Abstract
We developed and evaluated a recombinant Borrelia line immunoblot assay based on 18 homologues of seven different antigens, i.e., p100, p58, p41i, BmpA, VlsE, OspC, and DbpA. Each recombinant antigen can be detected separately and is distinct even from homologues with identical molecular weights. This blot was compared to the recently described recombinant Borrelia Western immunoblot assay (U. Schulte-Spechtel, G. Lehnert, G. Liegl, V. Fingerle, C. Heimerl, B. J. Johnson, and B. Wilske, J. Clin. Microbiol. 41:1299-1303, 2003). To verify sensitivity and specificity, both blots were evaluated for reactivity with Borrelia-specific immunoglobulin G (IgG) and IgM antibodies with 85 sera from patients with different manifestations of Lyme borreliosis and 110 controls. According to European interpretation criteria for Borrelia Western blots, which define a serum as positive when it recognizes at least two bands, sensitivity increased significantly from 70.6% (Western blot) to 84.7% (line blot) for IgG (P = 0.042) and from 40.0% (Western blot) to 73.8% (line blot) for IgM (P < 0.005). The increased sensitivity for IgG detection is due to the new line blot technique, whereas the improvement in detection of IgM is mainly achieved through incorporation of the additional antigens. Notably, the recombinant VlsE of Borrelia garinii strain PBi displayed the highest sensitivity of all antigens tested for IgG detection and is also one of the most useful antigens for IgM. Due to its excellent sensitivity and specificity combined with ease of evaluation, this line immunoblot assay offers a useful improvement in serodiagnosis of Lyme borreliosis.
Lyme borreliosis, the most common tick-borne disease in the northern hemisphere, is a multisystem disorder involving predominantly the skin, nervous system, joints, and heart (4, 26). In Europe, the etiologic agent, Borrelia burgdorferi sensu lato, comprises at least three human pathogenic species, i.e., B. burgdorferi sensu stricto, Borrelia afzelii, and Borrelia garinii (2, 6). Diagnosis of Lyme disease typically is based on clinical signs but frequently requires confirmation by serology. Current problems with serodiagnosis of Lyme borreliosis are lack of standardization and poor evaluation regarding sensitivity and specificity, even of commercially available tests, due in part to the broad heterogeneity of Borrelia strains causing human disease in Europe (28, 32-34). To improve specificity, the Centers for Disease Control and Prevention (Atlanta, Ga.) and the German Society for Hygiene and Medical Microbiology (Würzburg, Germany) developed standard criteria for a positive immunoblot; a two-tiered approach to Lyme serology is recommended (7, 37). For the latter, an initial screening test such as an enzyme-linked immunosorbent assay with high sensitivity is followed by an immunoblot with high specificity for confirmation. However, an important problem that remains is how to handle the heterogeneity of immunodominant antigens, especially for the confirmation test. The whole-cell lysate immunoblot assay comprises a single strain only. Use of several strains of different species would considerably increase the cost of routine diagnosis, since every strain would need to be represented on a separate immunoblot strip. In addition, standardization of the whole-cell lysate blot assay has been hampered by differential expression of immunodominant proteins, and discrimination of specific from nonspecific antigens is difficult (11, 34). Recombinant antigens of B. burgdorferi sensu lato for serodiagnosis of Lyme borreliosis offer a promising alternative to resolve some of these issues. For example, blots can be designed to comprise only B. burgdorferi-specific antigens or combine various antigens of proven diagnostic value. These could include homologues from different strains, truncated proteins, or fusion proteins. Such “designer” immunoblots are expected to be highly standardized and more easily interpretable than those currently available.
We have continuously improved and standardized our immunoglobulin G (IgG) immunoblot assay using recombinant antigens of strains that belong to the three defined human-pathogenic European species, B. burgdorferi sensu stricto, B. afzelii, and B. garinii (25, 29-31). Our previous version (25) incorporated the following 14 antigens: p100 (B. afzelii strain PKo), p58 (B. garinii strain PBi), BmpA (B. burgdorferi sensu stricto strain B31, PKo, and PBi), VlsE (B. burgdorferi sensu stricto strain PKa2), OspC (B31, PKo, PBi, and B. garinii strain 20047), p41i (PKo and PBi), and DbpA (PKo and PBr). DbpA, OspC of strain 20047, VlsE, and p58 have so far been evaluated only for IgG.
Here, we describe a recombinant IgG and IgM line immunoblot assay for serodiagnosis of Lyme borreliosis that allows simultaneous assessment of antibody reactivity with individual homologous proteins from different strains, including new DbpA (B31 and PBi) and VlsE (PKo and PBi) homologues. The performance of this new line blot assay was compared to our recently described recombinant Western blot assay (25) using 85 sera from Lyme borreliosis patients and 110 control sera.
(This work is part of the dissertation of G. Goettner.)
MATERIALS AND METHODS
Serum panels.
Sera were obtained from patients or blood donors from all parts of Germany but with the largest number originating from southern Germany where all relevant B. burgdorferi species and OspA types are present (20). Only serum samples from clinically well-defined cases of Lyme borreliosis were included. The erythema migrans (EM) panel consisted of sera from 15 patients enrolled in a borreliosis treatment study (27). The median time period between the appearance of EM and the collection of serum samples was 8 weeks (range, 3 to 24 weeks). Sera from 50 neuroborreliosis patients (stage II) with intrathecal Borrelia-specific antibody production and 20 sera from patients with late manifestations (10 patients with acrodermatitis chronica atrophicans [ACA] and 10 patients with Lyme arthritis) were also included. Controls comprised 60 sera from blood donors without actual or anamnestic dermatological, rheumatological, or neurological disease or history of a tick bite. Ten additional control sera were from syphilis patients, 10 samples came from patients who were positive for rheumatoid factor, and 30 sera came from patients with fever of unknown origin.
PCR and cloning of DbpA and VlsE.
B. burgdorferi sensu lato strains were cultivated at 33°C in modified Kelly medium (MKP) as previously described (22). Sources of strains B31, PKo, and PBi as used for DNA extraction have been described previously (32, 36). Extraction of DNA was performed with the High Pure Template Preparation kit (Roche, Penzberg, Germany) according to the manufacturer's instructions. dbpA of strains B31 and PBi, GenBank accession no. AF069269 (23) and AJ841673, was amplified using primers described previously (25), and the following vlsE primers were designed to amplify the vlsE gene of B. burgdorferi sensu stricto strain B31 (GenBank accession no. U76405) (38): vlsE-A1-ol (5′-AAAAGCCAAGTTGCTGATAAGGACGACCC-3′), forward; and vlsE-K6R (5′-CTTC ACAGCAAACTTTCCATCC-3′), reverse. The amplified part of vlsE from strains PKo and PBi (GenBank accession numbers AJ630109 and AJ630106) is located between the leader sequence and the end of IR6 in the recombination cassette. The PCR products of the amplified genes were cloned into the pQE30 vector (QIAGEN, Hilden, Germany) and used to transform Escherichia coli strain XL1-blue for protein expression.
Recombinant antigens.
Recombinant antigens in this study, strains used as source for DNA, and references for antigens described in previous studies are summarized in Table 1. Expression and purification of recombinant proteins was performed as described previously (25, 31) In short, expression of the cloned genes was induced by adding 0.5 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) to transformed E. coli; cells were incubated at 37°C for 18 h. E. coli cells were ruptured using a French press (Sim Aminco, Bowman, NY), and antigens were purified by affinity chromatography on a NiSO4-loaded IMAC column followed by ion exchange chromatography on a MonoQ column. All purification steps were performed with a fast-performance liquid chromatography system (Pharmacia Biotech, Freiburg, Germany).
TABLE 1.
Recombinant antigens used in the line immunoblot assay
| Antigen | Strain | Species | Reference or source for recombinant antigen |
|---|---|---|---|
| p100 | PKo | B. afzelii | 29 |
| p58 | PBi | B. garinii | 31 |
| BmpA | B31 | B. burgdorferi sensu stricto | 24 |
| PKo | B. afzelii | 24 | |
| PBi | B. garinii | 24 | |
| VlsE | PKa2 | B. burgdorferi sensu stricto | 25 |
| PKo | B. afzelii | This study | |
| PBi | B. garinii | This study | |
| OspC | B31 | B. burgdorferi sensu stricto | 30 |
| PKo | B. afzelii | 29 | |
| PBi | B. garinii | 30 | |
| 20047 | B. garinii | 25 | |
| p41i | PKo | B. afzelii | 29 |
| PBi | B. garinii | 30 | |
| DbpA | B31 | B. burgdorferi sensu stricto | This study |
| PKo | B. afzelii | 14 | |
| PBr | B. garinii | 25 | |
| PBi | B. garinii | This study |
SDS gel electrophoresis and immunoblotting.
For the previously used recombinant Western immunoblot, mixtures of the recombinant antigens were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12.5% polyacrylamide gel and electroblotted onto a nitrocellulose membrane (25).
For the new line immunoblot assay, each recombinant antigen was individually dissolved in 250 mM Tris, pH 7.5, and transferred into a separate line of a slot blot apparatus (Miniblotter 45, Immunetics; Cambridge, MA). Proteins were then allowed to bind to the membrane. Homologous antigens of different Borrelia species or OspA types were combined in groups on the line immunoblot (compare Fig. 1 and 2). With both blotting techniques, protein binding was assessed by staining membranes with Ponceau-S. After the membranes were destained and blocked, they were cut into 3- to 4-mm-wide strips and incubated overnight at room temperature with human sera diluted 1:200. Bound antibody was detected with horseradish peroxidase-labeled anti-human IgG or IgM antibodies (Dakopatts, Glostrup, Denmark) and visualized with diaminobenzidine.
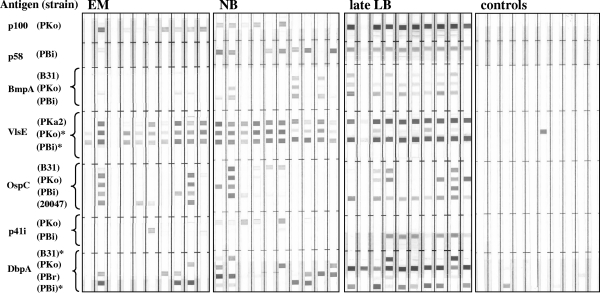
FIG. 1.
Representative IgG line immunoblots of patient and control sera. Strains belong to the following species: B31 and PKa2 to B. burgdorferi sensu stricto, PKo to B. afzelii, PBr to B. garinii OspA type 3, PBi to B. garinii OspA type 4, and 20047 to B. garinii unknown OspA type. Sera were obtained from patients with EM, early neuroborreliosis (NB), acrodermatitis chronica atrophicans or Lyme arthritis (late LB), and controls. *, additional antigen on the IgG line blot.
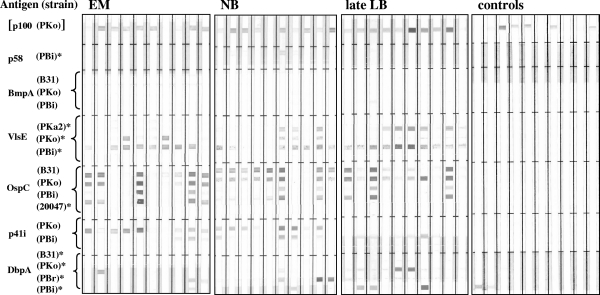
FIG. 2.
Representative IgM line immunoblots of patient and control sera. Strains belong to the following species: B31 and PKa2 to B. burgdorferi sensu stricto, PKo to B. afzelii, PBr to B. garinii OspA type 3, PBi to B. garinii OspA type 4, and 20047 to B. garinii unknown OspA type. Sera were obtained from patients with EM, early neuroborreliosis (NB), acrodermatitis chronica atrophicans or Lyme arthritis (late LB), and controls. [p100], not included in the valuation of the IgM blot results (note p100 reactivity of control sera); *, additional antigen on the IgM line blot.
Statistics.
For statistical analysis, Fisher's exact test for dichotomous variables was performed. A P value of ≤0.05 was regarded as significant.
RESULTS
Expression and purification of new recombinant proteins.
The following new antigens were efficiently produced and purified: DbpA of strains B31 (B. burgdorferi sensu stricto) and PBi (B. garinii) and VlsE of strains PKo (B. afzelii) and PBi. Purity of all recombinant proteins was verified by Western blotting as previously described (25), and a high-titer anti-E. coli serum did not detect any contaminating antigens (data not shown).
Assessment criteria and specificity.
Representative immunoreactivity patterns obtained with the line immunoblot assay for patient and control sera are shown in Fig. 1 and 2. Distinct bands for the corresponding protein can easily be identified. According to the interpretation criteria recommended by the German Society for Hygiene and Medical Microbiology for Borrelia Western blots, a serum sample is defined as positive when there are at least two reactive bands. Therefore, this two-band criterion was used for the evaluation (37). For IgM, a strong OspC band alone was also considered to identify a positive serum sample, while p100 was not regarded as a diagnostic antigen as it often exhibited nonspecific reactivity with controls (note the p100 reactivity of control samples shown in Fig. 2). Furthermore, assessment of IgM reactivity was restricted to sera from early stages of Lyme disease (EM and neuroborreliosis) because IgM is not useful for establishing a diagnosis in late-stage disease (37). Reactivity with homologous proteins of different strains was judged as one single band. Specificity of the line blot based on these criteria was 99.1% for the IgG blot and 98.2% for the IgM blot. By contrast, use of a one-band criterion resulted in low specificity, because for both immunoglobulin classes 11 sera of the control group were reactive (specificity of only 90.0% each).
Sensitivity of the line immunoblot assay.
The results that were obtained for the IgG and IgM line immunoblots are summarized in Tables 2 and 3, respectively. Comparison to our in-house recombinant IgG and IgM Western blot assay (25) revealed a significant improvement in sensitivity for the line immunoblot assay using additional antigens. IgG detection increased from 70.6% to 84.7% (P = 0.042) and from 40.0% to 73.8% (P < 0.005) for IgM (using only EM and neuroborreliosis sera for the latter), while specificity remained unchanged. When the new line blot assay was used without the additional antigens, sensitivity rose from 70.6% to 81.2% for IgG and from 40.0% to 52.3% for IgM antibody detection, but in both cases the increase was not significant.
TABLE 2.
Comparison of the new recombinant IgG line blot assay with the previous recombinant IgG Western blot and line blot assays using the same antigens as in the recombinant IgG blot assay
| Groupa | No. of sera | No. (%) of positive sera in assay |
||
|---|---|---|---|---|
| Western blot | Line blotb | Line blot with additional antigens | ||
| EM | 15 | 5 (33.3) | 7 (46.7) | 8 (53.3) |
| NB | 50 | 36 (72.0) | 43 (86.0) | 44 (88.0) |
| ACA | 10 | 9 (90.0) | 10 (100.0) | 10 (100.0) |
| AT | 10 | 10 (100.0) | 9 (90.0) | 10 (100.0) |
| All LB cases | 85 | 60 (70.6) | 69 (81.2) | 72 (84.7) |
| Controls | 110 | 1 (0.9) | 0 (0.0) | 1 (0.9) |
EM, erythema migrans; NB, neuroborreliosis (stage II); ACA, acrodermatitis chronica atrophicans (stage III); AT, Lyme arthritis (stage III); all LB cases, Lyme borreliosis. Controls were blood donors (n = 60), patients with syphilis (n = 10), patients with positive rheumatoid factor (n = 10), and patients with fever of unknown origin (n = 30).
Using only antigens already evaluated in the previous recombinant IgG Western blot assay.
TABLE 3.
Comparison of the new recombinant IgG line blot assay with the previous recombinant IgG Western blot and line blot assays using the same antigens as in the recombinant IgG blot assay
| Groupa | No. of sera | No. (%) of positive sera in assay: |
||
|---|---|---|---|---|
| Western blot | Line blotb | Line blot with additional antigens | ||
| EM | 15 | 6 (40.0) | 11 (73.3) | 13 (86.7) |
| NB | 50 | 20 (40.0) | 23 (46.0) | 35 (70.0) |
| All early LB cases | 65 | 26 (40.0) | 34 (52.3) | 48 (73.8) |
| Controls | 110 | 2 (1.8) | 1 (0.9) | 2 (1.8) |
For abbreviations, see Table 2, footnote a.
Using only antigens already evaluated in the previous recombinant IgG Western blot assay.
The recombinant IgG line immunoblot assay with the additional antigens was further tested in comparison to the previous recombinant IgG Western blot assay (25) and to an IgG whole-cell lysate blot assay based on the B. afzelii strain PKo (11), with a subpanel of 36 sera from patients with neuroborreliosis and 67 control sera (Table 4). While the increase in sensitivity from the whole-cell lysate blot assay to the recombinant Western blot assay was not significant (P = 0.055), the sensitivity of the recombinant line immunoblot assay when tested against the whole-cell lysate blot assay was significantly improved (P = 0.011).
TABLE 4.
Comparison of the new recombinant IgG line blot assay with the previous recombinant IgG Western blot and IgG whole-cell lysate Western blot assays
| Groupa | No. of sera | No. (%) of positive sera in assay: |
||
|---|---|---|---|---|
| Whole-cell lysate Western blot | Recombinant Western blot | Recombinant line blot | ||
| NB | 36 | 23 (63.8) | 31 (86.1) | 33 (91.7) |
| Controls | 67 | 2 (3.0) | 0 (0.0) | 0 (0.0) |
NB, neuroborreliosis (stage II); controls were blood donors (n = 49), patients with syphilis (n = 8), and patients with positive rheumatoid factor (n = 10).
Reactivity of the various antigens.
The line blot technique has the advantage of revealing differences in the reactivity of sera with homologous proteins from different strains, and it thus permits an evaluation of the antigenicity of every single antigen. Tables 5 and 7 summarize the reactivities of the combined homologous antigens in the IgG and IgM line immunoblot assay, and Tables 6 and 8 present the reactivity of every single homologue. The most sensitive protein (including all homologues) for IgG antibody detection in all stages of Lyme disease was VlsE, reacting with 78 of 85 (91.8%) patient samples, followed by DbpA (72.9%), p100 (55.3%), and p58 (55.3%). BmpA, OspC, and p41i were less useful for the detection of specific IgG antibodies. VlsE is especially valuable for diagnosis of Lyme disease during early manifestations (EM and acute neuroborreliosis).
TABLE 5.
IgG reactivity of at least one of the homologous Borrelia proteins
| Groupa | No. of sera | No. (%) of sera reactive with: |
||||||
|---|---|---|---|---|---|---|---|---|
| DbpA | p41i | OspC | VlsE | BmpA | p58 | p100 | ||
| EM | 15 | 5 (33.3) | 1 (6.7) | 3 (20.0) | 12 (80.0) | 1 (6.7) | 1 (6.7) | 4 (26.7) |
| NB | 50 | 39 (78.0) | 17 (34.0) | 9 (18.0) | 46 (92.0) | 19 (38.0) | 27 (54.0) | 26 (52.0) |
| ACA | 10 | 8 (80.0) | 9 (90.0) | 4 (40.0) | 10 (100.0) | 1 (10.0) | 10 (100.0) | 8 (80.0) |
| AT | 10 | 10 (100.0) | 8 (80.0) | 6 (60.0) | 10 (100.0) | 6 (60.0) | 9 (90.0) | 9 (90.0) |
| Controls | 110 | 4 (3.6) | 0 (0.0) | 0 (0.0) | 4 (3.6) | 0 (0.0) | 1 (0.9) | 2 (1.8) |
For abbreviations, see Table 2, footnote a.
TABLE 7.
IgM reactivity of at least one of the homologous Borrelia proteins
| Groupa | No. of sera | No. (%) of sera reactive with: |
|||||
|---|---|---|---|---|---|---|---|
| DbpA | p41i | OspC | VlsE | BmpA | p58 | ||
| EM | 15 | 1 (6.7) | 9 (60.0) | 12 (80.0) | 8 (53.3) | 0 (0.0) | 0 (0.0) |
| NB | 50 | 22 (44.0) | 20 (40.0) | 27 (54.0) | 28 (56.0) | 5 (10.0) | 9 (18.0) |
| ACA | 10 | 4 (40.0) | 4 (40.0) | 5 (50.0) | 7 (70.0) | 0 (0.0) | 0 (0.0) |
| AT | 10 | 5 (50.0) | 4 (40.0) | 6 (60.0) | 6 (60.0) | 0 (0.0) | 0 (0.0) |
| Controls | 110 | 1 (0.9) | 2 (1.8) | 2 (1.8) | 6 (5.4) | 0 (0.0) | 0 (0.0) |
For abbreviations, refer to Table 2, footnote a.
TABLE 6.
Reactivity of every single recombinant antigens in the IgG line blot assay
| Groupa | No. of sera | No. (%) of sera reactive with: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DbpA |
p41i |
OspC |
VlsE |
BmpA |
p58 PBi | P100 PKo | |||||||||||||
| PBib | PBr | PKo | B31b | PBi | PKo | 20047 | PBi | PKo | B31 | PBib | PKob | PKa2 | PBi | PKo | B31 | ||||
| EM | 15 | 0 (0.0) | 2 (13.3) | 4 (26.7) | 1 (6.7) | 0 (0.0) | 1 (6.7) | 3 (20.0) | 1 (6.7) | 1 (6.7) | 1 (6.7) | 12 (80.0) | 9 (60.0) | 6 (40.0) | 0 (0.0) | 1 (6.7) | 1 (6.7) | 1 (6.7) | 4 (26.7) |
| NB | 50 | 19 (38.0) | 20 (40.0) | 17 (34.0) | 6 (12.0) | 6 (12.0) | 15 (30.0) | 5 (10.0) | 2 (4.0) | 4 (8.0) | 8 (16.0) | 44 (88.0) | 41 (82.0) | 41 (82.0) | 17 (34.0) | 12 (24.0) | 13 (26.0) | 27 (54.0) | 26 (52.0) |
| ACA | 10 | 1 (10.0) | 0 (0.0) | 8 (80.0) | 1 (10.0) | 3 (30.0) | 9 (90.0) | 0 (0.0) | 1 (10.0) | 3 (30.0) | 0 (0.0) | 10 (100.0) | 0 (0.0) | 10 (100.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 10 (100.0) | 8 (80.0) |
| AT | 10 | 7 (70.0) | 1 (10.0) | 9 (90.0) | 2 (20.0) | 5 (50.0) | 8 (80.0) | 4 (40.0) | 1 (10.0) | 5 (50.0) | 3 (30.0) | 10 (100.0) | 2 (20.0) | 9 (90.0) | 5 (50.0) | 2 (20.0) | 4 (40.0) | 9 (90.0) | 9 (90.0) |
| Controls | 110 | 2 (1.8) | 2 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 3 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 2 (1.8) |
For abbreviations, see Table 2, footnote a.
Additional antigen on the IgG line blot assay.
TABLE 8.
Reactivity of every single recombinant antigens in the IgM line blot assay
| Groupa | No. of sera | No. (%) of sera reactive with: |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DbpA |
p41i |
OspC |
VlsE |
BmpA |
P58 PBib | |||||||||||||
| PBib | PBrb | PKob | B31b | PBi | PKo | 20047b | PBi | PKo | B31 | PBib | PKob | PKa2b | PBi | PKo | B31 | |||
| EM | 15 | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 9 (60.0) | 9 (60.0) | 2 (13.3) | 11 (73.3) | 12 (80.0) | 8 (53.3) | 4 (26.7) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NB | 50 | 9 (18.0) | 3 (26.0) | 2 (4.0) | 0 (0.0) | 10 (20.0) | 19 (38.0) | 10 (20.0) | 3 (6.0) | 19 (38.0) | 26 (52.0) | 26 (52.0) | 7 (14.0) | 6 (12.0) | 5 (10.0) | 1 (2.0) | 3 (6.0) | 9 (18.0) |
| ACA | 10 | 0 (0.0) | 0 (0.0) | 4 (40.0) | 0 (0.0) | 0 (0.0) | 4 (40.0) | 2 (20.0) | 0 (0.0) | 5 (50.0) | 2 (20.0) | 7 (70.0) | 1 (10.0) | 4 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AT | 10 | 3 (30.0) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 1 (10.0) | 4 (40.0) | 3 (30.0) | 2 (20.0) | 6 (60.0) | 3 (30.0) | 5 (50.0) | 0 (0.0) | 4 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Controls | 110 | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 1 (0.9) | 0 (0.0) | 1 (0.9) | 2 (1.8) | 6 (5.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
For abbreviations, see Table 2, footnote a.
Additional antigen on the IgM line blot assay.
Regarding the different homologues in the IgG blot (Table 6), the most sensitive one in all patient groups overall was VlsE of strain PBi, which was reactive with 76 (89.4%) of the sera tested. Almost all of the VlsE-reactive sera (76 of 78 samples) bound to this homologue. Remarkably, VlsE of the B. afzelii strain PKo reacted poorly with sera from patients with late manifestations (ACA and arthritis). The most sensitive DbpA homologue was derived from strain PKo, which detected antibody in 38 (44.7%) sera. Of note, DbpAs derived from the B. garinii strains PBi and PBr were the best antigens in the neuroborreliosis panel. The combination of different DbpAs was highly effective in the IgG blot, since reactivity of the four homologues complemented each other and cross-reactivity was low. IgG antibodies directed against OspC were present only in 22 (25.9%) sera but showed a heterogeneous pattern: sera from patients with late-stage disease reacted best with PKo OspC, those from patients with neuroborreliosis with B31 OspC, and those from patients with EM with 20047 OspC. The most relevant BmpA homologue was from strain PBi, whereas p41i from strain PKo bound antibody more avidly than did other p41i homologues.
To evaluate the ability of the new line immunoblot assay to detect IgM, we selected only those sera from EM and neuroborreliosis patients, since IgM serology is not recommended for chronic Lyme borreliosis (37). Previous publications have demonstrated that OspC is the major antigen for specific IgM antibody detection (11, 29, 34). In this study, 39 of 65 (60.0%) patient sera reacted with this antigen. OspC of strains B31 and PKo were the most sensitive proteins among the four homologues (38 and 30 positive sera, respectively). Reactivity of the B. garinii strain PBi OspC was remarkably poor, especially for the neuroborreliosis panel. Interestingly, VlsE turned out to be a sensitive antigen in the IgM blot as well, reacting with 36 of 65 (55.4%) sera from early manifestations. Of these, 34 solely recognized the homologue of strain PBi, which was also the most sensitive antigen in the IgG blot. By contrast, only 11 and 9 sera, respectively, detected the PKo and PKa2 VlsE homologues. It is noteworthy that the B. garinii VlsE exhibited the lowest specificity in the IgM blot, as 6 (5.4%) of the 110 control sera reacted with this antigen. p41i of strain PKo showed acceptable sensitivity for EM and neuroborreliosis, while p41i reactivity of strain PBi was poor. DbpA detected IgM antibodies only in neuroborreliosis sera, except for one of the 15 EM samples. For this patient group, the B. garinii homologues presented by far the best reactivity and, in addition, complemented each other. BmpA homologues as well as p58 rarely reacted with specific IgM antibodies.
DISCUSSION
Previous studies revealed that serological assays based on recombinant B. burgdorferi antigens are a promising alternative to tests based on whole-cell lysates (5, 8, 12, 14, 17-19, 21, 24, 25, 29-31). For immunoblots, recombinant antigens offer several major advantages. (i) Inclusion of specific antigens only prevents confusion by closely located or comigrating nonspecific proteins (e.g., p58/p60). (ii) Truncated antigens that contain only the specific part (like the p41 internal fragment) can be used. (iii) Antigens, such as VlsE, that are expressed exclusively in vivo can be included. (iv) Homologues from different species (like OspC or DbpA) can be combined. (v) Standardization and evaluation are easier, since antigen composition and concentrations can be controlled and do not depend on the actual expression pattern of a cultured strain (11, 12, 14, 17-19, 24, 25, 28-31, 35).
Here, we describe a significant improvement in Lyme borreliosis serology compared to our previous assays (25, 29-31) that depends on a new technology combined with additional homologous antigens.
Comparison of immunoblots.
The new recombinant line immunoblot assay increases sensitivity significantly for IgG and IgM antibody detection compared to the previously described recombinant Western blot and the IgG whole-cell lysate blot assays, without loss of specificity. This improvement results in part from the inclusion of additional antigens but the major reason is that production of the line blot differs fundamentally from the way the recombinant or whole-cell lysate immunoblot is made. For the line blot assay, each antigen or homologue is applied separately to the membrane under optimal buffer conditions and at the optimal concentration. This allows discrimination between antigens of similar or identical molecular weight and improves sensitivity, because reactivity of individual antigens is not impaired by dilution with nonreactive proteins. Furthermore, it is not necessary to separate and transfer antigens beforehand by SDS-PAGE and blotting, steps that are carried out under denaturing conditions that possibly result in antigen degradation or incomplete resolution and transfer of proteins. Even the line blot assay that did not include additional antigens already resulted in improved sensitivity over the whole-cell Western blot assay (Tables 2 and 3). It is likely that the native, nondenatured proteins used in the line blot assay are better antigens because of their intact secondary structure (3, 9), as recently described for a line immunoblot assay for serodiagnosis of Epstein-Barr virus infections. Nevertheless, to achieve significant improvement of sensitivity, inclusion of the additional antigens was required for both the IgG and the IgM line blot assays.
Furthermore, the line blot assay is cost saving and easier to standardize, since sources of errors such as SDS-PAGE or electrophoretic transfer to nitrocellulose membranes are excluded. Antigens of high and low molecular weights can be applied to the membrane under the same conditions, because there is no risk that small molecules may pass through the membrane driven by electrical force. Finally, the line blot assay should lend itself to automated analysis, because every antigen is located at an exactly defined position on the blot strip. Therefore, the recombinant line immunoblot assay may be adaptable to routine laboratory analysis when large numbers of sera need to be tested.
Sensitivity of antigens in the line blot assay.
The last improvement of our recombinant IgG Western blot assay resulted from addition of DbpA (from strain PBr) and, even more importantly, VlsE from B. burgdorferi sensu stricto strain PKa2 (25). The broad heterogeneity of the B. burgdorferi sensu lato species complex is a confounding problem for the serologic diagnosis of Lyme disease. Hoping to find sensitive and specific antigens to complement our existing assay, we tested additional recombinant proteins, i.e., VlsE of B. afzelii and B. garinii (10) and DbpA of B. burgdorferi sensu stricto and another B. garinii strain (U. Schulte-Spechtel et al., unpublished results). All new recombinant antigens showed considerable differences in amino acid composition compared to the ones used thus far.
In line with the fact that in chronic Lyme borreliosis the antibody repertoire expands, most of the antigens exhibited high sensitivity for IgG antibody detection during late manifestations such as ACA and Lyme arthritis. ACA is clearly associated with infection by B. afzelii (6), which is underscored by the fact that in the United States, where B. afzelii is not endemic, ACA is not seen. While the 10 sera from ACA patients reacted well with DbpA, p41i, and OspC derived from B. afzelii, we were surprised to find that none of the serum samples recognized VlsE of the B. afzelii strain PKo. The reasons for this remain unclear, but failure of ACA sera to bind PKo VlsE could be due to atypical protein folding where epitopes are not accessible and possibly be related to immune evasion during the late stages of Lyme disease.
The VlsE proteins of B. burgdorferi sensu lato comprise a family of variable surface antigens that are thought to be involved in immune evasion. However, during the IgG response, antibodies recognize a conserved region of the molecule, which is of diagnostic significance. The conserved VlsE region of B. burgdorferi sensu stricto appears to be the main antigen to elicit the IgG immune response and to display high sensitivity for diagnosis of Lyme borreliosis (1, 15-17, 21). We have demonstrated that VlsE is a heterogenous protein among B. burgdorferi sensu lato strains (10), which explains the different reaction patterns seen with sera from Lyme disease patients. The present study clearly identifies VlsE of strain PBi as the most sensitive antigen for IgG, as well as IgM, detection when compared to other VlsEs. Likewise, Magnarelli et al. (19) described VlsE as the most sensitive antigen for IgG (80%) and one of the most sensitive ones for IgM (40%) detection in an enzyme-linked immunosorbent assay that evaluated 11 different recombinant proteins from B. burgdorferi sensu stricto for reactivity with 20 sera from American patients with EM. In our study, the B. garinii VlsE reached comparable detection levels, while the B. burgdorferi sensu stricto VlsE was less sensitive, especially in early disease. This discrepancy might be explained by the predominance of different species in Europe: B. burgdorferi sensu stricto is the only human pathogenic species in North America, which is rarely observed in ticks and patients in Germany (13, 20, 28, 32). At present, we do not have a satisfactory explanation for the good reactivity of the B. afzelii VlsE in early manifestations and poor reactivity in late manifestations of the disease. Such discrepancies are not observed with two other recombinant VlsEs. VlsE is a highly sensitive and specific antigen that should be included in future recommendations for Lyme disease testing. Nevertheless, further studies are necessary to determine if VlsEs from Borrelia species other than B. burgdorferi sensu stricto should be preferentially used for serologic tests in Europe.
DbpA, formerly also named p17 or Osp17 (14, 35), is a diagnostically relevant but heterogeneous protein among the human pathogenic B. burgdorferi sensu lato species in Europe (12, 21, 23) and can be arranged into five groups (Schulte-Spechtel et al., unpublished). We included four homologues of DbpA representing the three main human pathogenic species in our new line blot assay, resulting in considerable improvement in sensitivity for IgG and IgM detection, especially in neuroborreliosis sera. Notably, addition of DbpA from a B. garinii strain associated with OspA type 4 was highly effective. This is of special interest, since OspA type 4 strains are highly prevalent in European patients with neuroborreliosis (28). The predominant reactivity of B. afzelii DbpA in patients with ACA and of B. garinii DbpA in patients with neuroborreliosis suggests that our new line blot assay could aid in the diagnosis of the infecting species. Even though selective reactivity in our antigen panel is restricted to certain antigens, this feature could help to elucidate the epidemiologic situation in Europe and provide a tool for vaccine development strategies. However, before our new line blot assay can be used for such novel applications, it would have to be carefully validated for this purpose by using patient serum panels where the causative Borrelia species is known.
In conclusion, the new line immunoblot assay that we developed for serodiagnosis of Lyme borreliosis achieves high levels of specificity and sensitivity in all stages of Lyme disease. This was true for both IgG and IgM antibody detection. Due to its appearance, the line blot assay is easy to interpret and offers the possibility of automation. Because the line blot assay technique eliminates many sources of errors and inconsistencies, it is easier to standardize, highly reliable, and reproducible. Thus, this Borrelia line immunoblot assay, which has already been included in our routine diagnostic tests, offers a useful improvement in the serodiagnosis of Lyme borreliosis.
Acknowledgments
We thank Ulrike Munderloh for excellent discussion and suggestions and Cecilia Hizo-Teufel for excellent technical assistance.
This work was supported in part by the DFG (Graduiertenkolleg “Infektion und Immunität,” Jürgen Heesemann, head).
REFERENCES
- 1.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baranton, G., D. Postic, G. Saint, I., P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 3.Buisson, M., B. Fleurent, M. Mak, P. Morand, L. Chan, A. Ng, M. Guan, D. Chin, and J. M. Seigneurin. 1999. Novel immunoblot assay using four recombinant antigens for diagnosis of Epstein-Barr virus primary infection and reactivation. J. Clin. Microbiol. 37:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 5.Burkert, S., D. Rössler, P. Munchhoff, and B. Wilske. 1996. Development of enzyme-linked immunosorbent assays using recombinant borrelial antigens for serodiagnosis of Borrelia burgdorferi infection. Med. Microbiol. Immunol. (Berlin) 185:49-57. [DOI] [PubMed] [Google Scholar]
- 6.Canica, M. M., F. Nato, L. du Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand.J. Infect. Dis. 25:441-448. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal Wkly. Rep. 44:590. [PubMed] [Google Scholar]
- 8.Fikrig, E., E. D. Huguenel, R. Berland, D. W. Rahn, J. A. Hardin, and R. A. Flavell. 1992. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J. Infect. Dis. 165:1127-1132. [DOI] [PubMed] [Google Scholar]
- 9.Gartner, B. C., J. M. Fischinger, K. Roemer, M. Mak, B. Fleurent, and N. Mueller-Lantzsch. 2001. Evaluation of a recombinant line blot for diagnosis of Epstein-Barr virus compared with ELISA, using immunofluorescence as reference method. J. Virol. Methods 93:89-96. [DOI] [PubMed] [Google Scholar]
- 10.Goettner, G., U. Schulte-Spechtel, and B. Wilske. 2004. Heterogeneity of the immunodominant surface protein VlsE among the three genospecies of Borrelia burgdorferi pathogenic for humans. Int. J. Med. Microbiol. 293(Suppl. 37):172-173. [DOI] [PubMed] [Google Scholar]
- 11.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikkilä, T., I. Seppälä, H. Saxen, J. Panelius, H. Yrjänainen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrandt, A., K. H. Schmidt, B. Wilske, W. Dorn, E. Straube, and V. Fingerle. 2003. Prevalence of four species of Borrelia burgdorferi sensu lato and coinfection with Anaplasma phagocytophila in Ixodes ricinus ticks in central Germany. Eur. J. Clin. Microbiol. Infect. Dis. 22:364-367. [DOI] [PubMed] [Google Scholar]
- 14.Jauris-Heipke, S., B. Rossle, G. Wanner, C. Habermann, D. Rössler, V. Fingerle, G. Lehnert, R. Lobentanzer, I. Pradel, B. Hillenbrand, U. Schulte-Spechtel, and B. Wilske. 1999. Osp17, a novel immunodominant outer surface protein of Borrelia afzelii: recombinant expression in Escherichia coli and its use as a diagnostic antigen for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. (Berlin) 187:213-219. [DOI] [PubMed] [Google Scholar]
- 15.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of. Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnarelli, L. A., E. Fikrig, S. J. Padula, J. F. Anderson, and R. A. Flavell. 1996. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of lyme borreliosis. J. Clin. Microbiol. 34:237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnarelli, L. A., M. Lawrenz, S. J. Norris, and E. Fikrig. 2002. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J. Med. Microbiol. 51:649-655. [DOI] [PubMed] [Google Scholar]
- 20.Michel, H., B. Wilske, G. Hettche, G. Goettner, C. Heimerl, U. Reischl, U. Schulte-Spechtel, and V. Fingerle. 2003. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med. Microbiol. Immunol. (Berlin) 193:219-226. [DOI] [PubMed] [Google Scholar]
- 21.Panelius, J., P. Lahdenne, H. Saxen, S. A. Carlsson, T. Heikkilä, M. Peltomaa, A. Lauhio, and I. Seppälä. 2003. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J. Neurol. 250:1318-1327. [DOI] [PubMed] [Google Scholar]
- 22.Preac-Mursic, V., B. Wilske, and S. Reinhardt. 1991. Culture of Borrelia burgdorferi on six solid media. Eur. J. Clin. Microbiol. Infect. Dis. 10:1076-1079. [DOI] [PubMed] [Google Scholar]
- 23.Roberts, W. C., B. A. Mullikin, R. Lathigra, and M. S. Hanson. 1998. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect. Immun. 66:5275-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roessler, D., U. Hauser, and B. Wilske. 1997. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 35:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulte-Spechtel, U., G. Lehnert, G. Liegl, V. Fingerle, C. Heimerl, B. J. Johnson, and B. Wilske. 2003. Significant improvement of the recombinant Borrelia-specific immunoglobulin G immunoblot test by addition of VlsE and a DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J. Clin. Microbiol. 41:1299-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 27.Weber, K., B. Wilske, V. Preac-Mursic, and R. Thurmayr. 1993. Azithromycin versus penicillin V for the treatment of early Lyme borreliosis. Infection 21:367-372. [DOI] [PubMed] [Google Scholar]
- 28.Wilske, B., U. Busch, H. Eiffert, V. Fingerle, H. W. Pfister, D. Rössler, and V. Preac-Mursic. 1996. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med. Microbiol. Immunol. (Berlin) 184:195-201. [DOI] [PubMed] [Google Scholar]
- 29.Wilske, B., V. Fingerle, P. Herzer, A. Hofmann, G. Lehnert, H. Peters, H. W. Pfister, V. Preac-Mursic, E. Soutschek, and K. Weber. 1993. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med. Microbiol. Immunol. (Berlin) 182:255-270. [DOI] [PubMed] [Google Scholar]
- 30.Wilske, B., V. Fingerle, V. Preac-Mursic, S. Jauris-Heipke, A. Hofmann, H. Loy, H. W. Pfister, D. Rössler, and E. Soutschek. 1994. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med. Microbiol. Immunol. (Berlin) 183:43-59. [DOI] [PubMed] [Google Scholar]
- 31.Wilske, B., C. Habermann, V. Fingerle, B. Hillenbrand, S. Jauris-Heipke, G. Lehnert, I. Pradel, D. Rössler, and U. Schulte-Spechtel. 1999. An improved recombinant IgG immunoblot for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. (Berlin) 188:139-144. [DOI] [PubMed] [Google Scholar]
- 32.Wilske, B., V. Preac-Mursic, U. B. Göbel, B. Graf, S. Jauris, E. Soutschek, E. Schwab, and G. Zumstein. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 31:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilske, B., V. Preac-Mursic, G. Schierz, and K. V. Busch. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 263:92-102. [DOI] [PubMed] [Google Scholar]
- 35.Wilske, B., V. Preac-Mursic, G. Schierz, W. Gueye, P. Herzer, and K. Weber. 1988. Immunochemical analysis of the immune response in late manifestations of Lyme borreliosis. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 267:549-558. (In German.) [PubMed] [Google Scholar]
- 36.Wilske, B., V. Preac-Mursic, G. Schierz, R. Kuhbeck, A. G. Barbour, and M. Kramer. 1988. Antigenic variability of Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 539:126-143. [DOI] [PubMed] [Google Scholar]
- 37.Wilske, B., L. Zöller, V. Brade, H. Eiffert, U. B. Göbel, G. Stanek, and H. W. Pfister. 2000. MIQ 12, Lyme-Borreliose, p. 1-59. In H. Mauch and R. Lütticken (ed.), Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. Urban & Fischer Verlag, Munich, Germany.
- 38.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]