Abstract
A 54-year-old ranch hand presented to the emergency room with an alleged spider bite and multiple abscesses. Both wound and blood cultures grew Photorhabdus asymbiotica, an enteric gram-negative rod that was initially misidentified by the hospital's rapid identification system. Clinical laboratories should be aware of the limitations of their rapid identification systems and always use them as an adjunct to analysis of morphological and phenotypic traits.
A 54-year-old male presented to the emergency department of a local Houston hospital during July 2003. He was a ranch hand who believed that he was bitten by a spider on his left breast. He presented with multiple carbuncles on his left chest wall and multiple pustular nodular lesions over his extremities. The patient, who has a family history of diabetes, had a blood sugar level of 400 on admission. His temperature was 101°F, his blood pressure was 135/70, his respiratory rate was 20, and his pulse was 60. Culture of the left-breast abscess showed moderate numbers of methicillin-resistant Staphylococcus aureus and an unremarkable gram-negative rod identified by a MicroScan Neg Urine Combo Panel Type 34 on the MicroScan WalkAway (Dade Behring, Inc., MicroScan Division, West Sacramento, CA) as Pseudomonas oryzihabitans. An identical gram-negative rod was isolated from four of four blood culture bottles from two separate venipunctures. However, it was identified on the same system as Providencia rustigianii. Both isolates were sent to a local reference laboratory (Microbiology Specialists Incorporated). Each isolate produced two colony types, which exhibited annular hemolysis and swarming on blood agar (Fig. 1 and 2). Annular hemolysis is unusual in that there is no hemolysis immediately around the colony but there is a thin line (about 2 mm wide) of hemolysis about 12 mm from the edge of the colony. Each isolate was oxidase negative, catalase positive, and motile, with a nondiffusible yellow to dirty-brown pigment. Neither isolate reduced nitrate to nitrite, but both fermented glucose (Table 1). The isolate was finally identified as Photorhabdus asymbiotica (formerly Xenorhabdus luminescens) on the basis of weak bioluminescence when tryptic soy agar slants grown at either 25°C or 35°C were observed in a totally darkened room for 5 to 10 min. The organism identifications were subsequently confirmed by the Centers for Disease Control and Prevention using conventional biochemicals (1) and a number of other rapid identification systems.
FIG. 1.
Photorhabdus asymbiotica showing annular hemolysis (arrow) and yellow-brown color.
FIG. 2.
Photorhabdus asymbiotica exhibiting swarming (arrow).
TABLE 1.
Key biochemical and morphological characteristics of Photorhabdus asymbiotica
| Characteristic type | Characteristic (comment or method) |
|---|---|
| Morphological | Yellow-dirty-brown pigment |
| Annular hemolysis | |
| Swarming | |
| Weak bioluminescence when grown on tryptic soy agar | |
| Biochemicala | Oxidase negative |
| Catalase positive | |
| Glucose fermented; no gas produced | |
| Motile | |
| Nitrate not reduced to nitrite (unlike other Enterobacteriaceae) | |
| Gelatin hydrolyzed in 2 days | |
| Urea hydrolyzed (Christenson) | |
| Citrate utilized | |
| Acetate utilized | |
| DNase positive in 5 days | |
| Tartrate fermented (Jordan) |
The biochemical tests yielded the same results at both 25°C and 37°C, although time to positive reactions was reduced at 25°C.
Table 2 illustrates the problem with correctly identifying P. asymbiotica if a microbiologist fails to consider morphological characteristics in addition to commercial gram-negative rod identification results. Failure of clinical laboratories to identify Photorhabdus is understandable, as this organism does not appear in the databases of the MicroScan or Vitek automated systems or other manual bioMérieux products. In this case, a microbiologist (R. Kern, Katy, TX) questioned why the wound and blood isolates, which morphologically looked the same, were identified as two different organisms on the WalkAway system. Both isolates were finally identified by another clinical microbiologist (R. J. Halliday) using standard biochemicals and extensive detective work. Key reactions for the identification of this organism include yellow pigment, nitrate not reduced to nitrite (unlike most other Enterobacteriaceae), annular hemolysis, swarming, and weak bioluminescence. The misidentifications in Table 2 point out how important it is for a competent technologist to critically review data from a rapid system. Interestingly, the same colonial morphotypes (e.g., wound isolate no. 1 and blood isolate no. 1) gave different results, indicating no consistency in the rapid-system results. It seems preferable to get no identification (API 20E) or an unacceptable identification (BBL Crystal) rather than the wrong answer. It may also be prudent to inoculate triple-sugar iron agar to determine an isolate's ability to ferment versus oxidize glucose. The failure of rapid systems to elucidate this critical reaction in most cases was a key factor in the misidentification, as was failure to include an oxidase test. The New York State Department of Health Clinical Bacteriology Laboratory obtained a 100% match with Photorhabdus asymbiotica by 16S rRNA sequencing using the GenBank database.
TABLE 2.
Misidentification of Photorhabdus by a variety of rapid systemsd
| Isolate | Rapid system | Identification (%) | Is dextrose fermented on system? | Is dextrose oxidized on system? | Is oxidase part of result? |
|---|---|---|---|---|---|
| Wound isolate no. 1 | WalkAway Neg ID2a | Acinetobacter lwoffii (72.02) | − | + | = |
| Pseudomonas oryzihabitans (27.97) | |||||
| WalkAway Rapid Neg ID3a | Shewanella putrefaciens (98.58) | + | NT | = | |
| Pseudomonas aeruginosa (1.05) | |||||
| Pseudomonas mendocina (0.22) | |||||
| Vitek GNI+b | Unidentified | + | + | + | |
| Vitek ID-GNBb | Shewanella putrefaciens; acceptable identification | + | NT | = | |
| API 20E (25°C)b | No identification | + | NT | + | |
| Rapid ID 32E (25°C)b | Acinetobacter/Pseudomas spp.; very good identification | NT | NT | + | |
| BBL Crystal (25°C)c | Unacceptable identification | = | + | = | |
| BBL Phoenix NIDc | Chromobacterium violaceum (99) | = | + (w) | = | |
| Wound isolate no. 2 | WalkAway Neg ID2 Panel | Acinetobacter lwoffii (87.34) | = | + | = |
| Pseudomonas oryzihabitans (6.44) | |||||
| Acinetobacter baumanni (6.20) | |||||
| WalkAway Rapid Neg ID3 Panel | Chromobacterium violaceum (56.83); low-probability identification | + | NT | = | |
| Shewanella putrefaciens (40.90) | |||||
| Vibrio damsela (1.04) | |||||
| Burkholderia diminuta (0.70) | |||||
| Leminorella sp. (0.50) | |||||
| Vitek GNI+ | Shigella dysenteriae (68); serology required | + | + | + | |
| Cedecea lapagei (25) | |||||
| Vitek ID-GNB | Unidentified | + | NT | = | |
| API 20E (25°C) | No identification | + | NT | + | |
| Rapid ID 32E (25°C) | Acinetobacter/Pseudomonas spp.; very good identification | NT | NT | + | |
| BBL Crystal (25°C) | Unacceptable identification | = | + | = | |
| BBL Phoenix NID | Comamonas testosterone (99) | = | + (w) | = | |
| Blood isolate no. 1 | WalkAway Neg ID2 Panel | Acinetobacter lwoffii (87.34) | = | + | = |
| Pseudomonas oryzihabitans (6.44) | |||||
| Acinetobacter baumann/A. haem (6.20) | |||||
| WalkAway Rapid Neg ID3 Panel | Chromobacterium violaceum (99.32) | + | NT | = | |
| Leminorella spp. (0.27) | |||||
| Pseudomonas aeruginosa (0.27) | |||||
| Vibrio damsela (0.11) | |||||
| Vitek GNI+ | Unidentified | + | + | + | |
| Vitek ID-GNB | Chryseobacterium indologenes | + | NT | = | |
| Vibrio alginolyticus; low discrimination | |||||
| API 20E (25°C) | No identification | + | NT | + | |
| Rapid ID 32E (25°C) | Acinetobacter/Pseudomonas spp.; very good identification | NT | NT | + | |
| BBL Crystal (25°C) | Unacceptable identification | = | + | = | |
| BBL Phoenix NID | Chromobacterium violaceum (99) | = | + (w) | = | |
| Blood isolate no. 2 | WalkAway Neg ID2 Panel | Pseudomonas oryzihabitans (85.46) | = | + | = |
| Acinetobacter baumannii/A. haem (0.85) | |||||
| Pseudomonas luteola (3.68) | |||||
| WalkAway Rapid Neg ID3 Panel | Shewanella putrefaciens (99.87) | = | NT | = | |
| Ochrobacter anthropi (0.11) | |||||
| Vitek GNI+ | Providencia stuartii (64) | + | + | + | |
| Presumptive Acinetobacter lwoffii/A. junii (27); good identification, marginal separation | |||||
| Vitek ID-GNB | Shewanella putrefaciens; acceptable identification | + | NT | = | |
| API 20E (25°C) | No identification | + | NT | + | |
| Rapid ID 32E (25°C) | Acinetobacter/Pseudomonas spp; very good identification | NT | NT | + | |
| BBL Crystal (25°C) | Unacceptable identification | = | + | = | |
| BBL Phoenix NID | Chromobacterium violaceum (98) | = | + (w) | = |
MicroScan WalkAway (Dade Behring Inc., MicroScan Division, West Sacramento, CA).
bioMérieux Vitek (bioMérieux, Durham, NC).
Becton Dickinson Microbiology Systems (Sparks, MD).
=, negative; +, positive; NT, not tested; w, weak.
Table 3 shows the susceptibility profile for each isolate. Unfortunately, the organism identification is used by the MicroScan WalkAway computer to interpret the susceptibility pattern. The organism from the wound was determined to be a glucose nonfermenter, while the blood isolate was found to be an enteric glucose-fermenting organism. The susceptibility pattern from the WalkAway was unusual in that the blood isolate was listed as being susceptible to ampicillin and amoxicillin-clavulanic acid. In fact, when both isolates were retested using Kirby-Bauer disk diffusion, the results were different; all Kirby-Bauer results were repeated to ensure reproducibility. The amoxicillin-clavulanic acid seemed to be particularly affected by the temperature at which the Mueller-Hinton plate was incubated. At the environmental temperature (25°C) that P. asymbiotica prefers, the isolate was resistant to ampicillin, amoxicillin-clavulanic acid, and cephalothin. Although environmental organisms often grow better at 25°C, susceptibility tests performed at this temperature should carry a disclaimer identifying them as “nonstandardized.” Thus, it is critical to correctly identify gram-negative rods to ensure that the susceptibility results used to treat the patient produce a clinical cure.
TABLE 3.
Susceptibility profiles for wound and blood isolates by the MicroScan WalkAway test and Kirby-Bauer disk diffusion performed at two temperaturesa
| Drug | Wound |
Blood |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | Inter- pretation | KB 35°C zone size (mm) | Inter- pretation | KB 25°C zone size (mm) | Inter- pretation | MIC (μg/ml) | Inter- pretation | KB 35°C zone size (mm) | Inter- pretation | KB 25°C zone size (mm) | Inter- pretation | |
| Amoxicillin-clavulanate | 8/4 | NI | 19 | S | 13 | R | 8/4 | S | 21 | S | 6 | R |
| Ampicillin | 8 | NI | 6 | R | 6 | R | 16 | I | 6 | R | 6 | R |
| Cefazolin | 4 | NI | 4 | S | ||||||||
| Cefepime | 4 | S | 4 | S | ||||||||
| Ceftazidime | 4 | S | 4 | S | ||||||||
| Ceftriaxone | 8 | S | 8 | S | ||||||||
| Cefotaxime | NT | 36 | S | 40 | S | 40 | S | 40 | S | |||
| Cefoxitin | NT | 24 | S | 23 | S | 26 | S | 21 | S | |||
| Cephalothin | NT | 6 | R | 6 | R | 6 | R | 6 | R | |||
| Ciprofloxacin | 1 | S | 44 | S | 46 | S | 1 | NI | 45 | S | 46 | S |
| Gatifloxacin | 0.5 | NI | 0.5 | S | ||||||||
| Gentamicin | 2 | S | 26 | S | 28 | S | 2 | S | 29 | S | 28 | S |
| Tobramycin | 4 | S | 4 | S | ||||||||
| Levofloxacin | 1 | S | 1 | S | ||||||||
| Trimethoprim-sulfamethoxazole | 2/38 | S | 27 | S | 27 | S | 2/38 | S | 36 | S | 28 | S |
| Piperacillin-tazobactam | 16 | S | 16 | S | ||||||||
Results from MicroScan Neg Urine Combo Type 34 panel. Abbreviations: KB, Kirby-Bauer; NI, not interpreted; S, susceptible; R, resistant; I, intermediate; NT, not tested.
Photorhabdus (formerly Xenorhabdus) (2) was first described in the United States by Farmer et al. in 1989 (1). They described six cases, including four in San Antonio, Texas. Since our isolate is from Houston, Texas, and since Texas isolates represent 50% of cases worldwide, it makes sense that infection due to this species (especially in the Southwest) is probably underreported due to incorrect laboratory identification. The two isolates described here are only the 13th and 14th recovered worldwide. Patients usually present with either localized soft tissue infection or disseminated bacteremic infection, sometimes with spread to multiple skin/soft tissue sites (3, 4, 5). Although many patients report an antecedent spider bite, an actual bite cannot be proven in most cases. Thus, the source of human infection remains unknown, although it seems likely that there is an unidentified invertebrate vector, since Photorhabdus spp. colonize the guts of nematodes pathogenic for insects and human infection with P. asymbiotica is associated with outdoor activity. Some patients are diabetics, and some are on steroids; most have a history of working outdoors, especially during warm, rainy months.
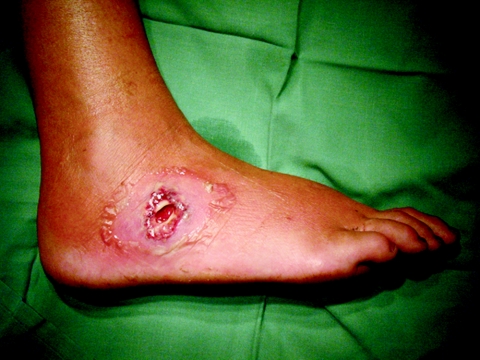
The ability to search the Internet for information regarding bioluminescent organisms and collaboration with colleagues in Australia sped up the identification of this organism. In fact, at the same time that we saw our Houston patient, our Australian colleagues were seeing their own patient, a 29-year-old woman with an intensely painful and swollen right foot (3). Two days before presentation, she had cleared debris and weeds from her country property while barefoot. She was started on oral amoxicillin-clavulanic acid in the emergency department, but by the next day her foot had become even more swollen, erythematous, and painful. She was admitted to the hospital and started on intravenous antistaphylococcal (flucloxacillin) antibiotics. Despite treatment, a local abscess formed (Fig. 3). This was incised, and pus was sent to the laboratory for culture. Three days later, a gram-negative rod was isolated in pure culture. The Vitek GNI card identified the organism as Flavobacterium sp. The microbiologist doubted this identification, as the colony morphology (small and round with entire edges and swarming, with a dirty-yellow pigment) did not fit the description of the genus Flavobacterium. The swarming growth and the unusual pigment raised the suspicion of the genus Photorhabdus. The isolate was confirmed as P. asymbiotica on the basis of (i) annular hemolysis on sheep blood agar, (ii) swarming growth, (iii) dirty-yellow pigment, (iv) weak bioluminescence, (v) inability to reduce nitrate to nitrite, (vi) positive citrate and urease tests, and (vii) fermentation of glucose and mannose. Subsequent Australian testing at another laboratory by the MicroScan WalkAway test using the Neg BP Combo 11 panel identified the organism as Pseudomonas oryzihabitans, and using the Rapid Neg BP 5A panel, it was identified as Shewanella putrefaciens. Although both the Vitek and MicroScan tests initially failed to identify this pathogen, the patient was switched to doxycycline and amoxicillin-clavulanate, and the wound was debrided (Fig. 4). The antimicrobial susceptibility of the Australian isolate was assessed using broth microdilution. The isolate was susceptible to a broad range of antimicrobial agents with activity against gram-negative bacteria, including fluoroquinolones, tetracyclines, and aminoglycosides, but it was resistant to ampicillin and cephalothin. The patient was ultimately treated with ciprofloxacin for 4 weeks, and her foot was completely healed 2 months later.
FIG. 3.
Abscess prior to debridement and antimicrobial therapy.
FIG. 4.
Abscess after debridement and antimicrobial therapy.
These cases, taken together, point out the importance of microbiologists who are alert enough to question organism identification and susceptibility results from commonly used automated systems. It is incumbent on all manufacturers of miniaturized or automated gram-negative rod systems to review their databases frequently to prevent these types of errors. P. asymbiotica should be added to the respective databases as soon as possible.
REFERENCES
- 1.Farmer, J. J., III, J. H. Jorgensen, P. A. D. Grimont, R. J. Akhurst, G. O. Poinar, Jr., E. Ageron, G. V. Pierce, J. A. Smith, C. P. Carter, K. L. Wilson, and F. W. Hickman-Brenner. 1989. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J. Clin. Microbiol. 27:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher-Le Saux, M., V. Viallard, B. Brunel, P. Normand, and N. E. Boemare. 1999. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. Int. J. Syst. Bacteriol. 49:1645-1656. [DOI] [PubMed] [Google Scholar]
- 3.Gerrard, J., N. Waterfield, R. Vohra, and R. ffrench-Constant. 2004. Human infection with Photorhabdus asymbiotica: an emerging bacterial pathogen. Microbes Infect. 6:229-237. [DOI] [PubMed] [Google Scholar]
- 4.Gerrard, J. G., S. McNevin, D. Alfredson, R. Forgan-Smith, and N. Fraser. 2003. Photorhabdus species: bioluminescent bacteria as emerging human pathogens? Emerg. Infect. Dis. 9:251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peel, M. M., D. A. Alfredson, J. G. Gerrard, J. M. Davis, J. M. Robson, R. J. McDougall, B. L. Scullie, and R. J. Aekhurst. 1999. Isolation, identification, and molecular characterization of strains of Photorhabdus luminescens from infected humans in Australia. J. Clin. Microbiol. 37:3647-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]