Abstract
The protozoan parasite Babesia equi replicates within erythrocytes. During the acute phase of infection, B. equi can reach high levels of parasitemia, resulting in a hemolytic crisis. Horses that recover from the acute phase of the disease remain chronically infected. Subsequent transmission is dependent upon the ability of vector ticks to acquire B. equi and, following development and replication, establishment of B. equi in the salivary glands. Although restriction of the movement of chronically infected horses with B. equi is based on the presumption that ticks can acquire and transmit the parasite at low levels of long-term infection, parasitemia levels during the chronic phase of infection have never been quantified, nor has transmission been demonstrated. To address these epidemiologically significant questions, we established long-term B. equi infections (>1 year), measured parasitemia levels over time, and tested whether nymphal Boophilus microplus ticks could acquire and, after molting to the adult stage, transmit B. equi to naive horses. B. equi levels during the chronic phase of infection ranged from 103.3 to 106.0/ml of blood, with fluctuation over time within individual horses. B. microplus ticks fed on chronically infected horses with mean parasite levels of 105.5 ± 100.48/ml of blood acquired B. equi, with detection of B. equi in the salivary glands of 7 to 50% of fed ticks, a range encompassing the percentage of positive ticks that had been identically fed on a horse in the acute phase of infection with high parasitemia levels. Ticks that acquired B. equi from chronically infected horses, as well as those fed during the acute phase of infection, successfully transmitted the parasite to naive horses. The results unequivocally demonstrated that chronically infected horses with low-level parasitemia are competent mammalian reservoirs for tick transmission of B. equi.
Babesia equi, a protozoan of the phylum Apicomplexa, is biologically transmitted by ixodid ticks and causes acute disease in equids with clinical signs characterized by fever, anemia, icterus, and hemoglobulinuria (7, 22). Horses that recover from the acute phase of infection remain chronically infected, apparently for life (22). The significance of the disease is sufficient that positive horses are prohibited from international movement, a requirement that has substantial economic impact (8, 22). This restriction is not limited to horses in the acute phase of infection but extends to chronically infected horses, as detected by serological assays (6, 14, 15, 23). This restriction is based on the presumption that chronically infected horses serve as reservoirs for vector ticks to acquire and subsequently transmit B. equi. This presumption, however, is untested.
Following acquisition tick feeding, sexual reproduction of B. equi occurs in the midgut of the tick vector, with subsequent development of parasite kinetes that invade salivary glands via the hemolymph (17, 20, 21, 27). Within the tick salivary glands, the developmental process culminates in infectious sporozoites, which can then be transmitted to a susceptible horse upon feeding (9, 12). Thus, the initial parasitemia in the horse during acquisition feeding may be a critical component of transmission efficacy, as there must be sufficient parasites in the blood to meet the threshold requirement for development within the tick. While there are clearly differences in the level of parasitemia between the acute phase of infection, during which B. equi parasitized erythrocytes are abundant when peripheral blood smears are examined microscopically, and the chronic phase of infection, in which levels are below those reliably detected microscopically, the actual levels of parasitemia during the chronic phase of infection are unknown.
In this study, we tested the hypothesis that chronically infected horses are a competent reservoir for tick transmission of B. equi, and we specifically addressed three components of transmission. The first was to quantify the levels of B. equi in peripheral blood during the chronic phase of infection and to determine whether these levels are static or dynamic over time during infection. The second was to determine the ability of Boophilus microplus to acquire B. equi by feeding on horses during the chronic phase of infection. B. microplus is a natural vector of B. equi with a widespread distribution in tropical and subtropical regions where B. equi infection is endemic. The third and most important component was to test whether B. microplus acquisition fed on chronically infected horses would transmit B. equi to susceptible horses. The use of B. microplus fed on horses in the acute phase of infection, previously shown to transmit B. equi (14, 24), provided the positive transmission control needed to definitively address the role of chronically infected horses as reservoirs for tick transmission.
MATERIALS AND METHODS
Animals, pathogen, and tick vector.
Fifteen horses (mixed-breed ponies), all geldings between 2.5 and 8 years of age and free of B. equi as determined by a nested PCR targeting ema-1 and serological testing using the equi merozoite antigen 1 (EMA-1) competitive inhibition enzyme-linked immunosorbent assay (14, 16), were utilized in this study. The B. equi isolate was obtained in 1976 from a horse in Florida and cryopreserved in liquid nitrogen as a blood stabilate containing 10% dimethyl sulfoxide. A horse with an intact spleen (H2) was intravenously inoculated with 30 ml of Florida isolate B. equi first-passage stabilate. This horse was monitored for clinical signs and parasitemia. At a parasitemia level of 0.64%, infected erythrocytes were collected and cryopreserved in liquid nitrogen as a stabilate containing packed erythrocytes 1:1 with a cryopreservative of 20% (wt/vol) polyvinylpyrrolidone and 2% (wt/vol) glucose in Puck's saline G, as previously described (15). The La Minta strain of B. microplus ticks was maintained at the U.S. Department of Agriculture Agricultural Research Service Animal Disease Research Unit, University of Idaho, Moscow (24). To rear B. microplus nymphs for acquisition of B. equi from infected horses, approximately 1 g of normal B. microplus larvae were fed for 5 days on Holstein-Friesian calves (26). Engorged larvae were removed using microsurgery forceps and incubated for 3 days at 26°C with 94% relative humidity and a 12-h photoperiod to allow molting to the nymphal stage (26).
Determination of B. equi levels in the peripheral blood by using real-time PCR.
To quantify B. equi levels in the peripheral blood of infected horses, a previously reported (26) real-time PCR assay based on the single-copy B. equi ema-1 gene (13) was used. Briefly, this assay amplifies a 155-bp fragment stretching from nucleotide 115 to 270 (numbering is based on the ema-1 sequence previously reported), with detection using a PE Applied Biosystems fluorogenic probe that anneals at bp 137 to 161 (26). The cycling conditions and preparation of the standard curve utilizing 102 to 107 copies of recombinant ema-1 (pema-1) were identical to those previously reported (26). All assays were run in triplicate.
To compare the results of the real-time PCR with parasite levels determined by microscopic examination of Giemsa-stained blood smears, horses H038 and H069 (26) were inoculated with the B. equi Florida isolate and levels determined daily during the ascending phase of parasitemia using both methods. When the microscopically determined B. equi level was 107.50/ml from one time point, real-time PCR demonstrated a mean of 107.6 parasites per ml of blood, indicating agreement between real-time PCR and microscopic examination. This sample was diluted to a level equivalent to 105.6 B. equi parasites per ml (based on PCR quantification), typical of the chronic phase of infection, and used as an internal standard in all subsequent real-time PCR assays.
To ensure that the sequence of ema-1 used as the target for the real-time PCR was stable during the chronic phase of infection, full-length ema-1 was cloned and sequenced from 11 time points over a period of 39 months after infection of horse H057. Genomic DNA from H057 was extracted at each time point and the 816-bp gene amplified as previously described (13). Amplified ema-1 fragments were cloned into the pCR-4 TOPO vector and competent TOP10 Escherichia coli cells transformed (Invitrogen Corporation, Carlsbad, CA). Approximately 10 individual transformed colonies were selected from each PCR, plasmid DNA was isolated, and the presence of inserts confirmed by restriction enzyme EcoRI digestion. The inserts were sequenced in both directions by using a Big Dye Kit and an ABI Prism automated sequencer. Sequencher (Gene Codes Corporation, Ann Arbor, MI) was utilized to assemble and edit ema-1 sequences. Plasmid clones that contained no changes in the ema-1 sequence and clones with changes at the real-time PCR primer and probe binding sites were compared to determine if the changes at the primers and probe binding sites altered the quantification of ema-1 by real-time PCR. Equivalent concentrations of clones were diluted to a known copy number and then quantified by real-time PCR.
The levels of B. equi in the peripheral blood during the chronic phase of infection of horses H057, H063, H065, and H066, infected by intravenous inoculation of approximately 8 × 107 parasitized erythrocytes, were determined by real-time PCR. Blood samples from H057 (650 to 720 days postinoculation), H063, H065, and H066 (480 to 550 days postinoculation) were collected in EDTA biweekly and genomic DNA extracted (Gentra Systems, Inc., Minneapolis, MN).
Tick acquisition feeding on acutely infected and chronically infected horses.
B. microplus nymphs were acquisition fed on horses H059, H072, and H076 for 8 days during the chronic phase of infection. As a positive control, B. microplus nymphs were identically fed on splenectomized horse H047 during the ascending phase of parasitemia and used as a comparative measurement for tick infection and subsequent transmission. During each of the 8 days of nymphal acquisition feeding, the levels of B. equi in the peripheral blood of all infected horses were quantified by real-time PCR. Engorged B. microplus nymphs acquisition fed on B. equi-infected horses were removed and incubated for 5 days at 26°C with 94% relative humidity and a 12-h photoperiod to allow molting to adulthood. Freshly molted adult ticks were utilized to determine the ability of adult ticks acquisition fed as nymphs on infected horses to transmit B. equi to naive horses.
Tick transmission feeding.
B. microplus adult ticks infected with B. equi as nymphs were transmission fed on naive horses for 8 days. The animal numbers and numbers of adult ticks that attached and fed were as follows: (i) 280 adult ticks fed as nymphs on chronically infected horse H059 were transmission fed on naive horses H072 (140 ticks) and H076 (140 ticks), with subsequent dissection of 127 adult ticks (64 and 63 obtained from H072 and H076, respectively) to identify the percentage of ticks with B. equi in the salivary glands; (ii) 233 adult ticks fed as nymphs on H072 during the chronic phase of infection were transmission fed on naive horse H073, with 98 ticks being dissected and examined; (iii) 213 adult ticks fed as nymphs on H076 during the chronic phase of infection were transmission fed on naive horse H068, and 98 ticks were dissected and examined; and (iv) 152 adult ticks fed as nymphs on horse H047 during the ascending phase of parasitemia were transmission fed on naive horses H045 and H064 (76 ticks per animal), with 31 (H045) and 47 (H064) ticks being dissected and examined.
To detect infected salivary glands, genomic DNA was extracted from the salivary gland pairs of individual adult ticks recovered following transmission feeding. Briefly, salivary glands were lysed in 450 μl of Tris-EDTA and sodium dodecyl sulfate (SDS) with 50 μl of proteinase K (2 mg/ml) and incubated overnight at 55°C. Following the incubation, 1 μl of glycogen (10 μg/ml) was added to the samples. The proteins were precipitated with ammonium acetate. Genomic DNA samples were precipitated in isopropanol, washed in 70% ethanol, and resuspended in Tris-EDTA. Duplex nested PCRs using primers targeting B. equi ema-1 and B. microplus α-tubulin, as previously described (4, 11, 26), were utilized to determine B. equi infection in the salivary glands and to control for the presence of tick genomic DNA. To confirm the identity of B. equi ema-1 amplicons, Southern hybridization was performed using a digoxigenin-labeled 226-bp ema-1 probe generated from pema-1. Following electrophoresis on a 1.5% agarose gel and transfer to a nylon membrane (Hybond; Amersham Biosciences, Piscataway, NJ), the membrane was hybridized with the ema-1 probe in DIG EASY Hyb (Roche Applied Science, Indianapolis, IN) at 42°C overnight. The membrane was washed four times: three times with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS (twice at room temperature and once at 68°C for 15 min) and once in 0.1× SSC-0.1% SDS at 68°C for 15 min. Detection of bound probe used alkaline phosphatase-labeled anti-digoxigenin antibody, followed by CDP-Star chemiluminescence (Roche Applied Science, Indianapolis, IN). Tick infection percentages were calculated by dividing the total number of infected ticks detected by the total number of ticks examined.
RESULTS
Determination of B. equi levels in peripheral blood by using real-time PCR.
The copy number and threshold cycle of the assay correlated with an r2 value of 0.93 to 0.99 in repeated assays using the standard curve based on known numbers of plasmid copies. In addition, there was close agreement, less than 0.5 log difference, between the number of B. equi parasites detected by real-time PCR and microscopic examination of blood smears during the ascending phase of parasitemia (Fig. 1). A sample of this parasitemic blood, diluted to the equivalent of 105.6 parasites per ml (based on PCR quantification) to approximate the levels predicted for the chronic phase of infection, was included in all assays as an internal control. There was minimal variation in the results of individual assays (coefficient of variability of 1.2%). The genetic stability of the ema-1 target sequences during long-term infection was examined by cloning and sequencing the full-length gene from infected horse H057 at 11 time points over a period of 39 months postinfection. The levels of identity among all clones compared to the previously described B. equi Florida ema-1 sequence (11) ranged from 99.7 to 100%. Of the total of 90 sequenced clones, 5 contained single nucleotide substitutions within the real-time PCR forward primer or at the probe binding sites. There were no changes within the reverse primer sequence. Direct comparison of the clones with or without these single changes revealed no differences in the efficiency of amplification and detection (data not shown).
FIG. 1.
Comparison of B. equi levels in the peripheral blood during the ascending phase of parasitemia detected by real-time PCR and by microscopic examination.
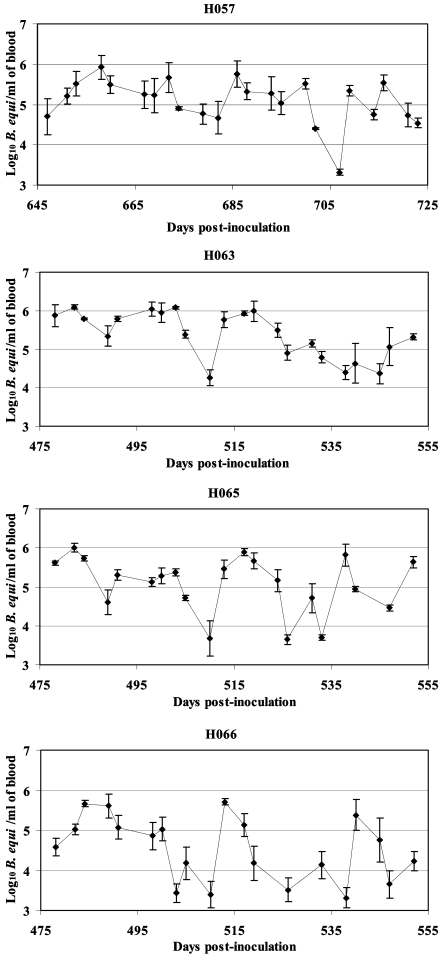
Application of the assay to four chronically infected horses during 10 weeks of evaluation revealed levels that varied from 103.3 to 106.0 parasites per ml of blood. The variation was derived from repeated fluctuations within individual horses, which occurred in all chronically infected horses examined (Fig. 2).
FIG. 2.
Determination of B. equi levels over time in four chronically infected horses.
Tick infection percentage in B. microplus fed on B. equi-infected horses.
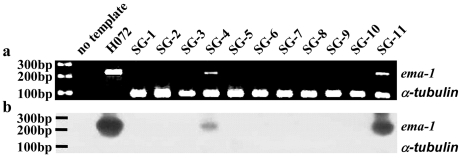
B. equi levels in the peripheral blood of infected horses were determined by real-time PCR during the 8 days of B. microplus nymphal acquisition feeding. The levels of B. equi within chronically infected horses H059, H072, and H076 ranged from 104.4 to 106.1 (mean parasite level of 105.5 ± 100.48/ml of blood) at the time of tick feeding (Table 1). In contrast, horse H047 in the acute phase of infection had a mean level of 108.8 parasites per ml, with a range of 104.1 to 109.4, during tick acquisition feeding (Table 1). The presence of B. equi in the tick salivary glands, which is requisite for transmission, was determined following transmission feeding using a duplex nested PCR (Fig. 3a). B. microplus α-tubulin was amplified from all samples to ensure that B. equi ema-1-negative salivary glands had amplifiable DNA. The identity of the B. equi ema-1 amplicons was confirmed by using Southern hybridization with a digoxigenin-labeled 226-bp ema-1 probe generated from pema-1 (Fig. 3b). B. microplus ticks acquisition fed as nymphs on chronically infected horses demonstrated a wide range, 7 to 50%, in the percentage of ticks with positive salivary glands (Table 1). The percentage of infected salivary glands in B. microplus ticks acquisition fed as nymphs on a horse during the ascending phase of parasitemia was 22% (Table 1).
TABLE 1.
Infection percentages within B. microplus adult ticks fed as nymphs on horses in the acute and chronic phases of infection
| Infection phase | Horse | B. equi levels (log10/ml of blood) in ticks acquisition fed as nymphs | Infection % of ticks transmission fed as adultsa |
|---|---|---|---|
| Chronic | H076 | 4.4-5.8 | 50 (47/94) |
| Chronic | H059 | 5.2-5.9 | 11 (14/127) |
| Chronic | H072 | 4.4-6.1 | 7.1 (7/98) |
| Acute | H047 | 4.1-9.4 | 22 (17/78) |
The number of positive ticks and the total number examined are in parentheses.
FIG. 3.
(a) Detection of B. equi-positive salivary glands from individual B. microplus adults fed as nymphs on a chronically infected horse. (b) Confirmation of amplicon identity of positive B. equi salivary glands from a duplex nested PCR with a digoxigenin-labeled ema-1 probe. SG1 to SG11, DNA amplified from salivary glands of individual ticks; H072, DNA amplified from B. equi Florida isolate-infected erythrocytes as a positive control. The predicted amplicon sizes for B. equi ema-1 and B. microplus α-tubulin are indicated in the right margin. Molecular size markers are in the left margin.
Transmission of B. equi to naive horses.
B. microplus adults that were acquisition fed as nymphs on horses in the acute (H047) or chronic (H059, H072, and H076) phase of infection successfully transmitted B. equi to naive recipients. Transmission was verified by microscopic detection of B. equi-parasitized erythrocytes in Giemsa-stained blood smears, seroconversion to competitive inhibition enzyme-linked immunosorbent assay-positive status, and ema-1 PCR. As ticks acquisition fed on a single horse could be allocated for transmission feeding on more than a single naive horse, the total number of successful transmissions was six (two of two using ticks acquisition fed during the acute phase of parasitemia, four of four using ticks acquisition fed during the chronic phase of infection). Adult B. microplus ticks that had been acquisition fed as nymphs on chronically infected horse H059 transmitted B. equi to naive horses H072 and H076. After resolution of the acute phase of parasitemia and establishment of chronic infection, H072 and H076 were utilized for nymphal acquisition feeding, and adult ticks acquisition fed as nymphs on these two horses transmitted the parasite to H073 and H068, respectively. As a positive control, adult B. microplus acquisition fed as nymphs on horse H047 during the ascending phase of parasitemia transmitted B. equi to H045 and H064.
DISCUSSION
Transmission studies using B. microplus adults acquisition fed as nymphs on horses with long-term infections and defined low levels of B. equi parasitemia establish that chronically infected horses, as well as acutely parasitemic horses, serve as mammalian reservoirs for tick transmission of B. equi. In contrast to the relatively short duration of the acute phase of parasitemia (22), the lifelong nature of B. equi infection in horses provides a continual source for pathogen acquisition by ticks, required for each tick generation as B. equi cannot be maintained solely in the tick. Despite a difference of >103 B. equi parasites per ml between peak levels during nymphal feeding in the acute (109.4/ml) versus the chronic (105.8 to 106.0/ml) phase of infection, there was no difference in the percentage of adult ticks that developed B. equi within the salivary gland. This is consistent with other studies of tick transmission of blood-borne pathogens in which, once a threshold level is reached, a further increase in the level of pathogens per milliliter has little or no effect on the percentage of ticks that acquire infection and are able to subsequently transmit the infection (5, 19, 25). Our data indicate that the threshold level for nymphal B. microplus to acquire B. equi and have development progress to salivary gland colonization following molting to the adult stage is ≤105.8 B. equi parasites per ml. Examination of parasitemia levels over time in chronically infected horses indicates that at 16% of the time points, levels were ≥105.8/ml (Fig. 2). The duration of the chronic phase of infection and the frequency with which horses maintain parasitemia levels at or above the maximal threshold to infect ticks are consistent with chronically infected horses being the most important reservoir for B. equi transmission.
The levels of parasitemia fluctuated over time in each of the chronically infected horses, ranging from a low of 103.3 to a high of 106.0 parasites per ml. The coefficients of variability derived from 20 time points per chronically infected horse for the real-time PCR were 11.1% (H059), 11.6% (H063), 14.4% (H065), and 17.6% (H066). In contrast, the coefficient of variability for the detection of the internal control of 105.8 parasites was 1.2%. The differences in the coefficient of variability between the time point assays of blood obtained from the chronically infected horses and the repeated assay of the internal control supports actual fluctuation in parasitemia levels, as opposed to assay-to-assay variation. This variation is also consistent with prior microscopic examination of Giemsa-stained blood smears from chronically infected horses, in which parasitized erythrocytes are observed at infrequent intervals, very likely corresponding to the peaks identified by quantitative PCR (Fig. 2). Fluctuation in the levels of parasitized erythrocytes during the chronic phase of infection is common with pathogens in the genera Babesia and Plasmodium (1, 3). In both Babesia bovis and Plasmodium falciparum, this fluctuation reflects intertwined processes of antigenic variation and cytoadhesion-sequestration (2, 10, 18). Having demonstrated significant fluctuation in the B. equi levels during the chronic phase of infection, the testing of specific hypotheses regarding the basis of this variation is a logical next step.
The number of infected ticks required to transmit B. equi is unknown. Although it was not a primary goal of this study to address this variable, examination of the number of fed ticks with B. equi in the salivary gland at the time of transmission provides some new information that may guide future studies, both in the field and in experimental transmission. The minimal number of ticks with detectable B. equi in the salivary gland at the time of successful transmission feeding was 4; 4/64 nymphs fed on H059 and transmission fed on H072 had positive salivary glands. Similar numbers of positive ticks were associated with positive transmission to H073 (7/98 positive ticks that had been acquisition fed on H072), H045 (8/31 positive ticks that had been acquisition fed on H047), H064 (9/47 positive ticks that had been acquisition fed on H047), and H072 (10/63 positive ticks that had been acquisition fed on H059). Thus, it appears that only a few positive ticks are required for transmission under the conditions described here. However, there remain many unknowns that may influence the association between PCR-positive ticks and transmission, including variables in the number, viability, and infectivity of sporozoites within the salivary gland, pathogen and tick strain differences, and duration of tick feeding in the field, that require additional investigation.
Currently, the international movement of equids is restricted in many countries, including the United States, to animals shown to be serologically negative for B. equi, with the goal of preventing the entry of chronically infected horses. The demonstration here that chronically infected horses commonly maintain parasitemia levels at or above the maximal threshold for ticks to acquire B. equi and that small numbers of infected ticks are capable of transmission supports this restriction on movement into areas where competent tick vectors are present or may become established.
Acknowledgments
We thank Ralph Horn, James Allison, and David Herndon for excellent technical assistance. Also, we thank Terry F. McElwain and Travis C. McGuire of the Department of Veterinary Microbiology and Pathology, Washington State University, for critical review of the manuscript. We thank Marian Marston for help through a high school independent study project.
This work was supported by U.S. Department of Agriculture-ARS-ADRU project 5348-32000-020D.
REFERENCES
- 1.Allred, D. R., and B. Al-Khedery. 2004. Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: different logics achieve the same goal. Mol. Biochem. Parasitol. 134:27-35. [DOI] [PubMed] [Google Scholar]
- 2.Allred, D. R., R. M. Cinque, T. J. Lane, and K. P. Ahrens. 1994. Antigenic variation of parasite-derived antigens on the surface of Babesia bovis-infected erythrocytes. Infect. Immun. 62:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crampton, A. L., C. Miller, G. D. Baxter, and S. C. Barker. 1998. Expressed sequenced tags and new genes from the cattle tick, Boophilus microplus. Exp. Appl. Acarol. 22:177-186. [DOI] [PubMed] [Google Scholar]
- 5.Eriks, I. S., D. Stiller, and G. H. Palmer. 1993. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J. Clin. Microbiol. 31:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frerichs, W. M., A. A. Holbrook, and A. J. Johnson. 1969. Equine piroplasmosis: production of antigens for the complement-fixation test. Am. J. Vet. Res. 30:1337-1341. [PubMed] [Google Scholar]
- 7.Friedhoff, K. T. 1988. Transmission of Babesia, p. 23-52. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 8.Friedhoff, K. T., A. M. Tenter, and I. Muller. 1990. Haemoparasites of equines: impact on international trade of horses. Rev. Sci. Tech. 9:1187-1194. [PubMed] [Google Scholar]
- 9.Guimaraes, A. M., J. D. Lima, and M. F. Ribeiro. 1998. Sporogony and experimental transmission of Babesia equi by Boophilus microplus. Parasitol. Res. 84:323-327. [DOI] [PubMed] [Google Scholar]
- 10.Horrocks, P., R. Pinches, Z. Christodoulou, S. A. Kyes, and C. I. Newbold. 2004. Variable var transition rates underlie antigenic variation in malaria. Proc. Natl. Acad. Sci. USA 101:11129-11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappmeyer, L. S., L. E. Perryman, and D. P. Knowles, Jr. 1993. A Babesia equi gene encodes a surface protein with homology to Theileria species. Mol. Biochem. Parasitol. 62:121-124. [DOI] [PubMed] [Google Scholar]
- 12.Karakashian, S. J., M. A. Rudzinska, A. Spielman, S. Lewengrub, J. Piesman, and N. Shoukrey. 1983. Ultrastructural studies on sporogony of Babesia microti in salivary gland cells of the tick Ixodes dammini. Cell Tissue Res. 231:275-287. [DOI] [PubMed] [Google Scholar]
- 13.Knowles, D. P., L. S. Kappmeyer, and L. E. Perryman. 1997. Genetic and biochemical analysis of erythrocyte-stage surface antigens belonging to a family of highly conserved proteins of Babesia equi and Theileria species. Mol. Biochem. Parasitol. 90:69-79. [DOI] [PubMed] [Google Scholar]
- 14.Knowles, D. P., Jr., L. S. Kappmeyer, D. Stiller, S. G. Hennager, and L. E. Perryman. 1992. Antibody to a recombinant merozoite protein epitope identifies horses infected with Babesia equi. J. Clin. Microbiol. 30:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles, D. P., Jr., L. E. Perryman, W. L. Goff, C. D. Miller, R. D. Harrington, and J. R. Gorham. 1991. A monoclonal antibody defines a geographically conserved surface protein epitope of Babesia equi merozoites. Infect. Immun. 59:2412-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles, D. P., Jr., L. E. Perryman, L. S. Kappmeyer, and S. G. Hennager. 1991. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlhorn, H., and E. Schein. 1998. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol. Res. 84:467-475. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor, R. M., T. J. Lane, S. E. Stroup, and D. R. Allred. 1997. Characterization of a variant erythrocyte surface antigen (VESA1) expressed by Babesia bovis during antigenic variation. Mol. Biochem. Parasitol. 89:259-270. [DOI] [PubMed] [Google Scholar]
- 19.Riek, R. F. 1964. The cycle of Babesia bigemina (Smith and Kilborne, 1893) in the tick vector Boophilus microplus (Canestrini). Aust. J. Agric. Res. 15:802-821. [Google Scholar]
- 20.Rudzinska, M. A., S. Lewengrub, A. Spielman, and J. Piesman. 1983. Invasion of Babesia microti into epithelial cells of the tick gut. J. Protozool. 30:338-346. [DOI] [PubMed] [Google Scholar]
- 21.Rudzinska, M. A., A. Spielman, S. Lewengrub, W. Trager, and J. Piesman. 1983. Sexuality in piroplasms as revealed by electron microscopy in Babesia microti. Proc. Natl. Acad. Sci. USA 80:2966-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schein, E. 1988. Equine babesiosis, p. 197-208. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 23.Shkap, V., I. Cohen, B. Leibovitz, Savitsky, E. Pipano, G. Avni, S. Shofer, U. Giger, L. Kappmeyer, and D. Knowles. 1998. Seroprevalence of Babesia equi among horses in Israel using competitive inhibition ELISA and IFA assays. Vet. Parasitol. 76:251-259. [DOI] [PubMed] [Google Scholar]
- 24.Stiller, D., W. L. Goff, L. W. Johnson, and D. P. Knowles. 2002. Dermacentor variabilis and Boophilus microplus (Acari: Ixodidae): experimental vectors of Babesia equi to equids. J. Med. Entomol. 39:667-670. [DOI] [PubMed] [Google Scholar]
- 25.Telford, S. R., III, and A. Spielman. 1993. Reservoir competence of white-footed mice for Babesia microti. J. Med. Entomol. 30:223-227. [DOI] [PubMed] [Google Scholar]
- 26.Ueti, M. W., G. H. Palmer, L. S. Kappmeyer, G. A. Scoles, and D. P. Knowles. 2003. Expression of equi merozoite antigen 2 during development of Babesia equi in the midgut and salivary gland of the vector tick Boophilus microplus. J. Clin. Microbiol. 41:5803-5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zapf, F., and E. Schein. 1994. The development of Babesia (Theileria) equi (Laveran, 1901) in the gut and the haemolymph of the vector ticks, Hyalomma species. Parasitol. Res. 80:297-302. [DOI] [PubMed] [Google Scholar]