Abstract
Bacillus cereus is a well-known cause of food-borne illness, but infection with this organism is not commonly reported because of its usually mild symptoms. A fatal case due to liver failure after the consumption of pasta salad is described and demonstrates the possible severity of the emetic syndrome.
CASE REPORT
In August 2003, five children of a family became sick after eating pasta salad. The pasta salad was prepared on a Friday and taken to a picnic on the following Saturday; the remainders had been stored in the fridge until the following Monday evening, when they were served for supper to the children. Because the pasta salad had an unusual smell, three children (B14, G10, and G9) ate only a small quantity. At 6 h after the meal the youngest girl (G7), 7 years old, started vomiting. She complained of respiratory distress and was taken to the emergency department of a local hospital. Upon arrival, her brothers and sisters started vomiting as well. Because the clinical condition of two children (G7 and B9) deteriorated rapidly, they were intubated and mechanically ventilated. All children were transported to the University Hospital in Leuven. During transfer, G7 had severe pulmonary hemorrhage and needed continuous resuscitation. Upon arrival she was moribund with coma, diffuse bleeding, and severe muscle cramps. She died within 20 min, at 13 h after the meal. On autopsy Bacillus cereus was detected in her gut content but also in the spleen, probably by postmortem translocation of the bacterium. A postmortem liver biopsy showed microvascular and extensive coagulation necrosis. Her initial laboratory values showed severe metabolic acidosis and liver failure. All four other children were affected, although to different degrees (Table 1). The 9-year-old boy (B9) was transferred to the pediatric intensive care unit, where mechanical ventilation and invasive hemodynamic monitoring were continued. After fluid resuscitation, his blood lactate levels gradually went down. Basic treatment for liver failure consisted of vitamin K supplementation, oral and rectal lactulose, oral neomycin, and high-dose acetylcysteine. At 24 h after the start of the treatment his aspartate transaminase and alanine transaminase levels peaked at 12,254 U/liter and 8,656 U/liter, respectively; his prothrombin time went down to 21.5%. Thereafter, the hepatic function recovered. He gradually recovered consciousness, and he was successfully extubated. Two sisters (G9 and G10) were treated with fluid resuscitation and bicarbonate substitution. Both gradually recovered. The 14-year-old brother (B14) was kept under observation. Subsequent blood samples showed no deterioration of hepatic function. The surviving children could leave the hospital within 8 days.
TABLE 1.
Laboratory values for the five children on admission
| Parameterb | Value for childa: |
||||
|---|---|---|---|---|---|
| G7 | B9 | G9 | G10 | B14 | |
| Arterial pH | 7.163 | 7.369 | 7.375 (7.35-7.45) | ||
| Bicarbonate (mmol/liter) | 9.1 | 10.2 | 13.8 | 10.8 (22.0-29.0) | 17.7 (22.0-29.0) |
| Lactate (mmol/liter) | 9.5 | 3.4 | 8.8 (0.5-1.6) | 5.95 (0.4-2.0) | |
| Ammonia (μmol/liter) | >2,000 | 107 | 30 | 112 (11-32) | |
| AP (U/liter) | 163 | 268 | 547 | 611 (<720) | 822 (<936) |
| AST (U/liter) | 3,123 | 1,858 | 239 | 5,327 (<32) | 42 (<38) |
| ALT (U/liter) | 2,636 | 5,181 | 447 | 4,101 (<31) | |
| γGT (U/liter) | 16 | 24 | 19 | 44 (<35) | |
| Total bilirubin | 0.28 | 1.23 | 0.52 | 1.94 (<1.00) | |
| Conjugated bilirubin | 0.66 | 1.49 (0.0-0.5) | |||
| LDH (U/liter) | 5,110 | 9,101 | 1,481 | 5,684 (240-480) | |
| Prothrombin time (%) | <10 | 23.1 | 83.7 | 30.6 (70-100) | >100 (70-100) |
Age specific normal values for our laboratory are in parentheses. Normal values for G7, B9, and G9 are identical to the values for G10.
AP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGT, γ-glutamyltransferase; LDH, lactate dehydrogenase.
In six food samples and the vomit of the deceased girl B. cereus was detected. The samples were not heat treated before analysis. After 24 h of incubation, the colonies were pink (mannitol negative) with a precipitation zone (lecithinase positive) on MYP agar (Oxoid Ltd., Basingstoke, England), beta-hemolytic on Columbia agar (Oxoid), and positive in motility agar. Gram staining on Columbia agar-grown colonies showed gram-positive rods with nondeforming subterminal spores. The highest B. cereus count (107 to 108 CFU/g) was found in the pasta salad, the lowest count in the vomit (2.0 × 102 CFU/g). From each positive sample, three (or four) isolates were phenotypically confirmed as B. cereus by use of the API 50 gallery. Further characterization of the 22 isolates obtained consisted of repetitive sequence-based PCR (rep-PCR) (6), pulsed-field gel electrophoresis (PFGE) of genomic DNA (5), and PCR analysis of a marker for emetic toxin (1). Rep-PCR has been shown before to be useful for outbreak investigation (8).
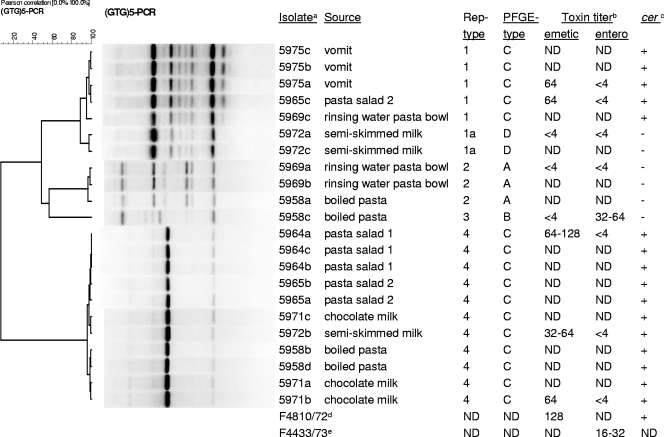
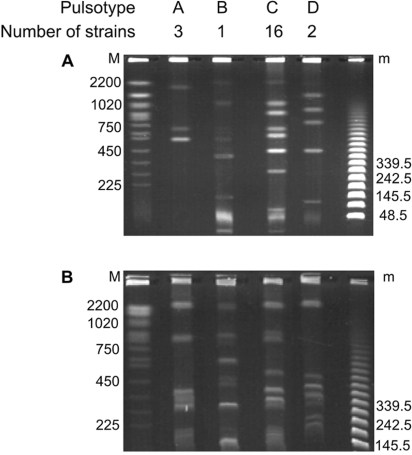
The typing of the isolates was focused on the food and vomit isolates, because the postmortem spleen isolate was not available at that moment. With rep-PCR these isolates could be divided into four groups representing rep types 1 to 4 (Fig. 1); all isolates obtained from the vomit, together with one isolate obtained from the pasta salad and one from the pasta bowl, were clustered within rep type 1. Rep type 4 consisted of 11 isolates, obtained from the pasta salad but also from another type of boiled pasta, chocolate milk, and semiskimmed milk, which indicates cross-contamination during handling of the food. PFGE revealed four distinct restriction patterns (PFGE types A to D) (Fig. 1 and 2). The majority of the isolates (n = 16) were classified as PFGE type C, which included isolates from the boiled pasta, the pasta salad, chocolate milk, vomit, semiskimmed milk, and the bowl. All these PFGE type C isolates corresponded to rep type 1 or 4 (Fig. 1), indicating a high discriminatory potential of rep-PCR. The above-described data indicate that more than one strain was present in this intoxication case, and they illustrate the importance of obtaining multiple isolates from even one food sample (Fig. 1). Detection of B. cereus emetic and enterotoxin production was performed with cytotoxicity assays (2, 4). Both tested isolates of rep type 1 (vomit and pasta salad) and the three tested isolates from rep type 4 produced the emetic toxin and were also positive in the emetic toxin-specific PCR assay (Fig. 1).
FIG. 1.
Cluster analysis of the (GTG)5 fingerprints and toxin production of B. cereus isolates. As listed in the Isolatea column, the following isolates have been deposited in the BCCM/LMG Bacteria Collection (Ghent University, Ghent, Belgium): 5975a (LMG 22728); 5965c (LMG 22729); 5972a (LMG 22730); 5969a (LMG 22731); 5958c (LMG 22732); 5964a (LMG 22733). Toxin titerb column: emetic and enterotoxin titers detected on the basis of cytotoxicity assays. cerc column: emetic toxin-specific marker detected in the PCR assay. The reference strain for emetic toxin production is identified with a superscript “d” suffix in the Isolatea column. The reference strain for enterotoxin production is identified with a superscript “e” suffix. ND, not determined.
FIG. 2.
Representative PFGE patterns (A to D) of AscI (A)- and NotI (B)-digested genomic DNA of B. cereus isolates. M, yeast chromosome PFGE marker; m, lambda 48.5-kb size marker. The values on the left and right are molecular mass markers in kilobases.
Although Bacillus cereus is a well-known cause of food-borne illness it is not commonly reported because of its usually mild symptoms. It can cause two types of food poisoning known as the emetic and the diarrheal types. The emetic type is caused by a heat-stable toxin, named cereulide, preformed in the food. Only one fatal case has been reported up to now (9). The present results provided evidence for B. cereus food poisoning of five children of one Belgian family. The clinical data and the rapid onset of symptoms, together with the microbiological and molecular study, pointed to B. cereus as the causative agent. It has been demonstrated that B. cereus from the pasta salad, the vomit of the deceased girl, and the pasta bowl produced identical patterns on the basis of both analyses (rep type 1 and PFGE type C).
Although the presence of cereulide in the pasta salad was not directly demonstrated, its production at a high level was indirectly proven in the cytotoxicity test of the isolates. These results were confirmed by PCR (1), which amplifies a DNA fragment whose presence is specific for cereulide-producing strains. All the isolates classified as PFGE type C and rep type 1 reacted positively with these primers, indicating the presence of cereulide-related genes. Also, the rest of the isolates of PFGE type C but pertaining to rep type 4 harbored the cereulide genetic determinants. Thus, although no isolate of rep type 4 was detected in the vomit, these isolates could also have produced the toxin in the pasta salad.
The present case illustrates the possible severity of the emetic syndrome and the importance of adequate refrigeration of prepared food. Because the emetic toxin is preformed in the food and not inactivated by heat treatment (7) it is important to prevent growth and the production of cereulide during storage. Some B. cereus strains are known to be psychrotrophic and to have the highest emetic toxin production between 12- and 15°C (3). In this case, the temperature of the fridge where the pasta salad was stored was 14°C. This allowed B. cereus to grow to a count of more than 108 CFU/g in 3 days with a probably very high toxin production that may explain the fatal outcome.
REFERENCES
- 1.Ehling-Schulz, M., M. Fricker, and S. Scherer. 2004. Identification of emetic toxin producing Bacillus cereus strains by a novel molecular assay. FEMS Microbiol. Lett. 232:189-195. [DOI] [PubMed] [Google Scholar]
- 2.Finlay, W. J., N. A. Logan, and A. D. Sutherland. 1999. Semiautomated metabolic staining assay for Bacillus cereus emetic toxin. Appl. Environ. Microbiol. 65:1811-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finlay, W. J., N. A. Logan, and A. D. Sutherland. 2000. Bacillus cereus produces most emetic toxin at lower temperatures. Lett. Appl. Microbiol. 31:385-389. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher, P., and N. A. Logan. 1999. Improved cytotoxicity assay for Bacillus cereus diarrhoeal enterotoxin. Lett. Appl. Microbiol. 28:394-400. [DOI] [PubMed] [Google Scholar]
- 5.Gaviria Rivera, A. M., and F. G. Priest. 2003. Pulsed field gel electrophoresis of chromosomal DNA reveals a clonal population structure to Bacillus thuringiensis that relates in general to crystal protein gene content. FEMS Microbiol. Lett. 223:61-66. [DOI] [PubMed] [Google Scholar]
- 6.Gevers, D., G. Huys, and J. Swings. 2001. Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiol. Lett. 205:31-36. [DOI] [PubMed] [Google Scholar]
- 7.Granum, P. E., and T. Lund. 1997. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 157:223-228. [DOI] [PubMed] [Google Scholar]
- 8.Healy, M., J. Huong, T. Bittner, M. Lising, S. Frye, S. Raza, R. Schrock, J. Manry, A. Renwick, R. Nieto, C. Woods, J. Versalovic, and J. R. Lupski. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 43:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahler, H., A. Passi, J. M. Kramer, P. Schulte, A. Scoging, W. Bär, and S. Krähenbühl. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336:1142-1148. [DOI] [PubMed] [Google Scholar]