom) at 200 rpm.
Chemicals, enzymes, radioisotopes, and oligonucleotides.
Analytical-grade or molecular biology-grade chemicals were purchased from Sigma-Aldrich Ireland Ltd. (Tallaght, Dublin, Ireland), BDH (Poole, Dorset, United Kingdom), or Roche Diagnostics Ltd. (Lewes, East Sussex, United Kingdom). Enzymes were purchased from the Promega Corporation (Madison, Wis.) and from New England Biolabs Inc. (Beverly, Mass.) and used according to the manufacturers' instructions. [α-32P]dATP (6,000 Ci mmol−1; 222 TBq mmol−1) was purchased from Amersham International Plc. (Little Chalfont, Buckinghamshire, United Kingdom). Custom-synthesized oligonucleotides were purchased from Sigma-Genosys Biotechnologies (Europe) Ltd. (Pampisford, Cambridgeshire, United Kingdom).
Southern blot hybridization and computer-assisted analysis.
Southern blot hybridization of restriction endonuclease-digested genomic DNA from C. dubliniensis isolates was performed as described previously (48). EcoRI-digested DNA was electrophoresed through 0.65% (weight/volume) agarose gels for 16 h at 65 V and transferred by capillary blotting to nylon membrane filters (Osmonics, Westborough, Mass.) as described previously (48). Hybridization reactions were carried out under high-stringency conditions with the Cd25 probe labeled with 32P by random primer labeling (18).
Computer-assisted analyses of hybridization patterns were performed using the DENDRON software package version 2.4 (Solltech, Iowa City, Iowa) as described previously (18, 22, 43). DNA from the C. dubliniensis isolate CM6 was used as a reference on each gel used for computer-assisted analysis as described previously by Joly et al. (22) and Gee et al. (18). SABs based on band position alone were calculated for each pairwise combination of isolate patterns according to the formula SAB = 2E/(2E + a + b), where E is the number of bands shared by strains A and B, a is the number of bands unique to A, and b is the number of bands unique to B (43). An SAB of 1.00 represents identical patterns, and SABs ranging from 0.01 to 0.99 represent patterns with increasing proportions of bands at the same positions. A selection of Cd25-generated fingerprint patterns from EcoRI-digested DNA from an international collection of C. dubliniensis isolates from the study of Gee et al. (18) from this laboratory were used with DENDRON to generate mixed dendrograms with the fingerprint pattern data generated with the Saudi Arabian, Egyptian, and Israeli isolates as described previously (5, 43).
Genotyping.
Template DNA from each of the Saudi Arabian, Egyptian, and Israeli C. dubliniensis isolates investigated in this study was tested in separate PCR amplification experiments with each of the primer pairs G1F/G1R, G2F/G2R, G3F/G3R, and G4F/G4R to identify the genotype of the isolate as described by Gee et al. (18). Genotypes are ascribed based on the nucleotide sequences of the internal transcribed spacer 1 (ITS1) and ITS2 regions and of the intervening 5.8S rRNA gene (51). Template DNAs from the four reference C. dubliniensis isolates (CD36, genotype 1; Can4, genotype 2; CD519, genotype 3; and p7718, genotype 4) previously described by Gee et al. (18) were used in control experiments. Each PCR was carried out with one pair of genotype-specific primers and the universal fungal primers RNAF/RNAR (15), which amplify approximately 610 bp from all fungal large-subunit rRNA genes and were used as an internal positive control. Two isolates (Eg207 and Is35 [Table 1]) failed to yield amplimers with any of the genotype-specific primer sets described above. The ITS region from each of these isolates was amplified using the ITS1/ITS2 (51) primer pair and cloned, and the nucleotide sequence was obtained and compared as described previously (18). pBluescript II KS(−) was used to clone the purified amplimers using standard procedures (40). Genotyping experiments were performed on a minimum of two occasions with each isolate tested with separately prepared C. dubliniensis template DNA.
Antifungal susceptibility testing.
The 22 Saudi Arabian, 8 Egyptian, and 16 Israeli C. dubliniensis isolates investigated in this study were tested for susceptibility to 5FC by the broth microdilution method described in the Clinical and Laboratory Standards Institute (CLSI) (formerly National Committee for Clinical Laboratory Standards [NCCLS]) document M27-A2 (31). In addition, 24 C. dubliniensis isolates from the study of Gee et al. (18), which were recovered from 12 different countries, were also tested (these included 10 Cd25 group I isolates [genotype 1] and 14 Cd25 group II isolates [8 of genotype 2, 5 of genotype 3, and 1 of genotype 4]). A stock solution of 5FC (Sigma-Aldrich) was prepared in water at a concentration of 1 mg/ml. Serial dilutions were then made in RPMI 1640 medium (Sigma-Aldrich) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid. Testing was carried out in a final volume of 100 μl in 96-well plates containing final concentrations of 5FC ranging from 0.12 to 128 μg/ml with inoculum densities of 1.5 × 103 ± 1 × 103 CFU. Drug-free and yeast-free controls were included in each plate. After 48 h of incubation at 30°C, the cell density was measured at 450 nm with a Spectra I automated plate reader (SLT Labinstruments, Grödig, Austria). The MICs were determined as the lowest concentrations of 5FC that reduced turbidity by 50% (MIC50) relative to the growth of the drug-free growth controls. All isolates were tested on a minimum of two occasions in separate experiments. The results were interpreted according to the criteria of the NCCLS using the following breakpoints: susceptibility, ≤4 μg per ml; intermediate resistance, 8 to 16 μg per ml; resistance, ≥32 μg per ml.
Amplification and sequencing of C. dubliniensis FCY1 and FUR1 genes.
The complete open reading frames (ORFs) of the C. dubliniensis genes encoding uracil phosphoribosyltransferase (FUR1) and cytosine deaminase (FCY1) were amplified by PCR from seven selected Middle Eastern isolates, including four 5FC-resistant (MIC50 ≥ 128 μg/ml) and three 5FC-susceptible (MIC50 ≤ 0.125 μg/ml) isolates (Table 1), and their nucleotide sequences were determined. These were compared with the corresponding sequences of the 5FC-susceptible reference strains CD36 and CM6 (genotype 1), CD506, Can6, and CD541 (genotype 2), CD519 and p6265 (genotype 3), and p7718 (genotype 4) described previously by Gee et al. (18). The sequences of the C. albicans genes FCY1 (GenBank accession no. U55194) and FUR1 (Stanford orf6.3823) were used in a BLAST search against the C. dubliniensis genome sequence database (the Wellcome Trust Sanger Institute C. dubliniensis genome sequence project; http://www.sanger.ac.uk/Projects/C_dubliniensis/) to identify the C. dubliniensis homologs. The C. dubliniensis FUR1 gene is 841 bp in length and exhibited 93.3% homology with the C. albicans gene. Similarly, the C. dubliniensis FCY1 gene is 529 bp in length and exhibited 100% homology with the C. albicans gene (data not shown). The primer pairs FCY1F1/FCY1R1 and FUR1F1/FUR1R1 were designed to amplify the complete ORFs of each of the C. dubliniensis genes, including some upstream and downstream sequences (Table 2). Reaction mixtures contained 100 ng of purified template DNA, 1× Expand High Fidelity buffer (Roche, Lewes, East Sussex, United Kingdom), deoxynucleoside triphosphates at concentrations of 0.2 mM each, 2.5 U of Expand High Fidelity PCR system enzyme mix (Roche), and 0.2 μM concentrations of either FCY1F1/FCY1R1 or FUR1F1/FUR1R1. The reaction mixtures were subjected to an initial denaturation for 7 min at 94°C and 30 cycles of 1 min at 94°C, 1 min of annealing at 50°C for FCY1 or at 52.5°C for FUR1, and 1 min at 74°C. The final extension was for 10 min at 72°C. Amplification products were purified using a Promega PCR extraction kit (Promega) and sequenced in both the forward and reverse directions with the primers used for their amplifications. PCRs with each isolate tested were performed a minimum of three times in separate experiments with separately prepared template DNA. At least three cloned amplimers from each separate PCR experiment were sequenced for each isolate tested.
TABLE 2.
Nucleotide sequences of PCR primers used to amplify the C. dubliniensis FUR1 and FCY1 genesa
| Primer | Sequence (5′ to 3′) | Nucleotide coordinatesb |
|---|---|---|
| FCY1F | CGGCATATTAATTCCGCTTG | −145 to −164 |
| FCY1R | CGAATTCTCTTCTGCTTCTG | +106 to +125 |
| FUR1F | ATGTGGGTTACATCAGAAGA | −198 to −217 |
| FUR1R | TCTCGCGACCCTCCTCTAAC | +205 to +224 |
The nucleotide sequences of the C. dubliniensis FUR1 and FCY1 genes were obtained from the C. dubliniensis genome sequence database (http://www.sanger.ac.uk/Projects/C_dubliniensis/).
Nucleotide coordinates for C. dubliniensis genes FUR1 and FCY1 have the first bases of the ATG start codons designated +1.
DNA sequence analysis.
Nucleotide sequence analysis was performed by the dideoxy chain-terminating method of Sanger et al. (41) by use of an automated Applied Biosystems 377 DNA sequencer and dye-labeled terminators (Applied Biosystems, Foster City, Calif.). The sequencing primers used for ITS1 and ITS2 and the intervening 5.8S rRNA gene of C. dubliniensis were the M13 forward and reverse primers. To sequence the FCY1 and FUR1 genes, the FCY1F1/FCY1R1 and FUR1F1/FUR1R1 primer pairs were used (Table 2). Sequence alignments were carried out using the CLUSTAL W sequence analysis computer software package (50).
RESULTS
Candida dubliniensis from Saudi Arabian and Egyptian patients.
Oral swab samples were obtained from 434 immunocompromised patients in four hospitals in Saudi Arabia and Egypt (Table 3). The proportions of oral Candida-positive individuals sampled from the two countries were similar (Saudi Arabia, 62.8%; Egypt, 70.4%), as were the proportions of individuals that yielded C. dubliniensis (Saudi Arabia, 5.5%; Egypt, 6.4%) (Table 3). Details of the Saudi Arabian and Egyptian cohorts sampled and the prevalence of oral C. dubliniensis and other Candida species recovered from these cohorts are shown in Table 3. In total, 17 Saudi Arabian and 8 Egyptian C. dubliniensis isolates were recovered from the patient cohorts sampled. Five additional Saudi Arabian C. dubliniensis isolates were included in the study, resulting in a collection of 30 Saudi Arabian and Egyptian isolates for this investigation (Table 1). An additional 16 C. dubliniensis isolates recovered in an Israeli hospital were included for comparison (Table 1).
TABLE 3.
Recovery of oral C. dubliniensis from Saudi Arabian and Egyptian patients
| Country and no. of patients | Underlying condition | No. (%) with oral candidiasisa | No. (%) oral Candida positive | No. (%) yielding C. dubliniensisb |
|---|---|---|---|---|
| Saudi Arabiac | ||||
| 160 | Renal transplant | 42 (26.3) | 104 (65) | 4 (2.5) |
| 132 | Diabetes | 9 (6.8) | 78 (59) | 13 (9.9) |
| 17 | HIV-infected | 0 (0) | 12 (70.6) | 0 (0) |
| Egypt | ||||
| 58 | Neoplasia | 12 (20.7) | 39 (67.2) | 4 (6.9) |
| 67 | Diabetes | 6 (8.9) | 49 (73.1) | 4 (6.0) |
| Total, 432 | 69 (16) | 282 (65.3) | 25 (5.8) |
Swabs from all individuals with clinical symptoms indicative of oral candidiasis yielded semiconfluent or confluent growth of Candida species.
Six of the 25 C. dubliniensis isolates were recovered as pure cultures, whereas the remaining 19 were isolated in mixed culture with other Candida species, predominantly C. albicans.
In addition to the isolates referred to above, five additional C. dubliniensis isolates, recovered from various specimens from separate Saudi Arabian individuals (SA100 to SA104 in Table 1), were included in the study, but details on whether they were isolated with other yeast species were not available.
Genotyping of C. dubliniensis isolates.
In a previous study in which 98 C. dubliniensis isolates from 15 different countries were investigated, it was found that C. dubliniensis consists of four separate genotypes based on the nucleotide sequence of the ITS region of the rRNA gene cluster (18). Interestingly, 4/6 genotype 3 and genotype 4 isolates included in this study were from Israel. In order to begin to investigate the population structure and diversity of C. dubliniensis isolates recovered in Saudi Arabia and Egypt compared to those of isolates recovered in other countries around the world, the 30 C. dubliniensis isolates (Table 1) recovered from separate Saudi Arabian (22 isolates) and Egyptian (8 isolates) patients were genotyped by PCR analysis. Sixteen C. dubliniensis isolates recovered from individual patients in Israel were used for comparison (Table 1). Template DNA from each isolate was tested separately in PCR experiments with each of the genotype-specific primer pairs GIF/G1R, G2F/G2R, G3F/G3R, and G4F/G4R previously described by Gee et al. (18). These primers allow C. dubliniensis isolates to be grouped into one of four genotypes based on the nucleotide sequences of ITS1 and ITS2 regions and of the intervening 5.8S rRNA gene region of the rRNA gene cluster. Isolates belonging to a particular genotype yield a single PCR amplimer only with the corresponding genotype-specific primers. Template DNA from each of the reference C. dubliniensis isolates, i.e., CD36 (genotype 1), Can4 (genotype 2), CD519 (genotype 3), and p7718 (genotype 4), was used in control experiments.
All of the C. dubliniensis isolates tested yielded the 610-bp product resulting from amplification with the fungal universal primers. Six (27.3%) of the Saudi Arabian and four (50%) of the Egyptian isolates tested were found to belong to genotype 1 (Table 1), whereas none of the Saudi Arabian or Egyptian isolates tested belonged to genotype 2. Thirteen (59.1%) of the Saudi Arabian isolates but none of the Egyptian isolates belonged to genotype 3. The remaining three Saudi Arabian isolates and three of the four remaining Egyptian isolates belonged to genotype 4 (Table 1). One Egyptian isolate (Eg207) failed to yield an amplimer with any of the genotype-specific primer pairs, i.e., GIF/G1R, G2F/G2R, G3F/G3R, and G4F/G4R. In order to investigate Eg207 further, the ITS region from this isolate was amplified by PCR using the ITS1/ITS2 primer pair (51) and cloned, and its nucleotide sequence was determined and compared with the corresponding ITS sequences of genotypes 1 to 4 previously reported by Gee et al. (18). The ITS sequence of Eg207 was found to differ from the C. dubliniensis genotype 4 consensus sequence at nucleotide position 82 (T-to-C transition) according to the numbering system of Gee et al. (18). Isolate Eg207 was deemed to be a genotype 4 variant, termed genotype 4A. Of the 16 C. dubliniensis isolates from Israel included in the study, 12/16 (75%) belonged to genotype 1, 1/16 (6.3%) belonged to genotype 2, and 2/16 (12.5%) belonged to genotype 3 (Table 1). The remaining isolate (Is35) failed to yield an amplimer with any of the genotype-specific primer pairs and had its ITS region amplified and sequenced as described above for Egyptian isolate Eg207. The ITS sequence of Is35 was found to differ from the C. dubliniensis genotype 4 ITS consensus sequence at nucleotide positions 63 (T-to-C transition) and 82 (T-to-C transition) according to the numbering system of Gee et al. (18) and was deemed to be a genotype 4 variant, termed genotype 4B.
These results demonstrated that genotype 3 and genotype 4 isolates predominated (20/30; 66.6%) among the combined Saudi Arabian and Egyptian isolates studied. This is in contrast with the Israeli isolates, where genotype 1 isolates predominated (12/16; 75%), and with the 98 C. dubliniensis isolates from the study of Gee et al. (18), which were recovered in 15 different countries from around the world where genotype 1 isolates predominated (71/98; 72%).
DNA fingerprint analysis of Saudi Arabian and Egyptian C. dubliniensis isolates.
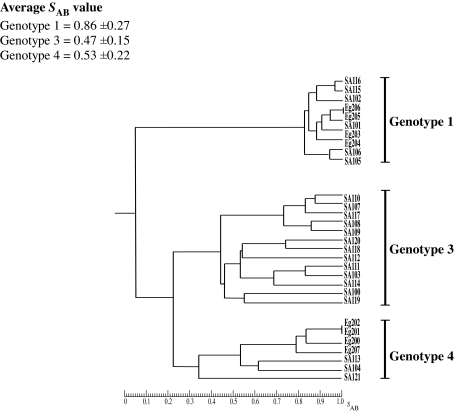
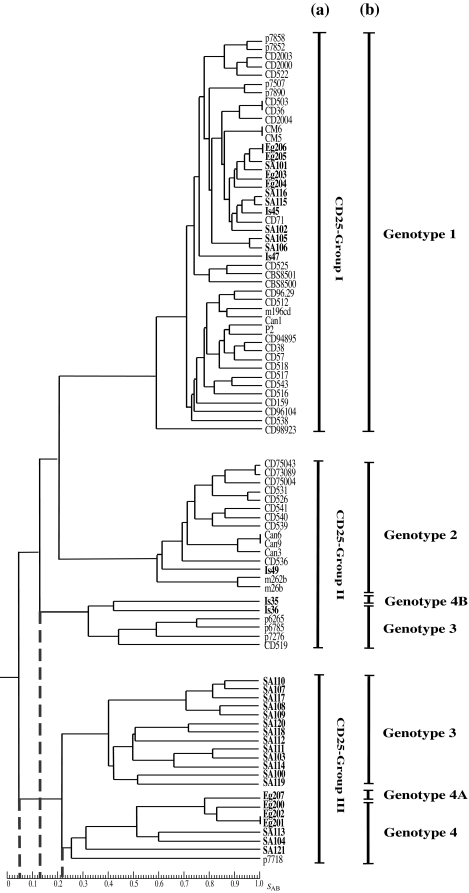
In order to investigate the unexpectedly high prevalence of genotype 3 and genotype 4 isolates and the range of genetic diversity among C. dubliniensis isolates from Saudi Arabia and Egypt, the 30 isolates included in the study were fingerprinted with the C. dubliniensis-specific complex DNA fingerprinting probe Cd25. Only 5 of the Israeli isolates were fingerprinted, because the majority (12/16) belonged to genotype 1 and a previous study from this laboratory demonstrated that genotype 1 Israeli C. dubliniensis isolates belong to fingerprint group Cd25-I (18). The Israeli isolates fingerprinted included the two genotype 1 isolates Is45 and Is47, the genotype 2 isolate Is49, the genotype 3 isolate Is36, and the genotype 4B variant isolate Is35 (Table 1). The Cd25 DNA fingerprint profiles were subjected to computer-assisted analysis with the fingerprint profile analysis software package DENDRON. SAB values were computed for every possible pairwise combination of isolates, and these data were used to construct dendrograms showing the relationships between the isolates. Examination of the dendrogram constructed from fingerprint data obtained with the Saudi Arabian and Egyptian isolates revealed that the 30 isolates could be clearly divided by a node at an SAB value of 0.05 into two distinct main populations of isolates, the first corresponding to genotype 1 and the second to genotypes 3 and 4 (Fig. 1). The total collection of Saudi Arabian and Egyptian isolates had an average SAB value of 0.28 ± 0.27. The first subpopulation (genotype 1) of isolates had an average SAB value of 0.86 ± 0.27, whereas the second subpopulation (genotype 3 [average SAB value, 0.47 ± 0.15] and genotype 4 [average SAB value, 0.53 ± 0.22]) had an average SAB value of 0.35 ± 0.19. In order to investigate the relationships between the two populations of Saudi Arabian and Egyptian isolates identified in this collection and the two groups (Cd25 group I and Cd25 group II) identified in isolates from countries around the world by Joly et al. (22) and verified by Gee et al. (18), a mixed dendrogram was generated from the 30 Saudi Arabian and Egyptian isolates, the 5 Israeli isolates, and 51 independent isolates from 13 countries from the study of Gee et al. (18) (which included 32 isolates from Cd25 group I and 19 isolates from Cd25 group II). Examination of the mixed dendrogram revealed that the isolates could be clearly divided at an SAB node of 0. 05 into three distinct populations (Fig. 2). The first group of isolates (average SAB value of 0.63 ± 0.12) consisted solely of genotype 1 isolates and corresponded to Cd25 group I, previously described by Joly et al. and Gee et al. (18, 22). This group contained the six Saudi Arabian (SA101, SA102, SA105, SA106, SA115, and SA116), the four Egyptian (Eg203, Eg204, Eg205, and Eg206), and the two Israeli (Is45 and Is47) genotype 1 isolates fingerprinted in this study mixed with isolates from the study of Gee et al. (18). The second group of isolates (average SAB value of 0.37 ± 0.30) corresponded to Cd25 group II, previously described by Joly et al. and Gee et al. (18, 22), and did not contain any Saudi Arabian or Egyptian isolates but did contain the other three Israeli isolates fingerprinted in this study, including Is49 (genotype 2), Is36 (genotype 3), and Is35 (genotype 4B) mixed with isolates from the study of Gee et al. (18). The third group of isolates (average SAB value of 0.35 ± 0.19) consisted of the 20 genotype 3 and genotype 4 Saudi Arabian and Egyptian isolates included in this study together with the genotype 4 Israeli isolate p7718 from the study of Gee et al. (18). This latter isolate was a distinct outlier within Cd25 group II in the study of Gee et al. (18). None of the Saudi Arabian or Egyptian genotype 3 or genotype 4 isolates mixed with any of the Cd25 group I or Cd25 group II isolates from the study of Gee et al. (18). These findings demonstrated that genotype 3 and genotype 4 Saudi Arabian and Egyptian C. dubliniensis isolates consist of a hitherto-undescribed clade of isolates that is prevalent in Saudi Arabia and Egypt but, apart from the Israeli isolate p7718, was absent from the international collection of isolates from the study of Gee et al. (18). The third group of C. dubliniensis isolates forming the novel Saudi Arabian and Egyptian clade was termed Cd25 group III (Fig. 2).
FIG. 1.
Dendrogram generated from the SABs computed for every possible pairwise combination of 30 C. dubliniensis isolates recovered from individual patients in Saudi Arabia and Egypt fingerprinted with Cd25. The provenances of the isolates are shown in Tables 1 and 3. At an SAB node of 0.05, the isolates are divided into two main populations. The first of these populations consists solely of genotype 1 isolates and are closely related, with an average SAB value of 0.86 ± 0.27. The second population consists of genotype 3 and genotype 4 isolates that are less closely related to each other than are genotype 1 isolates and have an average SAB value of 0.35 ± 0.19. At an SAB node of 0.22, the second population is divided into two subpopulations consisting of genotype 3 and genotype 4 isolates, respectively.
FIG.2.
Dendrogram generated from the SABs computed for every possible pairwise combination of independent C. dubliniensis isolates from Saudi Arabia and Egypt (n = 30), Israel (n = 5), and 13 other countries (n = 51) from the study of Gee et al. (18) fingerprinted with Cd25. At an SAB node of 0.05 (short dashed vertical line), the isolates are divided into three main populations, the first and second of which correspond to the major clades Cd25 group I and Cd25 group II described previously (18, 22). The third main population (Cd25 group III) corresponds to a third major clade identified in this study and contains 20/30 of the Saudi Arabian and Egyptian isolates investigated. The Cd25 group I major clade consists solely of closely related genotype 1 isolates with an average SAB value of 0.63 ± 0.12. At an SAB node of 0.13 (short dashed vertical line), the Cd25 group II major clade can be divided into two minor clades, the first of which consists solely of genotype 2 isolates. The second minor clade consists of genotype 3 isolates and the genotype 4B variant isolate Is35. At an SAB node of 0.22 (short dashed vertical line), the Cd25 group III major clade can also be divided into two minor clades, the first of which consists solely of genotype 3 isolates and the second solely of genotype 4 isolates, including the genotype 4A variant isolate Eg207. Within the Cd25 group III major clade, all of the isolates were from Saudi Arabia or Egypt and were resistant to 5FC (MIC50 ≥ 128 μg/ml), apart from the Israeli isolate, p7718 (18), which was susceptible to 5FC (MIC50 ≤ 0.125 μg/ml). All of the other Saudi Arabian, Egyptian, and Israeli isolates investigated in this study and a selection of 24 independent isolates from the study of Gee et al. (18) (10 Cd25 group I isolates and 14 Cd25 group II isolates) were susceptible to 5FC (MIC50 ≤ 0.125 μg/ml). The Saudi Arabian, Egyptian, and Israeli isolates investigated in this study are highlighted in boldface. The Cd25 groups (a) and the ITS genotypes (b) of the isolates are shown to the right of the dendrogram.
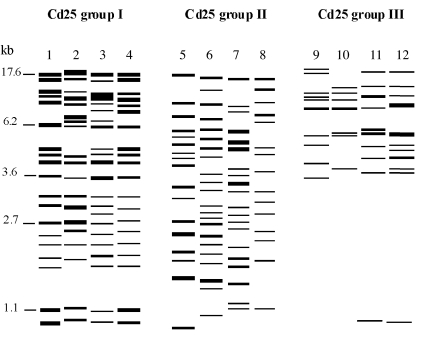
The Cd25-generated fingerprinted patterns of C. dubliniensis isolates within the Cd25 group III clade are very distinct from the corresponding patterns of Cd25 group I and Cd25 group II isolates and can be easily distinguished by direct visual comparison (Fig. 3). In particular, Cd25 group III isolates yield fingerprints with far fewer bands (i.e., 8 to 13 bands) compared with Cd25 group I and Cd25 group II fingerprint profiles (i.e., 15 to 20 bands). More strikingly, the majority of Cd25 group III isolates investigated in this study have no or very few bands below 3.5 kb (Fig. 3).
FIG. 3.
Model generated using the DENDRON software package showing band positions and intensities of Cd25-generated hybridization fingerprint patterns of EcoRI-digested genomic DNA of C. dubliniensis isolates belonging to the major clades Cd25 group I, Cd25 group II, and the novel 5FC-resistant Saudi Arabian and Egyptian clade Cd25 group III identified in this study. Molecular sizes in kilobases are shown on the left. The C. dubliniensis isolates from which the corresponding patterns in the lanes were obtained are as follows (genotypes are shown in parentheses): lane 1, CM6 (1); lane 2, CD518 (1); lane 3, SA102 (1); lane 4, Eg204 (1); lane 5, Can9 (2); lane 6, CD514 (2); lane 7, Is49 (2); lane 8, p6265 (3); lane 9, SA103 (3); lane 10, SA121 (4); lane 11, SA119 (3); lane 12, Eg201 (4).
Flucytosine resistance in Cd25 group III isolates.
Several previous studies have reported that the majority of C. dubliniensis clinical isolates reported in the literature are susceptible to commonly used antifungal drugs, including azoles, amphotericin B, and 5FC (29, 30, 32, 33, 39). However, two recent studies reported an unusually high prevalence of 5FC resistance among C. dubliniensis isolates from Saudi Arabia and Kuwait (1, 17). In order to investigate whether there is an association between the Cd25 group III isolates identified in the present study and resistance to 5FC, all of the Saudi Arabian, Egyptian, and Israeli C. dubliniensis isolates included in the study were tested for susceptibility to 5FC with the NCCLS-approved broth microdilution method (31). A selection of 24 independent C. dubliniensis isolates from 12 different countries from the study of Gee et al. (18) were also included for comparison (which included 10 Cd25 group I isolates [genotype 1] and 14 Cd25 group II isolates [8 of genotype 2, 5 of genotype 3, and 1 of genotype 4]). All 10 Cd25 group I (genotype 1) Saudi Arabian and Egyptian isolates, all 16 Israeli isolates, and all 24 reference isolates from the study of Gee et al. (18) were found to be 5FC susceptible (MIC50 ≤ 0.125 μg/ml) (Table 1). In contrast, all 20 of the Saudi Arabian and Egyptian isolates belonging to Cd25 group III (genotypes 3 and 4) were 5FC resistant (MIC50 ≥ 128 μg/ml) (Table 1). Interestingly, Israeli isolate p7718, which belongs to this group, was susceptible to 5FC (MIC50 ≤ 0.125 μg/ml). These findings demonstrate that the Saudi Arabian and Egyptian isolates forming the novel CD25 group III clade can be distinguished from C. dubliniensis isolates belonging to other groups on the basis of resistance to 5FC.
Analysis of C. dubliniensis FCY1 and FUR1 gene sequences.
Resistance to 5FC in C. albicans has been shown previously to be linked to a single clade (38). A recent study demonstrated that 5FC resistance in C. albicans is linked to a point mutation at nucleotide position 301 in the FUR1 gene encoding uracil phosphoribosyltransferase activity that results in the replacement of arginine with cysteine at amino acid position 101 in the translated protein (13). Another recent study reported that a point mutation in the C. albicans FCY1 gene (encoding cytosine deaminase) resulting in a glycine-to-aspartate substitution at position 28 in the translated protein may also be associated with 5FC resistance (20). In order to investigate whether mutations in the C. dubliniensis FUR1 and FCY1 genes could be associated with 5FC resistance in the Saudi Arabian and Egyptian Cd25 group III isolates identified in the present study, the complete ORFs of these genes together with flanking sequences were amplified by PCR with primers specific for the C. dubliniensis FUR1 and FCY1 genes (Table 2). Amplimers of both genes were obtained and sequenced from three 5FC-resistant (MIC50 ≥ 128 μg/ml) Saudi Arabian isolates, i.e., SA100, SA107 (both genotype 3), and SA113 (genotype 4), from three 5FC-susceptible (MIC50 ≤ 0.125 μg/ml) Saudi Arabian isolates, i.e., SA101, SA102, and SA105 (all genotype 1), and from the 5FC-resistant (MIC50 ≥ 128 μg/ml) Egyptian isolate Eg200 (genotype 4). These sequences were compared with the corresponding sequences obtained from selected 5FC-susceptible (MIC50 ≤ 0.125 μg/ml) reference isolates from the study of Gee et al. (18), including CD36 and CM6 (genotype 1), CD506, Can 6, and CD541 (genotype 2), CD519 and p6265 (genotype 3), and p7718 (genotype 4).
The sequences of the FCY1 gene and flanking sequences from all of the 5FC-susceptible reference isolates tested and the 5FC-susceptible Saudi Arabian genotype 1 isolates SA102 and SA105 were identical to the FCY1 consensus sequence of the C. dubliniensis type strain CD36 obtained from the C. dubliniensis genome sequence database (http://www.sanger.ac.uk/Projects/C_dubliniensis/). The 5FC-resistant Saudi Arabian isolates SA100, SA107, and SA113 and the 5FC-resistant Egyptian isolate Eg200 each contained a single identical nucleotide polymorphism (transition from A to T) at position 258 in the FCY1 gene sequence that did not result in an amino acid residue change in the predicted protein. Comparison of the nucleotide sequences of the FUR1 genes from the 5FC-susceptible reference isolates and the 5FC-susceptible and 5FC-resistant Saudi Arabian and Egyptian isolates revealed the presence of several single nucleotide polymorphisms, none of which resulted in changes in amino acids in the predicted protein (data not shown).
These results demonstrated that polymorphisms in the FCY1 and FUR1 genes could not be associated with 5FC resistance in the C. dubliniensis isolates tested.
DISCUSSION
There have been several reports describing the recovery of C. dubliniensis isolates in a variety of Middle Eastern countries (1, 17, 18, 26), but little is known about the population structure or the epidemiology of these isolates or their relationship to isolates recovered in the rest of the world. Gee et al. (18) included eight independent isolates of C. dubliniensis recovered in Israel in their study, four of which belonged to Cd25 group I (genotype 1), with the other four belonging to Cd25 group II. Interestingly, three of these four Cd25 group II isolates belonged to genotype 3 and one belonged to genotype 4. In fact, of the five genotype 3 isolates identified by Gee et al. (18) out of a total of 98 isolates investigated from 15 different countries, three (60%) were from Israel. The single genotype 4 isolate identified among the isolate collection of Gee et al. (18) was also from Israel. These findings suggested that distinct subpopulations of C. dubliniensis may be present in Israel and possibly in neighboring countries. The purpose of the present study was to investigate this possibility further by analyzing additional isolates of C. dubliniensis from Saudi Arabia, Egypt, and Israel.
In this study, we investigated a total of 46 independent C. dubliniensis isolates recovered from three countries in the Middle East, including 30 C. dubliniensis isolates recovered from Saudi Arabian and Egyptian patients with severe underlying conditions and 16 C. dubliniensis isolates from Israeli patients (Table 1).
Genotyping of the C. dubliniensis isolates demonstrated that the majority (12/16; 75%) of the sixteen Israeli isolates tested belonged to genotype 1 (Table 1), similar to what has been found in previous studies (8, 18). One of the remaining Israeli isolates belonged to genotype 2, two belonged to genotype 3, and the last was a genotype 4 variant, termed genotype 4B, which harbored two single nucleotide base substitutions compared with the C. dubliniensis genotype 4 ITS consensus sequence. In contrast, 4/8 (50%) of the Egyptian isolates and 6/22 (27.2%) of the Saudi Arabian isolates belonged to genotype 1 (Table 1). None of the Egyptian or Saudi Arabian isolates belonged to genotype 2. Interestingly, the majority (13/22; 59%) of the Saudi Arabian isolates belonged to genotype 3, with the remainder (3/22; 13.6%) belonging to genotype 4 (Table 1). None of the Egyptian isolates belonged to genotype 3, and three out of eight (37.5%) belonged to genotype 4. The remaining Egyptian isolate was found to be a genotype 4 variant, termed 4A, which harbored a single nucleotide base substitution compared with the C. dubliniensis genotype 4 ITS consensus sequence. These findings demonstrated that the prevalence and distribution of C. dubliniensis genotypes among the isolates from Saudi Arabia and Egypt compared to those of Israeli isolates are significantly different, at least among the subject cohorts from whom the isolates were recovered. Interestingly, of the 30 combined Saudi Arabian and Egyptian C. dubliniensis isolates included in the study, 20/30 (66.6%) belonged to genotypes 3 and 4 and none were of genotype 2. In contrast, in the previous study by Gee et al. (18), only 6/94 (6.4%) independent C. dubliniensis isolates from 15 different countries were identified as belonging to genotypes 3 and 4. These results indicated that the C. dubliniensis population present in Saudi Arabia and Egypt is quite different from the corresponding populations present in other countries around the world.
In order to further investigate the population structure of C. dubliniensis isolates from the Middle East, all 30 Saudi Arabian and Egyptian C. dubliniensis isolates and 5 selected Israeli isolates (two of genotype 1, one of genotype 2, one of genotype 3, and the genotype 4B variant) were subjected to DNA fingerprint analysis with the C. dubliniensis-specific fingerprinting probe Cd25. Computer-assisted analysis of the fingerprint patterns obtained from the combined Saudi Arabian and Egyptian isolate collection revealed that the isolates separated at a deep-rooted SAB node of 0.05 into two distinct main populations (Fig. 1 and 2). The first main population consisted solely of genotype 1 isolates that were closely related and had an average SAB value of 0.86. (Fig. 1). The second main population was much more diverse, with an average SAB value of 0.35, and could be separated at an SAB node of 0.22 into two equally diverse subpopulations, one of which consisted solely of genotype 3 isolates and the other consisting solely of genotype 4 isolates (Fig. 1). In order to investigate the relationships between the two main populations identified among this isolate collection and the Cd25 group I and Cd25 group II clades identified in previous studies by Joly et al. (22) and Gee et al. (18), a mixed dendrogram was generated from the Cd25-generated fingerprint patterns of the 30 Saudi Arabian and Egyptian isolates and the corresponding patterns of 51 independent isolates from 13 different countries from the study of Gee et al. (18). The fingerprint profiles of the five Israeli isolates fingerprinted in the present study were also included. Examination of the mixed dendrogram revealed that the isolates separated clearly into three distinct major populations or clades, the first of which corresponded to Cd25 group I, the second to Cd25 group II, and the third to a hitherto-undescribed major clade which we have termed CD25 group III (Fig. 2). In agreement with the findings of Gee et al. (18), all genotype 1 isolates were grouped within Cd25 group I, and all genotype 2 isolates were grouped in a subclade within Cd25 group II (Fig. 2). The Israeli genotype 3 isolate Is36 and the genotype 4B variant isolate Is35 fingerprinted in this study grouped within a second subclade within Cd25 group II, in agreement with the findings of a previous study by Gee et al. (18). The Cd25 group III clade contained all of the Saudi Arabian and Egyptian genotype 3 and 4 isolates and the single genotype 4 isolate (p7718) from the study of Gee et al. (18) (Fig. 2). The Cd25 group III clade also contained two subclades at an SAB node of 0.22, the first of which contained only Saudi Arabian isolates of genotype 3, with the second containing both Saudi Arabian and Egyptian genotype 4 isolates. All of these findings demonstrated that the population structure of C. dubliniensis isolates in Saudi Arabia and Egypt is significantly different from that of previously investigated populations from other countries around the world, possibly reflecting a more homogenous ethnic population in Saudi Arabia and Egypt.
All 20 Saudi Arabian and Egyptian C. dubliniensis isolates forming the Cd25 group III clade were found to be 5FC resistant (MIC > 125 μg/ml). The majority of C. dubliniensis clinical isolates reported to date are susceptible to commonly used antifungal drugs, including azoles, polyenes, and 5FC (29, 32, 33, 39). However, two recent studies reported that 18/32 (56%) and 2/7 (29%) C. dubliniensis isolates tested from Saudi Arabia (17) and Kuwait (1), respectively, were 5FC resistant (MIC > 32 μg/ml). On the basis of the results of the present study, it is interesting to speculate that the 5FC-resistant C. dubliniensis isolates described by Fotedar and Al Hedaithy (17) and Ahmad et al. (1) may also belong to the CD25 group III clade. In support of this suggestion, a recent study demonstrated that 5FC resistance in C. albicans is restricted to a single genetic clade (38).
A recent study by Dodgson et al. (13) demonstrated that clade-specific 5FC resistance in C. albicans is due to a single nucleotide change in the FUR1 gene that results in the substitution of arginine by cysteine at amino acid position 101 in the translated protein. Another recent study by Hope et al. (20) reported that a point mutation in the C. albicans FCY1 gene resulting in a glycine-to-aspartate substitution at position 28 in the translated protein may also be associated with 5FC resistance in this species. In order to determine whether mutations in the C. dubliniensis homologs of the FUR1 and FCY1 genes were associated with 5FC resistance in Cd25 group III clade isolates as reported for C. albicans (13, 20), the CdFUR1 and CdFCY1 ORFs and flanking sequences were cloned and sequenced from 5FC-resistant and 5FC-susceptible C. dubliniensis isolates (Tables 1 and 2). No mutations were observed in the ORFs that resulted in amino acid changes in 5FC-resistant isolates, demonstrating that mutations in these genes are not responsible for the 5FC-resistant phenotypes exhibited by these isolates (data not shown). Additional studies are required to elucidate the mechanism(s) of 5FC resistance in Cd25 group III C. dubliniensis.
The results of the present study demonstrate that C. dubliniensis isolates belonging to the Cd25 group III clade appear to be restricted to Saudi Arabia and Egypt, and with the exception of isolate p7718 from Israel (18), all are 5FC resistant. Interestingly, an examination of the records revealed that p7718 was recovered from an Arab individual in Jerusalem in 1999. If our speculation that the 5FC-resistant isolates reported from Saudi Arabia and Kuwait (1, 17) also belong to Cd25 group III is correct, this would suggest that Cd25 group III isolates may be predominant in and/or possibly restricted to persons of Arab demographic/ethnic groups. Associations between certain Candida species and particular geographical regions and racial groups have been reported previously. A recent study (5) suggested that in South Africa, C. dubliniensis colonization is influenced more by race than by HIV infection status. However, no South Africa-specific clade was identified. Another study (6) also identified a novel clade of C. albicans in black and white South Africans. Furthermore, a recent study (26) reported that the genotype distribution of C. albicans isolates from Arab and Druze ethnic groups in Israel were significantly different from that of global populations. Further studies to investigate the distribution of C. dubliniensis major clades and genotypes in healthy and diseased cohorts of individuals in Middle Eastern countries are in progress.
The identification of a novel clade of C. dubliniensis predominant in Saudi Arabia and Egypt, and possibly in other Arab countries, further extends our knowledge about the population structure of this yeast species. Additional studies are required to investigate how widespread the Cd25 group III clade is and whether additional clades are present among specific racial groups; such studies may be facilitated by the application of multilocus sequence typing analysis to C. dubliniensis epidemiological studies.
Acknowledgments
This work was supported by the Microbiology Research Unit, Dublin Dental School and Hospital. A.A.M. was supported by a grant from King Saud University, Riyadh, Saudi Arabia.
REFERENCES
- 1.Ahmad, S., Z. Khan, E. Mokaddas, and Z. U. Khan. 2004. Isolation and molecular identification of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Kuwait. J. Med. Microbiol. 53:633-637. [DOI] [PubMed] [Google Scholar]
- 2.Al Mosaid, A., D. Sullivan, I. F. Salkin, D. Shanley, and D. C. Coleman. 2001. Differentiation of Candida dubliniensis from Candida albicans on Staib agar and caffeic acid-ferric citrate agar. J. Clin. Microbiol. 39:323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Mosaid, A., D. J. Sullivan, and D. C. Coleman. 2003. Differentiation of Candida dubliniensis from Candida albicans on Pal's agar. J. Clin. Microbiol. 41:4787-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagg, J., M. P. Sweeney, M. A. Lewis, M. S. Jackson, D. Coleman, A. Al Mosaid, W. Baxter, S. McEndrick, and S. McHugh. 2003. High prevalence of non-albicans yeasts and detection of anti-fungal resistance in the oral flora of patients with advanced cancer. Palliat. Med. 17:477-481. [DOI] [PubMed] [Google Scholar]
- 5.Blignaut, E., C. Pujol, S. Joly, and D. R. Soll. 2003. Racial distribution of Candida dubliniensis colonization among South Africans. J. Clin. Microbiol. 41:1838-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blignaut, E., C. Pujol, S. Lockhart, S. Joly, and D. R. Soll. 2002. Ca3 fingerprinting of Candida albicans isolates from human immunodeficiency virus-positive and healthy individuals reveals a new clade in South Africa. J. Clin. Microbiol. 40:826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt, M. E., L. H. Harrison, M. Pass, A. N. Sofair, S. Huie, R. K. Li, C. J. Morrison, D. W. Warnock, and R. A. Hajjeh. 2000. Candida dubliniensis fungemia: the first four cases in North America. Emerg. Infect. Dis. 6:46-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brena, S., M. del Carmen Rubio, R. Salesa, I. Iglesias, J. Gil, A. Rezusta, M. D. Moragues, and J. Ponton. 2004. Genotypes of Candida dubliniensis in clinical isolates. Rev. Iberoam. Micol. 21:20-23. (In Spanish.) [PubMed] [Google Scholar]
- 9.Cardenes-Perera, C. D., A. Torres-Lana, R. Alonso-Vargas, M. D. Moragues-Tosantas, J. Ponton-San Emeterio, G. Quindos-Andres, and M. P. Arevalo-Morales. 2004. Evaluation of API ID 32C and VITEK-2 to identify Candida dubliniensis. Diagn. Microbiol. Infect. Dis. 50:219-221. [DOI] [PubMed] [Google Scholar]
- 10.Carr, M. J., S. Clarke, F. O'Connell, D. J. Sullivan, D. C. Coleman, and B. O'Connell. 2005. First reported case of endocarditis caused by Candida dubliniensis. J. Clin. Microbiol. 43:3023-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman, D., D. Sullivan, B. Harrington, K. Haynes, M. Henman, D. Shanley, D. Bennett, G. Moran, C. McCreary, and L. O'Neill. 1997. Molecular and phenotypic analysis of Candida dubliniensis: a recently identified species linked with oral candidosis in HIV-infected and AIDS patients. Oral Dis. 3(Suppl. 1):S96-S101. [DOI] [PubMed] [Google Scholar]
- 12.Coleman, D. C., D. J. Sullivan, D. E. Bennett, G. P. Moran, H. J. Barry, and D. B. Shanley. 1997. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS 11:557-567. [DOI] [PubMed] [Google Scholar]
- 13.Dodgson, A. R., K. J. Dodgson, C. Pujol, M. A. Pfaller, and D. R. Soll. 2004. Clade-specific flucytosine resistance is due to a single nucleotide change in the FUR1 gene of Candida albicans. Antimicrob. Agents Chemother. 48:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly, S. M., D. J. Sullivan, D. B. Shanley, and D. C. Coleman. 1999. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology 145:1871-1882. [DOI] [PubMed] [Google Scholar]
- 15.Fell, J. 1993. Rapid identification of yeast species using three primers in a polymerase chain reaction. Mol. Mar. Biol. Biotechnol. 3:174-180. [PubMed] [Google Scholar]
- 16.Fotedar, R., and S. S. Al Hedaithy. 2004. Prevalence of Candida dubliniensis among germ tube-positive yeasts recovered from the respiratory specimens in HIV-negative patients. Mycoses 47:150-155. [DOI] [PubMed] [Google Scholar]
- 17.Fotedar, R., and S. S. A. Al Hedaithy. 2003. Candida dubliniensis at a university hospital in Saudi Arabia. J. Clin. Microbiol. 41:1907-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gee, S. F., S. Joly, D. R. Soll, J. F. G. M. Meis, P. E. Verweij, I. Polacheck, D. J. Sullivan, and D. C. Coleman. 2002. Identification of four distinct genotypes of Candida dubliniensis and detection of microevolution in vitro and in vivo. J. Clin. Microbiol. 40:556-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb, G. S., A. P. Limaye, Y. C. Chen, and W. C. Van Voorhis. 2001. Candida dubliniensis fungemia in a solid organ transplant patient: case report and review of the literature. Med. Mycol. 39:483-485. [DOI] [PubMed] [Google Scholar]
- 20.Hope, W. W., L. Tabernero, D. W. Denning, and M. J. Anderson. 2004. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 48:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabra-Rizk, M. A., T. M. Brenner, M. Romagnoli, A. A. M. A. Baqui, W. G. Merz, W. A. Falkler, Jr., and T. F. Meiller. 2001. Evaluation of a reformulated CHROMagar Candida. J. Clin. Microbiol. 39:2015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joly, S., C. Pujol, M. Rysz, K. Vargas, and D. R. Soll. 1999. Development and characterization of complex DNA fingerprinting probes for the infectious yeast Candida dubliniensis. J. Clin. Microbiol. 37:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkpatrick, W. R., S. G. Revankar, R. K. McAtee, J. L. Lopez-Ribot, A. W. Fothergill, D. I. McCarthy, S. E. Sanche, R. A. Cantu, M. G. Rinaldi, and T. F. Patterson. 1998. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar Candida screening and susceptibility testing of isolates. J. Clin. Microbiol. 36:3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefler, E., M. J. McCullough, K. V. Clemons, and D. A. Stevens. 2001. Initial isolation of Candida dubliniensis from the Middle East. Int. J. Infect. Dis. 5:40-42. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi, M., M. J. McCullough, Z. M. Al-Karaawi, S. J. Hurel, and S. R. Porter. 2002. The isolation, identification and molecular analysis of Candida spp. isolated from the oral cavities of patients with diabetes mellitus. Oral Microbiol. Immunol. 17:181-185. [DOI] [PubMed] [Google Scholar]
- 26.McCullough, M. J., J. J. Jorge, F. Lejbkowicz, E. Lefler, F. Nassar, K. V. Clemons, and D. A. Stevens. 2004. Genotypic differences of Candida albicans and C. dubliniensis isolates related to ethnic/racial differences within the same geographic area. Mycopathologia 158:39-41. [DOI] [PubMed] [Google Scholar]
- 27.McMullan, R., J. Xu, J. E. Moore, B. C. Millar, M. J. Walker, S. T. Irwin, J. Price, J. Barr, and S. Hedderwick. 2002. Candida dubliniensis bloodstream infection in patients with gynaecological malignancy. Eur. J. Clin. Microbiol. Infect. Dis. 21:635-636. [DOI] [PubMed] [Google Scholar]
- 28.Meis, J. F., M. Ruhnke, B. E. De Pauw, F. C. Odds, W. Siegert, and P. E. Verweij. 1999. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg. Infect. Dis. 5:150-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran, G., D. Sullivan, J. Morschhäuser, and D. Coleman. 2002. The Candida dubliniensis CdCDR1 gene is not essential for fluconazole resistance. Antimicrob. Agents Chemother. 46:2829-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran, G. P., D. J. Sullivan, M. C. Henman, C. E. McCreary, B. J. Harrington, D. B. Shanley, and D. C. Coleman. 1997. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob. Agents Chemother. 41:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing for yeasts. Approved standard, 2nd ed. M27-A2. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 32.Pfaller, M. A., S. A. Messer, L. Boyken, H. Huynh, R J. Hollis, and D. J. Diekema. 2002. In vitro activities of 5-fluorocytosine against 8,803 clinical isolates of Candida spp.: global assessment of primary resistance using National Committee for Clinical Laboratory Standards susceptibility testing methods. Antimicrob. Agents Chemother. 46:3518-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller, M. A., S. A. Messer, S. Gee, S. Joly, C. Pujol, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pincus, D. H., D. C. Coleman, W. R. Pruitt, A. A. Padhye, I. F. Salkin, M. Geimer, A. Bassel, D. J. Sullivan, M. Clarke, and V. Hearn. 1999. Rapid identification of Candida dubliniensis with commercial yeast identification systems. J. Clin. Microbiol. 37:3533-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinjon, E., D. Sullivan, I. Salkin, D. Shanley, and D. Coleman. 1998. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J. Clin. Microbiol. 36:2093-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polacheck, I., J. Strahilevitz, D. Sullivan, S. Donnelly, I. F. Salkin, and D. C. Coleman. 2000. Recovery of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Israel. J. Clin. Microbiol. 38:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponton, J., R. Ruchel, K. V. Clemons, D. C. Coleman, R. Grillot, J. Guarro, D. Aldebert, P. Ambroise-Thomas, J. Cano, A. J. Carrillo-Munoz, J. Gene, C. Pinel, D. A. Stevens, and D. J. Sullivan. 2002. Emerging pathogens. Med. Mycol. 38:225-236. [DOI] [PubMed] [Google Scholar]
- 38.Pujol, C., M. A. Pfaller, and D. R. Soll. 2004. Flucytosine resistance is restricted to a single genetic clade of Candida albicans. Antimicrob. Agents Chemother. 48:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quindos, G., M. T. Ruesga, E. Martin-Mazuelos, R. Salesa, R. Alonso-Vargas, A. J. Carrillo-Munoz, S. Brena, R. San Millan, and J. Ponton. 2004. In-vitro activity of 5-fluorocytosine against 1,021 Spanish clinical isolates of Candida and other medically important yeasts. Rev. Iberoam. Micol. 21:63-69. (In Spanish.) [PubMed] [Google Scholar]
- 40.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebti, A., T. E. Kiehn, D. Perlin, V. Chaturvedi, M. Wong, A. Doney, S. Park, and K. A. Sepkowitz. 2001. Candida dubliniensis at a cancer center. Clin. Infect. Dis. 32:1034-1038. [DOI] [PubMed] [Google Scholar]
- 43.Soll, D. R. 2000. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 13:332-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staib, P., and J. Morschhäuser. 1999. Chlamydospore formation on Staib agar as a species-specific characteristic of Candida dubliniensis. Mycoses 42:521-524. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan, D., and D. Coleman. 1998. Candida dubliniensis: characteristics and identification. J. Clin. Microbiol. 36:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan, D., K. Haynes, J. Bille, P. Boerlin, L. Rodero, S. Lloyd, M. Henman, and D. Coleman. 1997. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J. Clin. Microbiol. 35:960-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan, D. J., G. P. Moran, E. Pinjon, A. Al-Mosaid, C. Stokes, C. Vaughan, and D. C. Coleman. 2004. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res. 4:369-376. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 49.Tekeli, A., I. Dolapci, R. Emral, and S. Cesur. 2004. Candida carriage and Candida dubliniensis in oropharyngeal samples of type-1 diabetes mellitus patients. Mycoses 47:315-318. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, T. J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sininsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 52.Willis, A. M., W. A. Coulter, D. J. Sullivan, D. C. Coleman, J. R. Hayes, P. M. Bell, and P. L. Lamey. 2000. Isolation of C. dubliniensis from insulin-using diabetes mellitus patients. J. Oral Pathol. Med. 29:86-90. [DOI] [PubMed] [Google Scholar]