Abstract
Background
Ruptured abdominal aortic aneurysm (rAAA) is a surgical emergency with a high mortality. Thus, it requires immediate intervention. While endovascular aneurysm repair (EVAR) has become the preferred approach, significant regional disparities remain in diagnosis, treatment accessibility, and outcomes. This study aimed to analyze rAAA repair trends in South Korea over a decade, highlighting inequities and proposing a centralized referral model.
Methods
This retrospective cohort study analyzed patients who underwent rAAA repair between 2007 and 2016 using the national Health Insurance Review and Assessment Service (HIRA) database. Patients were identified using ICD-10 and procedural codes for EVAR and open surgical repair (OSR). Regional differences in diagnosis, repair rates, 30-day mortality, and referral patterns were analyzed to propose an optimized hospital network.
Results
Of 13,832 AAA repairs, 1,812 (13.1%) were for rAAA. EVAR increased from 30.8% in 2007 to 73.7% in 2016, surpassing OSR since 2012. rAAA repairs were concentrated in metropolitan areas (86.6%), showing significant patient influx to Seoul and Daegu (+16.9% and +13.1%, respectively), while low-volume regions exhibited patient efflux rates exceeding 20%. The 30-day mortality rate for rAAA repair was 13.5% nationally, showing evident regional disparities. Low-volume regions demonstrated less than two rAAA repairs per million people, suggesting a potential selective treatment bias.
Conclusions
A 50-km referral system designating 28 vascular centers capable at least 4.5 rAAA repairs annually is proposed, considering transport networks and hospital distribution. National guidelines should implement a coordinated transfer system to ensure timely, equitable access to specialized care and improve survival outcomes.
Keywords: Abdominal aortic aneurysm (AAA), endovascular repair, healthcare disparities, vascular surgery, emergency transfer system
Highlight box.
Key findings
• The utilization of endovascular aneurysm repair (EVAR) for ruptured abdominal aortic aneurysm (rAAA) increased from 30.8% in 2007 to 73.7% in 2016, surpassing open surgical repair (OSR) since 2012.
• Regional disparities in rAAA management were observed, with 86.6% of repairs performed in metropolitan areas, while low-volume regions exhibited patient efflux exceeding 20%.
• The overall 30-day mortality rate for rAAA repair was 13.5%, with significant variation across regions.
• Low-volume regions recorded fewer than two rAAA repairs per million people, indicating potential limitations in access to specialized care.
What is known and what is new?
• rAAA is a life-threatening emergency requiring immediate intervention, with EVAR recognized as the preferred treatment when anatomically feasible.
• High surgical volume is associated with improved patient outcomes, yet disparities in access to care remain unaddressed.
• This study provides a nationwide analysis of rAAA repair in South Korea, highlighting systemic barriers and proposing a centralized referral model to reduce geographic inequities.
What is the implication, and what should change now?
• A standardized referral and transfer system is essential to optimize rAAA management.
• A 50-km referral system is proposed, designating 28 vascular centers with the capacity to perform at least 4.5 rAAA repairs annually, ensuring timely intervention and improved outcomes.
Introduction
Ruptured abdominal aortic aneurysm (rAAA) remains one of the most formidable challenges in vascular surgery. Due to its high mortality, immediate intervention is needed for rAAA to prevent catastrophic outcomes. Among available treatment options, ruptured endovascular aneurysm repair (rEVAR) has emerged as the preferred approach for patients with suitable anatomy, offering superior survival rates and relatively consistent outcomes across hospitals (1-3). However, successful rEVAR implementation hinges on a well-coordinated multidisciplinary team, rapid clinical decision-making, and seamless access to specialized endovascular devices.
In South Korea, several systemic barriers complicate timely rEVAR execution. Strict health insurance regulations make preemptive device stocking difficult, and stringent privacy laws hinder the rapid inter-hospital transfer of medical imaging, delaying critical decision-making. Moreover, when an rEVAR attempt fails and open conversion is required, patients must be transferred again to a facility capable of performing ruptured open surgical repair (rOSR), exacerbating delays in definitive treatment. Since high-volume centers have been consistently linked to better rOSR survival rates (4-6), delayed transfers to such facilities might result in preventable mortality.
Most existing studies on rAAA management have been conducted in countries with vast landmasses, such as the United States, Australia, Germany, and Sweden (7-9). These nations operate under healthcare models that differ fundamentally from South Korea’s system, where universal national health insurance dictates care delivery with patient transport subject to regional constraints rather than an unrestricted referral network. As a result, international findings might not be directly applicable to South Korea, necessitating a localized analysis of treatment trends, disparities, and outcomes.
Currently, South Korea lacks standardized guidelines for inter-facility transfers of rAAA patients. In the absence of a structured referral system, emergency transfers rely on the central emergency medical center, which directs patients to the nearest available hospital with sufficient capacity. However, no comprehensive national data exists on the efficiency of this system or its impact on clinical outcomes, leaving critical gaps in the optimization of rAAA management.
This study aimed to analyze rAAA repair trends in South Korea between 2007 and 2016, comparing utilization of endovascular aneurysm repair (EVAR) and open surgical repair (OSR). Additionally, regional disparities in initial diagnoses, repair distributions, and treatment outcomes were compared to identify inequities in access to life-saving interventions. Based on these findings, an optimized, centralized transfer and treatment model tailored to the unique healthcare landscape of South Korea is proposed, with goals of improving survival rates and standardizing rAAA care nationwide. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-301/rc).
Methods
Patient and public involvement
The Health Insurance Review and Assessment Service (HIRA) is a government-operated agency responsible for reviewing and assessing the quality of claims submitted under the National Health Insurance (NHI) and National Medical Aid (NMA) programs. It covers approximately 98% of the South Korean population. As participation in the HIRA system is mandatory for healthcare providers, the database offers a comprehensive and standardized dataset for nationwide epidemiological studies (10).
For this study, we utilized HIRA data including extensive healthcare service records such as diagnosis, treatment, procedures, and medical history coded according to the International Classification of Diseases, Tenth Revision (ICD-10). Data usage was approved by the HIRA Data Access Committee. All patient information was fully anonymized before transmission, ensuring that no personally identifiable details—such as names, addresses, or dates of birth—were accessible to researchers.
Using the HIRA database, patients diagnosed with abdominal aortic aneurysms (AAA) between January 1, 2007, and December 31, 2016 were identified. Patients were included if they had a diagnosis of either intact AAA (iAAA) or ruptured AAA (rAAA), as classified by the following ICD-10 codes:
❖ I71.3 (rAAA).
❖ I71.4 (AAA, without rupture).
❖ I71.5 (ruptured thoracoabdominal aortic aneurysm).
❖ I71.6 (thoracoabdominal aortic aneurysm, without rupture).
❖ I71.8 (ruptured aortic aneurysm of unspecified site).
❖ I71.9 (aortic aneurysm of unspecified site, without rupture).
Additionally, patients were included if they had undergone a related surgical or endovascular procedure, identified by the following procedure codes:
❖ O0223 (suprarenal or juxtarenal aneurysm resection).
❖ O0224 (infrarenal aneurysm resection).
❖ O2034 (abdominal aorta and iliac artery aneurysm resection).
❖ M6603 (percutaneous intravascular metallic stent placement; aorta).
❖ M6611 (percutaneous intravascular stent-graft placement; aorta).
❖ M6612 (percutaneous intravascular stent-graft placement; aortic and iliac arteries).
Clinical variables and outcomes
This study analyzed a range of demographic and clinical variables, including:
❖ Age, categorized into seven 5-year intervals (<65, 65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years).
❖ Sex (male or female).
❖ Health insurance status, classified into NHI (mandatory enrollment) or NMA (low-income assistance program).
❖ Comorbidities, identified using the Charlson Comorbidity Index (CCI) and classified into three groups: 0–1, 2, and ≥3.
This study also examined specific comorbidities that might influence rAAA outcomes, including:
❖ Hypertension (I10).
❖ Diabetes mellitus (E10, E11, E13, E14).
❖ Myocardial infarction (I21, I22).
❖ Heart failure (I50).
❖ Peripheral artery occlusive disease (PAOD; I70.2, I77.9, I74.4).
❖ Chronic obstructive pulmonary disease (COPD; J44).
❖ Cerebrovascular disease (CVD; I60–I64).
❖ Chronic kidney disease (N18.1–N18.4, N18.9).
❖ End-stage kidney disease requiring dialysis (N18.5).
Primary and secondary outcomes
The primary outcome of this study was to assess trends in AAA repair strategies, comparing the utilization of EVAR and OSR over the study period (2007–2016). Additionally, regional disparities in initial diagnosis and repair rates for rAAA stratified by province were analyzed.
As a secondary outcome, 30-day mortality rates following rAAA repair were evaluated. To contextualize regional variations, we calculated patients per million (PPM) for each province using 2021 demographic data from the Ministry of Public Administration and Security of South Korea (11).
Statistical analysis
Baseline characteristics of rAAA patients undergoing EVAR or OSR were compared to identify potential differences in clinical presentation and outcomes. Regional disparities in initial diagnosis rates, repair rates, and 30-day mortality were quantified at the provincial level.
To propose a structured patient transfer model, we analyzed rAAA diagnosis volumes at tertiary referral hospitals and simulated an optimized delivery system for these patients. Temporal trends in total AAA repairs, iAAA repairs, and rAAA repairs by EVAR and OSR were evaluated over the study period (2007–2016). Geospatial patterns of patient influx and efflux across provinces were visualized to identify high-volume referral centers. Population-adjusted rAAA repair rates (PPM) were calculated for each region to assess accessibility disparities. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB 2406-061-1543). Due to its retrospective nature, the requirement for informed consent was waived by the IRB.
Results
Patient characteristics
Between 2007 and 2016, a total of 13,832 patients underwent AAA repairs, of which 1,812 (13.1%) were for rAAA. Demographics of rAAA patients are summarized in Table 1. Both rEVAR and rOSR groups consisted predominantly males. The highest proportion of patients in both groups was under 65 years old (n=423, 23.3%), followed by those aged 75–79 years (n=383, 21.1%). Regarding CCI scores, patients undergoing iAAA repair had a higher proportion of those with CCI scores ≥3 (iEVAR: 45.3%, iOSR: 39.5%). In contrast, those undergoing rAAA repair predominantly had CCI scores of 0–1 (rEVAR: 40.1%, rOSR: 45.3%). Among all AAA patients, hypertension was the most common comorbidity, followed by diabetes mellitus. Characteristics of iAAA repair patients are described in Table S1.
Table 1. Baseline characteristics of patients with rAAA treated by EVAR or OSR.
| Variables | Ruptured EVAR (n=815) | Ruptured OSR (n=997) | All rAAA repairs (n=1,812) |
|---|---|---|---|
| Sex | |||
| Male | 614 (75.3) | 803 (80.5) | 1,417 (78.2) |
| Female | 201 (24.7) | 194 (19.5) | 395 (21.8) |
| Age, years | |||
| <65 | 193 (23.7) | 230 (23.1) | 423 (23.3) |
| 65–69 | 116 (14.2) | 169 (17.0) | 285 (15.7) |
| 70–74 | 144 (17.7) | 202 (20.3) | 346 (19.1) |
| 75–79 | 166 (20.4) | 217 (21.8) | 383 (21.1) |
| 80–84 | 133 (16.3) | 110 (11.0) | 243 (13.4) |
| 85–89 | 54 (6.6) | 58 (5.8) | 112 (6.2) |
| ≥90 | 9 (1.1) | 11 (1.1) | 20 (1.1) |
| Charlson Comorbidity Index | |||
| 0–1 | 327 (40.1) | 452 (45.3) | 779 (43.0) |
| 2 | 186 (22.8) | 226 (22.7) | 412 (22.7) |
| 3 or more | 302 (37.1) | 319 (32.0) | 621 (34.3) |
| Comorbidities | |||
| CAD (myocardial infarction) | 55 (6.7) | 41 (4.1) | 96 (5.3) |
| Congestive heart failure | 95 (11.7) | 103 (10.3) | 198 (10.9) |
| Cerebrovascular disease | 25 (3.1) | 262 (26.3) | 287 (15.8) |
| Peripheral vascular disease | 199 (24.4) | 218 (21.9) | 417 (23.0) |
| Hypertension | 654 (80.2) | 723 (72.5) | 1,377 (76.0) |
| Diabetes mellitus | 292 (35.8) | 286 (28.7) | 578 (31.9) |
| Chronic obstructive pulmonary disease | 155 (19.0) | 121 (12.1) | 276 (15.2) |
| Chronic kidney disease | 78 (9.6) | 88 (8.8) | 166 (9.2) |
| End-stage kidney disease (dialysis) | 17 (2.1) | 19 (1.9) | 36 (2.0) |
Data are presented as n (%). CAD, coronary artery disease; EVAR, endovascular aneurysm repair; OSR, open surgical repair; rAAA, ruptured abdominal aortic aneurysm.
Trends in total, intact, and ruptured AAA repairs by EVAR and OSR
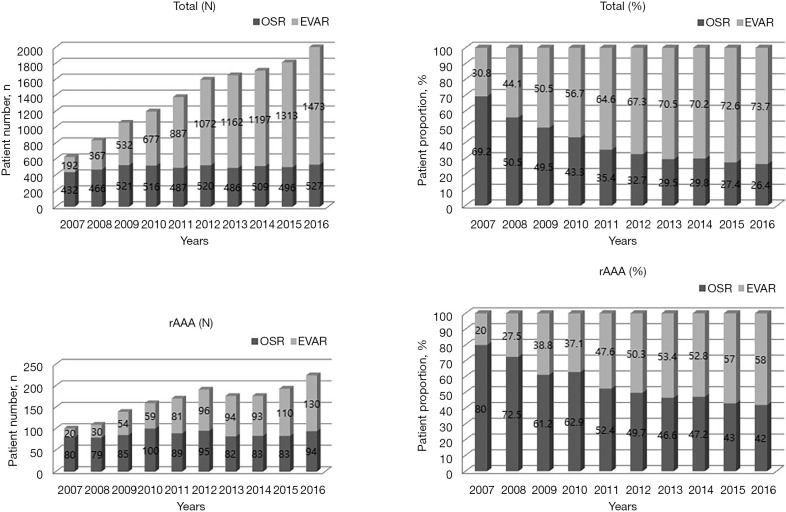
Over the ten years since 2007, the total number of AAA repairs increased by 220.5%. The proportion of EVAR rose from 30.8% in 2007 to 73.7% in 2016, surpassing OSR as the primary repair method in 2009. Meanwhile, the proportion of OSR declined from 69.2% to 26.4% over the same period. For iAAA, repair trends closely paralleled those observed for total AAA repairs (Figure S1). In contrast, the trend for rAAA repairs showed a different pattern. The total number of rAAA repairs increased from 2007 to 2012. It then stabilized until 2015. Since 2012, the number of rEVAR cases has consistently exceeded those of rOSR, reflecting a shift towards minimally invasive interventions for rAAA (Figure 1).
Figure 1.
Trends in total and ruptured abdominal aortic aneurysm repairs by EVAR and OSR from 2007 to 2016. EVAR, endovascular aneurysm repair; OSR, open surgical repair; rAAA, ruptured abdominal aortic aneurysm.
Difference between initial diagnosis and repair for rAAA by province
Table 2 presents distribution of initial diagnoses and subsequent rAAA repairs across provinces and major cities in South Korea from 2007 to 2016. Seoul recorded the highest number of initial diagnoses with 344 (28.4%) cases, which increased to 402 (33.2%) repairs, representing a net increase of 58 (16.9%) cases. Gyeonggi, the most populous province, had 223 (18.4%) initial diagnoses but experienced a net efflux of 17 (−7.6%) cases, resulting in 206 (17.0%) repairs. Among the six metropolitan cities, only Daegu showed a notable patient influx of 13.1%, whereas the others had patient influx or efflux of less than 10%. Jeju, the only island province, experienced a 12.5% patient efflux. Other regions exhibited patient efflux rates ranging from 9.0% to 83.3%. Notably, Chungbuk, Gyeongbuk, and Jeollanam had fewer than 10 repair cases each throughout the decade. Figure 2 visually presents patient influx and efflux rates between regions for initial diagnosis and rAAA repair.
Table 2. Difference between initial diagnosis and repair for ruptured abdominal aortic aneurysm repair by province.
| Region | Initial diagnosis, n (%) | Repair, n (%) | PPM | Repair-initial diagnosis, n | Patient influx ratio, % |
|---|---|---|---|---|---|
| Seoul metropolitan city | 344 (28.4) | 402 (33.2) | 3.57 | 58 | 16.9 |
| Gyeonggi | 223 (18.4) | 206 (17.0) | 1.66 | −17 | −7.6 |
| Busan metropolitan city | 143 (11.8) | 148 (12.2) | 4.22 | 5 | 3.5 |
| Daegu metropolitan city | 99 (8.2) | 112 (9.2) | 4.10 | 13 | 13.1 |
| Gyeongnam | 67 (5.5) | 61 (5.0) | 2.01 | −6 | −9.0 |
| Gwangju metropolitan city | 59 (4.9) | 63 (5.2) | 4.07 | 4 | 6.8 |
| Incheon metropolitan city | 59 (4.9) | 55(4.5) | 2.01 | −4 | −6.8 |
| Ulsan metropolitan city | 38 (3.1) | 37 (3.1) | 3.35 | −1 | −2.6 |
| Jeollabuk | 32 (2.6) | 29 (2.4) | 1.78 | −3 | −9.4 |
| Gangwon | 29 (2.4) | 23 (1.9) | 1.88 | −6 | −20.7 |
| Daejeon metropolitan city | 26 (2.2) | 27 (2.2) | 1.78 | 1 | 3.8 |
| Chungnam | 25 (2.1) | 20 (1.7) | 1.18 | −5 | −20.0 |
| Chungbuk | 19 (1.6) | 6 (0.5) | 1.19 | −13 | −68.4 |
| Gyeongbuk | 18 (1.5) | 3 (0.2) | 0.68 | −15 | −83.3 |
| Jeju | 16 (1.3) | 14 (1.2) | 2.37 | −2 | −12.5 |
| Jeollanam | 15 (1.2) | 6 (0.5) | 0.81 | −9 | −60.0 |
Patient influx ratio: repair-initial diagnosis/initial diagnosis. PPM, patients per million.
Figure 2.
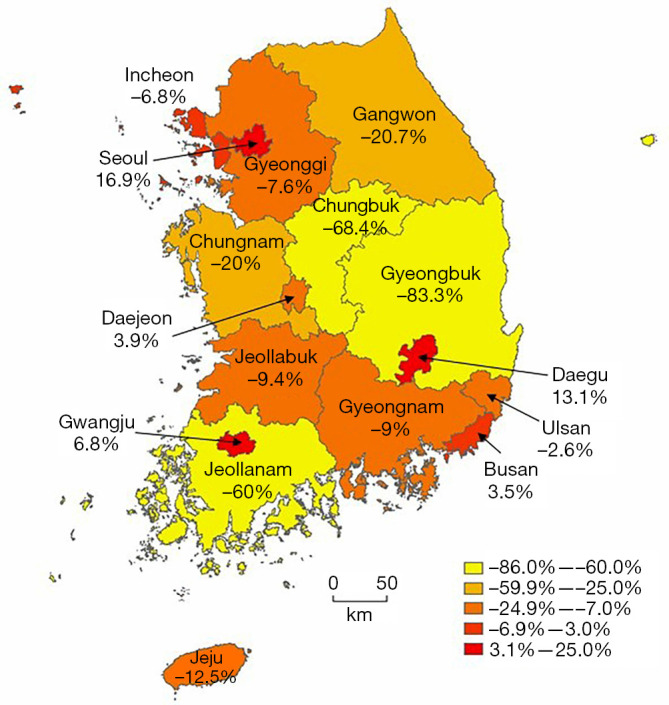
Visual representation of patients’ influx and efflux rates between regions for initial diagnosis and repair of ruptured abdominal aortic aneurysm.
Treatment outcomes of rAAA repairs
Thirty-day mortality rates following rAAA repair are presented in Table 3. The overall 30-day mortality rate was 13.5% nationally, showing evident regional differences. Regions with high patient influx, such as Seoul and Daegu where repairs exceeded initial diagnoses by more than 10%, had 30-day mortality rates of 24.1%. In regions where ratios of initial diagnoses to repairs were within 10%, 30-day mortality rates ranged from 20.6% to 37.8%. Conversely, regions with significant patient efflux, where initial diagnoses exceeded repairs by more than 10%, typically had mortality rates below 20%. These findings suggest that patients transferred to high-volume centers might have benefited from improved outcomes due to greater surgical expertise and more structured postoperative care.
Table 3. Thirty-day mortality rates for rAAA repairs by region in South Korea.
| Region | Repair (2007–2016), n |
30-day mortality for rAAA repair, n (%) |
|---|---|---|
| Seoul | 402 | 97 (24.1) |
| Gyeonggi | 206 | 68 (33.0) |
| Busan metropolitan city | 148 | 56 (37.8) |
| Daegu metropolitan city | 112 | 26 (23.2) |
| Gyeongnam | 61 | 18 (29.5) |
| Gwangju metropolitan city | 63 | 13 (20.6) |
| Incheon metropolitan city | 55 | 18 (32.7) |
| Ulsan metropolitan city | 37 | 11 (29.7) |
| Jeollabuk | 29 | 10 (34.5) |
| Gangwon | 23 | 5 (21.7) |
| Daejeon metropolitan city | 27 | 7 (25.9) |
| Chungnam | 20 | 3 (15.0) |
| Chungbuk | 6 | 1 (16.7) |
| Gyeongbuk | 3 | 0 |
| Jeju | 14 | 2 (14.3) |
| Jeollanam | 6 | 0 |
rAAA, ruptured abdominal aortic aneurysm.
Proposal for an rAAA patient transfer network
To optimize rAAA management and improve survival outcomes, we proposed to establish a centralized healthcare system within a 50-km radius based on annual number of rAAA diagnoses in each region. For Jeju Island, an exception was made to group it with the Seoul region, allowing for patient transfer directly to Seoul rather than within the island itself. From tertiary referral hospitals designated by South Korea’s Ministry of Health and Welfare, those that met the criteria for elective AAA repairs and could perform rAAA repairs were selected (Table 4). Each of the final 28 designated hospitals is expected to perform approximately 4.5 rAAA repairs annually. Figure 3 visually presents this distribution, with each circle indicating a 50-km radius around designated hospitals. Additionally, it presents the population and rAAA PPM by region in South Korea from 2007 to 2016. This system was also designed to promote efficient allocation and utilization of limited medical resources.
Table 4. Proposal for a rAAA patient delivery system based on regional rAAA diagnosis volumes among tertiary referral hospital in South Korea.
| Region | rAAA diagnosis per year | 4.5 rAAA repairs per year | Referral centers, n | 28 designated hospitals (beds) |
|---|---|---|---|---|
| Seoul, Jeju | 36 | 8 | 8 | A (1,997), B (1,762), C (2,715), D (803), E (1,356), F (1,048), G (2,458), H (689) |
| Gyeonggi | 22.3 | 4.96 | 5 | I (809), J (1,450), K (1,334), L (1,172), M (867) |
| Busan metropolitan city | 14.3 | 3.18 | 3 | N (997), O (1,191), P (1,204) |
| Daegu metropolitan city, Gyeongbuk | 11.7 | 2.6 | 3 | Q (912), R (1,012), S (874) |
| Daejeon metropolitan city, Chungbuk, Chungnam | 7 | 1.56 | 2 | T (1,329), U (793) |
| Gwangju metropolitan city, Jeollanam | 7.4 | 1.64 | 2 | V (1,078), W (849) |
| Incheon metropolitan city | 5.9 | 1.31 | 1 | X (925) |
| Ulsan metropolitan city | 3.8 | 0.84 | 1 | Y (998) |
| Jeollabuk | 3.2 | 0.71 | 1 | Z (1,143) |
| Gangwon | 2.9 | 0.64 | 1 | A’ (866) |
| Gyeongnam | 6.7 | 1.49 | 1 | B’ (893) |
rAAA, ruptured abdominal aortic aneurysm.
Figure 3.
Population and ruptured abdominal aortic aneurysm patients per million by region in South Korea (2007–2016). Each box in the figure display region, population and total incidence (patients per million) from 2007 to 2016.
Discussion
This study analyzed a decade of ruptured AAA (rAAA) repair trends in South Korea, focusing on epidemiology, regional disparities in diagnosis and treatment, and associated outcomes. Our findings highlight significant geographic variations in rAAA management, with 86.6% of repairs concentrated in metropolitan areas such as Seoul, Gyeonggi, and other high-volume centers. This concentration underscores the critical need for a structured and optimized patient transfer and treatment model to ensure equitable access to life-saving interventions.
Over the ten-year study period, the 30-day mortality rate for rAAA repairs was 13.5% nationally, showing substantial regional variations. Seoul and Daegu, which experienced high patient influx (>10%), reported 30-day mortality rates of 24.1% and 23.2%, respectively. These rates were notably lower than those observed in other studies (12,13), suggesting that high-volume centers might provide survival benefits. However, despite performing a high volume of surgeries, Gyeonggi and Busan exhibited significantly higher mortality rates (33% and 37.8%, respectively). This indicates that mere patient volume is insufficient. Efficient patient transfer and system-wide coordination also play a pivotal role in improving survival. Additionally, the fact that Gyeonggi and Busan had similarly high mortality rates despite different patient flow patterns suggests that other factors such as hospital capacity, treatment delays, or care coordination may also affect outcomes
Regions with high patient efflux (>20%), where many rAAA patients were transferred out, displayed lower mortality rates (~10%). However, these areas had fewer than two PPM. Some regions recorded fewer than ten rAAA repairs throughout the decade. As our analysis included only patients who received both diagnosis and repair, many rAAA cases in low-volume regions may have underdiagnosed or untreated. Based on an estimated national incidence of 18.2 cases per million population (2012–2016) (14), this analysis suggests substantial underdiagnosis in these areas and highlights a potential treatment bias, where patients in resource-limited regions may not have had equal access to care.
The Society for Vascular Surgery (SVS) recommends a door-to-intervention time of <90 minutes for rAAA, following the 30-30-30 framework—30 minutes for initial medical contact and stabilization, 30 minutes for rapid transfer to a regional vascular center, and 30 minutes for evaluation and intervention, including the use of an aortic occlusion balloon (1). However, current rAAA management in South Korea lacks clear national guidelines for inter-facility transfers, leading to inconsistent care pathways. While SVS criteria require centers performing elective OSR for AAA to conduct ≥10 open aortic operations per year with a perioperative mortality rate ≤5%, equivalent benchmarks do not exist for rAAA repairs. Based on our findings, we proposed a threshold of ≥4.5 rAAA repairs annually per designated center as a feasible benchmark to ensure proficiency and optimize outcomes (Figure 3). This threshold was derived from region-specific modeling using actual rAAA diagnosis volumes and hospital distribution. We confirmed that designating centers performing an average of 4.5 rAAA repairs per year allowed sufficient coverage of the population within a 50-km radius, thereby ensuring both geographic accessibility and institutional competency.
The proposed figure takes into account South Korea’s road conditions, as provided by the National Transport Information Center, as well as geographic distribution of tertiary referral hospitals. In this study, we determined the number of medical institutions that could handle regional rAAA case volumes while maintaining an appropriate balance. If too many institutions are involved, managing treatment quality and coordination becomes challenging. On the other hand, if too few institutions are designated, excessive patient loads might overwhelm the system. Thus, our selection process aimed to optimize both accessibility and efficiency in rAAA management.
While some remote regions—including Jeju Island, the Taebaek Mountain region, and areas near the Demilitarized Zone (DMZ)—fall outside the proposed referral network, a centralized transfer-based system may offer a more cost-effective and feasible alternative to establishing new vascular centers. The annual operating cost of a university tertiary hospital exceeds 1.5 billion USD, whereas the total annual cost of structured patient transfer, including ground and air transport, is estimated at only 5,500 to 42,500 USD in these low-incidence areas (15). Emerging technologies such as urban air mobility (UAM) may further enhance timely access to specialized care in geographically isolated regions. Equipping high-volume referral centers with necessary infrastructure, trained personnel, and real-time inter-hospital communication systems is essential for standardizing rAAA care. A centralized registry system should be implemented to track annual case volumes, repair methods, 30-day mortality, complications, and healthcare costs (16,17). Furthermore, standardizing emergency room (ER) to operating room (OR) transfer protocols could minimize delays and ensure consistent treatment outcomes nationwide (18).
Limitations
This study has several limitations. First, prior research has reported that 57% of rAAA diagnoses in HIRA data might have been misclassified, potentially leading to overestimation of incidence and underestimation of mortality (19). However, by restricting our analysis to patients who had both an rAAA diagnosis and a corresponding repair procedure, potential bias was minimized. Second, HIRA data do not capture critical health behaviors such as smoking, alcohol consumption, exercise, or diet, nor do they account for socioeconomic disparities, all of which might have influenced rAAA outcomes. Third, our study did not differentiate between rEVAR and rOSR, which limits interpretation of survival differences across regions. Variation in access to EVAR-such as logistical challenge in device availability, may have influenced both treatment decisions and outcomes. Further studies incorporating procedural data are needed to clarify the impact of surgical approach on survival. Lastly, although our dataset spans from 2007 to 2016, the COVID-19 pandemic has since altered patient transfer protocols, complicating direct comparisons with more recent data. However, given that no major changes in rAAA management have been reported in South Korea over the past two decades, our findings remain highly relevant to ongoing policy discussions (20-22).
Conclusions
This study underscores the urgent need for a centralized and standardized approach to rAAA management in South Korea. By addressing regional disparities in diagnosis and treatment, optimizing inter-hospital transfer protocols, and ensuring that high-volume centers are properly equipped and staffed, patient outcomes can be significantly improved. Future research should aim to differentiate rEVAR and rOSR outcomes across regions and explore the influence of modifiable risk factors and socioeconomic disparities on rAAA survival. Ultimately, this study provides crucial insights into resource allocation, healthcare system restructuring, and establishment of an optimized interregional referral network for this highly lethal condition.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB 2406-061-1543). Due to its retrospective nature, the requirement for informed consent was waived by the IRB.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-301/rc
Funding: This study was supported by the Lee Yong Gak Research Fund of the Korean Society for Vascular Surgery. The funding body had no role in the study design, data collection, data analysis, manuscript preparation, or decision to submit the manuscript for publication.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-301/coif). S.A. reports that this study was supported by the Lee Yong Gak Research Fund of the Korean Society for Vascular Surgery. The funding had no role in the study design, data collection, data analysis, manuscript preparation, or decision to submit the manuscript for publication. The other authors have no conflicts of interest to declare.
References
- 1.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2-77.e2. 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 2.Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57:8-93. 10.1016/j.ejvs.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline NG156. Accessed 20 March 2020. Available online: https://www.nice.org.uk/guidance/NG156 [PubMed]
- 4.Greenleaf EK, Hollenbeak CS, Aziz F. Outcomes after ruptured abdominal aortic aneurysm repair in the era of centralized care. J Vasc Surg 2020;71:1148-61. 10.1016/j.jvs.2019.06.187 [DOI] [PubMed] [Google Scholar]
- 5.Lim S, Kwan S, Colvard BD, et al. Impact of interfacility transfer of ruptured abdominal aortic aneurysm patients. J Vasc Surg 2022;76:1548-1554.e1. 10.1016/j.jvs.2022.05.020 [DOI] [PubMed] [Google Scholar]
- 6.Behrendt CA, Kölbel T, Larena-Avellaneda A, et al. Ten Years of Urgent Care of Ruptured Abdominal Aortic Aneurysms in a High-Volume-Center. Ann Vasc Surg 2020;64:88-98. 10.1016/j.avsg.2019.09.035 [DOI] [PubMed] [Google Scholar]
- 7.Lundgren F, Troëng T. Treatment choice and survival after ruptured abdominal aortic aneurysm: A population-based study. J Vasc Surg 2020;72:508-517.e11. 10.1016/j.jvs.2019.11.060 [DOI] [PubMed] [Google Scholar]
- 8.Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg 2019;69:378-384.e2. 10.1016/j.jvs.2018.03.435 [DOI] [PubMed] [Google Scholar]
- 9.Sawang M, Paravastu SCV, Liu Z, et al. The Relationship Between Operative Volume and Peri-operative Mortality After Non-elective Aortic Aneurysm Repair in Australia. Eur J Vasc Endovasc Surg 2020;60:519-30. 10.1016/j.ejvs.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 10.Ahn S, Min JY, Kim HG, et al. Outcomes after aortic aneurysm repair in patients with history of cancer: a nationwide dataset analysis. BMC Surg 2020;20:85. 10.1186/s12893-020-00754-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korea Statistical Information Service (KOSIS). Causes of Death Statistics. Accessed July 18, 2025. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1BPA002&vw_cd=MT_ZTITLE&list_id=8389
- 12.Jones M, Koury H, Faris P, et al. Impact of an emergency endovascular aneurysm repair protocol on 30-day ruptured abdominal aortic aneurysm mortality. J Vasc Surg 2022;76:663-670.e2. 10.1016/j.jvs.2022.02.050 [DOI] [PubMed] [Google Scholar]
- 13.Daviú-Molinari T, Chin-Bong Choi J, Roberts MC, et al. In-hospital mortality risk after endovascular and open aortic aneurysm repairs for ruptured abdominal aortic aneurysms. J Vasc Surg 2024;80:1448-1454.e1. 10.1016/j.jvs.2024.07.022 [DOI] [PubMed] [Google Scholar]
- 14.Choi C, Ahn S, Min SI, et al. Nationwide Epidemiologic Study of Abdominal Aortic Aneurysms in Korea: A Cross-Sectional Study Using National Health Insurance Review and Assessment Service Data. Vasc Specialist Int 2019;35:193-201. 10.5758/vsi.2019.35.4.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ALIO (All Public Information in-One). Search Results for “Seoul National University Hospital”. Accessed July 18, 2025. Available online: https://www.alio.go.kr/search/searchTabPage.do?word=%EC%84%9C%EC%9A%B8%EB%8C%80+%EB%B3%91%EC%9B%90&apbaNm=&sortType=LATEST&tab=attach
- 16.Budtz-Lilly J, Björck M, Venermo M, et al. Editor's Choice - The Impact of Centralisation and Endovascular Aneurysm Repair on Treatment of Ruptured Abdominal Aortic Aneurysms Based on International Registries. Eur J Vasc Endovasc Surg 2018;56:181-8. 10.1016/j.ejvs.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 17.Capó XF, García Reyes ME, Cánovas ÁS, et al. Hospital Volume of Elective Abdominal Aortic Aneurysm Repair as a Predictor of Mortality After Ruptured Abdominal Aortic Aneurysm Repair. Eur J Vasc Endovasc Surg 2024;68:30-8. 10.1016/j.ejvs.2024.02.034 [DOI] [PubMed] [Google Scholar]
- 18.Takei Y, Tezuka M, Saito S, et al. A protocol-based treatment for ruptured abdominal aortic aneurysm contributed to improving aorta-related mortality: a retrospective cohort study. BMC Cardiovasc Disord 2023;23:436. 10.1186/s12872-023-03473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo EA, Seong S, Ahn S, et al. Validation of I71.3 code for ruptured abdominal aortic aneurysm in Korea: misplaced diagnosis in claims data. Ann Surg Treat Res 2023;104:170-5. 10.4174/astr.2023.104.3.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Kwon TW, Cho YP, et al. Treatment Outcomes of Patients With Ruptured Abdominal Aortic Aneurysms. J Korean Med Sci 2023;38:e321. 10.3346/jkms.2023.38.e321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JA, Yoon S, Kim LY, et al. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J Korean Med Sci 2017;32:718-28. 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo EA, Ahn S, Mo H, et al. A 20-Year Analysis of Ruptured Abdominal Aortic Aneurysm Outcomes and Associated Factors in Korea. Ann Vasc Surg 2024;102:152-9. 10.1016/j.avsg.2023.10.033 [DOI] [PubMed] [Google Scholar]