Abstract
A sensitive, simple, and instrument-independent method for the visual detection and identification of multiple nucleic acid amplicons by dipstick has been developed. This method is based on nucleic acid hybridization on the dipstick membrane and a signal amplification system to allow visual detection. With hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus type 1 (HIV-1) as model analytes, it is demonstrated that the visual dipstick test combined with multiplex reverse transcription (RT)-PCR for the amplification of viral nucleic acid provides a specific and sensitive detection method. The RT-PCR products were detected by the dipstick with an efficiency similar to that of a complex, expensive, and instrument-dependent method based on fluorogenic oligonucleotide probes. The detection limits of the dipstick combined with multiplex RT-PCR were 50, 125, and 500 IU/ml for HBV DNA, HCV RNA, and HIV-1 RNA, respectively. The dipstick assay detected with similar efficiencies amplicons derived from strains of HBV genotypes A through F, HCV genotypes 1 to 6, and HIV-1 subtypes A through H as well as CRF02 circulating recombinant forms of HIV-1. Analysis of 295 clinical samples and 19 pools of 10 plasma specimens from blood donors revealed that multiplex dipstick detection was reproducible, sensitive, and specific. The visual dipstick detection of multiple amplicons thus provides an attractive alternative to complex, instrument-dependent detection methods currently in use for nucleic acid testing. This new and sensitive method for nucleic acid detection should increase the availability of genomic screening in resource-limited settings and its applicability to near-patient testing.
The importance of nucleic acid testing (NAT) has become increasingly evident during the last decade for many purposes, such as diagnosing viral infections, monitoring antiviral therapy, and improving the safety of blood supplies. NAT combines the advantages of direct and highly sequence-specific detection of the genome of an infectious agent with an analytic sensitivity that is several orders of magnitude greater than that of antigen detection or virus isolation methods. NAT also reduces the risks of viral transmission during the period between infection and seroconversion (34), of infection with immunovariant viruses (20), of immunosilent carriage (6), and of occult carriage (3, 34). Unfortunately, a high investment cost for instruments, a high running cost for reagents, a lack of maintenance support, complex and often labor-intensive procedures that require highly trained personnel, and the need for cold-chain transport and storage of reagents render NAT unaffordable in the settings where it is needed most. These settings include predominantly the resource-limited countries of Africa, Asia, and Latin America with high prevalence of infectious diseases. Consequently, two complementary approaches to lower the costs of NAT have been proposed: pool testing for large-scale screening (26, 35) and the development of multiplex assays for the simultaneous detection of several infectious agents (11).
Multiplex assays have recently been developed for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus type 1 (HIV-1), which are three blood-borne pathogenic viruses of major epidemiological and clinical importance (9, 19, 21, 22). The AMPLINAT MPX assay (21) and the multiplex real-time quantitative reverse transcription (RT)-PCR assay described by Candotti et al. (9) are based on TaqMan fluorescence technology, whereas the transcription-mediated amplification-based Procleix Ultrio assay (19) relies on chemiluminescence technology for detection.
NAT-based assays consist of three basic steps: extraction of nucleic acid, genome amplification mediated by a procedure such as PCR or RT-PCR, and amplicon detection. The first two steps can be performed with relatively inexpensive equipment, including a centrifuge, a water bath, and a thermal cycler. In contrast, the last step requires either time-consuming and labor-intensive electrophoretic separation or expensive equipment to detect fluorescence or chemiluminescence signals. The rapid visual detection of nucleic acid hybridization on a test strip (dipstick) would offer a simple and cost-effective alternative to these highly complex and instrument-dependent commercial methods. Serological testing performed with rapid dipstick tests is instrument independent and both simpler and cheaper than that achieved by classical enzyme immunoassay; it is therefore an attractive option for resource-limited settings and near-patient diagnosis. A substantial limitation of these rapid dipstick immunoassays, however, is their low sensitivities of detection. The ability to combine the advantage of rapid visual dipstick-based detection with the high sensitivity of NAT would allow these powerful technologies to be used in resource-limited settings.
We now describe a sensitive and simple multiplex dipstick-based NAT assay for the simultaneous visual detection and identification of HBV, HCV, and HIV-1 genomes in plasma samples. The performance of multiplex dipstick detection was evaluated by comparison with fluorescent TaqMan probe detection with the Mx4000 multiplex quantitative PCR (QPCR) system.
MATERIALS AND METHODS
Viral standards and clinical specimens.
To establish the analytic sensitivity of the multiplex dipstick detection system, we used WHO international standards for HBV (National Institute for Biological Standards and Controls [NIBSC] code 97/746), HCV (NIBSC code 96/790), and HIV-1 (NIBSC code 97/656) from the NIBSC (Potters Bar, United Kingdom). HBV genotypes A to F (HBsAg-subtyped panel) were obtained from the Institut National de Transfusion Sanguine (Paris, France). Two genotype panels comprising calibrated HCV genotypes 1 to 6 (NIBSC code 02/202) and HIV-1 subtypes A to H (NIBSC code 01/466) were also tested. The viral loads for the WHO international standards and for samples of HCV and HIV-1 genotype panels were provided by NIBSC in international units/ml. The conversion factor from IU/ml to copies/ml remains controversial and genotype dependent. However, it has been estimated to be 1 IU to 5.4 copies or genome equivalents/ml for HBV genotype A (29), 1 IU to 4 copies per ml for HCV genotype 3 (30), and 1 to 1 for HIV-1 subtype B (14).
Individual as well as pooled plasma samples from blood donors at the Komfo Anokye Teaching Hospital Blood Bank in Kumasi, Ghana, were assayed. These samples were tested before donation with rapid tests for HBV surface antigen (HBsAg) and for antibodies to HIV-1, to HIV-2, and to HCV as previously described (2, 9). Plasma samples (200 μl) from donors who tested negative by these rapid tests were mixed in pools of 10 samples and stored at −80°C; those from donors who tested positive were frozen individually. Both pools and individual samples were transferred to the Laboratory of Molecular Virology, Division of Transfusion Medicine (University of Cambridge), for further molecular and serological investigations. This study was approved by the committee on human research publication and ethics of the University of Science and the Technology School of Medical Sciences in Kumasi, Ghana. In addition to those from blood donors, plasma samples were obtained from Ghanaian, United Kingdom, and French individuals infected with HBV, HCV, or HIV-1.
A total of 32 HBV-positive (genotypes A and E; viral load range, 18 to 5.4 × 108 IU/ml), 34 HCV-positive (genotypes 1 to 4; viral load range, 3.0 × 102 to 1.6 × 107 IU/ml), 36 HIV-1-positive (subtypes A, B, CRF02, CRF02/A, and CRF02/G; viral load range, 2.4 × 102 to 2.3 × 106 IU/ml), and 201 negative individual samples as well as 19 pools, composed of 10 samples per pool, were tested.
Isolation of viral nucleic acid.
HBV DNA, HCV RNA, and HIV-1 RNA were isolated from 200 μl of individual or pooled plasma samples with the use of a High Pure viral nucleic acid kit (Roche Diagnostics, Mannheim, Germany). Viral nucleic acid was eluted from the filter column with 50 μl of nuclease-free double-distilled water and stored at −80°C until analysis.
RT-PCR amplification of viral nucleic acid.
Viral DNA and RNA were simultaneously amplified with a TaqMan technology-based real-time RT-PCR assay as previously described (9). In brief, amplification was performed with a Brilliant two-step quantitative RT-PCR core reagent kit and a Mx4000 multiplex quantitative PCR system (Stratagene, La Jolla, CA). The sequences of the primers and detection probes were based on the conserved regions of the HBV surface gene, the 5′ untranslated region of the HCV genome (7), and the long terminal repeat region of the HIV-1 genome (9). The HBV fluorogenic probe was labeled at the 5′ end with Cy5 and at the 3′ end with Black Hole Quencher 2. The HCV and HIV-1 probes were labeled at the 5′ ends with 6-carboxyfluorescein and VIC, respectively, and at the 3′ ends with 6-carboxy-N-tetramethylrhodamine. All probes were obtained from Proligo France SAS (Paris, France).
To simultaneously amplify HBV DNA and HCV and HIV-1 RNA in a standard non-real-time RT-PCR, the same amplification primers and conditions were used as in the real-time RT-PCR assay described above, with the exception that the fluorogenic detector probes were not added to the amplification mixture.
Viral nucleic acid quantification.
Viral loads of the clinical specimens and samples included in the HBV genotype panel were determined using an in-house single HBV QPCR assay and single HCV and HIV-1 RT-QPCR assays as previously described (2, 8, 9). The WHO international standards for HBV, HCV and HIV-1 were used to construct the reference curves.
Dipstick-based detection of viral amplicons.
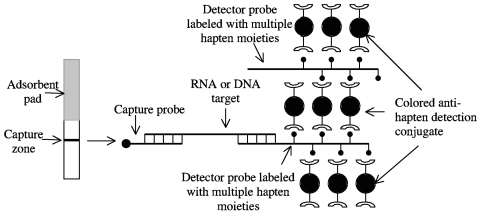
Detection of amplified products on the dipstick membrane was achieved by sandwich nucleic acid hybridization (23); that is, by the hybridization of the target with two target-specific oligonucleotide probes, one for capture and one for detection. The low sensitivity of dipstick assays was overcome with the use of detector probes labeled with multiple hapten moieties for signal amplification. The detector probes thus also specifically bind to multiple anti-hapten antibody molecules that are conjugated with colored particles. The design of the dipstick (Fig. 1) ensures the formation of large lattice-like structures that give rise to a visual signal at the capture zone for samples that contain target RNA or DNA. Detection is simple and rapid (signal develops within 15 min), and it does not require the addition of reagents or washing steps.
FIG. 1.
Design of the nucleic acid-based dipstick test. Detection of the amplification products of viral nucleic acid is achieved with two target-specific oligonucleotide probes, one for capture and one for detection. The detector probe is labeled with multiple hapten moieties and forms large lattices by specifically binding to multiple colored particle-conjugated antibodies to hapten.
Preparation of detector and capture probes.
Solid-phase synthesis of modified capture and detector probes was performed with an Expedite 8900 nucleic acid synthesis system (PerSeptive Biosystems, Framingham, MA) and standard β-cyanoethyl phosphoramidite chemistry. The detector and capture probes were labeled with multiple biotin molecules or derivatized with an amino modifier at the 5′ end, respectively. 5′-Amino-modifier [6-(trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite] and biotin-labeling reagent [1-dimethoxytrityloxy-3-O-(N-biotinyl-3-aminopropyl)-triethyleneglycolyl-glyceryl-2-O-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite], both from Glen Research, were used as modified phosphoramidite monomers for incorporation into the capture and detector probes, respectively, by coupling and deprotection reactions following procedures recommended by the manufacturers. The synthesized probes were purified (>95% homogeneity) by reversed-phase high-pressure liquid chromatography (HPLC) on a Prodigy 5-μm octyldecyl silane HPLC column (Phenomenex, Torrance, CA) by use of a gradient of acetonitrile in 0.1 M tetraethylammonium acetate (pH 7.5). The fractions corresponding to the labeled DNA probes were evaporated to dryness with a Jouan centrifugal evaporator (Jouan, France), and the residue was dissolved in Tris-EDTA buffer (pH 8.0). Probe concentration was determined by measurement of absorbance at 260 nm. Probe purity and structural modification were monitored by mass spectrophotometry, capillary electrophoresis, and reversed-phase and anion exchange HPLC. Before application to the dipstick membrane, 20 nmol amino-modified capture probes were conjugated to bovine serum albumin as a carrier with the use of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) in 25 mM (2-N-morpholino)ethanesulfonic acid (MES) buffer (pH 5.5). The conjugate was purified with a Microcon YM-10 centrifugal device (Millipore, Watford, United Kingdom) and adjusted to a volume of 100 μl with 25 mM MES (pH 5.5).
Preparation of colored particle-conjugated anti-hapten.
Colloidal gold-conjugated antibodies to biotin were used for visual detection in the dipstick assay. They were prepared by passive adsorption of mouse monoclonal anti-biotin to colloidal gold (EMGC 30; British Biocell International, Cardiff, United Kingdom) in 2 mM borax buffer (pH 8) containing 0.1% bovine serum albumin. The conjugation mixture was centrifuged at 7,000 × g for 1 h, and the resulting pellet was then washed with and resuspended in 2 mM borax buffer (pH 8).
Preparation of test strips.
For the preparation of test strips, a CN90 nitrocellulose membrane (Sartorius, Goettingen, Germany) and an adsorbent pad (Advanced Microdevices [PVT], Ambala Cantt, India) were mounted on an adhesive-coated backing sheet (G&L Precision Die Cutting Inc., San Jose, CA). The capture reagents specific for HBV, HCV, or HIV-1 were each applied in a line and separated from each other by a distance of 3 mm with the use of a Matrix 1600 reagent dispensing module (Kinematic Automation, Twain Harte, CA). Strips of the assembled membrane were cut at widths of 5 mm with an automatic cutter (Matrix 2360 Programmable Shear, Kinematic Automation, Twain Harte, CA). The prepared test strips were stored in vacuum-sealed bags with desiccant at room temperature until use.
Detection protocol.
After the addition of 20 pmol of each detector probe to the amplified sample obtained by use of the RT-PCR amplification as described above, the resulting solution was heated at 100°C for 5 min and then mixed with 20 μl of detection reagent (6 μl of colloidal gold-conjugated anti-biotin plus 14 μl of 2 mM borax buffer [pH 8]). The dipstick was placed in contact with the resulting mixture of target, probes, and detection reagent, and the fluid was allowed to migrate up the dipstick. Positive signals were visually read and assigned intensity scores of 1 to 5 with the use of a chart with standards.
Amplicons derived from the WHO international standard reagents for the three viral nucleic acids and negative samples were used as positive and negative controls to validate the detection protocol and detection reagents.
RESULTS
Analytic sensitivity of the dipstick test.
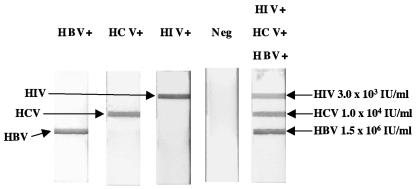
To determine the ability of the dipstick assay to detect and identify individual viral amplicons, we tested WHO international standards for HBV DNA (104 IU/ml) and HCV RNA (5 × 104 IU/ml) with the dipstick in parallel with a multiplex real-time RT-PCR assay. A sample of HIV-1-infected plasma (104 IU/ml) calibrated against the WHO international standard for HIV-1 was tested rather than the standard itself, because the latter was previously found to be contaminated with HBV DNA (33). The amplification of viral nucleic acid was monitored during the reaction by detection of the fluorescence emitted by the three virus-specific TaqMan probes (data not shown); at the end of the reaction, the accumulated amplicons were detected with the triplex dipstick assay. Virus-specific amplified products were readily detected with the dipstick and were identified on the basis of the position of the signal on the test strip (Fig. 2).
FIG. 2.
Triplex dipstick detection of individual and multiple virus amplicons. Nucleic acid standards for HBV, HCV, and HIV-1 were individually amplified by RT-PCR and then tested with the dipstick to determine its ability to identify individual viruses. In addition, a mixture of plasma specimens from three individuals, each infected with one of the three viruses, was used to test the ability of the triplex dipstick to detect HBV DNA, HCV RNA, and HIV-1 RNA in the same sample simultaneously. The viral loads of the combined sample are indicated. A negative plasma sample (Neg) was used to test for the specificity of triplex dipstick detection.
Amplicons obtained by the non-real-time RT-PCR amplification were tested by dipstick detection in parallel. The presence or absence of the fluorogenic probes used during the PCR amplification process had no influence on the dipstick detection (data not shown).
Serial dilutions of the WHO international standards for HBV DNA, HCV RNA, and HIV-1 RNA were prepared in human plasma negative for antibodies and for nucleic acids of all three viruses and were then tested in quadruplicate to assess the analytic sensitivity of the dipstick assay. The detection limit of the triplex dipstick test was 50 IU/ml for HBV DNA, 125 IU/ml for HCV RNA, and 500 IU/ml for HIV-1 RNA. The corresponding detection limits determined in parallel with TaqMan probes were 47, 132, and 680 IU/ml, respectively. Therefore, the visual dipstick detection method showed an analytical sensitivity equivalent to that of fluorescence TaqMan probe-based detection.
The ability of the dipstick assay to detect amplified products derived from the entire range of HBV, HCV, and HIV-1 genotypes was tested with three genotype panels. Compared to TaqMan probe-based detection, the dipstick assay detected with similar sensitivities all reference viral strains included in each genotype panel. The detection limit observed ranged from 10 to 300 IU/ml for the HBV panel and from 300 to 2,000 IU/ml for the HIV-1 panel and was 200 IU/ml for the HCV panel (Table 1), although more specimens from each of the viral subtypes would be needed to reach accurate estimates of the detection limits. The dipstick test was slightly more sensitive than were TaqMan probes in detecting amplified products of HBV genotypes B, C, and F (Table 1). For HBV genotype C, the limit of detection by the TaqMan probe was found to be 30 IU/ml, while the dipstick assay detection limit was 10 IU/ml. A similar difference in sensitivities was obtained for HIV-1 subtype A/E. No differences in sensitivities between the two detection systems were apparent for HCV genotypes.
TABLE 1.
Detection limit of HBV, HCV, and HIV-1 genotypes by the triplex dipstick assay or with fluorogenic TaqMan probes
| Virus | Viral load (IU/ml) | Result for amplicon detection witha: |
|
|---|---|---|---|
| Dipstick assay | TaqMan probes | ||
| HBV genotype | |||
| A | 10 | + | + |
| B | 20 | + | +/− |
| C | 10 | + | − |
| D | 10 | + | + |
| E | 300 | + | + |
| F | 20 | + | +/− |
| HCV genotype | + | + | |
| 1 | 200 | + | + |
| 2 | 200 | + | + |
| 3 | 200 | + | + |
| 4 | 200 | + | + |
| 5 | 200 | + | + |
| 6 | 200 | + | + |
| HIV-1 subtype | |||
| A | 300 | + | + |
| B | 300 | +/− | +/− |
| C | 800 | + | + |
| D | 2,000 | + | + |
| A/E | 600 | + | +/− |
| F | 800 | + | + |
| G | 900 | + | + |
| AG-GH | 1,500 | + | + |
+, positive signal in both duplicates from two independent experiments; −, negative signal in both duplicates from two independent experiments; +/−, one replicate negative and one positive in at least one of two independent experiments.
Simultaneous detection of HBV, HCV, and HIV-1 amplicons with the dipstick test.
The ability of the dipstick assay to detect and identify HBV, HCV, and HIV-1 amplicons in the same sample simultaneously was evaluated with serial 10-fold dilutions of a mixture of three plasma samples from patients infected with a single virus. Viral loads ranged between 1.5 × 107 and 1.5 × 104 IU/ml for HBV DNA, between 105 and 102 IU/ml for HCV RNA, and between 3 × 104 and 3 × 101 IU/ml for HIV-1 RNA. Virus-specific signals were simultaneously detected with the triplex dipstick (Fig. 2) at the same detection limit as that achieved with TaqMan probes (Table 2). Irrespective of the detection method used, no reproducible amplification signals were obtained for HCV RNA at 1 × 102 IU/ml and for HIV-1 RNA at 3 × 102 IU/ml, consistent with the analytic sensitivity data. In addition, dipstick detection of genomic amplicons of one virus at a low viral load was unaffected by the simultaneous detection of amplicons of the other two viruses present at higher (up to 500×) viral loads. Although the capture line for HIV-1 (the lowest viral load in the sample tested) was the most distant from the start site of the dipstick capillary flow and that for HBV (the highest viral load) was the closest, the ability to detect HIV-1 at low concentrations was not impaired (Fig. 2). Positioning of the capture lines for the three analytes in a different order did not affect amplicon detection.
TABLE 2.
Simultaneous detection of HBV DNA, HCV RNA, and HIV-1 RNA by the triplex dipstick test or with fluorogenic TaqMan probes
| Viral load (IU/ml) for: | Result for detection of amplicons derived from the following viruses witha: |
|||||||
|---|---|---|---|---|---|---|---|---|
| HBV | HCV | HIV-1 | TaqMan probes |
Dipstick assay |
||||
| HBV | HCV | HIV-1 | HBV | HCV | HIV-1 | |||
| 1.5 × 107 | 1 × 105 | 3 × 104 | + | + | + | + | + | + |
| 1.5 × 106 | 1 × 104 | 3 × 103 | + | + | + | + | + | + |
| 1.5 × 105 | 1 × 103 | 3 × 102 | + | + | − | + | + | − |
| 1.5 × 104 | 1 × 102 | 3 × 101 | + | − | − | + | − | − |
+, positive signal in both duplicates in two independent experiments; −, negative signal in both duplicates in two independent experiments.
Screening of clinical samples with the dipstick test.
The performance of the triplex dipstick assay with clinical samples was investigated by blind testing of a panel of plasma specimens from blood donors and nontreated individuals infected with HBV, HCV, or HIV-1 (Table 3). All HCV RNA-positive samples were detected both by the triplex dipstick test and by the TaqMan probe. One HBV DNA-positive sample and one HIV-1 RNA-positive sample were not detected with either method; the viral loads of these two samples were 18 IU/ml for HBV DNA and 210 IU/ml for HIV-1 RNA. In these experiments, the limits of detection were 21, 300, and 240 IU/ml for HBV DNA, HCV RNA, and HIV-1 RNA, respectively. Seven of these clinical samples were previously shown to be infected with more than one virus by single quantitative RT-PCR and enzyme immunoassay, one sample was infected with all three viruses, five samples were infected with both HCV and HIV-1, and one sample was coinfected with HBV and HIV-1. For each of these seven samples, the expected viral genomes were simultaneously detected and identified by the triplex dipstick test (Fig. 3).
TABLE 3.
Summary of test results with clinical samples
| Sample characteristic | No. of samples | Viral load range (IU/ml) | No. of samples (%) detected by: |
|
|---|---|---|---|---|
| TaqMan probes | Dipstick assay | |||
| HIV-1 positive | 36 | 2.4 × 102-2.3 × 106 | 35 (97.2) | 35 (97.2) |
| HCV positive | 34 | 3.0 × 102-1.6 × 107 | 34 (100) | 34 (100) |
| HBV positive | 32 | 1.8 × 101-5.4 × 108 | 31 (96.9) | 31 (96.9) |
| HIV-1/HCV/HBV negative | 201 | 198 (98.5) | 201 (100) | |
FIG. 3.
Triplex dipstick detection of clinical specimens from African individuals coinfected with HIV-1, HCV, and HBV (sample identification [ID] 32), with HIV-1 and HCV (sample ID 04008), or with HIV-1 and HBV (sample ID KWADO).
As a control for specificity, we assayed with the dipstick test 201 blood donor samples negative for HBsAg and for antibodies to HCV and to HIV-1, as well as for nucleic acids of the three viruses. All these samples tested negative with the triplex dipstick. In contrast, borderline HCV and HIV-1 signals were obtained in one or both replicates for two and one samples, respectively, with TaqMan probes (Table 3).
The feasibility of the use of the triplex dipstick test for screening plasma pools was examined by testing 19 pools composed of 10 plasma specimens per pool from West African blood donors. Duplicate testing of pooled samples by the dipstick assay revealed that 11 pools were positive for HBV DNA alone, one was positive for HCV RNA alone, and two were positive for both HBV and HCV (Table 4). No HIV-1-positive signals were observed. Identical results were obtained with TaqMan probes, with the exception of two pools that tested borderline positive for HBV but were dipstick negative.
TABLE 4.
Detection of HBV, HCV, and HIV-1 amplicons derived from 19 pools of 10 plasma samples with TaqMan probes and with the triplex dipstick
| Sample pool no. | Result for detection of amplicons derived from the following viruses witha: |
|||||
|---|---|---|---|---|---|---|
| TaqMan probes |
Dipstick assay |
|||||
| HBV | HCV | HIV-1 | HBV | HCV | HIV-1 | |
| 59 | − | + | − | − | + | − |
| 67 | + | − | − | + | − | − |
| 68 | + | − | − | + | − | − |
| 136 | + | − | − | + | − | − |
| 137 | − | − | − | − | − | − |
| 138 | + | − | − | + | − | − |
| 143 | +/− | − | − | − | − | − |
| 146 | +/− | − | − | − | − | − |
| 154 | + | − | − | + | − | − |
| 157 | − | − | − | − | − | − |
| 158 | + | + | − | + | + | − |
| 161 | + | + | − | + | + | − |
| 177 | + | − | − | + | − | − |
| 178 | − | − | − | − | − | − |
| 179 | + | − | − | + | − | − |
| 180 | + | − | − | + | − | − |
| 181 | + | − | − | + | − | − |
| 182 | + | − | − | + | − | − |
| 187 | + | − | − | + | − | − |
+, positive signal in both duplicates in two independent experiments; −, negative signal in both duplicates in two independent experiments; +/−, one replicate negative and one positive in at least one of two independent experiments.
Individual samples included in plasma pools were tested for HBV DNA and HCV RNA by single quantitative RT-PCR (2, 8); this analysis could not be performed with pool 161, because individual samples were not available. The individual samples included in the pools tested negative for HIV-1 RNA by the HIV-1 real-time RT-QPCR assay (9). In addition, they also tested negative for antibodies to HIV-1, HIV-2, and HIV-O by the HIV Determine test (Abbott Laboratories, Delkenheim, Germany).
All pools that tested HBV positive by both methods contained one or more individual samples positive for HBV; the HBV loads in these individual specimens ranged between 10 and 84 IU/ml. No HBV DNA-positive samples were found in pools 143 and 146, which were negative by the dipstick test and borderline positive by triplex TaqMan probe detection. One HCV RNA-positive sample (viral load, 104 IU/ml) was present in pool 59. Resolution of pool 158 revealed that it contained eight HBV DNA-positive samples (average viral load, 25 IU/ml) and one HCV RNA-positive sample (viral load, 7 × 103 IU/ml). No positive individual samples were detected in negative pools 137, 157, and 178.
DISCUSSION
We have investigated the possibility of combining multiplex RT-PCR with a multiplex rapid dipstick assay for simultaneous detection and identification of HBV, HCV, and HIV-1. Detection of specific nucleic acid hybridization on the dipstick was based on a signal amplification system (SAS) for the enhancement of visual signals on test strips. Previously described membrane-based nucleic acid hybridization assays have relied on reporter probes tagged with an enzyme (24), with dye-encapsulating liposomes (4, 12, 27, 28), or with submicrometer-sized phosphor particles (10) and require a reader or reflectometer for the detection and correction of the detection signal for the background of the test strip. Moreover, these assays entail complex multistep procedures that include stepwise addition of various components and both incubation and washing steps after the addition of each new component. In contrast, the application of SAS in the present study yielded a clear background on the test strip that allowed visual reading of the signal without equipment. Furthermore, the SAS-based dipstick detection step is rapid (result within 15 min) and is a one-step procedure that does not require further addition of reagents and washing steps.
Although multiplex NAT for the detection of HBV, HCV, and HIV-1 has been previously demonstrated, these tests require expensive equipment and complex procedures for the detection of fluorescence or chemiluminescence signals (9, 19, 21). The two commercially available semiautomated multiplex assay systems, the AMPLINAT MPX test based on TaqMan real-time PCR with the ABI PRISM 7700 analyzer (21) and the Procleix Ultrio assay based on transcription-mediated amplification (19), do not discriminate between the three viruses. A secondary discrimination step based on virus-specific amplification is thus necessary to identify the origin of a positive test signal. In contrast, triplex RT-PCR with dipstick detection provides direct, single-step identifications of amplified viral genomes. Analysis of the data obtained with calibrated standard reagents and multiply infected clinical samples demonstrated that simultaneous detection and identification of each of the three viruses was possible on a single test strip with good specificity. Although dipstick detection performed after PCR is potentially associated with an increased risk of carryover contamination compared with real-time PCR, no evidence of cross-contamination was observed in the present study, probably because of our compliance with standard PCR safety requirements and the simplicity of the dipstick detection procedure.
HCV and HIV-1 are both characterized by high genetic variability, with the many genotypes (six HCV genotypes) or subtypes (11 subtypes in HIV-1 major group M) differing from each other by ∼30% over the complete genome (15, 36). Seven HBV genotypes, exhibiting 8 to 15% divergence, have also been identified (16). Our results obtained with WHO standards and genotype panels for each virus showed that, although the dipstick test is visual and instrument independent, it detected the standards and all reference viral strains included in each genotype panel with sensitivity comparable to that of fluorescence-based TaqMan detection. Indeed, the dipstick assay appeared to have a slight sensitivity advantage in detecting HBV genotypes B, C, and F and HIV-1 subtype A/E. The fact that dipstick detection is based on sandwich hybridization of the target nucleic acid with two sequence-specific probes (capture and detection), whereas real-time detection relies on only one TaqMan probe, may account for this difference in sensitivities. For HIV-1 detection, the use of two capture probes would further improve the detection of viral variants. Although the use of combinations of multiple sequence-specific probes for the detection of each analyte might increase assay sensitivity and specificity, it might also interfere with the simultaneous detection of different viruses. The latter concern was one of the challenges faced in the development of the multiplex dipstick detection assay.
The window period between infection and seroconversion remains the main residual risk for viral transmission by blood transfusion after serological testing. Viral loads of HBV DNA, HCV RNA, and HIV-1 RNA have been found to range between 10 and 1 × 104 IU/ml, between 1 × 105 and 1 × 106 IU/ml, and between 2 × 102 and 1 × 106 IU/ml (or copies/ml), respectively, during this window (5, 21). The sensitivity of the triplex dipstick for HCV should be sufficient to detect HCV during this period when samples are tested individually or in pools of various sizes and performed rationally for blood donation screening. As a result of its lower sensitivity for HIV-1, the dipstick is likely to be slightly less effective for the detection of HIV-1 in the window period, as has been previously demonstrated with fluorogenic TaqMan probe-based detection (9). In regions of high endemicity and in blood bank settings, HBV infection is commonly monitored by testing for HBsAg. The residual risk of HBV infection is related not only to the window period between infection and the detectability of HBsAg but also to the existence of chronic carriers that are positive for antibodies to HBV core antigen (HBc) but no longer so for detectable HBsAg (1, 34). Previous studies have shown that a low level of HBV DNA was still detectable in such HBsAg-negative, anti-HBc-positive individuals (17, 31). Because of its high level of sensitivity for HBV detection, the dipstick assay has the potential to reduce the risk associated with both the window period and that of late-stage chronic infection with HBV.
The results of testing 201 clinical samples negative for HIV-1, HCV, and HBV showed 100% specificity for triplex dipstick detection of the three viruses. In a previous study, 100% and 98.9% specificities were observed for HBV and HIV-1 detection and for HCV detection, respectively, with multiplex TaqMan-based real-time RT-PCR assays (9). A false-positive rate of 0.17% has also been reported with the AMPLINAT MPX assay (22). In addition, our results revealed that the dipstick assay was able to detect complex recombinant HIV-1 strains (CRF02, CRF02/A, and CRF02/G) circulating in West Africa (13).
In Western countries and Japan, NAT is applied to pools of plasma of various sizes in order to reduce the cost of screening donated blood for the three major transfusion-transmitted viruses. The feasibility of applying the triplex dipstick detection format to the screening of plasma pools was evaluated by testing 19 pools of 10 plasma samples from West Africa. In agreement with the high prevalence of HBV infection previously reported in African countries (2), 68% of the tested pools were HBV positive. Previous clinical trials have documented a lack of sensitivity of pool testing as a result of dilution by samples with low viral loads (35). However, individual samples with HBV viral loads of <100 IU/ml included in the pools of 10 plasma samples were successfully detected by the triplex dipstick test. Dipstick detection also showed a higher specificity for HBV than did TaqMan probe detection. The two pools that gave false-positive results by TaqMan detection were borderline positive, suggesting that the risk of false-positive results may increase above the 40 amplification cycles used in the present study, as previously observed (9, 18, 32). The inclusion of an internal control is important for achieving a higher degree of confidence in negative results. The internal control should be added before sample processing to control for nucleic acid preparation, amplification, and detection steps of viral nucleic acid-based assays (25). The design and integration of a relevant internal control for the triplex dipstick test are in progress.
The multiplex dipstick assay was developed for simultaneous detection of HBV, HCV, and HIV-1 amplification products. The sensitivity and specificity of the dipstick test with both standards and clinical samples were comparable to those of TaqMan probe-based detection. Our results thus demonstrate the feasibility of an instrument-independent and sensitive method for the visual detection of amplicons derived from the three major viruses relevant to blood donation. Combination testing of multiple infectious agents in a simple, rapid, and robust test format at reduced cost is desirable for the screening of blood samples by laboratory workers in blood banks or diagnostic laboratories in developing countries. Integration of this new visual rapid-detection technology with nucleic acid amplification in a closed system format would enable the application of NAT in resource-limited settings and in near-patient testing.
Acknowledgments
This study has been supported in part by the Wellcome Trust and the National Blood Service, England.
We thank Shirley Owusu-Ofori and Francis Sarkodie for the preparation of plasma pools and individual samples from blood donors.
REFERENCES
- 1.Allain, J.-P., P. E. Hewitt, R. S. Tedder, and L. M. Williamson. 1999. Evidence that anti-HBc but not HBV DNA testing may prevent some HBV transmission by transfusion. Br. J. Haematol. 107:186-195. [DOI] [PubMed] [Google Scholar]
- 2.Allain, J.-P., D. Candotti, K. Soldan, F. Sarkodie, B. Phelps, C. Giachetti, V. Shyamala, F. Yeboah, M. Anokwa, S. Owusu-Ofori, and O. Opare-Sem. 2003. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 101:2419-2425. [DOI] [PubMed] [Google Scholar]
- 3.Allain, J.-P. 2004. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 86:83-91. [DOI] [PubMed] [Google Scholar]
- 4.Baeumner, A. J., N. A. Schlesinger, N. S. Slutzki, J. Romano, E. M. Lee, and R. A. Montagna. 2002. Biosensor for dengue virus detection: sensitive, rapid, and serotype specific. Anal. Chem. 74:1442-1448. [DOI] [PubMed] [Google Scholar]
- 5.Biswas, R., E. Tabor, C. C. Hsia, D. J. Wright, M. E. Laycock, E. W. Fiebig, L. Peddada, R. Smith, G. B. Schreiber, J. S. Epstein, G. J. Nemo, and M. P. Busch. 2003. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion 43:788-798. [DOI] [PubMed] [Google Scholar]
- 6.Candotti, D., Y. Adu-Sarkodie, F. Davies, E. Baldrich-Rubio, K. Stirrups, H. Lee, and J.-P. Allain. 2000. AIDS in an HIV-seronegative Ghanaian woman with intersubtype A/G recombinant HIV-1 infection. J. Med. Virol. 62:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Candotti, D., A. Richetin, B. Cant, J. Temple, C. Sims, I. Reeves, J. A. Barbara, and J.-P. Allain. 2003. Evaluation of a transcription-mediated amplification-based HCV and HIV-1 RNA duplex assay for screening individual blood donations: a comparison with a minipool testing system. Transfusion 43:215-225. [DOI] [PubMed] [Google Scholar]
- 8.Candotti, D., J. Temple, F. Sarkodie, and J.-P. Allain. 2003. Frequent recovery and broad genotype 2 diversity characterize hepatitis C virus infection in Ghana, West Africa. J. Virol. 77:7914-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candotti, D., J. Temple, S. Owusu-Ofori, and J.-P. Allain. 2004. Multiplex real-time quantitative RT-PCR assay for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. J. Virol. Methods 118:39-47. [DOI] [PubMed] [Google Scholar]
- 10.Corstjens, P., M. Zuiderwijk, M. Nilsson, H. Feindt, R. S. Niedbala, and H. J. Tanke. 2003. Lateral-flow and up-converting phosphor reporters to detect single-stranded nucleic acids in a sandwich-hybridization assay. Anal. Biochem. 312:191-200. [DOI] [PubMed] [Google Scholar]
- 11.Elnifro, E. M., A. M. Ashshi, R. J. Cooper, and P. E. Klapper. 2000. Multiplex PCR: optimization and application in diagnostic virology. Clin. Microbiol. Rev. 13:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esch, M. B., A. J. Baeumner, and R. A. Durst. 2001. Detection of Cryptosporidium parvum using oligonucleotide-tagged liposomes in a competitive assay format. Anal. Biochem. 73:3162-3167. [DOI] [PubMed] [Google Scholar]
- 13.Fischetti, L., O. Opare-Sem, D. Candotti, F. Sarkodie, H. Lee, and J.-P. Allain. 2004. Molecular epidemiology of HIV in Ghana: dominance of CRF02-AG. J. Med. Virol. 73:158-166. [DOI] [PubMed] [Google Scholar]
- 14.Holmes, H., C. Davis, A. Heath, I. Hewlett, and N. Lelie. 2001. An international collaborative study to establish the 1st international standard for HIV-1 RNA for use in nucleic acid-based techniques. J. Virol. Methods 92:141-150. [DOI] [PubMed] [Google Scholar]
- 15.Janssens, W., A. Buve, and J. N. Nkengasong. 1997. The puzzle of HIV-1 subtypes in Africa. AIDS 11:705-712. [DOI] [PubMed] [Google Scholar]
- 16.Kidd-Ljunggren, K., Y. Miyakawa, and A. H. Kidd. 2002. Genetic variability in hepatitis B viruses. J. Gen. Virol. 83:1267-1280. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman, S. H., M. C. Kuhns, D. S. Todd, S. A. Glynn, A. McNamara, A. DiMarco, and M. P. Busch. 2003. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion 43:696-704. [DOI] [PubMed] [Google Scholar]
- 18.Lanciotti, R. S., and A. J. Kerst. 2001. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J. Clin. Microbiol. 39:4506-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linnen, J., A. Broulik, A. Umali, D. Kolk, J. Dockter, S. McDonogh, L. Mimms, and C. Giachetti. 2002. Analytical sensitivity of the TMA-based Procleix Ultrio assay and effect of assay sensitivity on closing the HBV detection window. Transfusion 42(Suppl.):8S. [Google Scholar]
- 20.Loussert-Ajaka, I., T. D. Ly, M. L. Chaix, D. Ingrand, S. Saragosti, A. M. Courouce, F. Brun-Vezinet, and F. Simon. 1994. HIV-1/HIV-2 seronegativity in HIV-1 subtype O infected patients. Lancet 343:1393-1394. [DOI] [PubMed] [Google Scholar]
- 21.Meng, Q., C. Wong, A. Rangachari, S. Tamatsukuri, M. Sasaki, E. Fiss, L. Cheng, T. Ramankutty, D. Clarke, H. Yawata, Y. Sakakura, T. Hirose, and C. Impraim. 2001. Automated multiplex assay system for simultaneous detection of hepatitis B virus DNA, hepatitis C virus RNA, and human immunodeficiency virus type 1 RNA. J. Clin. Microbiol. 39:2937-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mine, H., H. Emura, M. Miyamoto, T. Tomono, K. Minegishi, H. Murokawa, R. Yamanaka, A. Yoshikawa, and K. Nishioka. 2003. High throughput screening of 16 million serologically negative blood donors for hepatitis B virus, hepatitis C virus and human immunodeficiency virus type-1 by nucleic acid amplification testing with specific and sensitive multiplex reagent in Japan. J. Virol. Methods 112:145-151. [DOI] [PubMed] [Google Scholar]
- 23.Nichollas, P. J., and A. D. Malcolm. 1989. Nucleic acid analysis by sandwich hybridization. J. Clin. Lab. Anal. 3:122-135. [DOI] [PubMed] [Google Scholar]
- 24.Reinhartz, A., S. Alajem, A. Samson, and M. Herzberg. 1993. A novel rapid hybridization technique: paper chromatography hybridization assay (PACHA). Gene 136:221-226. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstraus, M., Z. Wang, S.-Y. Chang, D. DeBonville, and J. P. Spadoro. 1998. An internal control for routine diagnostic PCR: design, properties, and effect on clinical performance. J. Clin. Microbiol. 36:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth, W. K., M. Weber, and E. Seifried. 1999. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet 353:359-363. [DOI] [PubMed] [Google Scholar]
- 27.Rule, G. S., R. A. Montagna, and R. A. Durst. 1996. Rapid method for visual identification of specific DNA sequences based on DNA-tagged liposomes. Clin. Chem. 42:1206-1209. [PubMed] [Google Scholar]
- 28.Rule, G. S., R. A. Montagna, and R. A. Durst. 1997. Characteristics of DNA-tagged liposomes allowing their use in capillary-migration, sandwich-hybridization assays. Anal. Biochem. 244:260-269. [DOI] [PubMed] [Google Scholar]
- 29.Saldanha, J., W. Gerlich, N. Lelie, P. Dawson, K. Heermann, A. Heath, and The W.H.O. Collaborative Study Group. 2001. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 80:63-71. [DOI] [PubMed] [Google Scholar]
- 30.Saldanha, J., A. Heath, and the Collaborative Study Group. 2003. Collaborative study to calibrate hepatitis C virus genotypes 2-6 against the HCV International Standard, 96/790 (genotype 1). Vox Sang. 84:20-27. [DOI] [PubMed] [Google Scholar]
- 31.Sato, S., W. Ohhashi, H. Ihara, S. Sakaya, T. Kato, and H. Ikeda. 2001. Comparison of the sensitivity of NAT using pooled donor samples for HBV and that of a serologic HBsAg assay. Transfusion 41:1107-1113. [DOI] [PubMed] [Google Scholar]
- 32.Schutten, M., B. van den Hoogen, M. E. van der Ende, R. A. Gruters, A. D. M. E. Osterhaus, and H. G. M. Niesters. 2000. Development of a real-time quantitative RT-PCR for the detection of HIV-2 RNA in plasma. J. Virol. Methods 88:81-87. [DOI] [PubMed] [Google Scholar]
- 33.Shyamala, V., J. Cottrell, P. Arcangel, D. Madriaga, B. Phelps, and D. Chien. 2004. Detection and quantification of HBV DNA in the W.H.O. International Standard for HIV-1 RNA (NIBSC code: 97/656). J. Virol. Methods 118:69-72. [DOI] [PubMed] [Google Scholar]
- 34.Soldan, K., J. A. Barbara, M. E. Ramsay, and A. J. Hall. 2003. Estimation of the risk of hepatitis B virus, hepatitis C virus and human immunodeficiency virus infectious donations entering the blood supply in England, 1993-2001. Vox Sang. 84:274-286. [DOI] [PubMed] [Google Scholar]
- 35.Stramer, S. L., K. T. Waldman, J. P. Brodsky, and J. T. Davis. 2000. Comparison of HIV-1/HCV nucleic acid test reactivities of diluted and undiluted donations. Transfusion 40(Suppl.):265-275. [Google Scholar]
- 36.Zein, N. N. 2000. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 13:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]