Abstract
Background
Respiratory syncytial virus (RSV) infection is a significant burden for children under 5 years of age. While RSV is a common cause of acute lower respiratory tract infection (ALRTI), routine confirmatory testing is not widely practiced. Identifying risk factors for RSV infection can help inform clinical decision-making and guide appropriate management in emergency departments. The objective of this study is to determine the risk factors associated with RSV infection in children hospitalized with ALRTI.
Methods
A retrospective case-control study was conducted among pediatric patients under 5 years of age diagnosed with ALRTI at Naresuan University Hospital between January 2018 and December 2020. Data were extracted from electronic medical records. RSV diagnoses were confirmed using a rapid RSV antigen test. Potential risk factors—including prematurity, family history of atopic disease, past respiratory illness, history of ALRTI contact, and underlying disease—were analyzed using a multivariable logistic model in Stata version 17.0.
Results
Among 3,579 children hospitalized with ALRTI, 459 (13%) were diagnosed with RSV infection. Peaks in RSV-ALRTI were observed in September, October, and November across 2018–2020. Significant risk factors identified through multivariable logistic regression analysis included the rainy season [odds ratio (OR) =15.51; 95% confidence interval (CI): 1.74–138.42; P=0.01], daycare attendance (OR =3.00; 95% CI: 1.27–7.10; P=0.01), lung crepitations (OR =2.60; 95% CI: 1.23–5.50; P=0.01), and oxygen supplementation (OR =2.38; 95% CI: 1.07–5.30; P=0.03).
Conclusions
This study highlights the significant clinical burden of RSV in ALRTI, with RSV-ALRTI accounting for 13% of all ALRTI cases. Notably, RSV-ALRTI cases peaked during the rainy season, specifically in September, October, and November. The identified risk factors for RSV-ALRTI include the rainy season, daycare attendance, lung crepitations, and the need for oxygen supplementation. These findings underscore the importance of early identification and management of high-risk patients, particularly during peak seasonal periods. Clinically, these risk factors can help guide targeted prevention and treatment strategies, improving patient outcomes and optimizing resource allocation in healthcare settings.
Keywords: Lower respiratory tract infection, respiratory syncytial virus (RSV), risk factors
Highlight box.
Key findings
• The peak of respiratory syncytial virus-related acute lower respiratory tract infection (RSV-ALRTI) occurred from September to November, with risk factors including the rainy season, daycare attendance, lung crepitations, and oxygen supplementation.
What is known and what is new?
• The global prevalence of RSV-ALRTI varies widely across studies, with multiple risk factors identified.
• This study provides prevalence data from Thailand, identifies peak outbreak months, and further confirms key risk factors associated with RSV-ALRTI.
What is the implication, and what should change now?
• Understanding the outbreak pattern and risk factors of RSV-ALRTI enables clinicians to optimize treatment strategies and outbreak management, leading to more effective control. It also supports planning for preventive interventions using palivizumab or nirsevimab, which are currently effective prophylactic options.
Introduction
Respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory traction infection (ALRTI) in children and represents the most common reason for pediatric hospitalizations. Globally, RSV-ALRTI affects 34 million children annually, resulting in over 3 million hospitalizations and between 66,000 and 199,000 deaths, with 99% of which occur in low- and middle-income countries (1). The long-term sequelae of RSV infection, including increased asthma risk and allergen sensitization, further underscore its clinical significance. For example, one cohort study found that 41% of children under one year of age with prior RSV infection showed allergen sensitization, while 39% developed asthma (2). In contrast, other respiratory viruses aside from RSV are rarely associated with the development of asthma. Evidence regarding the contribution of non-RSV respiratory infections-such as influenza, human coronavirus, and parainfluenza-to the development of asthma later in life remains scarce and inconclusive. According to previous studies, rhinovirus is the only non-RSV virus identified as a potential contributor, and even then, the available evidence is significantly more limited compared to that for RSV (3-6).
Between 2015 and 2019, over 1.1 million children under the age of five were hospitalized with acute lower respiratory tract infections (ALRTIs) in Thailand, with RSV identified as the causative agent in approximately 19.5% to 74% of cases (7-9). Given the substantial burden of RSV on pediatric health and the limited availability of RSV antigen testing in many Thai hospitals, the identification of risk factors associated with RSV-ALRTI is essential to enhance clinical decision-making and optimize the allocation of healthcare resources, particularly in emergency settings. While previous studies have investigated potential risk factors—such as young age, atopic history, maternal smoking exposure, seasonal variation, contact with individuals with respiratory illness, daycare attendance, and underlying comorbidities—the findings have been inconsistent (10-12). Current treatment options for RSV infection are limited; although aerosolized ribavirin is used in some contexts, it remains unavailable in Thailand. Preventive measures primarily rely on passive immunization with monoclonal antibodies, notably palivizumab and nirsevimab, both of which are approved by the United States Food and Drug Administration (U.S. FDA). However, their widespread use is constrained by high costs and the requirement for seasonal administration. An understanding of the epidemiological pattern of RSV infection in Thailand—both prior to and during the coronavirus disease 2019 (COVID-19) pandemic—is of particular importance, given that the seasonal circulation of RSV is known to vary by country and region. These variations are influenced by local climatic and environmental conditions. In this context, the early identification and appropriate management of modifiable risk factors may offer a practical and cost-effective approach to alleviating the clinical and public health burden of RSV-ALRTI. The present study was conducted to identify the risk factors associated with RSV infection in children under 5 years of age who were hospitalized with ALRTI at Naresuan University Hospital. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2170/rc).
Methods
Study design and setting
A retrospective case-control analytic study was conducted on children aged 5 years or younger who were admitted to Naresuan University Hospital between 1 January 2018 and 31 December 2020, a tertiary-care university hospital situated in the lower northern region of Thailand. The hospital comprises a 410-bed facility, including an 85-bed pediatrics ward and 8 single-patient rooms designated for isolation or patients requiring specialized care. All hospital admissions are documented in the hospital’s database, with clinical diagnoses entered by coders using the International Classification of Diseases, 10th revision (ICD-10).
Data collection
This research utilized data collected from electronic medical records and inpatient medical records, which were transferred to case record forms spanning the period between 1 January 2018 and 31 December 2020. Subjects were identified based on a principal diagnosis of bronchitis, bronchiolitis, pneumonia, laryngotracheobronchitis, or epiglottitis using ICD-10 codes (J05, J12, J13, J14, J15, J16, J17, J18, J20, J21, J22, J40 and B97.4). RSV diagnoses were confirmed using a rapid RSV antigen test. Data collected included admission date, age, gender, and potential risk factors such as prematurity, family history of atopic disease, past respiratory illnesses, previous exposure to individuals with respiratory tract infections, and underlying diseases (congenital heart disease, bronchopulmonary dysplasia, atopic disease). Illness-related data, such as fever, rhinorrhea, cough, feeding difficulty, lung sounds, chest wall retraction, SpO2 levels, laboratory tests [complete blood count (CBC) and chest X-ray], oxygen supplementation, onset of illness, and length of stay (LOS), were also recorded.
Population and selection of cases and controls
The study population comprised children aged 5 years old or younger who were diagnosed with ALRTI based on clinical manifestations and laboratory findings as confirmed by the attending physician. All chest films were re-evaluated by at least two out of three experienced pulmonologists and radiologists, who were blinded to the clinical data. All patients were retrospectively re-diagnosed based on a combination of coded diagnoses, clinical data extracted from medical records, and chest radiographic findings.
Inclusion criteria
Children aged 5 years or younger who were admitted to Naresuan University Hospital during the study period were considered eligible for inclusion. The diagnosis of ALRTI was retrospectively confirmed based on the consistency between the coded diagnoses, clinical documentation in the medical records, and findings from chest radiographs. Additionally, only those children who underwent rapid RSV antigen testing using an immunochromatographic assay (Quick NaviTM-RSV2, Denka Seiken Co., Ltd., Tokyo, Japan) were included in the study.
Exclusion criteria
Children were excluded if their ALRTI was of nosocomial origin, defined as infections acquired during hospitalization for non-respiratory conditions. Such cases were excluded to avoid confounding from hospital-acquired infections. Furthermore, patients with viral-induced asthma exacerbations were not included, as it is often difficult to clearly distinguish these episodes from true lower respiratory tract infections based on clinical presentation alone.
Case and control selection
All participants included in this study were hospitalized and underwent a CBC, blood culture for bacterial pathogens, and a rapid antigen test for RSV. In cases identified as viral infections other than RSV, the diagnosis was made by the attending physicians based on clinical presentation in conjunction with CBC findings. Bacterial infections were determined through a combination of clinical evaluation, CBC results, and positive blood culture findings. Cases classified as having unknown etiologies were those in which the clinical features were inconclusive, or where the attending physicians were unable to definitively distinguish between viral and bacterial infections. These patients were empirically treated for both viral and bacterial infections, and their blood cultures yielded no identifiable organisms. Children with a positive rapid RSV antigen test were categorized as cases (RSV-ALRTI group). Conversely, children with a negative rapid RSV antigen test were classified as controls (non-RSV-ALRTI group). Cases and controls were matched 1:4 within the same season (3-month period) to ensure comparability.
Laboratory method for rapid RSV antigen test
Nasopharyngeal swab specimens were analyzed using the Quick NaviTM-RSV2 immunoassay (Denka Seiken Co., Ltd.), an immunochromatographic assay technique, to identify RSV. This test has a sensitivity of 79% and specificity of 100% (13). All pediatric patients who were either suspected of having, or diagnosed with, ALRTIs were tested for RSV using a rapid antigen test prior to admission to the inpatient ward.
Study size estimation
Based on pilot data, the proportion of males was 40.0% in the RSV-ALRTI group and 60% in the non-RSV-ALRTI group. To achieve a statistical power of 0.8 with an alpha error of 0.05, a study size of 69 participants was required for the RSV-ALRTI group, and 276 participants for the non-RSV-ALRTI group. A computer-generated random selection process was used to select 69 participants from the 459 RSV-ALRTI cases for inclusion in the study. A 1:4 case-control matching ratio was employed, resulting in the selection of 276 controls. Control subjects were time-matched to cases within the same 3-month period (Figure 1).
Figure 1.
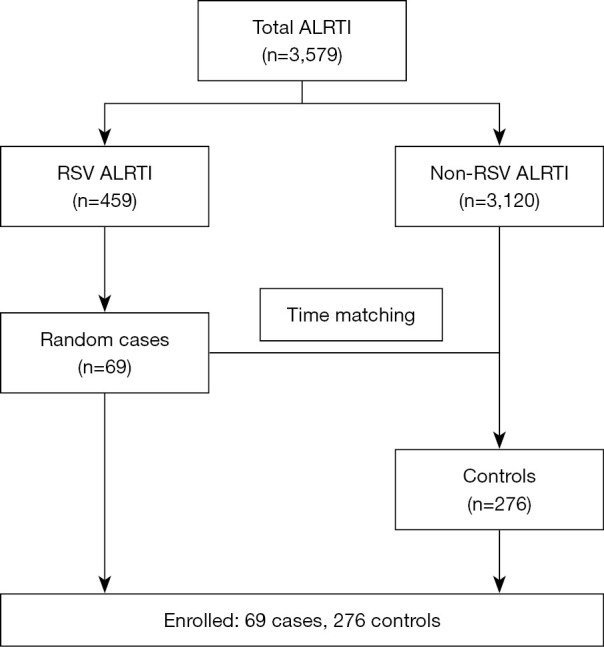
Selection process of study participants. ALRTI, acute lower respiratory tract infection; RSV, respiratory syncytial virus.
Statistical analysis
Categorical data were summarized as frequencies and percentages and analyzed using exact probability tests. Normally distributed continuous data were presented as means and standard deviations and tested using independent t-tests. Non-normally distributed continuous data were described as medians and interquartile ranges (IQRs) and analyzed using the Mann-Whitney U test. Statistical uncertainties were expressed as 95% two-sided confidence intervals (CIs), with a P value of <0.05 will indicating statistical significance. Multivariable binary logistic regression was employed to adjust for potential cofounders based on literature, including prematurity, family history of atopic disease, past respiratory illnesses, previous exposure to individuals with respiratory tract infections, and underlying diseases.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Naresuan University Institutional Review Board (COA No. 384/2022, IRB No. P3-0077/2565) and individual consent for this retrospective analysis was waived.
Results
A total of 3,579 patients were diagnosed ALRTI at Naresuan University Hospital from January 2018 to December 2020. Among these, 459 (12.8%) were confirmed to have RSV infection. The remaining cases comprised 1,085 (30.4%) other viral infections, 608 (17.0%) bacterial infections, and 1,427 (39.8%) cases with unknown etiologies. A computer-generated random selection process was used to select 69 participants from the 459 RSV-ALRTI cases for inclusion in the study. A 1:4 case-control matching ratio was employed, resulting in the selection of 276 controls. Control subjects were time-matched to cases within the same 3-month period (Figure 1).
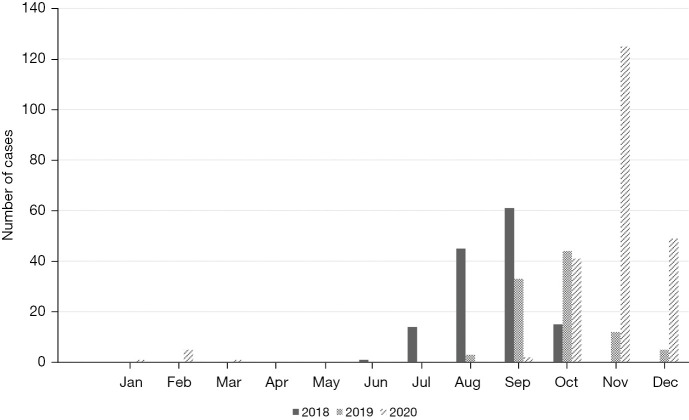
RSV was detected year-round, except in April and May, with notable peaks during certain months. Major RSV surges were recorded occurred from July to October in 2018, September to December in 2019, and October to December in 2020. In 2018, RSV cases peaked in September with 61 cases (2.9%), followed by August with 45 cases (2.1%), and July with 14 cases (0.6%). In 2019, the highest number of RSV cases was observed in October with 44 cases (1.9%), followed by September with 33 cases (1.5%), and November with 12 cases (0.5%). In 2020, November saw the highest incidence with 125 cases (7%), followed by December with 49 cases (2.7%) and October with 41 cases (2.3%). The overall incidence of RSV infections exhibited a consistent upward trend, culminating in the highest incidence during the final year of the study (Figure 2).
Figure 2.
Monthly distribution of RSV-ALRTI cases over the study period. ALRTI, acute lower respiratory tract infection; RSV, respiratory syncytial virus.
The proportion of male patients was 44.9% in the RSV-ALRTI group and 41.3% in the non-RSV-ALRTI group (P=0.59). The median age of patients in the RSV-ALRTI group was considerably lower than in the non-RSV-ALRTI group [12.0 (IQR, 8.0, 24.0) vs. 24.0 (IQR, 12.0, 36.0) months, P=0.02]. Distinct clinical and demographic characteristics were noted between children with RSV-ALRTI and those with non-RSV-ALRTI. A higher proportion of children in the RSV-ALRTI group were younger than 12 months (52.2% vs. 41.1%, P=0.17), preterm (8.7% vs. 5.4%, P=0.40), or had a family history of atopic disease (7.2% vs. 3.3%, P=0.17). Additionally, contact history with individuals diagnosed with ALRTI (23.2% vs. 19.7%, P=0.51), daycare attendance (21.7% vs. 17.5%, P=0.49), and personal history of atopic disease (8.7% vs. 6.9%, P=0.61) were also more frequently reported in the RSV-ALRTI group. Clinically, these children were more likely to require oxygen supplementation (72.5% vs. 63.4%, P=0.20), had a longer duration from symptom onset to admission [median: 3.0 (IQR, 2.0–4.0) vs. 2.0 (IQR, 1.0–4.0) days], and presented more frequently with cough (94.2% vs. 87.3%; P=0.14). However, none of these differences reached statistical significance. These included a lower prevalence of previous ALRTIs (17.4% vs. 32.2%, P=0.02), lower white blood cell counts [(10.9±3.6)×103/µL vs. (12.6±5.5)×103/µL, P=0.02], and a lower percentage of neutrophils [(45.1±19.2)% vs. (53.5±18.6)%, P=0.001]. Conversely, the RSV-ALRTI group demonstrated a longer length of hospital stay [median: 4.0 (IQR, 3.0–6.0) vs. 4.0 (IQR, 3.0–5.0) days, P=0.01], a higher prevalence of feeding difficulties (73.8% vs. 59.8%, P=0.045), a higher frequency of crepitations (56.5% vs. 36.6%, P=0.004), and significantly higher lymphocyte counts [(44.1±17.1)% vs. (36.7±17.8)%, P=0.002] compared to the non-RSV-ALRTI group (Table 1).
Table 1. Baseline characteristics and investigations at enrollment.
| Data | Missing | RSV-ALRTI (n=69) | Non-RSV-ALRTI (n=276) | P value |
|---|---|---|---|---|
| Male | 0 | 31 (44.9) | 114 (41.3) | 0.59 |
| Age (months) | 0 | 12.0 [8.0, 24.0] | 24.0 [12.0, 36.0] | 0.02 |
| >36–60 | 0 | 7 (10.1) | 51 (18.5) | 0.17 |
| >24–36 | 0 | 9 (13.0) | 52 (18.8) | |
| >12–24 | 0 | 17 (24.6) | 58 (21.0) | |
| <12 | 0 | 36 (52.2) | 115 (41.7) | |
| Preterm | 0 | 6 (8.7) | 15 (5.4) | 0.40 |
| Family history of atopic disease | 0 | 5 (7.2) | 9 (3.3) | 0.17 |
| Past history of ALRTI | 0 | 12 (17.4) | 89 (32.2) | 0.02 |
| History of ALRTI contact | 2 | 16 (23.2) | 54 (19.7) | 0.51 |
| Daycare attendance | 2 | 15 (21.7) | 48 (17.5) | 0.49 |
| Congenital heart disease | 0 | 3 (4.3) | 21 (7.6) | 0.44 |
| Bronchopulmonary dysplasia | 0 | 1 (1.4) | 7 (2.5) | >0.99 |
| Atopic disease | 0 | 6 (8.7) | 19 (6.9) | 0.61 |
| O2 supplementation | 3 | 50 (72.5) | 175 (63.4) | 0.20 |
| Onset of illness (days) | 5 | 3.0 [2.0, 4.0] | 2.0 [1.0, 4.0] | 0.14 |
| Length of hospital stay (days) | 0 | 4.0 [3.0, 6.0] | 4.0 [3.0, 5.0] | 0.01 |
| Clinical | ||||
| Fever | 2 | 55 (79.7) | 219 (79.9) | >0.99 |
| Rhinorrhea | 0 | 50 (72.5) | 227 (82.2) | 0.09 |
| Cough | 0 | 65 (94.2) | 241 (87.3) | 0.14 |
| Dyspnea | 0 | 25 (36.2) | 129 (46.7) | 0.14 |
| Feeding difficulty | 9 | 48 (73.8) | 162 (59.8) | 0.045 |
| Wheezing | 0 | 15 (21.7) | 80 (29.0) | 0.29 |
| Chest retraction | 0 | 18 (26.1) | 90 (32.6) | 0.31 |
| Crepitations | 0 | 39 (56.5) | 101 (36.6) | 0.004 |
| Rhonchi | 0 | 26 (37.7) | 122 (44.2) | 0.34 |
| Laboratory | ||||
| White blood cell (×103/μL) | 1 | 10.9±3.6 | 12.6±5.5 | 0.02 |
| Neutrophil (%) | 1 | 45.1±19.2 | 53.5±18.6 | 0.001 |
| Lymphocyte (%) | 1 | 44.1±17.1 | 36.7±17.8 | 0.002 |
| Eosinophil (%) | 2 | 0.6 [0.2, 1.3] | 0.6 [0.1, 2.2] | 0.95 |
| Eosinophil >4% | 2 | 4±5.8 | 33±12.0 | 0.19 |
Data are presented as n, n (%), median [interquartile range] or mean ± standard deviation. ALRTI, acute lower respiratory tract infection; RSV, respiratory syncytial virus.
Our study identified ICD-10-coded diagnoses among patients with ALRTIs. In the RSV-ALRTI group, the most common diagnosis was RSV pneumonia (J121), accounting for 48 cases (69.6%), followed by RSV bronchitis (J205) with 19 cases (27.5%), and RSV bronchiolitis (J210) with 2 cases (2.9%). In the non-RSV-ALRTI group, viral pneumonia (J100, J110, J129) was the most prevalent diagnosis, found in 113 cases (40.9%), followed by bacterial pneumonia (J154, J157, J159) with 76 cases (27.5%), and unspecified pneumonia (J186, J208), which accounted for 12.0% of cases (Table 2).
Table 2. ICD-10 diagnostic codes in ALRTI patients.
| Diagnosis (ICD-10) | RSV-ALRTI (n=69), n (%) | Non-RSV-ALRTI (n=276), n (%) |
|---|---|---|
| RSV pneumonia (J121) | 48 (69.6) | 0 (0.0) |
| Bacterial pneumonia (J154, J157, J159) | 0 (0.0) | 76 (27.5) |
| Viral pneumonia (J100, J110, J129) | 0 (0.0) | 113 (40.9) |
| Pneumonia, unspecified (J186, J208) | 0 (0.0) | 33 (12.0) |
| RSV bronchiolitis (J210) | 2 (2.9) | 0 (0.0) |
| Bronchiolitis, unspecified (J219) | 0 (0.0) | 10 (3.6) |
| RSV bronchitis (J205) | 19 (27.5) | 0 (0.0) |
| Viral bronchitis (J111, J108) | 0 (0.0) | 17 (6.2) |
| Acute bronchitis, unspecified (J209) | 0 (0.0) | 20 (7.3) |
| Viral croup (J050) | 0 (0.0) | 7 (2.5) |
ALRTI, acute lower respiratory tract infection; ICD-10, International Classification of Diseases, 10th revision; RSV, respiratory syncytial virus.
Univariable analysis identified several potential risk factors for RSV-ALRTI, including feeding difficulties [odds ratio (OR) =1.90; 95% CI: 1.04–3.48; P=0.04], the presence of lung crepitations (OR =2.25; 95% CI: 1.32–3.85; P=0.003), and the rainy season (OR =11.27; 95% CI: 1.49–85.06; P=0.02). On the contrary, a past history of ALRTIs (OR =0.44; 95% CI: 0.23–0.87; P=0.02) and the onset of illness (OR =0.21; 95% CI: 0.14–0.33; P<0.001) were identified as reducing the risk.
Multivariable logistic regression identified several further highlighted potential risk factors for RSV-ALRTI, including daycare attendance (OR =3.00; 95% CI: 1.27–7.10; P=0.01), oxygen supplementation (OR =2.38; 95% CI: 1.07–5.30; P=0.03), bilateral crepitations (OR =2.60; 95% CI: 1.23–5.50; P=0.01), and the rainy season (OR =15.51; 95% CI: 1.74–138.42; P=0.01). Conversely, a history of prior ALRTIs (OR =0.39; 95% CI: 0.17–0.92; P=0.03) was associated with a decreased risk (Table 3).
Table 3. Multivariable logistic regression analysis.
| Potential risk factors | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Male | 1.09 | 0.5–2.38 | 0.83 |
| Age (months) | |||
| >36–60 | Reference | Reference | |
| >24–36 | 0.84 | 0.26–2.76 | 0.78 |
| >12–24 | 2.2 | 0.82–5.89 | 0.12 |
| <12 | 2.41 | 0.87–6.69 | 0.09 |
| Preterm | 2.13 | 0.48–9.4 | 0.32 |
| Family history of atopic disease | 1.94 | 0.26–14.66 | 0.52 |
| Past history of ALRTI | 0.39 | 0.17–0.92 | 0.03 |
| History of ALRTI contact | 1.42 | 0.59–3.45 | 0.43 |
| Daycare attendance | 3 | 1.27–7.1 | 0.01 |
| Atopic disease | 0.9 | 0.27–2.99 | 0.86 |
| Congenital heart disease | 1.2 | 0.24–6.08 | 0.83 |
| Bronchopulmonary dysplasia | 1.97 | 0.17–23.39 | 0.59 |
| O2 supplementation | 2.38 | 1.07–5.30 | 0.03 |
| Lung crepitations | 2.60 | 1.23–5.50 | 0.01 |
| Season | |||
| Rainy season | 15.51 | 1.74–138.42 | 0.01 |
| Winter season | 6.98 | 0.76–63.9 | 0.09 |
ALRTI, acute lower respiratory tract infection; CI, confidence interval.
Discussion
The prevalence of RSV-ALRTI in our study was 13%, consistent with findings from other hospitals in Thailand (7), but lower than those reported in several international studies. Teck et al. reported an RSV prevalence of 22.6% in northeastern Peninsular Malaysia, where the rainy season strongly influences transmission patterns, using nasopharyngeal antigen testing and seasonal questionnaires (14). In Sri Lanka, Divarathna et al. found a prevalence of 28% among hospitalized children under 5 years between 2016 and 2018, based on immunofluorescence assay and real-time reverse transcription polymerase chain reaction (RT-PCR) (15). A longitudinal birth cohort study by Okiro et al. in coastal Kenya identified 362 episodes of RSV—acute respiratory infections and 92 episodes of RSV—lower respiratory tract infections among 469 children, highlighting both the high disease burden and potential for reinfection over time (16). In Egypt, Fattouh et al. reported an RSV prevalence of 16.4% among children ≤5 years with ALRTI in outpatient and emergency settings, detected using immunofluorescence assay (17). A broader perspective from Europe is provided by Suleiman-Martos et al., who conducted a systematic review and meta-analysis of 20 studies, estimating a pooled RSV prevalence of 46% (95% CI: 34–59%) among over 16,000 children under 5 years of age (18). These variations reflect the influence of multiple factors, including geographic location, climate, study design, diagnostic techniques, and healthcare access. Taken together, these findings underscore the substantial global burden of RSV and highlight the importance of region-specific epidemiological surveillance and preventive strategies, particularly in high-risk pediatric populations.
Based on our findings, RSV was prevalent throughout the year, with notable peaks from July to October in 2018, September to December in 2019, and October to December in 2020, consistent with prior studies (7-9,14). RSV is a leading respiratory virus affecting children and causes widespread outbreaks globally, irrespective of climate or geographical region. While RSV activity can may occur year-round in most climates, peak periods typically align with specific months. In tropical and subtropical climates, RSV outbreaks are often linked to the rainy season, with peak activity commencing shortly after the rains begin (8,13-17). However, some studies have reported RSV infections peaking during the winter season (from December to February) (18,19), suggesting that cold weather and high humidity may increase the risk of respiratory tract infections. The findings of our study reveal a noticeable seasonal shift in the peak incidence of RSV infections, with the epidemic period progressively extending later into the calendar year over the study period. This trend appears to correspond with the delayed onset of the rainy and winter seasons in Thailand, highlighting the strong association between RSV activity and climatic factors, particularly lower temperatures and higher humidity. Such seasonal variation supports the widely accepted notion that RSV transmission is closely linked to environmental conditions, and that the timing of peak activity can vary across geographic regions depending on local seasonal patterns.
Importantly, our study provides prevalence data on RSV-ALRTI from both before and during the onset of the COVID-19 pandemic. The prevalence observed during the pre-pandemic years [2018–2019] was consistent with previous literature (7). However, a notably higher prevalence of RSV-ALRTI was observed in 2020, which contrasts with international reports of suppressed RSV circulation during the pandemic period (20-23). This discrepancy may be explained by Thailand’s gradual relaxation of COVID-19 control measures and school reopening during the latter half of 2020, which likely facilitated increased viral transmission. Furthermore, meteorological data indicated that the winter season in Thailand during 2020 was unusually cold, which may have further contributed to the heightened RSV activity. These observations underscore the importance of ongoing epidemiological surveillance and climate monitoring to better anticipate RSV outbreaks. They also highlight the need for region-specific planning, particularly in tropical and subtropical countries, where seasonal patterns may differ from those in temperate regions. Early recognition of these shifts may aid in optimizing resource allocation and implementing timely preventive strategies, especially in high-risk pediatric populations.
Clinically, RSV-ALRTI was associated with greater feeding difficulties, lung crepitation, higher oxygen supplementation needs, prolonged hospital stays, lower white blood cell counts, and reduced neutrophil percentages. Multivariate logistic regression analysis confirmed the statistical significance of lung crepitations and the need for oxygen supplementation as risk factors for RSV ALRTI. Notably, patients in the RSV-ALRTI group were 2.60 times more likely to exhibit lung crepitation compared to the non-RSV-ALRTI group. This aligns with previous studies conducted in Thailand (8,9,15), which identified pneumonia as the most common diagnosis among children with RSV-ALRTI. In contrast, research in other regions often found that RSV primarily caused bronchiolitis (16,18,19,24), likely due to differences in nutritional status, healthcare access, and the vulnerability of young children’s underdeveloped immune systems.
Our study also revealed that RSV-ALRTI patients required significantly higher oxygen supplementation (2.38 times more) compared to a previous study (18). This finding may be explained by the progression of RSV infection in the lower respiratory tract, characterized by necrosis and disorganized proliferation of bronchiolar epithelial cells, destruction of ciliated epithelial cells, and accumulation of sloughed epithelial debris in the airway spaces, leading to atelectasis and hypoxia (25).
Socio-demographic factors, particularly daycare attendance, showed a significant association with RSV-ALRTI, consistent with previous studies (14,19,24-27). Crowded environments like daycares increase the likelihood of RSV transmission due to close and prolonged contact between individuals.
Patients in the RSV-ALRTI group were generally younger age more than those in the non-RSV-ALRTI group. Although multivariate analysis showed no statistically significant difference, previous studies (14-19,24,26) have consistently identified younger age, particularly below 12 months, as a risk factor for RSV infection. Our findings suggest a potential association for this age group, but the small sample size may have limited statistical power.
Interestingly, a previous history of ALRTIs was associated with a decreased risk of RSV-ALRTI. This finding may appear counterintuitive, as prior respiratory illness is often considered a risk factor for subsequent infections. However, this observation should be interpreted in light of the younger mean age observed in the RSV-ALRTI group, which likely resulted in fewer opportunities for prior exposure to respiratory pathogens. In contrast, the older age of children in the non-RSV-ALRTI group may explain the higher proportion of patients with a history of ALRTI. This age-related difference highlights the importance of considering patient demographics when evaluating clinical risk factors and supports the notion that host factors such as immune maturity and cumulative pathogen exposure may influence susceptibility to RSV infection.
Several variables were not identified as risk factors in this study, including male gender, exposure to individuals with respiratory infections, underlying conditions (e.g., congenital heart disease or chronic lung disease), and family history of atopic diseases. These findings align with other research (1,14-16,18,19,24,25,28), but differences in study methodologies and covariate adjustments may account for discrepancies. Despite these variations, our robust study design and database provide valuable insights into RSV-ALRTI hospitalization.
The authors possibility of false negatives in RSV testing due to the use of an antigen rapid test (immunochromatographic assay technique) must be considered. This test has a sensitivity of 79% and specificity of 100% (13), potentially affecting our results. In addition, differences in study populations with this study focusing exclusively on pediatric patients with ALRTI may have contributed to variations from other literature, which included both patients with ALRTI and healthy controls.
Limitations
This study has several limitations. First, the retrospective nature of the study and the reliance on medical records may have resulted in incomplete or inaccurate data. Second, the RSV detection method relied on an antigen rapid test, which may yield false negative results. Finally, the interpretation of RSV epidemiological patterns should be contextualized according to the climate and geographical characteristics of each country, as the timing and seasonal peaks of RSV outbreaks may differ across regions. Future research should increase the sample size and use prospective data collection to ensure completeness and minimize data loss. RT-PCR should also be employed for RSV diagnosis, as it offers greater diagnostic accuracy than the antigen rapid test.
Conclusions
The prevalence of RSV-associated ALRTI was 13%. Peak infection periods were observed from July to October in 2018, September to December in 2019, and October to December in 2020. Risk factors for RSV-ALRTI in children included the rainy season, daycare attendance, lung crepitation, and a greater higher need for oxygen supplementation.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to express their gratitude to Miss Daisy Gonzales for her assistance in editing and revising this manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Naresuan University Institutional Review Board (COA No. 384/2022, IRB No. P3-0077/2565) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2170/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2170/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2170/dss
References
- 1.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047-64. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010;65:1045-52. 10.1136/thx.2009.121582 [DOI] [PubMed] [Google Scholar]
- 3.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol 2017;140:895-906. 10.1016/j.jaci.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DJ, Gern JE. Rhinovirus Infections and Their Roles in Asthma: Etiology and Exacerbations. J Allergy Clin Immunol Pract 2022;10:673-81. 10.1016/j.jaip.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmdahl I, Lüning S, Gerdin SW, et al. Rhinovirus-induced wheeze was associated with asthma development in predisposed children. Acta Paediatr 2024;113:1376-84. 10.1111/apa.17158 [DOI] [PubMed] [Google Scholar]
- 6.Proud D. Role of rhinovirus infections in asthma. Asian Pac J Allergy Immunol 2011;29:201-8. [PubMed] [Google Scholar]
- 7.Sitthikarnkha P, Uppala R, Niamsanit S, et al. Epidemiology of acute lower respiratory tract infection hospitalizations in Thai children: A 5-year national data analysis. Influenza Other Respir Viruses 2022;16:142-50. 10.1111/irv.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasan R, Rhodes J, Thamthitiwat S, et al. Incidence and etiology of acute lower respiratory tract infections in hospitalized children younger than 5 years in rural Thailand. Pediatr Infect Dis J 2014;33:e45-52. 10.1097/INF.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunjoungmanee P, Rotejaratpaisan A, Tangsathapornpong A. Epidemiology, clinical manifestation and risk factors of respiratory syncytial virus infection in children at Thammasat University Hospital. Thammasat Medical Journal 2016;16:370-8. [Google Scholar]
- 10.Ma HY, Lin IF, Liu YC, et al. Risk Factors for Severe Respiratory Syncytial Virus Infection in Hospitalized Children. Pediatr Infect Dis J 2024;43:487-92. 10.1097/INF.0000000000004270 [DOI] [PubMed] [Google Scholar]
- 11.Deng S, Cong B, Edgoose M, et al. Risk factors for respiratory syncytial virus-associated acute lower respiratory infection in children under 5 years: An updated systematic review and meta-analysis. Int J Infect Dis 2024;146:107125. 10.1016/j.ijid.2024.107125 [DOI] [PubMed] [Google Scholar]
- 12.Vartiainen P, Jukarainen S, Rhedin SA, et al. Risk factors for severe respiratory syncytial virus infection during the first year of life: development and validation of a clinical prediction model. Lancet Digit Health 2023;5:e821-30. 10.1016/S2589-7500(23)00175-9 [DOI] [PubMed] [Google Scholar]
- 13.Thuy Tien TT, Park H, Tuong HT, et al. Development of a Rapid Fluorescent Immunochromatographic Test to Detect Respiratory Syncytial Virus. Int J Mol Sci 2018;19:3013. 10.3390/ijms19103013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teck KS, Mac Guad R, Van Rostenberghe AH, et al. Prevalence, risk factors and clinical characteristics of respiratory syncytial virus-associated lower respiratory tract infections in Kelantan, Malaysia. J Med Virol 2019;91:1608-15. 10.1002/jmv.25500 [DOI] [PubMed] [Google Scholar]
- 15.Divarathna MVM, Rafeek RAM, Morel AJ, et al. Epidemiology and risk factors of respiratory syncytial virus associated acute respiratory tract infection in hospitalized children younger than 5 years from Sri Lanka. Front Microbiol 2023;14:1173842. 10.3389/fmicb.2023.1173842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okiro EA, Ngama M, Bett A, et al. Factors associated with increased risk of progression to respiratory syncytial virus-associated pneumonia in young Kenyan children. Trop Med Int Health 2008;13:914-26. 10.1111/j.1365-3156.2008.02092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattouh AM, Mansi YA, El-Anany MG, et al. Acute lower respiratory tract infection due to respiratory syncytial virus in a group of Egyptian children under 5 years of age. Ital J Pediatr 2011;37:14. 10.1186/1824-7288-37-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suleiman-Martos N, Caballero-Vázquez A, Gómez-Urquiza JL, et al. Prevalence and Risk Factors of Respiratory Syncytial Virus in Children under 5 Years of Age in the WHO European Region: A Systematic Review and Meta-Analysis. J Pers Med 2021;11:416. 10.3390/jpm11050416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Na'amnih W, Kassem E, Tannous S, et al. Incidence and risk factors of hospitalisations for respiratory syncytial virus among children aged less than 2 years. Epidemiol Infect 2022;150:e45. 10.1017/S0950268822000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lastrucci V, Pacifici M, Puglia M, et al. Seasonality and severity of respiratory syncytial virus during the COVID-19 pandemic: a dynamic cohort study. Int J Infect Dis 2024;148:107231. Erratum in: Int J Infect Dis 2024;149:107286. 10.1016/j.ijid.2024.107231 [DOI] [PubMed] [Google Scholar]
- 21.Trigueros Montes JB, Montes D, Miele A, et al. The Impact of COVID-19 Pandemic on Respiratory Syncytial Virus Infection in Children. Pulm Med 2024;2024:2131098. 10.1155/2024/2131098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg I, Shekhar R, Sheikh AB, et al. Impact of COVID-19 on the Changing Patterns of Respiratory Syncytial Virus Infections. Infect Dis Rep 2022;14:558-68. 10.3390/idr14040059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zven S, Dorr M, Malloy AMW, et al. Predicting the RSV Surge: Pediatric RSV Patterns of the COVID Pandemic. Pediatr Infect Dis J 2023;42:e349-51. 10.1097/INF.0000000000003980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi GA, Medici MC, Arcangeletti MC, et al. Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr 2007;166:1267-72. 10.1007/s00431-007-0418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan PW, Lok FY, Khatijah SB. Risk factors for hypoxemia and respiratory failure in respiratory syncytial virus bronchiolitis. Southeast Asian J Trop Med Public Health 2002;33:806-10. [PubMed] [Google Scholar]
- 26.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr 2003;143:S118-26. 10.1067/s0022-3476(03)00511-0 [DOI] [PubMed] [Google Scholar]
- 27.Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J Glob Health 2015;5:020416. 10.7189/jogh.05.020416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tungsupreechameth A, Srisingh K. Factors Associated with Severe Lower Respiratory Tract Infection from Respiratory Syncytial Virus (RSV) in Thai Children. Siriraj Medical Journal 2021;73:808-14. [Google Scholar]