Abstract
ECa 233 is a standardized extract of Centella asiatica that contains the major active compounds madecassoside (MDS) and asiaticoside (ASS), which can strengthen hippocampal synapses, resulting in a memory-enhancing effect. This study explored the possible mechanisms of ECa 233 related to synaptic enhancement via proteomic analysis. Differentiated SH-SY5Y cells and hippocampal slices obtained from healthy male Wistar rats were treated with dimethyl sulfoxide, ECa 233, or the MDS + ASS mixture. Mitochondrial function and protein expression were examined via proteomic, western blot, and immunofluorescence analyses. Effects on synaptic plasticity were confirmed via electrophysiology using acute hippocampal slices. The treated groups exhibited a significant increase in mitochondrial activity and synaptic plasticity–related protein expression. Upstream Akt-mTOR signaling was significantly enhanced, with the most pronounced effects observed in the MDS + ASS group. However, the ECa 233 group still showed the highest hippocampal synaptic response. Based on our data, both ECa 233 and MDS + ASS were involved in mitochondrial metabolism, with the Akt-mTOR pathway as one of the targets promoting these activities. Furthermore, the synergistic effect of MDS + ASS was essential to achieve the same level of response obtained with ECa 233. Ultimately, the MDS + ASS mixture may enhance cellular plasticity, while the remaining components of ECa 233 may promote specific proteins that play a role in maintaining biological homeostasis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-12622-2.
Keywords: Triterpenoid glycosides, Madecassoside, Asiaticoside, Mitochondria, SH-SY5Y, Proteomic
Subject terms: Molecular biology, Neuroscience, Physiology
Introduction
ECa 233 is a standardized extract of Centella asiatica. It contains two main active components, namely madecassoside (MDS) and asiaticoside (ASS), which help achieve final triterpenoid concentrations of at least 85% of the extracted powder. The concentrations of MDS and ASS are maintained at a ratio of 1.5 ± 0.5:1. Both MDS and ASS are pentacyclic triterpenoid glycosides with five-membered ring structures. However, MDS has a hydroxyl (OH) group instead of a hydrogen atom at C61. Studies have shown that these triterpenoid glycosides exert neuroprotective effects due to their antiapoptotic, antioxidant, and anti-inflammatory actions in injured cells2,3.
ECa 233 can enhance spatial memory retention and increase the expression of hippocampal synapses and synaptic plasticity–associated proteins such as BDNF, TrkB, NR2A, and NR2B4. The synaptic-enhancing capability of ECa 233 most likely depends on the action of the triterpenoid glycosides, which are the major compounds in the extract. Moreover, in a previous study, ECa 233 at a concentration of 10 µg/mL produced a maximal long-term potentiation (LTP) effect in healthy hippocampal slices5 compared with the control and ECa 233 at 100 µg/mL. This finding emphasizes the inverted U-shaped dose–response of ECa 233 and the complex interplay between the constituents of the extract.
Previous studies on the neuroprotective effects of ASS and MDS in memory-related deficit models—both in vitro and in vivo—have focused on their association with mitochondrial function and synaptic plasticity. These effects are attributed to their antioxidant and antiapoptotic activities. Additionally, most studies on neurogenesis and synaptic plasticity have examined the downstream pathway of BDNF/TrkB, a key mechanism of synaptic plasticity, along with associated pathways such as cAMP/PKA, MAPK, PI3K/Akt, and mTOR. Studies have shown that ASS can promote synaptic function enhancement by activating the mTOR, ERK/CREB, PI3K/Akt, and Akt–GSK3β pathways6,7. However, there is limited evidence regarding the underlying mechanisms by which MDS influences synaptic function. MDS has been shown to promote neurite outgrowth via the MEK/ERK pathway3,6.
Therefore, this study aimed to explore the underlying mechanisms of ECa 233 related to synaptic plasticity by comparing its effects with those of a combined MDS and ASS mixture at equivalent concentrations. The outcomes were assessed by investigating synaptic function–associated protein expression in differentiated SH-SY5Y cells, determining the direct impact on mitochondrial function, and examining cellular synaptic plasticity using LTP recording data from rat hippocampal slice preparations. Gaining a deeper understanding of the effects of MDS and ASS may have significant implications for the therapeutic potential of ECa 233 in the future.
Results
Proteomic analysis of differentiated SH-SY5Y cells after drug treatments
ECa 233 and a mixture of MDS and ASS enhanced rat hippocampal LTP. Therefore, their mechanisms of action in the differentiated SH-SY5Y cell line were further examined via proteomic analysis. In this study, data were collected from three biologically independent samples, each analyzed in three technical replicates. After removing contaminants, the proteomic results showed 2,241 identified master proteins with valid gene symbols across the untreated control, ECa 233, and MDS + ASS groups. The raw proteomics files were deposited in the ProteomeXchange Consortium via the PRIDE partner repository under the dataset accession code PXD057572 .
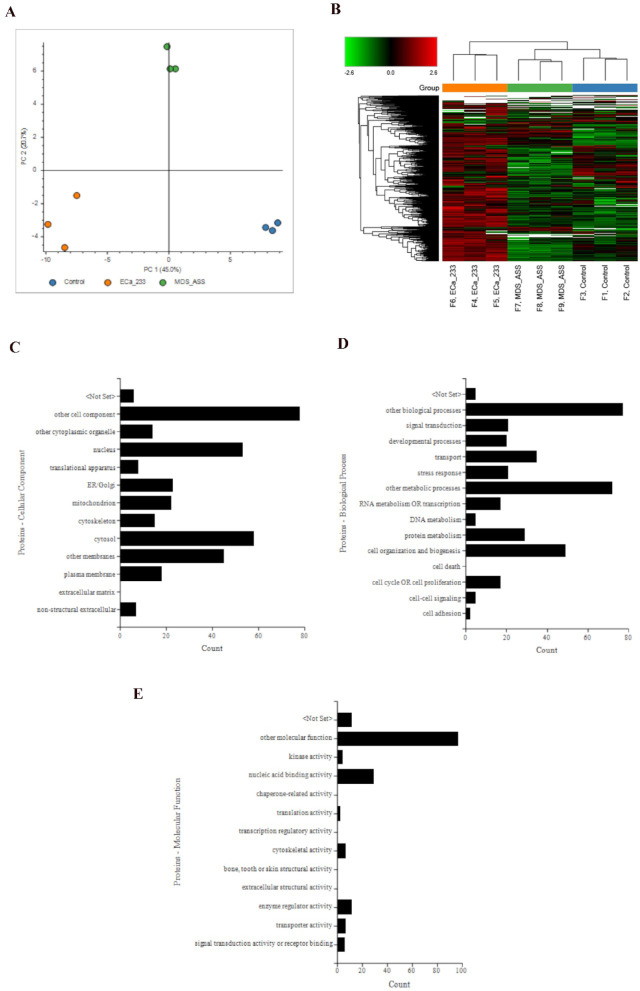
Principal component analysis showed three distinct groupings, color-coded as blue, orange, and green for the control, ECa 233, and MDS + ASS groups, respectively. PC1 and PC2 accounted for 45.0% and 20.7% of the total variation, respectively. A hierarchical cluster analysis heatmap also showed clustering of each replicate within each group, with the MDS + ASS group clustering closer to the control group compared with the ECa 233 group (Figs. 1A–1B). Supplementary File 1A shows the abundance of all 2,241 proteins. Differentially expressed proteins (DEPs)—defined as proteins with a log2 fold change > 2 and a p-value < 0.05—between the ECa 233 and control groups were profiled according to ontology: cellular component, biological process, and molecular function (Figs. 1C–1E). Supplementary File 1B lists all 121 DEPs between the ECa 233 and control groups.
Fig. 1.
Proteomics data analysis of differentiated SH-SY5Y cells. (A) Principal component analysis plot of all identified master proteins. (B) Heatmap showing the clustering of all master proteins. (C–E) Gene ontology classification of differentially expressed proteins between the ECa 233 and control groups: (C) cellular components, (D) biological processes, and (E) molecular functions. Data were obtained from three biological replicates, each analyzed in triplicate.
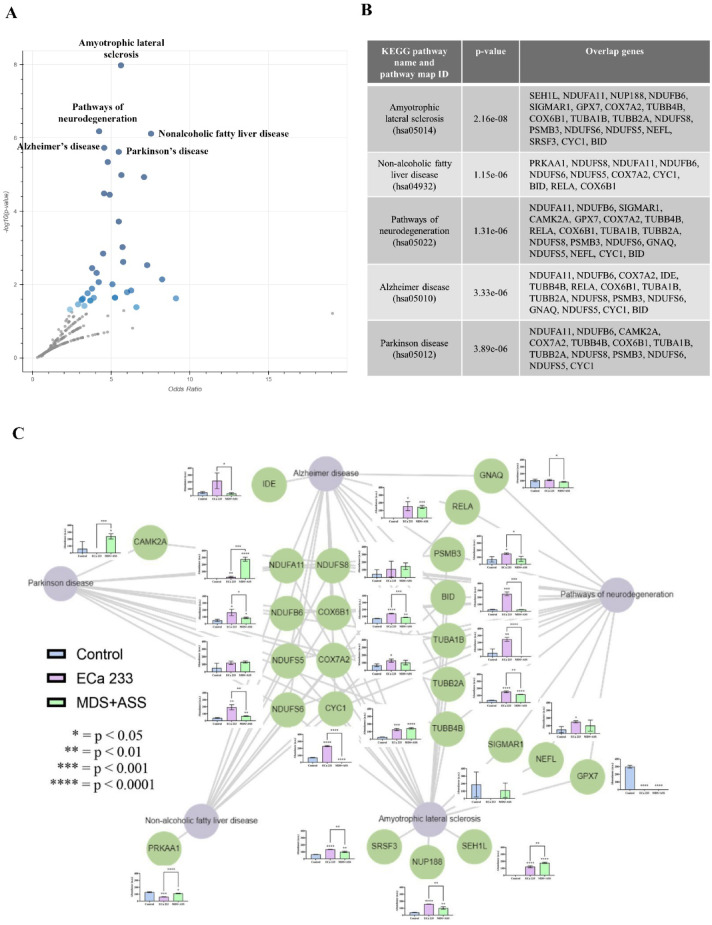
A pathway analysis of DEPs between the ECa 233 and control groups was performed using Enrichr. The top five most significant pathways were as follows: (1) amyotrophic lateral sclerosis, (2) nonalcoholic fatty liver disease, (3) pathways of neurodegeneration, (4) Alzheimer’s disease (AD), and (5) Parkinson’s disease (PD). These pathways are shown in a volcano plot, with their respective overlapping protein-coding genes summarized in a table (Figs. 2A–2B). This was further visualized in a network using Enrichr-KG, where purple circles represent pathways and green circles indicate protein-coding genes. Normalized protein abundance is shown in a bar chart next to each respective protein node (Fig. 2C).
Fig. 2.
Pathway analysis of differentially expressed proteins (DEPs). (A) Volcano plot of KEGG pathways generated from DEPs between the ECa 233 and control groups, visualized using Appyter. Gray points represent non-significant pathways, whereas blue points indicate significant pathways (p < 0.05). Darker blue points denote higher statistical significance. Data are expressed as means ± SEM (n = 3, triplicate). (B) Table listing the KEGG pathways and overlapping protein-coding genes identified via enrichment analysis using Enrichr. (C) Pathway network generated from the enrichment analysis of DEPs between the ECa 233 and control groups. Purple bubbles represent KEGG pathways, and green bubbles indicate DEPs associated with each pathway. Abundance bar graphs are displayed for each protein, ordered from left to right as: control, ECa 233, and MDS+ASS.
Our analysis of the pathway network (Fig. 2C) revealed differences in protein expression among all groups. However, the results highlighted only those proteins that were significantly differentially expressed between the ECa 233 and MDS + ASS groups. Compared with the MDS + ASS group, the ECa 233 group had significantly higher levels of several proteins, including CYC1 [F(2,6) = 1423.197, p < 0.001], BID [F(2,6) = 193.801, p < 0.001], COX6B1 [F(2,6) = 245.135, p < 0.001], TUBA1B [F(2,6) = 40.201, p < 0.001], NDUFS6 [F(2,6) = 42.131, p < 0.001], TUBB2A [F(2,6) = 241.542, p < 0.001], NUP188 [F(2,6) = 103.905, p < 0.001], SRSF3 [F(2,6) = 98.489, p < 0.001], IDE [F(2,6) = 7.185, p = 0.026], NDUFB6 [F(2,6) = 12.067, p = 0.008], GNAQ [F(2,6) = 3.831, p = 0.039], and PSMB3 [F(2,6) = 6.252, p = 0.034]. Table 1 summarizes their functions. Meanwhile, the protein levels of CAMK2A [F(2,6) = 11.439, p = 0.009], NDUFA11 [F(2,6) = 238.522, p < 0.001], PRKAA1 [F(2,6) = 132.821, p < 0.001], and SEH1L [F(2,6) = 301.386, p < 0.001] were significantly higher in the MDS + ASS group than in the ECa 233 group. Table 2 presents their functions. These prominent proteins are associated with several biological activities (Table 3).
Table 1.
Proteins with significantly higher expression in cells treated with ECa 233 than in those treated with MDS + ASS.
| Protein | Function | Protein expression (compared with the control) | |
|---|---|---|---|
| ECa | MDS + ASS | ||
| CYC1 (cytochrome C1 or cytochrome bc1 complex) |
- Promotes mitochondrial complex III activity and ROS production in neurons under stress33 - Promotes cancer cell proliferation34 |
↑↑↑↑ | ↓↓↓↓ |
| BID (pro-apoptotic Bcl-2 protein) | - Regulates the intrinsic apoptosis pathway, leading to programmed cell death35 | ↑↑↑ | ↔ |
| COX6B1 (cytochrome c oxidase subunit 6B1) |
- Subunit of mitochondrial complex IV (cytochrome c oxidase) - Overexpression protects neurons by reducing calcium levels and preventing cell death36 |
↑↑↑↑ | ↑ |
| TUBA1B (tubulin alpha 1b protein) | - Major component of neuronal microtubules37 | ↑↑↑↑ | ↔ |
| NDUFS6 (NADH: ubiquinone oxidoreductase subunit S6) | - Mitochondrial complex I subunit involved in axonal transport. Mutations linked to mitochondrial disease38 | ↑↑↑ | ↑ |
| TUBB2A (tubulin beta 2a protein) | - Neuronal microtubule component39 | ↑↑↑↑ | ↑↑↑ |
| NUP188 (nucleoporin 188) | - An essential part of the nuclear pore complex; regulates mRNA and protein transport from nucleus to cytoplasm40 | ↑↑↑↑ | ↑↑ |
| SRSF3 (serine/arginine-rich splicing factor 3 or SRp20) | - Inhibits cellular senescence and promotes cell growth41 | ↑↑↑↑ | ↑↑ |
| IDE (insulin-degrading enzyme) |
- Dampens the microglial response to oxidative stress and may reduce the vicious cycle of neuroinflammation. However, it does not influence Aβ degradation42 - Promotes proliferation of SH-SY5Y cells and microglia42 |
↑ | ↔ |
| NDUFB6 (NADH: ubiquinone oxidoreductase subunit B6) | - Subunit of mitochondrial complex I; it is depleted in patients with both early- and late-onset AD within the medial frontal gyrus—a brain region associated with spatial memory retrieval43 | ↑↑ | ↑ |
| GNAQ (guanine nucleotide-binding protein G(q) subunit alpha) |
- Highly expressed in the mouse hippocampus and decreases with age44 - Involved in synaptic formation, upregulation of genes associated with memory formation, and improvement in memory performance in both aged mice and Caenorhabditis elegans models44 - Promotes antioxidant and antiapoptotic activities in H2O2-treated SH-SY5Y cells45 |
↑ | ↔ |
| PSMB3 (proteasome 20 S subunit beta 3) |
- Involved in the ubiquitin–proteasome pathway46 - Associated with LTP formation by degrading transcriptional suppressors and facilitating CREB-dependent gene transcription46 - Its expression is reduced in AD and PD47 |
↑ | ↔ |
Table 2.
Proteins with significantly higher expression in cells treated with MDS + ASS than in those treated with ECa 233.
| Protein | Function | Protein expression (compared with the control) | |
|---|---|---|---|
| ECa | MDS + ASS | ||
| CAMK2A (calcium/calmodulin-dependent protein kinase II alpha) |
- A multifunctional serine/threonine kinase highly expressed in neurons48 - Involved in neurotransmitter synthesis, synaptic organization, synaptic plasticity, and LTP48 |
↔ | ↑↑↑ |
| NDUFA11 (NADH: ubiquinone oxidoreductase subunit A11) | - A subunit of mitochondrial complex I49 | ↑ | ↑↑↑↑ |
| PRKAA1 (protein kinase AMP-activated catalytic subunit alpha 1) |
- A catalytic subunit of AMP-activated protein kinase (AMPK); promotes gastric cancer cell proliferation via JNK1 and Akt pathways21 - AMPK senses cellular energy status, maintains homeostasis, and enhances mitochondrial respiration during synaptic transmission50 |
↓↓↓ | ↑ |
| SEH1L (SEH1-like nucleoporin) |
- A component of nuclear pore complexes51 - Important for oligodendrocyte proliferation and myelination51 - Plays a key role in neuronal development and neurogenesis in mice52 |
↑↑ | ↑↑↑↑ |
Table 3.
Biological activities of prominent proteins following treatment with ECa 233 and MDS + ASS.
| Biological activity | Protein | |
|---|---|---|
| ECa 233 | MDS + ASS | |
| Mitochondrial activity | CYC1, BID, COX6B1, NDUFS6, NDUFB6 | NDUFA11 and PRKAA1 |
| Cytoskeletal stability | TUBA1B, TUBB2A | |
| Synaptic activity | GNAQ | CAMK2A |
| Post-transcriptional modification | SRSF3 | |
| Nucleocytoplasmic transport of macromolecules (such as transcriptional protein, RNA) | NUP188 | SEH1L |
| Protein degradation | IDE, PSMB3 | |
| Cell proliferation | IDE, SRSF3 | PRKAA1, SEH1L |
Enhanced expression of synaptic-related proteins in differentiated SH-SY5Y cells induced by both ECa 233 and the MDS + ASS mixture
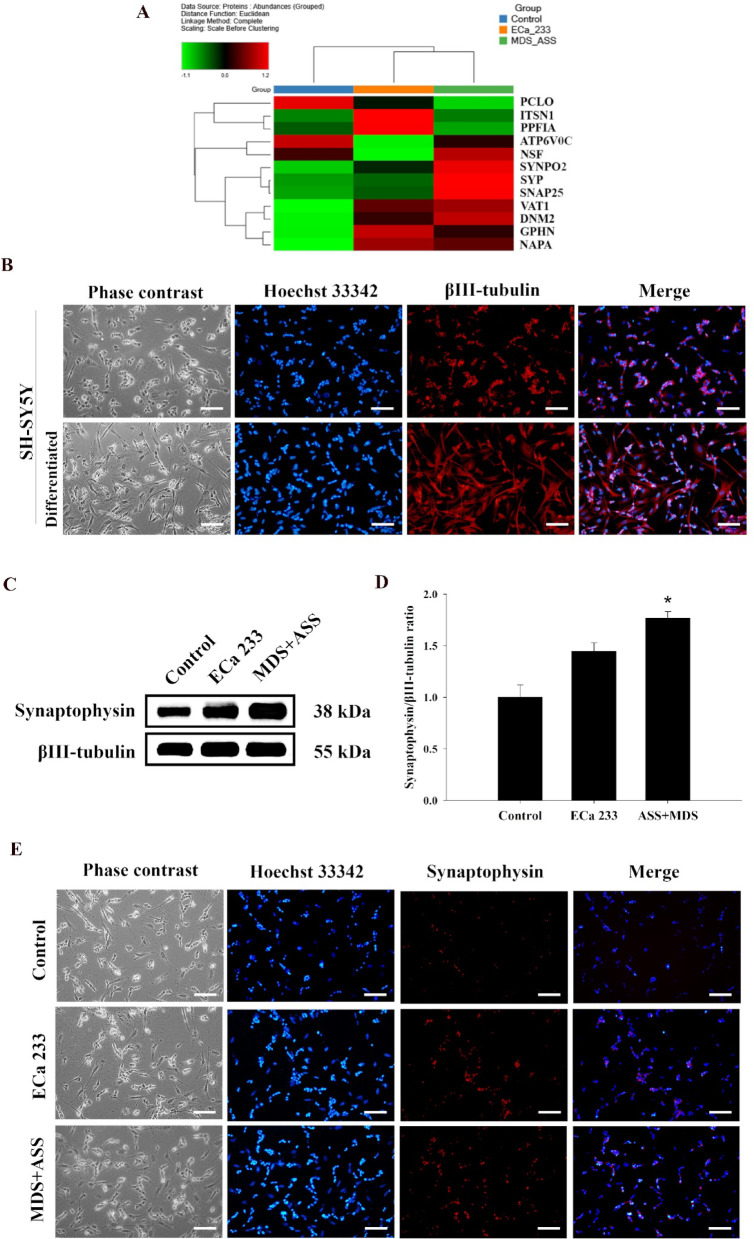
Proteomic analysis revealed that both ECa 233 and the MDS + ASS mixture altered the expression of 2,241 identified master proteins in distinct patterns. In total, 12 synaptic proteins (PCLO, ITSN1, PPFIA, ATP6V0C, NSF, SYNPO2, SYP, SNAP25, VAT1, DNM2, GPHN, and NAPA) were identified. Their relative abundances are shown in a heatmap (Fig. 3A; Table 4) and their normalized abundance values are provided in Supplementary File 1C.
Fig. 3.
Effect of ECa 233 and the MDS+ASS mixture on the expression of synaptic markers in differentiated SH-SY5Y cells. (A) Heatmap of synaptic proteins identified through proteomic analysis. (B) Immunofluorescence imaging of βIII-tubulin in differentiated and undifferentiated SH-SY5Y cells (scale bar= 100 µm). (C, D) Western blot analysis of synaptophysin expression in differentiated SH-SY5Y cells from the control, ECa 233, and MDS+ASS groups. Data are expressed as means ± SEM (n = 4) and normalized to βIII-tubulin before group comparisons (*p < 0.05 vs. control group). (E) Immunofluorescence imaging of synaptophysin in differentiated SH-SY5Y cells from the control, ECa 233, and MDS+ASS groups (scale bar = 100 µm).
Table 4.
Top 12 synaptic proteins and their roles in synaptic transmission.
| Protein | Function | Protein expression (compared with the control) | |
|---|---|---|---|
| ECa | MDS + ASS | ||
| PCLO (piccolo presynaptic cytomatrix protein, also known as aczonin) |
- Involved in the endo- and exocytosis of synaptic vesicles at presynaptic active zones53 - Involved in hippocampal LTP and spatial learning and memory54 |
↓ | ↓↓ |
| ITSN1 (intersectin 1) |
- Involved in the endocytosis of glutamate receptor 1, a non-NMDA receptor homolog55 - Involved in dendritic spine formation, hippocampal LTP, and spatial learning and memory16 |
↑ | ↔ |
| PPFIA (PPFIA-binding protein) | - Involved in presynaptic bouton formation, synaptic vesicular docking, and neurotransmitter release17 | ↑ | ↔ |
| ATP6V0C (V-type proton ATPase 16-kDa proteolipid subunit c) | - Regulates the pH gradient across synaptic vesicle membranes, which is important for neurotransmitter uptake56 | ↓↓ | ↓ |
| NSF (N-ethylmaleimide-sensitive fusion protein, NEM-sensitive fusion protein, or vesicular-protein NSF) |
- Regulates exocytosis by disassembling the SNARE complex after vesicle fusion57 - Involved in AMPA receptor–mediated synaptic transmission58 |
↓ | ↑ |
| SYNPO2 (synaptopodin 2) | - Involved in mitophagy (degradation of dysfunctional mitochondria)59 | ↑ | ↑↑ |
| SYP (synaptophysin) | - Involved in the endo- and exocytosis of synaptic vesicles and synaptic formation60,61 | ↔ | ↑ |
| SNAP25 (synaptosomal-associated protein 25) |
- Supports postsynaptic compartment stability and dendritic spine formation62 - Promotes NMDAR surface insertion during synaptic transmission63 |
↔ | ↑ |
| VAT1 (vesicle amine transport 1) |
- Involved in dopamine biosynthesis and synaptic release64 - Involved in axonal growth in DRG neurons65 - Works with mitofusin to modulate mitochondrial fusion66 |
↑ | ↑ |
| DNM2 (dynamin 2) | - Involved in synaptic vesicle endocytosis, postsynaptic membrane protein turnover, and neurite outgrowth67 | ↑ | ↑↑ |
| GPHN (gephyrin) | - Anchors the γ-aminobutyric acid type A (GABAA) and glycine receptors at inhibitory synapses and modulates inhibitory synaptic transmission68 | ↑↑ | ↑ |
| NAPA (alpha-soluble NSF attachment protein or α-SNAP) | - Cooperates with NSF to mediate SNARE complex assembly and disassembly69 | ↑ | ↑ |
Synaptophysin, a key protein in the synaptic vesicle machinery and commonly used as a presynaptic marker, was selected to elucidate protein expression in terms of localization and expression levels. Immunofluorescence labeling of differentiated SH-SY5Y cells showed enhanced synaptophysin expression in the MDS + ASS mixture group (Fig. 3B and E). This finding was consistent with both the proteomic data and western blot analysis results. Western blot analysis of four biologically independent samples revealed that the MDS + ASS group exhibited increased synaptophysin expression, with a ratio of 1.77 ± 0.06:1 compared with the control group [F(2,9) = 11.626, p = 0.015] (Fig. 3C-3D).
Enhancement of mitochondrial functions in differentiated SH-SY5Y cells induced by ECa 233 and the MDS + ASS mixture
According to the previous proteomic analysis, mitochondrial oxidative phosphorylation (OXPHOS) showed an increase, indicating its important role in cellular energy generation. The oxygen consumption rate (OCR) is a potential indicator of mitochondrial function, as OXPHOS consumes nearly all the oxygen in cells to produce ATP8. In this study, data were collected from four biologically independent samples, with each sample analyzed in three technical replicates (Figs. 4A and 4B). Compared with the control group, both the ECa 233 and MDS + ASS mixture groups exhibited significantly increased basal respiration [F(2,9) = 7.795; ECa 233: p = 0.003; MDS + ASS: p = 0.016], ATP generation [F(2,9) = 4.943; ECa 233: p = 0.019; MDS + ASS: p = 0.018], and maximal respiration [F(2,9) = 16.045; ECa 233: p = 0.006; MDS + ASS: p = 0.000]. Notably, the MDS + ASS mixture group exhibited higher maximal respiration than the ECa 233 group. Interestingly, among the three groups, only the MDS + ASS group showed a significant increase in spare respiratory capacity (SRC) [F(2,9) = 11.444; ECa 233: p = 0.589; MDS + ASS: p = 0.001].
Fig. 4.
ECa 233 and the MDS+ASS mixture enhance mitochondrial activity in differentiated SH-SY5Y cells. (A) Mitochondrial respiration profile of differentiated SH-SY5Y cells treated with ECa 233 or the MDS+ASS mixture. (B) Quantification of basal respiration, ATP production, maximal respiration, and spare respiratory capacity. Data are presented as mean ± SEM (n = 4, triplicate). *p < 0.05 compared with the control group; #p < 0.05 compared with the ECa 233 group.
mTOR signaling is activated by ECa 233 and the MDS + ASS mixture, thereby modulating mitochondrial and synaptic-related protein expression
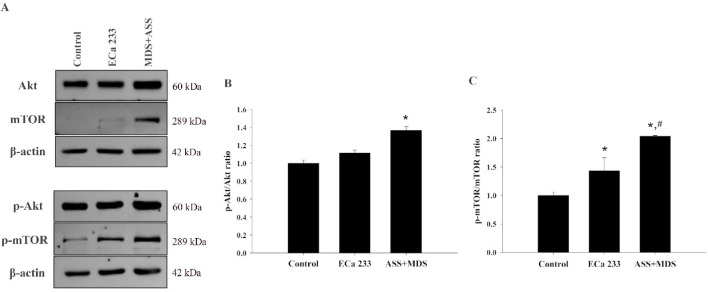
Akt/mTOR signaling plays a crucial role in regulating various neuronal functions, including cell proliferation, survival, and apoptosis. Protein expression data related to this signaling pathway were obtained from four biologically independent samples (Figs. 5A-5C). Activation of mTOR signaling was significantly observed in both the ECa 233 and MDS + ASS groups compared with the control group (ratios of 1.43:1 and 2.04:1, respectively) [F(2,9) = 17.718; ECa 233: p = 0.049; MDS + ASS: p = 0.001]. However, only the MDS + ASS group showed a significantly increased p-Akt/Akt ratio relative to the control group (ratio of 1.37:1) [F(2,9) = 6.348; ECa 233: p = 0.315; MDS + ASS: p = 0.013].
Fig. 5.
Akt/mTOR signaling protein expression in differentiated SH-SY5Y cells following treatment with ECa 233 and the MDS+ASS mixture.Western blot analysis of Akt, p-Akt, mTOR, and p-mTOR expression in the control, ECa 233, and MDS+ASS groups. (A) Representative western blot images for each protein. (B) Densitometric analysis of protein expression levels. Data are presented as mean ± SEM (n = 4) and were normalized to βIII-tubulin before group comparisons. *p < 0.05 vs. control group; #p < 0.05 vs. ECa 233 group.
ECa 233 and the MDS + ASS mixture have no impact on basal synaptic transmission
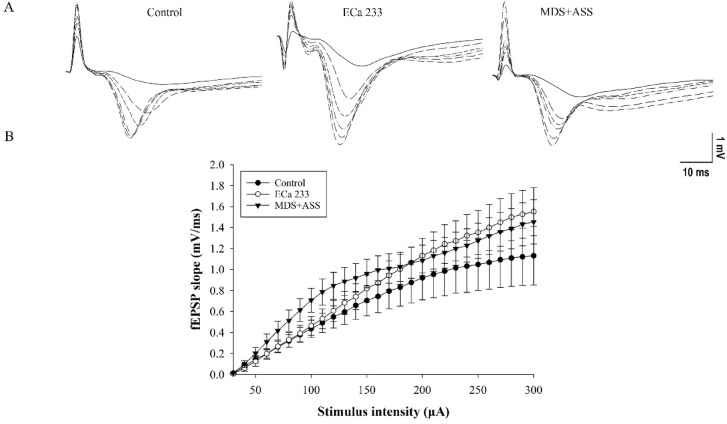
Input–output (I-O) recordings were used to assess basal synaptic transmission. The field excitatory postsynaptic potential (fEPSP) slopes were expected to increase with greater stimulus intensities. ECa 233 and the MDS + ASS mixture were dissolved in 0.01% DMSO to obtain the final artificial cerebrospinal fluid (aCSF) concentrations: 10 µg/mL of ECa 233 and 5 µg/mL of MDS mixed with 3.6 µg/mL of ASS. The tested substances were perfused onto the hippocampal slices throughout the experiment. Results from six biologically independent samples showed that the substances had no significant effect on the interaction between group and stimulus intensity [F(10,90) = 0.210, p = 0.995]. However, the fEPSP slopes significantly differed across stimulus intensities [F(5,90) = 42.380, p < 0.001] (Fig. 6). Therefore, neither of the tested substances altered the basal synaptic transmission in the Schaffer collateral pathway.
Fig. 6.
ECa 233 and the MDS+ASS mixture did not alter basal synaptic transmission. (A) Input–output curves recorded from hippocampal slices following stimulation at intensities of 30–300 µA. (B) Representative fEPSP traces recorded at various stimulus intensities. Data are presented as mean ± SEM (n = 6).
ECa 233 requires the synergistic effect of the equivalent mixture of MDS and ASS for synaptic enhancement
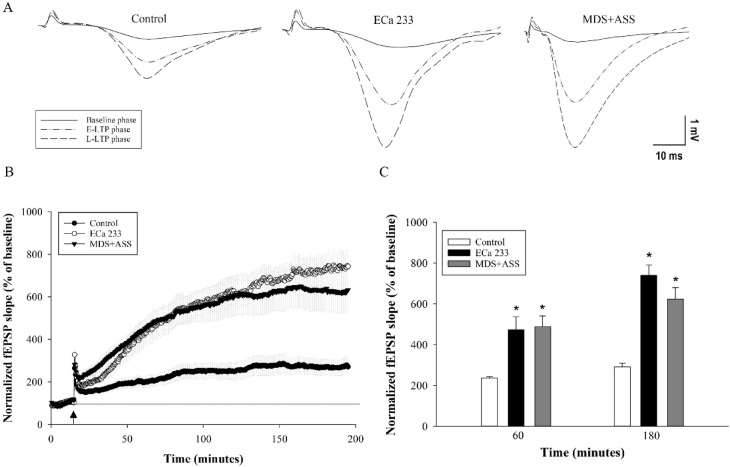
To validate the effects of the active components of ECa 233, hippocampal slices were perfused with either ECa 233 or the MDS + ASS mixture at the same concentrations found in ECa 233. Results from six biologically independent samples revealed significant differences in fEPSP levels between the control group and both the MDS + ASS and ECa 233 groups at 60 and 180 min after high-frequency stimulation (HFS) [group effect: F(2,30) = 16.336, p < 0.001; time effect: F(1,30) = 31.980, p < 0.001; group × time interaction: F(2,30) = 2.679, p = 0.085]. The least significant difference (LSD) post hoc test showed no significant difference in fEPSP levels between the ECa 233 and MDS + ASS groups at both 60 and 180 min after HFS (p = 0.828 and p = 0.082, respectively) (Fig. 7).
Fig. 7.
Synaptic enhancement by ECa 233 requires triterpenoid glycosides (MDS and ASS). (A) Representative fEPSP traces recorded before high-frequency stimulation (HFS) and at 60 min (early LTP) and 180 min (late LTP) after HFS. (B) Time course of synaptic responses in the ECa 233 and MDS+ASS groups, showing comparable enhancement. The black dotted line indicates baseline percentage values before HFS. The arrow marks the time point of HFS application. (C) Average fEPSP slopes during the last 10 min at 60 and 180 min after HFS. Data are presented as mean ± SEM (n = 6). *p< 0.05 compared with the control group.
The control group showed LTP expression at 60 and 180 min after HFS as 236.53% ± 5.84% and 291.91% ± 17.07%, respectively. ECa 233 enhanced fEPSP levels to 473.78% ± 63.54% and 741.33% ± 50.19%, whereas the MDS + ASS mixture increased fEPSP levels to 488.15% ± 52.83% and 623.51% ± 56.43%, respectively. Based on these results, triterpenoid glycosides (MDS and ASS at concentrations equivalent to those in ECa 233) are required for the strong synaptic-enhancing activity of ECa 233. Moreover, both ECa 233 and the MDS + ASS mixture demonstrated rapid synaptic-enhancing effects by promoting both early and late phases of LTP at the CA3–CA1 synapses of the hippocampus.
Discussion
Previous studies have shown that ECa 233, a standardized extract of C. asiatica, enhances memory retention and increases the expression of synaptic plasticity–associated proteins in normal rats4. In this study, both ECa 233 and its active components (MDS + ASS mixture) altered protein expression in distinct patterns. The MDS + ASS group exhibited predominantly enhanced mitochondrial function and may exert notable neuroenhancement effects. Nonetheless, the MDS + ASS mixture demonstrated synaptic plasticity potential comparable to that of ECa 233, as evidenced by LTP recordings.
Using proteomic analysis, this study investigated the protein profiles of substance-treated differentiated SH-SY5Y cells, which have been proposed to represent a neuronal phenotype9. Our findings showed that ECa 233 and the MDS + ASS mixture altered the expression of numerous proteins, predominantly located in the cytosol and nucleus, respectively. Proteins such as GNAQ, PSMB3, CAMKII, SYP, ITSN1, and SNAP25 play significant roles in cell organization, organelle biogenesis, and nucleic acid binding activity, thereby supporting gene transcription and protein translation, which are key steps in neuronal plasticity (Tables 1, 2, 3 and 4).
ECa 233 and the MDS + ASS mixture also altered the expression of numerous proteins linked to neurodegenerative pathways such as AD, Huntington’s disease, PD, and prion disease, all of which are associated with memory impairment. Many of these proteins, including NDUFS6 and NDUFA11, are closely associated with the mitochondrial respiratory chain, particularly complexes I, III, and IV. These complexes play a critical role in ATP production. Mitochondria-derived ATP is essential for maintaining membrane potential, generating action potentials, and supporting synaptic transmission by supplying energy for ion channels and neurotransmitter release10. Beyond energy production, mitochondria are crucial for regulating calcium (Ca2+) homeostasis, a vital process for proper neuronal signaling11. A previous study in Drosophila melanogaster demonstrated that complex I dysfunction led to reduced synaptic transmission and impaired neural plasticity at the Drosophila neuromuscular junction12. Additionally, mitochondria play an essential role in neurotransmitter synthesis. The production of acetylcholine—a key neurotransmitter involved in learning and memory—depends on acetyl coenzyme A, which is generated from pyruvate, the end product of mitochondrial glycolysis13. Neuronal glutamate, another critical neurotransmitter involved in LTP, is synthesized from its precursor glutamine, which is produced by mitochondria within astrocytes14. Consequently, mitochondrial dysfunction can contribute to impairments in learning and memory.
Further investigation of mitochondrial function using the Seahorse assay revealed that both ECa 233 and the MDS + ASS mixture enhanced the mitochondrial capacity for ATP generation. This improvement is consistent with the observed increase in ATP production and maximal respiration, enabling cells to meet their energy demands more effectively. However, at the tested concentration of ECa 233, which contains the same amounts of MDS and ASS as in the MDS + ASS mixture, only the MDS + ASS mixture significantly enhanced mitochondrial function under stress conditions, as evidenced by a significant increase in SRC. The increase in ATP production, however, led to mitochondrial hyperpolarization and a subsequent increase in reactive oxygen species production, which can have detrimental effects15. Examination of the proteomic data revealed that ECa 233 enhanced the expression of BID (a proapoptotic protein) and PSMB3 (a subunit of the 20S proteasome). This mechanism may serve to mitigate mitochondrial dysfunction during stress, potentially leading to a reduction in SRC.
Proteomic analysis of synaptic proteins showed that the expression of synaptophysin and SNAP25, key presynaptic proteins involved in synapse formation16, was significantly enhanced following treatment with the MDS + ASS mixture. However, no such changes were observed in the ECa 233 group. Conversely, ECa 233 promoted the expression of ITSN1, which contributes to LTP formation, PPFIA, which is involved in presynaptic bouton formation17, and GPHN, which may support the anxiolytic activity of ECa 233 by modulating inhibitory synaptic transmission18.
The MDS + ASS mixture contains the two major active ingredients of the ECa 233 extract; however, its pharmacological actions are not identical to those of the full extract. This difference may be attributed to the remaining 15% of constituents in the ECa 233 extract, indirectly highlighting their potential role in contributing to ECa 233’s synaptic enhancement effects. These remaining components might interact with the main constituents (MDS and ASS) or exert independent effects that modify or confound the primary actions of MDS and ASS. This hypothesis is supported by findings showing that ECa 233 exhibits different pharmacokinetics compared with the MDS + ASS mixture19.
The different patterns of synaptic protein expression observed between ECa 233 and the MDS + ASS mixture may be attributed to the activation of the Akt/mTOR signaling pathway, which promotes the translation of various synaptic proteins involved in neurogenesis, synapse formation, and processes related to learning and memory20. Activation of the Akt/mTOR pathway was predominantly observed in the MDS + ASS mixture group. One proposed mechanism, based on our proteomic profile, suggests that Akt activation may be driven by increased levels of PRKAA1, a protein that acts as an energy sensor to maintain energy homeostasis by influencing mitochondrial function21.
However, the activation of the Akt/mTOR signaling pathway following ECa 233 treatment was unexpected. ECa 233 altered the expression of the mTOR protein but did not affect that of Akt (Fig. 6B). The mTOR activation by ECa 233 may result from crosstalk with the Ras–ERK signaling pathway, which enhances cell survival and proliferation via the actions of RSK and TSC1/2, respectively22. Another pathway that may interact with ERK and Akt/mTOR is focal adhesion kinase (FAK) signaling. Activation of FAK signaling mediates the phosphorylation of both ERK and PI3K/Akt, which in turn promotes the transcription of various synaptic proteins, including synaptophysin, glutamate receptor 1 (GluR1), and synapsin II23. Therefore, ECa 233 and its active compounds may also influence this signaling pathway, as indicated by the expression of synaptophysin and intersectin 1 (a protein relevant to GluR1). Moreover, the synaptic functions promoted by mTOR may be associated with mitochondrial SRC enhancement, as inhibition of this pathway has been shown to reduce SRC levels24. Thus, activation of mTOR may contribute to increased mitochondrial SRC.
Nevertheless, both ECa 233 and the MDS + ASS mixture similarly enhanced synaptic function in hippocampal slices. Our findings highlighted the performance of ECa 233 and the active compound mixture in two key aspects: the rapid onset of action and strong synaptic enhancement, as evidenced by late-phase LTP expression. This study demonstrated the synergistic effect of MDS and ASS, with the MDS + ASS mixture producing robust fEPSP magnitudes comparable to those observed with ECa 233. Proteomic analysis revealed distinct patterns of synaptic-related protein expression between the two groups. However, the proteins expressed in both groups were associated with several key biological activities, including mitochondrial function (promoting ATP production and synapse stability), cytoskeletal stability (facilitating axonal transport and ion channel trafficking), nucleocytoplasmic transport (supporting DNA transcription via protein synthesis), and cell proliferation. These biological functions likely interact and contribute to the regulation of synaptic plasticity.
ECa 233 and the MDS + ASS mixture influence the protein profiles of differentiated SH-SY5Y cells. Both treatments alter mitochondrial function and the expression of synaptic-related proteins, each with distinct expression patterns. Increased hippocampal LTP serves as a potential indicator of these effects. Notably, this study is the first to provide evidence of the synergistic activity between MDS and ASS in enhancing synaptic plasticity. Additionally, it reveals the proteomic profiles of ECa 233 and the MDS + ASS mixture. Several altered proteins identified through proteomic analysis are associated with synaptic plasticity and may serve as promising targets for future research on memory-enhancing compounds.
Thus, this work emphasizes the pivotal role of ECa 233 and its major components in synaptic enhancement, which may have implications for the development of therapies for neurological diseases in which synaptic dysfunction is a key pathophysiological process.
Methods
Tested compound characteristics
ECa 233, which contains 86% total triterpenoid glycosides with an MDS-to-ASS ratio of 1.5 ± 0.5:1 (50% MDS and 36% ASS), was obtained from Siam Herbal Innovation Ltd., Thailand (lot number MRA051401). Standardized compounds—MDS (≥ 95%, HPLC, product number M6949) and ASS (≥ 98.5%, HPLC, product number 43191)—and DMSO (product number D8418) were purchased from Sigma-Aldrich.
SH-SY5Y cell culture and differentiation
The human neuroblastoma cell line SH-SY5Y (ATCC–CRL-2266), purchased from the American Type Culture Collection (ATCC), was cultured in Modified Essential Medium (MEM) supplemented with 1% GlutaMAX™, 2% MEM amino acid solution (50X), 10% fetal bovine serum, and 100 U/mL penicillin–streptomycin (Gibco, Thermo Fisher Scientific) in an incubator maintained at 37 °C with 5% CO2 in a humidified atmosphere. SH-SY5Y cells were seeded in 6-well plates (5 × 105 cells/well for protein identification and western blot analysis, and 3 × 105 cells/well for immunofluorescence staining) and cultured for 48 h. Differentiation was induced by treatment with 10 µM retinoic acid (Sigma-Aldrich) in medium containing 1% fetal bovine serum for 4 days. On day 5, the cells were treated with 0.01% DMSO, 10 µg/mL ECa 233, or 5 µg/mL MDS mixed with 3.6 µg/mL ASS for 24 h. The concentrations of ECa 233, MDS, and ASS were based on the effective dose of ECa 233 reported by Boondam et al. in 20215.
Protein identification via liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis
Protein extraction from cell samples
After treatment of the differentiated SH-SY5Y cells, as described previously, the cells were collected and lysed via sonication. Protein lysates were prepared for proteomic analysis using the EasyPep™ Mini MS Sample Prep Kit (A40006, Thermo Fisher Scientific), following the manufacturer’s instructions. Briefly, protein concentrations of the cell lysates were determined using the Pierce™ BCA Protein Assay Kit (23225, Thermo Fisher Scientific) and adjusted to 1 µg/µL in a final volume of 100 µL. Reduction and alkylation solutions were added, and the mixtures were incubated at 95 °C for 10 min. After cooling to room temperature, the trypsin/Lys-C protease mixture was added, and the samples were incubated at 37 °C for 3 h to digest the proteins. Digestion was stopped by adding the Digestion Stop Solution. The digested protein samples were transferred to Peptide Clean-Up Columns for washing, using the provided wash solution and centrifugation at 1,500 × g for 2 min, repeated for a total of three cycles. The columns were then placed in new 2-mL microcentrifuge tubes, and Elution Solution was added. After centrifugation at 1,500 × g for 2 min, the cleaned peptide samples were collected and dried via vacuum centrifugation25. Finally, samples were reconstituted in 0.1% formic acid (Fisher Scientific) in water for LC-MS/MS analysis.
Proteomic analysis with LC-MS/MS
LC-MS/MS analysis was performed using the RSLCnano MS system coupled to the Orbitrap Exploris™ 480 mass spectrometer, equipped with a PepMap™ RSLC C18 column (2 μm, 100 Å, 75 μm × 25 cm) (Thermo Fisher Scientific). The analytical method was adapted from a previously established procedure26. Briefly, the elution gradient began with 3% mobile phase B (0.1% formic acid in acetonitrile; product number A117-50, Fisher Scientific) maintained for 7 min. It was then increased to 6% B over 1 min, followed by a gradual increase to 40% B over 35 min. The gradient was subsequently raised to 80% B within 1 min and held for 4 min. Mobile phase A consisted of 0.1% formic acid in water. The flow rate was maintained at 0.4 µL/min. A voltage of 1.2 kV was applied to the nanospray ion source, with the ion transfer tube temperature set at 280 °C. MS1 data were acquired at an Orbitrap resolution of 120,000 over a scan range of 350–1200 m/z, using high-field asymmetric waveform ion mobility spectrometry (FAIMS) Pro with a compensation voltage of − 60 V and the RF lens set at 50%27. MS2 scans were performed at an Orbitrap resolution of 15,000 using higher-energy collisional dissociation with a collision energy of 27% and an isolation window of 1.2 m/z.
The LC-MS/MS files were subjected to proteomic analysis using Proteome Discoverer software version 2.5.0.400, with strict and relaxed target false discovery rates for peptide-spectrum matches set at 0.01 and 0.05, respectively. Contaminant proteins were excluded using the protein contaminant list provided by the Max Planck Institute of Biochemistry, Martinsried (https://www.biochem.mpg.de/en). Enrichment analysis was performed using Enrichr28–30 on August 21, 2023 (accessible at https://maayanlab.cloud/Enrichr/). Pathway analysis was conducted using the KEGG 2021 Human database (https://www.genome.jp/kegg/pathway.html), Enrichr-KG (https://maayanlab.cloud/enrichr-kg), and Appyters (https://appyters.maayanlab.cloud/#/)31,32. The raw proteomics files were deposited in the ProteomeXchange Consortium via the PRIDE partner repository under the dataset accession code PXD057572.
Western blot analysis
After the specific treatments, cellular protein lysates were prepared by incubating the cells on ice for 30 min in RIPA lysis buffer (Sigma-Aldrich) containing protease and phosphatase inhibitor cocktails (Roche Molecular Biochemicals). The lysates were centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was collected, and protein concentrations were measured using the Qubit Protein Assay Kit (33211, Thermo Fisher Scientific). Equal amounts of protein from each sample were mixed with 4× Laemmli sample buffer and denatured by heating at 95 °C for 5 min. Each sample was loaded onto a 12% SDS–polyacrylamide gel for electrophoresis and then transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (25 mM Tris–HCl [pH 7.5], 125 mM NaCl, 0.1% Tween-20) for 1 h. Following blocking, membranes were incubated overnight at 4 °C with primary antibodies against synaptophysin (1:1000, #36406, Cell Signaling Technology), PI3K (1:1000, #4257, Cell Signaling Technology), Akt (1:1000, #4685, Cell Signaling Technology), p-Akt (1:1000, #4060, Cell Signaling Technology), mTOR (1:1000, #2972s, Cell Signaling Technology), p-mTOR (1:1000, #2971, Cell Signaling Technology), β-actin (1:10000, ab8226, Abcam), or βIII-tubulin (1:10000, ab215037, Abcam). The membranes were then washed and incubated with either secondary anti-mouse (1:10000, ab6789, Abcam) or anti-rabbit (1:10000, ab6721, Abcam) antibodies. Detection of antibody–protein complexes was performed using a chemiluminescent substrate (SuperSignal™ West Pico PLUS Chemiluminescent Substrate, 34579, Thermo Fisher Scientific) and visualized with the ImageQuant LAS4000 system (GE Healthcare Life Sciences). Band intensities were quantified using ImageJ software.
Immunofluorescence staining
After treatment, the cells were washed three times with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min at room temperature. They were then blocked with a solution containing 5% bovine serum albumin (Sigma-Aldrich) and 0.1% Triton X-100 (Merck) for 1 h at room temperature. Following blocking, the cells were washed and incubated overnight at 4 °C with primary antibodies against synaptophysin (1:200, #36406, Cell Signaling Technology) or βIII-tubulin (1:1000, ab215037, Abcam). After primary antibody incubation, the cells were washed with PBS and incubated for 2 h at room temperature with fluorescent-conjugated secondary antibodies: anti-rabbit IgG (H + L), F(ab’)2 fragment Alexa Fluor® 594 conjugate (1:500, #8889, Cell Signaling Technology); anti-rabbit IgG (H + L), F(ab’)2 fragment Alexa Fluor® 488 conjugate (1:500, #4412, Cell Signaling Technology); or anti-mouse IgG (H + L), F(ab’)2 fragment Alexa Fluor® 488 conjugate (1:500, #4408, Cell Signaling Technology). The nuclei were counterstained with 10 µg/mL Hoechst 33,342 (H3570, Thermo Fisher Scientific) for 15 min at room temperature. Images were captured using an Olympus fluorescence microscope (IX83, Evident Life Sciences) equipped with CellSens imaging software (Olympus CellSens Dimension 3.1.1).
Mitochondrial function analysis
OCR and other bioenergetic parameters were assessed using the Seahorse XFe96 Extracellular Flux Analyzer and the Cell Mito Stress Kit (103015-100, Agilent Technologies). Following cell treatment, the culture medium was replaced with assay medium composed of XF Base Medium, pH 7.4 (103575-100, Agilent Technologies), supplemented with 10 mM glucose, 10 mM pyruvic acid, and 1 mM L-glutamine. OCR was measured at baseline and after the sequential addition of the following compounds: 1 µM oligomycin (an ATP synthase inhibitor), 0.5 µM FCCP (carbonyl cyanide-4-[trifluoromethoxy] phenylhydrazone, an oxidative phosphorylation uncoupler), and 1 µM rotenone + 1 µM antimycin (complex I and III inhibitors).
Hippocampal LTP
Animals
This study used male Wistar rats aged 8 weeks, purchased from Nomura Siam International Co., Ltd. (Thailand). All rats were housed under controlled conditions with constant humidity and temperature, a 12-h light–dark cycle, and free access to food and water. The study was conducted in accordance with the ARRIVE guidelines. All animal protocols adhered to institutional and international standards for the care and use of laboratory animals and were approved by the Animal Ethics Committee of the Faculty of Medicine Siriraj Hospital (approval number: SI-ACUP 010/2563) and the Faculty of Pharmacy (approval number: PYR003/2021), Mahidol University, Thailand.
Hippocampal slice preparation
The rats were deeply anesthetized by inhalation of 5% isoflurane (Forane, USP) and immediately decapitated using a guillotine. The brain was quickly removed and transferred to ice-cold (4 °C) aCSF containing 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.30 mM MgSO4−7H2O, 26 mM NaHCO3, 10 mM D-glucose, and 2.4 mM CaCl2−2H2O, with added sucrose. The aCSF was saturated with carbogen gas (95% O2 and 5% CO2). Transverse hippocampal slices were cut at a thickness of 350 μm. All slices were then transferred to a carbogen-saturated interface chamber at room temperature and allowed to recover for at least 1 h.
Electrophysiology recordings
To assess the synaptic plasticity–enhancing effects of the substances, extracellular fEPSP recordings were performed. A recording electrode filled with aCSF was placed in the dendritic layer of the CA1 region. To stimulate the Schaffer collateral–commissural fibers, a tungsten-stimulating electrode was positioned 1,000 μm away from the recording electrode within the same dendritic layer of the CA1 region (Fig. 8). fEPSPs were recorded using PowerLab equipment and LabChart 7 software (AD Instruments, Dunedin, New Zealand).
Fig. 8.
Experimental design of the electrophysiological study. The upper right panel illustrates the placement of the stimulating and recording electrodes in a hippocampal slice. The tested substances were continuously perfused over the hippocampal slices throughout the experiment. The input–output curve was assessed after 15 min of perfusion using stimulus intensities of 30–300 µA. Baseline activity was recorded for 15 min, followed by high-frequency stimulation to induce long-term potentiation. Synaptic activity was then monitored for 180 min.
Extracellular application of the substances
ECa 233, MDS, and ASS were dissolved in 0.01% DMSO to achieve the following final concentrations in aCSF: 10 µg/mL of ECa 233 and 5 µg/mL of MDS mixed with 3.6 µg/mL of ASS. Based on the treatment group, the test substance (DMSO, ECa 233, or MDS + ASS) was directly perfused onto the hippocampal slices throughout the recording period (Fig. 8) at a flow rate of approximately 1.5 mL/min. The temperature of the solutions was maintained at 31–32 °C throughout the experiment.
I-O recordings
I-O recordings were used to evaluate the effects of the tested substances on basal synaptic transmission at the CA3–CA1 hippocampal synapses. After 15 min of perfusion with the test substance, stimulus intensities of 30–300 µA (1-ms duration) were applied to the Schaffer collateral–commissural fibers. fEPSPs were recorded from the dendritic layer of the CA1 region. The average fEPSP slopes (n = 6) at each stimulus intensity were calculated and analyzed to assess the relationship between stimulus strength and synaptic response. The stimulus intensity that elicited 50% of the maximal response in the I-O recordings was used for subsequent hippocampal LTP recordings.
LTP recordings
The synaptic enhancement properties of the tested substances were assessed using hippocampal LTP recordings, a widely used cellular model for evaluating learning and memory. The stimulus intensity was selected based on the intensity that produced a half-maximal response in the I-O curve. Stable baseline fEPSP values were recorded for 15 min. Subsequently, HFS was applied, consisting of two trains of 100 Hz tetanic stimulation, each delivering 100 pulses, using the same stimulus intensity as in the baseline phase. fEPSPs were recorded continuously for 180 min to assess LTP formation in both the early and late phases. The peak slope of the rising phase of the fEPSPs (1 ms duration) was calculated to evaluate synaptic potentiation.
Statistical analysis
All statistical analyses were performed using SPSS software version 21 (IBM Inc.). Electrophysiological data are presented as the mean ± standard error of the mean (SEM). The Shapiro–Wilk test was used to assess the normality of data distribution. To analyze protein expression and mitochondrial function, data were evaluated using one-way analysis of variance (ANOVA), followed by the LSD post hoc test. For validation of basal synaptic transmission, fEPSP slopes were analyzed using two-way ANOVA followed by the LSD post hoc test. To assess synaptic potentiation after HFS, fEPSP slopes were normalized to the average baseline response and statistically analyzed using the appropriate t-test and two-way ANOVA, followed by the LSD post hoc test. A p-value of < 0.05 was considered statistically significant.
Limitations of the study
Although the number of biological replicates was limited in some experiments, the statistical significance and consistency of the observed effects indicate that the findings are robust. However, we acknowledge this limitation and recommend that future studies replicate these results using larger sample sizes and a broader range of cell models.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful for the grant support from Mahidol University, Thailand. Moreover, this study would not have been possible without the support of the Mahidol-Liverpool Joint Unit for Aging Research, which provided many research instruments.
Author contributions
Conceptualization, Y.B. and N.P.; Methodology, Y.B.; Validation, Y.B. and N.P.; Formal analysis, Y.B.; Electrophysiology setup, S.C. and Y.B.; Electrophysiology investigation, Y.B. and J.R.; Cell culture, Y.B.; Western blotting analysis, Y.B. and K.P.; Mitochondrial function analysis, Y.B. and J.S.; Proteomic analysis, discussion, and writing of the proteomic results, A.M. and M.C.Y.; Immunofluorescence staining, N.S.; Writing—original draft preparation, Y.B.; Writing—review and editing, Y.B., C.C., and N.P.; Visualization, N.P.; Supervision, N.P.; Funding acquisition, Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Mahidol University, Thailand (grant number: A27/2563).
Data availability
The raw proteomics files were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier accession code PXD057572.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hengjumrut, P., Anukunwithaya, T., Tantisira, M. H., Tantisira, B. & Khemawoot, P. Comparative pharmacokinetics between madecassoside and Asiaticoside presented in a standardised extract of Centella asiatica, ECa 233 and their respective pure compound given separately in rats. Xenobiotica48, 18–27. 10.1080/00498254.2016.1273562 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Liu, S. et al. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci. Lett.709, 134386. 10.1016/j.neulet.2019.134386 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Lin, X. et al. Protective effect of madecassoside against cognitive impairment induced by D-galactose in mice. Pharmacol. Biochem. Behav.124, 434–442. 10.1016/j.pbb.2014.07.014 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Boondam, Y. et al. Inverted U-shaped response of a standardized extract of Centella Asiatica (ECa 233) on memory enhancement. Sci. Rep.9, 8404. 10.1038/s41598-019-44867-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boondam, Y., Tantisira, M. H., Tilokskulchai, K., Tapechum, S. & Pakaprot, N. Acute enhancing effect of a standardized extract of Centella Asiatica (ECa 233) on synaptic plasticity: an investigation via hippocampal long-term potentiation. Pharm. Biol.59, 367–374. 10.1080/13880209.2021.1893348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalinratana, N., Meksuriyen, D. & Ongpipattanakul, B. Differences in neuritogenic activity and signaling activation of madecassoside, asiaticoside, and their aglycones in neuro-2a cells. Planta Med.84, 1165–1173. 10.1055/a-0619-5710 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Yin, Z. et al. Asiaticoside attenuates diabetes-induced cognition deficits by regulating PI3K/Akt/NF-κB pathway. Behav. Brain Res.292, 288–299. 10.1016/j.bbr.2015.06.024 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Nolfi-Donegan, D., Braganza, A. & Shiva, S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol.37, 101674. 10.1016/j.redox.2020.101674 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simões, R. F. et al. Refinement of a differentiation protocol using neuroblastoma SH-SY5Y cells for use in neurotoxicology research. Food Chem. Toxicol.149, 111967. 10.1016/j.fct.2021.111967 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Song, N., Mei, S., Wang, X., Hu, G. & Lu, M. Focusing on mitochondria in the brain: from biology to therapeutics. Transl Neurodegener. 13, 23. 10.1186/s40035-024-00409-w (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte, F. V., Ciampi, D. & Duarte, C. B. Mitochondria as central hubs in synaptic modulation. Cell. Mol. Life Sci.80, 173. 10.1007/s00018-023-04814-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallik, B. & Frank, C. A. Roles for mitochondrial complex I subunits in regulating synaptic transmission and growth. Front. Neurosci.16, 846425. 10.3389/fnins.2022.846425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronowska, A. et al. The regulatory effects of acetyl-coa distribution in the healthy and diseased brain. Front. Cell. Neurosci.12, 169. 10.3389/fncel.2018.00169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuellar-Santoyo, A. O. et al. Revealing the contribution of astrocytes to glutamatergic neuronal transmission. Front. Cell. Neurosci.16, 1037641. 10.3389/fncel.2022.1037641 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttemann, M. et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion11, 369–381. 10.1016/j.mito.2011.01.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malakooti, N. et al. The long isoform of intersectin-1 has a role in learning and memory. Front. Behav. Neurosci.10.3389/fnbeh.2020.00024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul, M. S. et al. A syndromic neurodevelopmental disorder caused by rare variants in PPFIA3. Am. J. Hum. Genet.111, 96–118. 10.1016/j.ajhg.2023.12.004 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanasuntronwong, A., Tantisira, M. H., Tantisira, B. & Watanabe, H. Anxiolytic effects of standardized extract of Centella Asiatica (ECa 233) after chronic immobilization stress in mice. J. Ethnopharmacol.143, 579–585. 10.1016/j.jep.2012.07.010 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Anukunwithaya, T., Tantisira, M. H., Tantisira, B. & Khemawoot, P. Pharmacokinetics of a standardized extract of Centella Asiatica ECa 233 in rats. Planta Med.83, 710–717. 10.1055/s-0042-122344 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y., Guan, W., Wang, M. L. & Lin, X. Y. PI3K-AKT/mTOR signaling in psychiatric disorders: a valuable target to stimulate or suppress?. Int. J. Neuropsychopharmacol.10.1093/ijnp/pyae010 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, Y. et al. PRKAA1 promotes proliferation and inhibits apoptosis of gastric cancer cells through activating JNK1 and Akt pathways. Oncol. Res.28, 213–223. 10.3727/096504019x15668125347026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza, M. C., Er, E. E. & Blenis, J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci.36, 320–328. 10.1016/j.tibs.2011.03.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karanian, D. A., Brown, Q. B., Makriyannis, A. & Bahr, B. A. Blocking cannabinoid activation of FAK and ERK1/2 compromises synaptic integrity in hippocampus. Eur. J. Pharmacol.508, 47–56. 10.1016/j.ejphar.2004.12.009 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Marchetti, P., Fovez, Q., Germain, N., Khamari, R. & Kluza, J. Mitochondrial spare respiratory capacity: mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J.34, 13106–13124. 10.1096/fj.202000767R (2020). [DOI] [PubMed] [Google Scholar]
- 25.Varnavides, G. et al. In search of a universal method: a comparative survey of bottom-up proteomics sample Preparation methods. J. Proteome Res.21, 2397–2411. 10.1021/acs.jproteome.2c00265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stejskal, K., Op de Beeck, J., Dürnberger, G., Jacobs, P. & Mechtler, K. Ultrasensitive NanoLC-MS of subnanogram protein samples using second generation micropillar array LC technology with orbitrap exploris 480 and FAIMS PRO. Anal. Chem.93, 8704–8710. 10.1021/acs.analchem.1c00990 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong, R. H., Raederstorff, D. & Howe, P. R. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients10.3390/nu8070425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform.14, 128. 10.1186/1471-2105-14-128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie, Z. et al. Gene set knowledge discovery with enrichr. Curr. Protoc.1, e90. 10.1002/cpz1.90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res.44, W90–97. 10.1093/nar/gkw377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evangelista, J. E. et al. Enrichr-KG: bridging enrichment analysis across multiple libraries. Nucleic Acids Res.51, W168–W179. 10.1093/nar/gkad393 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke, D. J. B. et al. Appyters: turning Jupyter notebooks into data-driven web apps. Patterns2, 100213. 10.1016/j.patter.2021.100213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, Z. et al. Roles of neuroglobin binding to mitochondrial complex III subunit cytochrome C1 in oxygen-glucose deprivation-induced neurotoxicity in primary neurons. Mol. Neurobiol.53, 3249–3257. 10.1007/s12035-015-9273-4 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Li, G. et al. CYC1 Silencing sensitizes osteosarcoma cells to TRAIL-induced apoptosis. Cell. Physiol. Biochem.34, 2070–2080. 10.1159/000366402 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Culmsee, C. & Plesnila, N. Targeting bid to prevent programmed cell death in neurons. Biochem. Soc. Trans.34, 1334–1340. 10.1042/bst0341334 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Yang, S., Wu, P., Xiao, J. & Jiang, L. Overexpression of COX6B1 protects against I/R–induced neuronal injury in rat hippocampal neurons. Mol. Med. Rep.19, 4852–4862. 10.3892/mmr.2019.10144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snelleksz, M. & Dean, B. Lower levels of tubulin alpha 1b in the frontal pole in schizophrenia supports a role for changed cytoskeletal dynamics in the aetiology of the disorder. Psychiatry Res.303, 114096. 10.1016/j.psychres.2021.114096 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Armirola-Ricaurte, C. et al. Alternative splicing expands the clinical spectrum of NDUFS6-related mitochondrial disorders. Genet. Med.26, 101117. 10.1016/j.gim.2024.101117 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai, S., Li, J., Wu, Y. & Jiang, Y. De Novo mutations of TUBB2A cause infantile-onset epilepsy and developmental delay. J. Hum. Genet.65, 601–608. 10.1038/s10038-020-0739-5 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Muir, A. M. et al. Bi-allelic loss-of-function variants in NUP188 cause a recognizable syndrome characterized by neurologic, ocular, and cardiac abnormalities. Am. J. Hum. Genet.106, 623–631. 10.1016/j.ajhg.2020.03.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita, K. p53 isoforms in cellular senescence- and ageing-associated biological and physiological functions. Int. J. Mol. Sci.20, 6023. 10.3390/ijms20236023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corraliza-Gomez, M. et al. Insulin-degrading enzyme (IDE) as a modulator of microglial phenotypes in the context of alzheimer’s disease and brain aging. J. Neuroinflamm. 20, 233. 10.1186/s12974-023-02914-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adav, S. S., Park, J. E. & Sze, S. K. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in alzheimer’s disease. Mol. Brain. 12, 8. 10.1186/s13041-019-0430-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson, M. E. et al. Neuronal activation of G(alphaq) EGL-30/GNAQ late in life rejuvenates cognition across species. Cell. Rep.42, 113151. 10.1016/j.celrep.2023.113151 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia, N. et al. Protective role and related mechanism of Gnaq in neural cells damaged by oxidative stress. Acta Biochim. Biophys. Sin. 49, 428–434. 10.1093/abbs/gmx024 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Davidson, K. & Pickering, A. M. The proteasome: a key modulator of nervous system function, brain aging, and neurodegenerative disease. Front. Cell. Dev. Biol.11, 1124907. 10.3389/fcell.2023.1124907 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Cruz, I. & Reynaud, E. Proteasome subunits involved in neurodegenerative diseases. Arch. Med. Res.52, 1–14. 10.1016/j.arcmed.2020.09.007 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Rostas, J. A. P. & Skelding, K. A. Calcium/calmodulin-stimulated protein kinase II (CaMKII): different functional outcomes from activation, depending on the cellular microenvironment. Cells10.3390/cells12030401 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wirth, C., Brandt, U., Hunte, C. & Zickermann, V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta (BBA). 1857, 902–914. 10.1016/j.bbabio.2016.02.013 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Marinangeli, C. et al. AMP-activated protein kinase is essential for the maintenance of energy levels during synaptic activation. iScience9, 1–13. 10.1016/j.isci.2018.10.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, Z. et al. Nucleoporin Seh1 interacts with Olig2/Brd7 to promote oligodendrocyte differentiation and myelination. Neuron102, 587–601e7. 10.1016/j.neuron.2019.02.018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai, W. et al. Nucleoporin Seh1 controls murine neocortical development via transcriptional repression of p21 in neural stem cells. Dev. Cell.59, 482–495e486. 10.1016/j.devcel.2024.01.002 (2024). [DOI] [PubMed] [Google Scholar]
- 53.Fenster, S. D. et al. Interactions between piccolo and the actin/dynamin-binding protein abp1 link vesicle endocytosis to presynaptic active zones*. J. Biol. Chem.278, 20268–20277. 10.1074/jbc.M210792200 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Ibi, D. et al. Piccolo knockdown-induced impairments of Spatial learning and long-term potentiation in the hippocampal CA1 region. Neurochem Int.56, 77–83. 10.1016/j.neuint.2009.09.004 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Glodowski, D. R., Chen, C. C., Schaefer, H., Grant, B. D. & Rongo, C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol. Biol. Cell.18, 4387–4396. 10.1091/mbc.e07-05-0486 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodzeta, A., Kahms, M. & Klingauf, J. The presynaptic v-ATPase reversibly disassembles and thereby modulates exocytosis but is not part of the fusion machinery. Cell. Rep.20, 1348–1359. 10.1016/j.celrep.2017.07.040 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Littleton, J. T. et al. SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc. Natl. Acad. Sci. USA. 98, 12233–12238. 10.1073/pnas.221450198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimune, A. et al. NSF binding to GluR2 regulates synaptic transmission. Neuron21, 87–97. 10.1016/s0896-6273(00)80517-6 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Zheng, Z. & Song, Y. Synaptopodin-2: a potential tumor suppressor. Cancer Cell. Int.23, 158. 10.1186/s12935-023-03013-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon, S. E. & Chapman, E. R. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron70, 847–854. 10.1016/j.neuron.2011.04.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarsa, L. & Goda, Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. U S A. 99, 1012–1016. 10.1073/pnas.022575999 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fossati, G. et al. Reduced SNAP-25 increases PSD-95 mobility and impairs spine morphogenesis. Cell. Death Differ.22, 1425–1436. 10.1038/cdd.2014.227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irfan, M. et al. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci. Rep.9, 6403. 10.1038/s41598-019-42833-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma, Z., Guo, W., Guo, X., Wang, X. & Kang, L. Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc. Natl. Acad. Sci. USA. 108, 3882–3887. 10.1073/pnas.1015098108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia, L. et al. MiR-34a regulates axonal growth of dorsal root ganglia neurons by targeting FOXP2 and VAT1 in postnatal and adult mouse. Mol. Neurobiol.55, 9089–9099. 10.1007/s12035-018-1047-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eura, Y., Ishihara, N., Oka, T. & Mihara, K. Identification of a novel protein that regulates mitochondrial fusion by modulating Mitofusin (Mfn) protein function. J. Cell. Sci.119, 4913–4925. 10.1242/jcs.03253 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Jamett, A. M. et al. Dynamin-2 in nervous system disorders. J. Neurochem. 128, 210–223. 10.1111/jnc.12455 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Pizzarelli, R. et al. Tuning GABAergic inhibition: gephyrin molecular organization and functions. Neuroscience439, 125–136. 10.1016/j.neuroscience.2019.07.036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma, L. et al. alpha-SNAP enhances SNARE Zippering by stabilizing the SNARE four-helix bundle. Cell. Rep.15, 531–539. 10.1016/j.celrep.2016.03.050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw proteomics files were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier accession code PXD057572.