Abstract
The lungs of patients with cystic fibrosis (CF) are colonized initially by Pseudomonas aeruginosa, which is associated with progressive lung destruction and increased mortality. The pathogenicity of P. aeruginosa is caused by a number of virulence factors, including exotoxin A (ETA) and the type III cytotoxins (ExoS, ExoT, ExoU, and ExoY). P. aeruginosa contacts the plasma membrane to deliver type III cytotoxins through a channel formed by PopB, PopD, and PcrV; ETA enters mammalian cells via receptor-mediated endocytosis. The Wisconsin CF Neonatal Screening Project is a longitudinal investigation to assess the potential benefits and risks of newborn screening for CF; the project was the source of serum samples used in this study. Past studies evaluated the longitudinal appearance of antibodies to ETA and elastase and P. aeruginosa infections in patients with CF. The current study characterized the longitudinal appearance of antibodies to components of the type III system in children with CF. Western blot analyses showed that serum antibodies to PopB, PcrV, and ExoS were common. Longitudinal enzyme-linked immunosorbent assays determined that the first detection of antibodies to pooled ExoS/PopB occurred at a time similar to those of detection of antibodies to a P. aeruginosa cell lysate and the identification of oropharyngeal cultures positive for P. aeruginosa. This indicates that children with CF are colonized early with P. aeruginosa expressing the type III system, implicating it in early pathogenesis, and implies that surveillance of clinical symptoms, oropharyngeal cultures, and seroconversion to type III antigens may facilitate early detection of P. aeruginosa infections.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein (28, 37). Despite advances in understanding its molecular organization and pathophysiology, CF remains one of the most common chronic genetic diseases in the United States. More than 90% of CF patients die of lung disease due to chronic endobronchial infections by Pseudomonas aeruginosa (13).
Several studies comparing oropharyngeal cultures and bronchoalveolar lavage (BAL) cultures found that the sensitivity of oropharyngeal cultures in predicting lower-airway P. aeruginosa infection was poor. Although more sensitive, BAL is an invasive procedure that requires sedation and is often performed on a single lobe, which may miss regional disease (1, 27, 35, 38). The timing of collection of BAL specimens in CF patients is unlikely to reveal information about the acquisition of specific pathogens or the evolution of the genotypic or phenotypic changes in P. aeruginosa (40).
P. aeruginosa produces a number of virulence factors, which are cell surface components or secreted toxins. An ADP-ribosylating exotoxin, exotoxin A (ETA), is the most toxic protein secreted by P. aeruginosa (23). The type III system of P. aeruginosa consists of three coordinately functional protein complexes: the secretion apparatus; the translocation or targeting apparatus; the secreted toxins and cognate chaperones (22). P. aeruginosa secretes four cytotoxins via the type III secretion system: ExoS, ExoT, ExoU, and ExoY (44). These cytotoxins have been implicated in increased cellular and animal toxic effects in experimental models of P. aeruginosa infection (14, 39, 41, 44). Feltman and coworkers have reported the common occurrence of popB, indicating that the type III secretion system was present in clinical isolates and that exoS was more prevalent than exoU in CF isolates of P. aeruginosa (12).
P. aeruginosa is the most significant pathogen in cystic fibrosis, and based on the immune responses in children with CF, infection appears to occur earlier than diagnosed by culture techniques (4, 42). The early diagnosis of P. aeruginosa infections has been sought to allow treatment or eradication of the pathogen before irreversible lung damage has occurred. Johansen et al. monitored the development of an immune response to P. aeruginosa cell lysates as an early indication of infection (26). While there is controversy regarding the ability to use seroconversion to specific Pseudomonas antigens as an early indication of infection, West et al. (42) evaluated the longitudinal relationship between the antibody responses against P. aeruginosa and clinical factors associated with P. aeruginosa infections in children with CF and reported that seroconversion to the cell lysate and ETA occurred before the isolation of P. aeruginosa. This suggested the potential utility of longitudinal monitoring of P. aeruginosa titers, along with the patient's Wisconsin Chest X-Ray (WCXR) score, for early detection and treatment of P. aeruginosa infection in children with CF. Immune responses to components of the type III system in adults and children with CF have been reported (2, 34). In the current study, the hypothesis being tested is that identification of the immune response to type III antigens will lead to earlier detection of infection than has been measured for the analysis of other Pseudomonas antigens. If this hypothesis is true, then detection of seroconversion to type III antigens may allow early therapeutic intervention and improved clinical outcome for patients with CF.
MATERIALS AND METHODS
Study participants;
The Wisconsin CF Neonatal Screening Project is a longitudinal investigation designed to assess the potential benefits and risks of newborn screening for CF; the design and purpose have been described in detail elsewhere (10, 31). Serum samples used in the current study were from children with CF who were enrolled in the CF Neonatal Screening Project. Forty-eight patients who were randomized in the early-diagnosis (screened) group or the standard-diagnosis (control) group, and who were followed at the Milwaukee CF Center, had their serum specimens analyzed. To screen for CF, an immunoreactive trypsinogen assay was used from 15 April 1985 to 30 June 1991 and in combination with DNA analysis for the ΔF508 CFTR mutation from 1 July 1991 to 30 June 1994. Presumptive diagnosis of CF was confirmed with a positive sweat test. After consent for participation was obtained from their parents, patients were enrolled at the Milwaukee CF Center and managed clinically with an evaluation and treatment protocol. The Research and Publications Committee/Human Rights Board at Children's Hospital of Wisconsin, Milwaukee, approved the original Wisconsin CF Neonatal Screening Project and the Pseudomonas study reported here.
Bacterial cultures.
Samples of oropharyngeal secretions were obtained and cultured for P. aeruginosa every 6 months as part of the longitudinal evaluation protocol. Additional samples were obtained as needed at the request of the examining physician, resulting in a median culture interval of 2.7 ± 2.3 months (11). For infants and young children who could not cough on instruction, a tongue depressor was used and the oropharyngeal area was aggressively swabbed using the BD Culturette Collection and Transport system (Becton Dickinson and Co., Franklin Lakes, NJ). For patients who could cough on instruction, the oropharyngeal area was vigorously swabbed while they were coughing. Expectorated sputum samples (<10% of specimens) were cultured from patients who could produce such samples.
Quantitation of anti-Pseudomonas type III antibodies.
Serum samples were obtained at intervals of approximately 6 months and stored at −80°C. The ExoS used in the analysis was the full-length form from which residues 51 to 72 had been deleted to yield a soluble, nonaggregative protein that was purified from Escherichia coli (33). An expression vector for glutathione S-transferase (GST)-PcrV was obtained from Dara Frank (Medical College of Wisconsin, Milwaukee) and purified as a GST fusion protein in E. coli (16). PopB, comprising the N-terminal 170 amino acids of PopB (396 amino acids), was engineered for expression in E. coli as a His6 fusion protein by standard PCR techniques. P. aeruginosa cell lysate was prepared from a 6-h or overnight culture supernatant of P. aeruginosa PA01 that was provided by Steven Lory (Harvard Medical School, Boston, MA). The bacterium was grown in a deferrated dialysate of Trypticase soy broth supplemented with 10 mM nitrilotriacetic acid (Sigma Chemical Company, St. Louis, MO), 1% glycerol, and 100 mM monosodium glutamate at 32°C (15). The cells were pelleted (14,000 × g for 40 min), and the spent culture supernatant was subjected to ammonium sulfate precipitation (65% final concentration). The cells were broken by a French press, and the soluble and membrane fractions were separated by centrifugation (27,000 × g for 20 min). The supernatant (∼5 μg total protein) was used for subsequent analysis. Exotoxin A was purchased from List Biological Laboratories, Inc. (Campbell, CA). Preliminary analyses of four sera from the protocol patients showed that immune reactivity to antigens in the cell lysate (supernatant) was equal to or greater than that of the membrane or cytosol fractions, and the cell lysate was used as a source of P. aeruginosa PA01 antigens for Western blotting and enzyme-linked immunosorbent assay (ELISA) analyses (R. Corech and J. T. Barbieri, unpublished data).
Western blots were performed on polyvinylidene difluoride (PVDF) membranes that had the indicated proteins transferred from sodium dodecyl sulfate (SDS)-polyacrylamide gels. After being blocked in buffer containing 0.01% Triton X-100 and 2% dry milk (wt/vol), sera (1/4,000 final dilution or 1/2,000 final dilution if the initial probe was negative) were incubated in buffer containing 0.01% Triton X-100 and 2% dry milk for 1 h at room temperature (RT). The blots were washed three times with Western blotting buffer containing 0.05% Triton X-100 before incubation with secondary antibody (goat anti-human immunoglobulin G-horseradish peroxidase [α-human IgG-HRP] [catalog no. 31410; Pierce Chemicals, Rockford, IL]) at a 1:100,000 dilution for 1 h at RT. The blots were washed three times with buffer containing 0.05% Triton X-100, incubated in SuperSignal West Pico (Pierce, Rockford, IL) for 5 min, and exposed to X-ray film. Signals were scored relative to that of serum from a patient with CF that reacted to PopB, which was probed with each analysis. Each set of Western blots included the analysis of the reactivity of a human serum that contained antibodies to PopB. Western blot signals were scored as either positive or negative on X-ray film when the reactivity of this human serum to PopB was not saturated. Signals were scored in a blinded manner where both the identity of the patient and the absolute age of the patient when the serum samples were drawn were coded.
ELISA was performed in 96-well plates (Corning), each well containing 250 ng purified antigen or 1 μg of cell lysate in 50 mM sodium carbonate binding buffer, pH 9.6 (100 μl), and the plates were incubated overnight at 4°C. The plates were rinsed four times with 350 μl of phosphate-buffered saline (PBS) and blocked with 200 μl of 50 mM sodium carbonate, pH 9.6, plus 1% bovine serum albumin (BSA) for 1 h at RT. Next, serum (100 μl of a 1/500 dilution in PBS plus 1% BSA) was added, and the plates were incubated for 1 h at 37°C, followed by four PBS washes. Next, secondary antibody (goat α-human IgG-HRP; 100 μl of a 1/8,000 dilution in PBS plus 1% BSA) was added, and the plates were incubated for 1 h at RT. The plates were rinsed four times with PBS and incubated with 100 μl of 1-Step Slow TMB (Pierce, Rockford, IL) for 30 min at RT, after which 100 μl 1.0 M H2SO4 was added to stop the reaction. Absorbance was read at 450 nm. ELISA was performed on duplicate sample sets, and the results reported were the averages of duplicate, independent analyses. An analysis was repeated if the duplicate determinations differed by more than ∼20% (0.2 optical density [OD] units). Seroconversion was scored positive when the OD increased to 0.2 OD units above background.
Statistical methods.
The age at the first antibody titer above 1/500 for ELISA and above 1/4,000 or 1/1,000 for Western blotting (cell lysate, ETA, ExoS, PopB, and PcrV) compared to the age at the first positive culture for P. aeruginosa and the age at the first significant clinical event (coughing or WCXR of ≥5) were summarized with Kaplan-Meier estimates of the cumulative distribution function to account for right censoring of the data (29). Comparisons between event-time distributions were made with a paired log rank test (5), using a robust variance estimate for the many individuals who typically experienced both events being compared.
RESULTS
Patient characteristics and antigens used in the analyses.
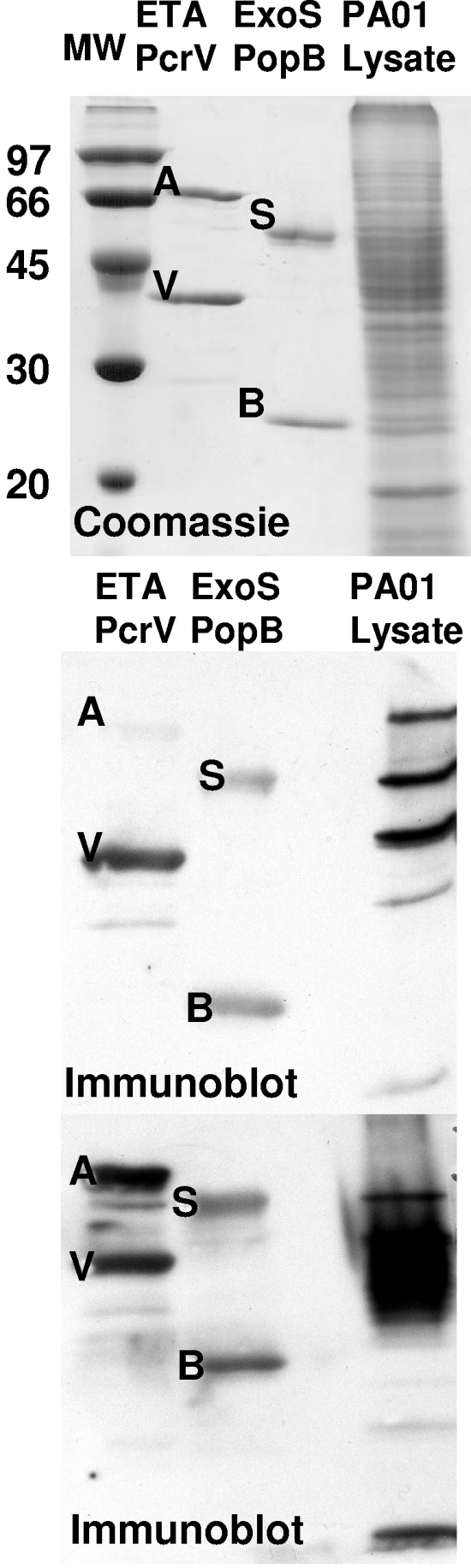
Table 1 shows the baseline characteristics of 48 patients recruited from the Milwaukee site of the Wisconsin CF Neonatal Screening Project. Twenty-four patients were from the newborn screened group, and 24 patients were from the traditional-diagnosis group. The antigens used in this study are shown in Fig. 1, along with two Western blots using serum that was reactive to proteins of the type III secretion system. Serum reactivity to a distinct set of proteins in the cell lysate was detected (Fig. 1, middle panel), or serum reactivity was less clear in resolving proteins in the cell lysate (Fig. 1, lower panel). The ExoS antigen (an internal-deletion protein that lacks residues 51 to 72 of ExoS), is a soluble, monomeric protein. The PopB antigen (the N-terminal 170 amino acids of PopB) was expressed as a soluble, monomeric protein. PcrV is expressed as a GST fusion protein in E. coli. The utilization of E. coli-derived antigens reduces immunoreactivity due to P. aeruginosa lipopolysaccharide contamination. ETA is a commercial product.
TABLE 1.
Characteristics of patients with CF followed at the Milwaukee CF Center (n = 48) participating in the Wisconsin CF Newborn Screening Study with sera analyzed for type III antibodies
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 29 (60) |
| Female | 19 (40) |
| Genotype | |
| ΔF508/ΔF508 | 27 (56) |
| ΔF508/other | 19 (40) |
| Other/other | 2 (4) |
| Meconium ileus | |
| Yes | 13 (27) |
| No | 35 (73) |
| Pancreatic status | |
| Sufficient | 4 (8) |
| Insufficient/probably insufficient | 44 (92) |
FIG. 1.
Antigens used for analysis of seroconversion in children with CF. The indicated purified proteins (1 μg), ExoS (S), PcrV (V), ETA (A), PopB (B), and a P. aeruginosa PA01 cell lysate, were subjected to SDS-polyacrylamide gel electrophoresis. One gel (upper panel) was stained with Coomassie blue. Molecular weight (MW) markers are shown in the left lane. Proteins from two other gels were transferred to PVDF membranes and incubated with sera from two patients with CF. The membrane was then probed with goat α-human IgG-HRP and developed by enhanced chemiluminescence. Exposed X-ray films are shown (middle and lower panels).
Western blot analyses of sera from children with CF.
Figure 2 shows a Western blot analysis of the serum samples against components of the type III system, ETA, and a cell lysate from P. aeruginosa PA01. Seroconversion to all antigens or subsets of antigens was typically observed. In the longitudinal Western blot analyses, 100% of the sera converted for the PA01 cell lysate, 94% for PopB, 90% for ExoS, 85% for PcrV, and 58% for ETA. Seroconversion was measured at a final serum dilution of 1/4,000; if this did not show seroconversion, the experiment was performed at a final serum dilution of 1/1,000. Serum reactivity was measured relative to immune reactivity to PopB in a standardized serum preparation from an adult with CF. The median age of seroconversion for the 48 patients was 42 months for cell lysate, 60 months for ExoS, 59 months for PopB, 60 months for PcrV, and 131 months for ETA. The median age for an oropharyngeal culture positive for P. aeruginosa was 30 months. Statistical analyses showed that seroconversion to antigens in the cell lysate appeared earlier than that for PopB, which was earlier than ExoS, PcrV, or ETA (Table 2).
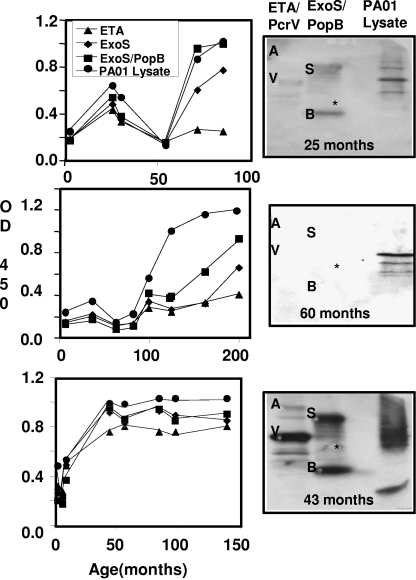
FIG. 2.
Representative immunoreactivities of sera from children with CF. Longitudinal ELISA assessments of sera from three patients with CF are represented in the top, middle, and bottom rows, showing probes with the indicated antigens with a 1/500 dilution of sera, followed by incubation with α-human IgG-HRP. Antibody reactivity was detected with TMB. Western blots of the sera collected at the indicated times were performed, using the indicated purified proteins (1 μg), ExoS (S), PcrV (V), ETA (A), PopB (B), and a P. aeruginosa PA01 cell lysate subjected to SDS-PAGE, and they are shown to the right of the ELISA plot. OD 450, OD at 450 nm.
TABLE 2.
Median age of seroconversion to the indicated immunogen for patients with CF by Western blottingaand ELISAb
| Antigen | Age (mo) | P value to PopB | P value to lysate |
|---|---|---|---|
| Western blotting | |||
| ETA | 131 | <0.001c | <0.001c |
| PcrV | 60 | 0.11 | <0.001c |
| ExoS | 60 | 0.85 | <0.001c |
| PopB | 59 | <0.001c | |
| PA01 lysate | 42 | <0.001c | |
| ELISA | |||
| ETA | 78 | <0.001c | <0.001c |
| ExoS | 60 | 0.05c | 0.003c |
| ExoS/PopB | 40 | 0.33 | |
| PA01 lysate | 27 | 0.33 |
Western blotting was performed after the transfer of SDS-polyacrylamide gel electrophoresis-separated proteins to PVDF membranes. The membranes were probed with longitudinal sera (diluted 1/4,000; if negative, diluted to 1/1,000) from 48 patients with CF. The sera were analyzed in at least two independent experiments, and the averages are shown. Positive seroconversion was determined relative to an internal positive control that detected an immune reaction to PopB.
ELISA was performed using 0.25 μg of purified proteins or 1 μg of PA01 cell lysate. Wells were probed with longitudinal sera (1/500 dilution) from 48 patients with CF. The sera were analyzed in at least two independent experiments with similar results; the results from one set of data are shown. Positive seroconversion was determined when the OD was 0.2 units above the negative controls (minus primary serum and minus secondary antibody).
Statistically significant (P ≤ 0.05) difference between the indicated samples.
ELISA analyses of sera from children with CF.
Based upon Western blot analyses, the four antigens chosen for the ELISA testing included the PA01 cell lysate, a pool of ExoS and PopB (ExoS/PopB), ExoS, and ETA. Figure 2 shows the longitudinal ELISA for three sera in which seroconversion was observed to all antigens (Fig. 2, lower panel) or a subset of antigens (Fig. 2, middle panel) or where the ELISA titer fluctuated in intensity with time (Fig. 2, upper panel). The fluctuating pattern of the antibody titer was observed with serum samples from several patients and probably reflects the ability of the host to temporarily reduce the Pseudomonas load. Ninety-six percent of the seroconversion detected was to antigens in the cell lysate, while 94% was against ExoS/PopB, 83% against ExoS, and 75% against ETA. The median age of seroconversion to antigens was 27 months for the cell lysate, 40 months for ExoS/PopB, 60 months for ExoS, and 78 months for ETA (Table 2). Statistical analyses showed that the ages of seroconversion to antigens in the cell lysate and ExoS/PopB were not significantly different, whereas seroconversion to ExoS and ETA occurred later than that to antigens in the cell lysate or ExoS/PopB (Table 2).
Detection of an immune response to P. aeruginosa antigens and clinical correlations.
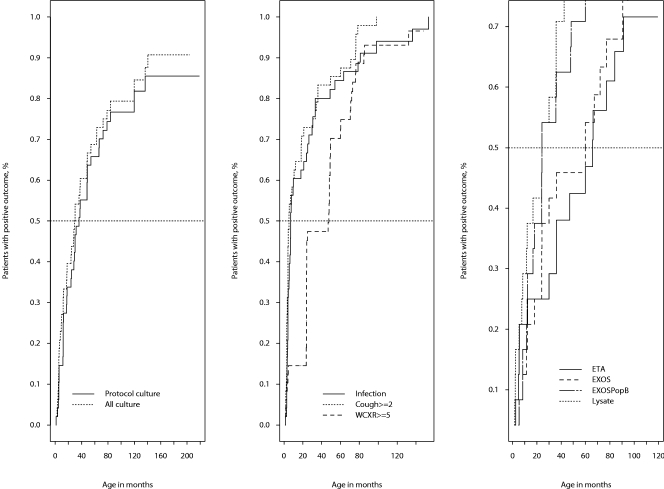
Longitudinal relationships were plotted as the percentage of patients with a specific event versus the time of first acquisition of that event using the Kaplan-Meier plot (Fig. 3). Each Kaplan-Meier plot utilized data from the results for the 48 patients. Potential early indicators of infection included the first report of significant cough; the first pulmonary infection or course of antibiotics; a WCXR score of 5 or greater; the first report of an antibody response against cell lysate, ETA, PcrV, ExoS, or PopB; and the first isolation of P. aeruginosa from an oropharyngeal culture. A category 2 cough was defined as a cough occurring in the morning or with postural drainage. The median time for a WCXR score of ≥5 was 48 months, which was later than the isolation of P. aeruginosa in any culture or the detection of the type III antibody response to ExoS/PopB.
FIG. 3.
Kaplan-Meier survival curve results comparing first acquisition of clinical profiles and seroconversion to Pseudomonas aeruginosa antigens in children with CF. (Left) First acquisition of P. aeruginosa from oropharyngeal cultures in screened patients versus all patients (median, 36.1 months for screened patients; median, 29.5 months for all patients). (Middle) Time to first cough score was ≥2 months (median, 5.3 months) for all patients; median time to a WCXR score of ≥5 was 47.5 months for all patients. (Right) First occurrences of ELISA-positive reactions to PA01 cell lysate, ExoS, ExoS/PopB, and ETA at 1:500 serum dilution; ETA, median = 77.8 months; ExoS, median = 59.8 months; ExoS/PopB, median = 39.8 months; lysate PA01, median = 27.1 months.
DISCUSSION
There is no approved therapy for correction of the underlying genetic defect in CF or for reversal of the ion transport abnormalities associated with dysfunctional CFTR. Current therapies are directed toward slowing the progression of secondary organ dysfunction and its sequelae. The chronic lung infection with mucoid strains of P. aeruginosa that develops in most patients with CF is difficult to eradicate and results in progressive lung destruction and increased mortality (31, 32).
The rank orders for seroconversion to Pseudomonas antigens were similar using ELISA or Western blotting, with earlier detection of seroconversion by ELISA. The greater sensitivity of ELISA was likely due to the use of lower serum dilutions relative to Western blotting. The largest discrepancy in the time to first antibody detection was observed for ETA, where ELISA was positive at 78 months and Western blotting was positive at 131 months. ELISA may detect antibodies against conformational epitopes that are not present in proteins subjected to Western blot analyses. An earlier study reported an earlier time to seroconversion to ETA than was observed in the current study (42). There are several differences between the ELISA described in the early study and that used in the current study, which could contribute to the differences in the observed times to serum conversion, including the serum dilution used in the analysis, the secondary antibody reactivity and antibody detection system, threshold scoring for seroconversion, and storage time of samples prior to analysis. Sera from a subset of patients that were analyzed in both studies for seroconversion to ETA showed first seroconversion in the West et al. study at 33 months and first seroconversion to ETA in the current study at 63 months, with 19 of the 24 samples having earlier or identical times to seroconversion in the earlier study and in the current study (Z. Li, unpublished analysis). While experimental conditions define the absolute sensitivity for the detection of the immune response to an antigen, the ranked times of detection should be constant between studies. Under conditions where patients with CF had detectable titers to Pseudomonas antigens, sera from normal controls did not have detectable reactivity by ELISA (42) or Western blotting (34).
Type III-dependent secretion is a common mechanism used by gram-negative pathogens for delivery of antihost factors. This implies either that during early colonization P. aeruginosa contacts host cells to stimulate expression of type III components or that the CF lung environment is sufficient to stimulate type III secretion in a contact-independent mechanism, similar to the indication observed with nitrilotriacetic acid in culture (14). Yersinia and P. aeruginosa utilize a common core of proteins for the secretion of the type III cytotoxins from the bacterium into the host cell. YopB and YopD of Yersinia and PopB and PopD of P. aeruginosa induce pore formation in the target cell membrane (18). Benner et al. (3) evaluated the humoral immune responses of mice that survived a lethal Yersinia pestis aerosol challenge after antibiotic treatment. The major antigens recognized by murine convalescent-phase sera included YopD, indicating the expression of the type III system. LcrV in Yersinia is part of the translocation apparatus and is required to deliver antihost effector proteins into host cells and, like PcrV in P. aeruginosa, is also essential for translocation of the cytotoxins. Antibodies directed against PcrV (39) and LcrV (20, 21) protect animals against infections, which has implicated these immunogens as possible candidates for vaccine therapy.
Serologic detection of P. aeruginosa infection has been used as a diagnostic tool in Europe. A 30-year study (26) investigated the effects of increasingly intensive treatment regimens on anti-Pseudomonas antibody levels and survival in five successive cohorts of Danish patients after acquisition of chronic P. aeruginosa lung infection. Cystic fibrosis patients treated intensively with antibiotics had lower antibody titers, less lung inflammation, and longer survival, even after acquisition of chronic P. aeruginosa lung infection. The European consensus on treatment of patients suffering from chronic P. aeruginosa infection is therapeutic intervention with antibiotics with specific activity against P. aeruginosa (8). In the United States, early diagnosis and early, aggressive treatment with anti-Pseudomonas antibiotics delays the onset of chronic P. aeruginosa infection (4), and the characteristics of early P. aeruginosa isolates infecting the CF airway appear to be more favorable for eradication and delayed reinfection with appropriate antibiotic therapy (17, 36, 43).
A rise in the serum titers of antibodies against P. aeruginosa exoproteins may serve as an early sign of infection for therapeutic intervention (19, 24). Several previous studies detected antibody responses to the P. aeruginosa exoproteins (e.g., ETA, proteases, and phospholipase C) by immune blotting techniques and established correlations between elevated titers and poor clinical status (9, 19, 24, 30). More recent studies have implicated the presence of an immune response to components of the type III secretion system of P. aeruginosa in patients with CF. Moss et al. (34) observed that antibodies to PopB were common in the sera of adults with CF, and a screen of sera from children with CF showed that 7/13 infants had antibodies against ExoS and PopB (2). The current study shows that children with CF have serum antibodies to the type III apparatus (PopB) at an early stage of P. aeruginosa infection. This early antibody response implies expression of type III cytotoxins during the initial and acute phases of P. aeruginosa infection in the lungs of children with CF. Other evidence implying a role for type III cytotoxins in P. aeruginosa infections in patients with CF includes the determination that the P. aeruginosa CF clinical isolate, CHA, showed toxicity for human polymorphonuclear neutrophils via a type III-dependent pathway (7) and that P. aeruginosa isolates from patients with CF that did not show type III-dependent cytotoxicity toward phagocytes were defective in the expression of exsA (6), a positive regulator for type III expression. In addition, a molecular analysis of clinical and environmental P. aeruginosa isolates reported that the gene encoding PopB was common and that the majority of isolates contained the gene encoding ExoS in clinical and environmental isolates (12). Measurement of seroconversion against PopB and or the combination of ExoS/PopB provides a sensitive and early indication of infection, and when combined with oropharyngeal culturing for P. aeruginosa, it does not miss CF patients with P. aeruginosa infections.
In this study, children with CF developed an early immune response to components of the type III apparatus, and the immune response to type III components occurred earlier than that to ETA and seroconversion to type III components occurred within a time similar to that of the seroconversion to antigens in the cell lysate of PA01. A recent characterization of environmental and clinical isolates of P. aeruginosa observed that while most isolates from the environment were positive for type III secretion, approximately half of the isolates from newly infected children secreted type III cytotoxins, while lower percentages of isolates from chronically infected children or chronically infected adults with CF showed the ability to secrete type III cytotoxins (25). The current study observed early seroconversion at 94% to Pop/ExoS in children with CF by ELISA. Thus, expression of the type III system is common at the earliest stages of P. aeruginosa infections, with the prevalence reduced during the chronic stages of infections. This suggests that the most important role of the type III system may be during the initial stages of colonization of P. aeruginosa in the CF lung and either that type III cytotoxins, such as ExoS, are less important in chronic infection or that the prevalence of isolates that express the type III system fluctuates to counter the host response to infection.
Acknowledgments
Research conducted by the Medical College of Wisconsin was supported by a grant from the NIH, HL68912, and General Clinical Research Center Grant M01-RR00058 from the NIH. Research conducted by the Wisconsin Newborn CF Neonatal Screening Study Group was supported by grants DK34108 and RR03186 from the NIH.
We thank Martha Vaughan for critical reading of the manuscript.
REFERENCES
- 1.Armstrong, D. S., K. Grimwood, J. B. Carlin, R. Carzino, A. Olinsky, and P. D. Phelan. 1996. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr. Pulmonol. 21:267-275. [DOI] [PubMed] [Google Scholar]
- 2.Banwart, B., M. L. Splaingard, P. M. Farrell, M. J. Rock, P. L. Havens, J. Moss, M. E. Ehrmantraut, D. W. Frank, and J. T. Barbieri. 2002. Children with cystic fibrosis produce an immune response against exoenzyme S, a type III cytotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 185:269-270. [DOI] [PubMed] [Google Scholar]
- 3.Benner, G. E., G. P. Andrews, W. R. Byrne, S. D. Strachan, A. K. Sample, D. G. Heath, and A. M. Friedlander. 1922. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infect. Immun. 67:1922-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 5.Cottle, R., J.-S. Pang, and R. E. Stone. 1992. The linear complementarity problem, p. 265-288. Academic Press, New York, N.Y.
- 6.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doring, G., S. P. Conway, H. G. Heijerman, M. E. Hodson, N. Hoiby, A. Smyth, and D. J. Touw. 2000. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur. Respir. J. 16:749-767. [DOI] [PubMed] [Google Scholar]
- 9.Doring, G., and N. Hoiby. 1983. Longitudinal study of immune response to Pseudomonas aeruginosa antigens in cystic fibrosis. Infect. Immun. 42:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell, P. M., M. R. Kosorok, A. Laxova, G. Shen, R. E. Koscik, W. T. Bruns, M. Splaingard, E. H. Mischler, et al. 1997. Nutritional benefits of neonatal screening for cystic fibrosis. N. Engl. J. Med. 337:963-969. [DOI] [PubMed] [Google Scholar]
- 11.Farrell, P. M., G. Shen, M. Splaingard, C. E. Colby, A. Laxova, M. R. Kosorok, M. J. Rock, and E. H. Mischler. 1997. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics 100:E2. [DOI] [PubMed] [Google Scholar]
- 12.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 13.FitzSimmons, S. C. 1993. The changing epidemiology of cystic fibrosis. J. Pediatr. 122:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 15.Frank, D. W., and B. H. Iglewski. 1991. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 173:6460-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank, D. W., A. Vallis, J. P. Wiener-Kronish, A. Roy-Burman, E. G. Spack, B. P. Mullaney, M. Megdoud, J. D. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 17.Frederiksen, B., C. Koch, and N. Hoiby. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23:330-335. [DOI] [PubMed] [Google Scholar]
- 18.Frithz-Lindsten, E., A. Holmstrom, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and A. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 19.Granstrom, M., A. Ericsson, B. Strandvik, B. Wretlind, O. R. Pavlovskis, R. Berka, and M. L. Vasil. 1984. Relation between antibody response to Pseudomonas aeruginosa exoproteins and colonization/infection in patients with cystic fibrosis. Acta Paediatr. Scand. 73:772-777. [DOI] [PubMed] [Google Scholar]
- 20.Hill, J., C. Copse, S. Leary, A. J. Stagg, E. D. Williamson, and R. W. Titball. 2003. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect. Immun. 71:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill, J., S. E. Leary, K. F. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglewski, B. H., P. V. Liu, and D. Kabat. 1977. Mechanism of action of Pseudomonas aeruginosa exotoxin A-adenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun. 15:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagger, K. S., D. L. Robinson, M. N. Franz, and R. L. Warren. 1982. Detection by enzyme-linked immunosorbent assays of antibody specific for Pseudomonas proteases and exotoxin A in sera from cystic fibrosis patients. J. Clin. Microbiol. 15:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain, M., D. Ramirez, R. Seshadri, J. F. Cullina, C. A. Powers, G. S. Schulert, M. Bar-Meir, C. L. Sullivan, S. A. McColley, and A. R. Hauser. 2004. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J. Clin. Microbiol. 42:5229-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen, H. K., L. Norregaard, P. C. Gotzsche, T. Pressler, C. Koch, and N. Hoiby. 2004. Antibody response to Pseudomonas aeruginosa in cystic fibrosis patients: a marker of therapeutic success? A 30-year cohort study of survival in Danish CF patients after onset of chronic P. aeruginosa lung infection. Pediatr. Pulmonol. 37:427-432. [DOI] [PubMed] [Google Scholar]
- 27.Jung, A., I. Kleinau, G. Schonian, A. Bauernfeind, C. Chen, M. Griese, G. Doring, U. Gobel, U. Wahn, and K. Paul. 2002. Sequential genotyping of Pseudomonas aeruginosa from upper and lower airways of cystic fibrosis patients. Eur. Respir. J. 20:1457-1463. [DOI] [PubMed] [Google Scholar]
- 28.Kerem, B., J. M. Rommens, J. A. Buchanan, D. Markiewicz, T. K. Cox, A. Chakravarti, M. Buchwald, and L. C. Tsui. 1989. Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073-1080. [DOI] [PubMed] [Google Scholar]
- 29.Klein, J. P., and M. L. Moeschberger. 1997. Survival analysis: techniques for censored and truncated data. Springer, New York, N.Y.
- 30.Klinger, J. D., D. C. Straus, C. B. Hilton, and J. A. Bass. 1978. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: demonstration by radioimmunoassay. J. Infect. Dis. 138:49-58. [DOI] [PubMed] [Google Scholar]
- 31.Kosorok, M. R., M. Jalaluddin, P. M. Farrell, G. Shen, C. E. Colby, A. Laxova, M. J. Rock, and M. Splaingard. 1998. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr. Pulmonol. 26:81-88. [DOI] [PubMed] [Google Scholar]
- 32.Li, Z., M. R. Kosorok, P. M. Farrell, A. Laxova, S. E. West, C. G. Green, J. Collins, M. J. Rock, and M. L. Splaingard. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581-588. [DOI] [PubMed] [Google Scholar]
- 33.Maresso, A. W., and J. T. Barbieri. 2002. Expression and purification of two recombinant forms of the type-III cytotoxin, Pseudomonas aeruginosa ExoS. Protein Expr. Purif. 26:432-437. [DOI] [PubMed] [Google Scholar]
- 34.Moss, J., M. E. Ehrmantraut, B. D. Banwart, D. W. Frank, and J. T. Barbieri. 2001. Sera from adult patients with cystic fibrosis contain antibodies to Pseudomonas aeruginosa type III apparatus. Infect. Immun. 69:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey, B. W., K. R. Wentz, A. L. Smith, M. Richardson, J. Williams-Warren, D. L. Hedges, R. Gibson, G. J. Redding, K. Lent, and K. Harris. 1991. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am. Rev. Respir. Dis. 144:331-337. [DOI] [PubMed] [Google Scholar]
- 36.Ratjen, F., G. Doring, and W. H. Nikolaizik. 2001. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 358:983-984. [DOI] [PubMed] [Google Scholar]
- 37.Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld, M., J. Emerson, F. Accurso, D. Armstrong, R. Castile, K. Grimwood, P. Hiatt, K. McCoy, S. McNamara, B. Ramsey, and J. Wagener. 1999. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr. Pulmonol. 28:321-328. [DOI] [PubMed] [Google Scholar]
- 39.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 40.Speert, D. P., S. W. Farmer, M. E. Campbell, J. M. Musser, R. K. Selander, and S. Kuo. 1990. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J. Clin. Microbiol. 28:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West, S. E., L. Zeng, B. L. Lee, M. R. Kosorok, A. Laxova, M. J. Rock, M. J. Splaingard, and P. M. Farrell. 2002. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA 287:2958-2967. [DOI] [PubMed] [Google Scholar]
- 43.Wiesemann, H. G., G. Steinkamp, F. Ratjen, A. Bauernfeind, B. Przyklenk, G. Doring, and H. von der Hardt. 1998. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr. Pulmonol. 25:88-92. [DOI] [PubMed] [Google Scholar]
- 44.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]