Abstract
Background and Objective
Bronchiectasis is a chronic respiratory disease characterized by predominantly neutrophilic inflammation, recurrent infection and pathological dilatation of the airways. Current therapeutic strategies primarily target infections and improving mucus clearance. However, no current treatment can directly ameliorate neutrophilic inflammation. Recently, dipeptidyl peptidase 1 (DPP-1) inhibitors effectively abrogated neutrophilic inflammation in bronchiectasis. This narrative review aimed to analyze the structural characteristics and functional effects of DPP-1, explore the mechanism of DPP-1 inhibitors in bronchiectasis, and highlight major clinical trial findings.
Methods
We performed an online electronic search for relevant English literature on PubMed and Web of Science databases, with the keywords of “dipeptidyl peptidase 1”, “cathepsin C”, “cathepsin C structure”, “cathepsin C maturation”, “cathepsin C loss-of-function”, “neutrophil serine proteases and bronchiectasis”, “neutrophil serine proteases and cathepsin C”, “neutrophil serine proteases and DPP-1”, “DPP-1 inhibitors”, “cathepsin C inhibitors”, “DPP-1 inhibitors and bronchiectasis”, “cathepsin C inhibitors and bronchiectasis”. Two authors (R.D.T. and J.Q.Y.) independently searched and reviewed the articles.
Key Content and Findings
Excessive and uncontrolled release of neutrophil serine proteases (NSPs) can lead to airway inflammatory responses and structural damage in bronchiectasis. Elevated levels of neutrophil elastase (NE) and other neutrophil markers in the airway are associated with exacerbations and lung function decline. In light of the pivotal role of DPP-1 in eliciting neutrophilic inflammation, several DPP-1 inhibitors have been developed and entered clinical trials for safety and efficacy assessment in bronchiectasis. Of these, brensocatib markedly prolonged the time to the first exacerbation and decreased the exacerbation frequency via decreasing the activity of NSPs. The phase III ASPEN trial has been completed and confirms reduced exacerbations and slower lung function decline with DPP-1 inhibitor treatment. Treatment with BI 1291583 reduced the risk of bronchiectasis exacerbations in the phase II Airleaf® trial. HSK31858 significantly decreased the exacerbation frequency and prolonged the time to the first exacerbation in the latest phase II trial among the Chinese population.
Conclusions
DPP-1 inhibitors have exhibited promising effects in improving several major clinical outcomes in bronchiectasis via suppressing the activity of NSPs.
Keywords: Dipeptidyl peptidase 1 inhibitors (DPP-1 inhibitors), neutrophilic inflammation, bronchiectasis
Introduction
Bronchiectasis is a common chronic structural lung disease, characterized by a vicious vortex of airway infection, mucus clearance impairment, neutrophilic inflammation, and structural damage (1). Currently, the prevalence and incidence of bronchiectasis are increasing globally, with a major economic burden for hospitalized patients and outpatients (2,3). Long-term and repeated use of antibiotics may readily predispose to drug resistance (4). Bronchiectasis is an inflammatory disease but there are currently no treatment approaches proven to tackle the inflammatory component of the disease.
Neutrophils mediate the host immune defense through the secretion of proteases, the formation of neutrophil extracellular traps (NETs), and phagocytosis (5,6). However, excessive and sustained activation of neutrophils can lead to chronic inflammation and airway destruction. The degree of neutrophilic inflammation has been shown to be closely associated with the severity of bronchiectasis and long-term prognosis, including the risk of exacerbations and lung function decline (7,8).
Serine proteases are synthesized in an inactive form, known as zymogens, and are selectively activated through targeted proteolysis (9). Typically, neutrophils contain a distinctive group of eleven single-domain serine proteases, with their precursors being actively transformed into the functional enzymes by dipeptidyl peptidase 1 (DPP-1) throughout the process of synthesis, targeting, and sequestration within the cytoplasmic granules. DPP-1 [also known as cathepsin C (CatC)] is the ubiquitously expressed lysosomal cysteine exopeptidase that belongs to the papain family of cysteine proteases. DPP-1 plays a crucial role in activating various granular serine proteases including cathepsin G (CatG), neutrophil elastase (NE) and proteinase 3 (PR3).
Neutrophil serine proteases (NSPs) are primarily stored within the azurophilic granules of neutrophils and modulate airway inflammation and tissue remodeling, whereas uncontrolled NSPs release may result in degenerative tissue damage (10-12). High NSPs activity has been associated with mucus hypersecretion, lung destruction and rapid lung function decline (13). NE is the most widely reported NSPs member in bronchiectasis, and its expression level has been closely correlated with the frequency of acute exacerbation and disease severity (7,14). Elevated levels of NE, PR3, and CatG that exceed the neutralizing capacity of natural inhibitors such as α1-antitrypsin and secretory leukoproteinase inhibitor can alter the airway microenvironment and increase the risk of infection (15). When these pro-inflammatory NSPs are activated and released, they can degrade a variety of extracellular matrix components (16,17), leading to tissue damage and chronic airway inflammation.
Currently, there are no regulatory approved treatment that directly targets neutrophilic inflammation which predominate in bronchiectasis. Achieving a balance between preserving an effective immune response to bacterial infections and minimizing inflammatory damage is crucial for developing targeted neutrophil inflammatory therapeutic approaches. Accumulating evidence has suggested that DPP-1 is a promising target for the treatment of neutrophil-mediated inflammatory diseases. DPP-1 inhibitors, by inhibiting NSPs activity, can alleviate neutrophilic inflammation, mitigate the aberrant host immune response, reduce mucus production, and ameliorate bronchiectasis progression (13). Individuals with loss-of-function mutations of the dpp-1 gene, causing a rare disease called Papillon-LeFevre syndrome (PLS), usually do not exhibit significant immune deficiencies (18). DPP-1 knock-out mice exhibited minimal accumulation of neutrophils upon the flares of acute arthritis (19), highlighting the therapeutic potential of DPP-1 inhibition in neutrophilic inflammation. Recent clinical trials pertaining to DPP-1 inhibitors have further substantiated its therapeutic potential in bronchiectasis through the targeted modulation of neutrophilic inflammation (20,21).
In this review, we summarize the structural characteristics, biosynthesis, and maturation of DPP-1, and delineate its physiological roles and pathological effects. We also assess DPP-1 as a therapeutic target and present the latest advances in the development and clinical evaluation of DPP-1 inhibitors. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-289/rc).
Methods
We searched PubMed and Web of Science databases for relevant articles published in journals up to 5th January 2025. The detailed search strategy can be found in Table 1.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search | 5th January 2025 |
| Databases and other sources searched | PubMed, Web of Science |
| Search terms used | “dipeptidyl peptidase 1”, “cathepsin C”, “cathepsin C structure”, “cathepsin C maturation”, “cathepsin C loss-of-function”, “neutrophil serine proteases and bronchiectasis”, “neutrophil serine proteases and cathepsin C”, “neutrophil serine proteases and DPP-1”, “DPP-1 inhibitors”, “cathepsin C inhibitors”, “DPP-1 inhibitors and bronchiectasis”, “cathepsin C inhibitors and bronchiectasis” |
| Timeframe | 1980–2025 |
| Inclusion and exclusion criteria | Inclusion criteria: original article, review, meeting abstract; language restrictions: English only |
| Exclusion criteria: letter to the editor, editorial | |
| Selection process | R.D.T. and J.Q.Y. independently searched and reviewed articles |
DPP-1, dipeptidyl peptidase 1.
Overview of DPP-1 and its inhibitors
DPP-1: characteristics and functions
Biosynthesis, maturation and structural characteristics
In mammals, DPP-1 is primarily expressed in the lungs, spleen, kidneys, liver, and myeloid cells, especially in neutrophils, mast cells, monocytes, macrophages, and their precursors (9,22). Initially, DPP-1 is synthesized as a single-chain glycosylated monomeric proenzyme, consists of four regions: the “exclusive” domain (Asp1-Gly119), the pro-peptide region (Thr120-His206), a heavy chain (Leu207-Arg370), and a light chain (Asp371-Leu439) (23). The maturation of DPP-1 is primarily achieved through a two-step process involving the processing mediated by CatL-like cysteine cathepsins (24,25). Following biosynthesis, the pro-peptide acts as an intramolecular chaperone to assist in the folding and stabilization of the DPP-1 precursor into a dimeric form (26). The dimeric form of Pro-DPP-1 is further processed into a catalytically active mature homo-tetrameric form (23).
DPP-1 is the only member of the papain family that uniquely operates as a tetrameric complex, characterized by a distinctive “exclusive” domain that facilitates the recognition and anchoring of specific catalytic substrates (27). The four identical subunits are connected through the non-covalent interactions, forming a stable quaternary structure essential for maintaining the enzymatic function (28). The matured DPP-1 consists of an N-terminal fragment (termed the residual pro-part domain), a heavy chain, and a light chain (23,27). The active site where DPP-1 exerts its catalytical function comprises the heavy and light chains on the surface of the protein complex. The thiolate-imidazolium ion pair formed by Cys234 on the heavy chain and His381 on the light chain are the key catalytic residues related to the physiological function (29). The heavy and light chains interweave to form an active site cleft, creating a site known as the S2 pocket. Above the S2 pocket, there exists a broad and shallow S1 site located on the surface of the protein’s exposed region (29). The N-terminal Asp1 is located at the entrance of the S2 pocket (27).
The Asp1 in the exclusive domain is responsible for recognizing and anchoring of the specific catalytic substrates, thereby maintaining the activity of aminopeptidase (27). The exclusion domain blocks the enzyme’s active sites outside the S2 subsite, allowing it to only access the N-terminus of the substrate (30). Three amino acid residues (Asp1, Cys234, His381) collectively form a catalytic triad complex, which is responsible for recognizing the type of substrate, restricting the length of the cleaved polypeptide, and slicing the polypeptide (28,31).
The physiological function and pathological roles of DPP-1
DPP-1 catalyzes the cleavage of amide bonds in peptide substrates and is highly selective for the hydrolysis substrates. This is also important for its capacity to modulate a series of physiological or pathological processes. DPP-1 activates many granule-associated serine proteases with pro-inflammatory and immune functions such as NSPs, mast cell chymase, tryptase, and lymphocyte granzymes (22,32). Because of its role in activating NSPs, DPP-1 is implicated in the pathogenesis and progression of various inflammatory and autoimmune diseases such as acute pancreatitis, inflammatory bowel disease, rheumatoid arthritis, glomerulonephritis, chronic obstructive pulmonary disease (COPD) and bronchiectasis (19,33,34).
Loss-of-function mutations in the human dpp-1 (ctsc) gene can cause PLS (35). PLS is a rare autosomal recessive genetic disorder caused by mutations in the ctsc gene that may result in DPP-1 dysfunction, leading to diffuse palmoplantar hyperkeratosis, severe prepubertal periodontitis, and a premature loss of the primary and permanent teeth (36,37). Despite some functional deficiencies in neutrophil activity, such as abnormal release of pro-inflammatory factors, impaired chemotaxis and an inability to form NETs, the bactericidal capacity of neutrophils remains intact in PLS and patients typically do not appear to have evidence of increased susceptibility to severe infections. Additionally, loss-of-function mutations in the dpp-1 gene can lead to Haim-Munk syndrome, which is characterized by palmoplantar hyperkeratosis, severe early onset periodontitis, onychogryphosis, pes planus, arachnodactyly, and acro-osteolysis (38,39). The absence of severe immunodeficiency in these disorders provides solid evidence for the safety of targeting DPP-1 as the therapeutic strategies for inflammatory diseases.
NSPs in bronchiectasis
NSPs are produced during the differentiation of neutrophils in the bone marrow and are synthesized as zymogens, requiring two separate N-terminal processing steps to become enzymatically activated (11). Immediately after synthesis, the signal peptide is cleaved off by signal peptidase, resulting in an N-terminal pro-sequence with two amino acid residues, and the subsequent removal of this N-terminal pro-dipeptide is dependent on the normal expression of DPP-1 (11). NSPs are stored in an active form within the neutrophil granules and are released when neutrophils are activated at the site of inflammation. Upon activation, NSPs are essential for modulating the inflammatory responses and tissue remodeling (40). However, uncontrolled NSPs release and heightened NSPs activity can lead to prominent tissue destruction.
Chronic neutrophilic inflammation is a major driver of bronchiectasis and is associated with heightened release of NSPs (including NE, CatG, and PR3), which facilitates the development of the pathogenic airway environment of bronchiectasis. Sputum NE activity has been demonstrated to be associated with the severity of bronchiectasis, the frequency of acute exacerbations, microbiome diversity, and future lung function decline (7,14). Furthermore, the expression levels of PR3 and CatG are significantly correlated with levels of NE in bronchiectasis (41).
NE accounts for approximately 80% of the total proteolytic activity and is the most destructive protein-degrading enzyme within the body. NE can up-regulate the expression of airway mucins, particularly mucin 5AC, leading to impaired mucociliary clearance (42). NE, which targets the elastin and other pulmonary components, can degrade elastin and collagen, and irreversibly damages the airway structure by cleaving structural proteins such as laminin and fibronectin, leading to irreversible airway dilatation and early-stage bronchiectasis (43). NE is also capable of cleaving host defense proteins, including secretory leucoprotease inhibitor and elafin (44), which suggests that NE could compromise the airway defense to bacterial colonization and infection.
PR3 is a long serine protease expressed by the activated neutrophils, and its biological functions are similar to those of NE. Specifically, PR3 is responsible for the proteolytic degradation and activation of interleukin (IL)-8 within the truncated peptides, thereby promoting the chemoattractive effects on neutrophils (45). This leads to the accumulation of neutrophils in the airways, creating a vicious cycle where neutrophils induce the overexpression of IL-8 through PR3, and IL-8 in turn may amplify the recruitment of neutrophils to the airways. PR3 has also been demonstrated to activate the downstream inflammatory pathways, including the activation of tumor necrosis factor-alpha and IL-1β. Therefore, PR3 may potently induce or amplify airway inflammation (46).
CatG is a serine protease enzyme primarily expressed in the azurophilic granules of neutrophils and is implicated in the immune response and inflammation. High levels of NE and CatG cause the degradation of the extracellular matrix by activating the matrix metalloproteinases (47), contributing to tissue remodeling and structural lung damage. NE, PR3 and CatG are also implicated in the release of pro-inflammatory effectors such as IL-36α, IL-36β, IL-36γ, IL-1α, IL-1β and IL-33 (48,49).
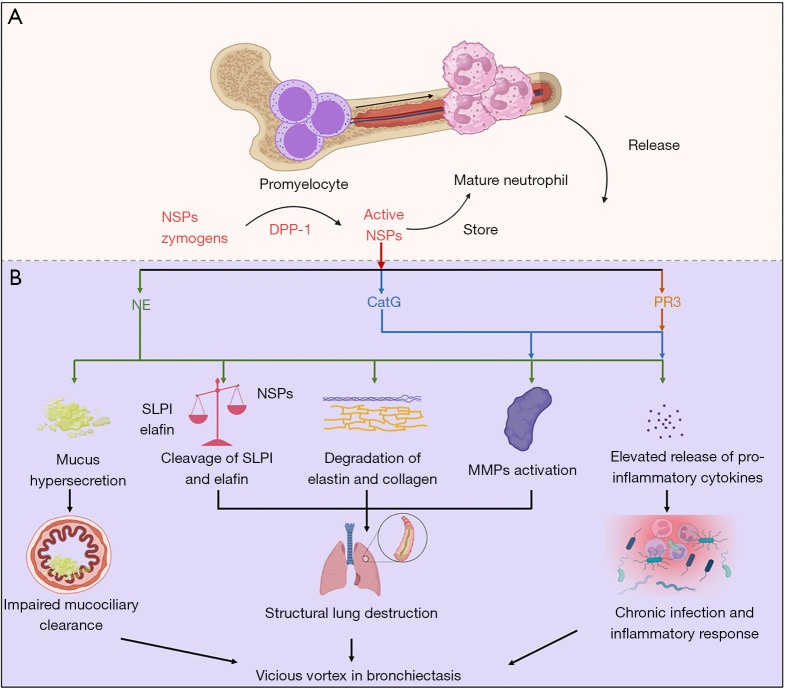
Consequently, the serine proteases NE, PR3, and CatG activated by DPP-1 may impact on the various stages of chronic inflammation and airway destruction in bronchiectasis (Figure 1). This has pointed to the opportunity to develop novel interventions of NSPs via DPP-1 inhibitors as a promising treatment option in bronchiectasis.
Figure 1.
NSPs processing and their role in bronchiectasis. (A) NSPs are activated by DPP-1 in the bone marrow and stored in neutrophil granules. (B) NSPs which are released by activated neutrophils can impair mucociliary clearance, exacerbate airway inflammation and structural destruction in bronchiectasis. CatG, cathepsin G; DPP-1, dipeptidyl peptidase 1; MMP, matrix metalloproteinase; NE, neutrophil elastase; NSP, neutrophil serine protease; PR3, proteinase 3; SLPI, secretory leucoprotease inhibitor.
DPP-1 inhibitors: a novel therapeutic option
Pharmacological mechanisms
In neutrophil-driven inflammatory airway diseases, circulating neutrophils are loaded with high levels of activated NSPs which is then responsible for tissue destruction (9). Mature DPP-1 has been identified in the sputum of patients with chronic inflammatory lung diseases (e.g., COPD, cystic fibrosis, bronchiectasis, neutrophilic asthma), characterized by the dominance of neutrophils (9,24). Previous studies have demonstrated that plasma deficiency of the NSPs inhibitor alpha-1 antitrypsin (AAT) can lead to COPD or emphysema (50,51). Notably, there is a correlation between the quantity of DPP-1 and the abundance of neutrophils (24).
NETs serve as a pivotal mediator of inflammation in various diseases, and its excessive release can induce airway epithelial cells to secrete inflammatory cytokines such as IL-6 and IL-8 (52,53). NETs formation is believed to the dominant mechanism for the release of NSPs in the bronchiectasis airway. Inhibition of DPP-1 activity may theoretically attenuate the formation of NETs (9). The absence of DPP-1 activity leads to the nearly complete elimination of NSPs from patients with PLS, as well as the diminished NETs formation (18,54,55).
Overall, the inhibition of DPP-1 activity provides protection against various inflammatory and immune-related diseases, which is associated with a reduction of neutrophil infiltration at the site of inflammation, inactivation of NSPs, and decreased secretion of NETs (19,22,56). Additionally, despite the lack of DPP-1 expression, patients with PLS do not exhibit substantial immune deficiency, suggesting that temporary inhibition of DPP-1 activity in the bone marrow precursor cells may be safe for treating various neutrophil-dominated inflammatory diseases (57). A range of potent, selective, reversible, and irreversible chemical inhibitors targeting DPP-1 have been synthesized, with some currently undergoing preclinical and clinical trials.
Research progress of DPP-1 inhibitors
Given the correlation between DPP-1 inhibition and inflammatory response, efforts have been made to pursue DPP-1 inhibitors as a therapeutic strategy. Currently, multiple inhibitors have already been developed at different stages of clinical trials. The analysis of DPP-1 protein structure has expedited the rational target-based design of DPP-1 inhibitors.
Reversible nitrile DPP-1 inhibitors
The development of DPP-1 inhibitors typically involves the incorporation of a “warhead” structure capable of forming covalent bonds with Cys234 on the peptidyl skeleton, thereby yielding peptidyl covalent compounds exhibiting potent inhibitory effects against DPP-1 and a high selectivity. The most extensively studied inhibitors of cysteine cathepsins are nitrile compounds (34,58). Cyano-group can form the covalent effect, with mild chemical properties, and no obvious cytotoxic effect, rendering it an excellent “warhead” structure.
The initial report on the DPP-1 inhibitor Gly-phe-CHN2 dates back to 1981, when George Green et al. reported multiple dipeptide-derived diazoketones as specific inactivating factors of thiol proteases including DPP-1 (59). In 1997, University of Texas patent application involving a small molecule DPP-1 inhibitor (US5602102) mentioned the dipeptide-derived diazoketone GLy-Phe-CHN2 as a highly effective and selective DPP-1 inhibitor (60). The development of nitrile DPP-1 inhibitors has been ongoing, but due to some key issues such as the metabolic instability, there are still no drugs that can be explored in clinical trials. To increase the metabolic stability, a cyclization strategy has been implemented to target the residual side chain of the peptidyl structure, resulting in the emergence of morpholine rings, and ultimately AZD5248 was selected as a candidate compound suitable for in vivo studies (61).
AZD5248 and AZD7986
AZD5248 has demonstrated low clearance rates and high bioavailability in rat, mouse, and dog models, along with the potent inhibitory activity and selectivity. Gardiner and colleagues evaluated the effects of AZD5248 (at a dose of 10 mg/kg) on the inhibition of DPP-1 downstream of NSPs activation in untreated rats (62). AZD5248 or a control was administered orally twice daily for eight consecutive days. After the 8-day treatment period, the inhibitory actions of NSPs culminated in the bone marrow, with activities of NE, PR3, and CatG being reduced by 90%, 64%, and 88%, respectively. However, AZD5248 exhibited binding to the aorta in a quantitative whole-body autoradiography assessment in rats (63). Therefore, AZD5248 was not subjected to further clinical trials, but was further modified for aortic binding toxicity, resulting in the formation of another new drug AZD7986 (64). AZD7986 was stable in aldehyde reactivity tests and showed no aortic binding in in vitro and in vivo aortic tissue homogenate tests. In vivo studies have shown that rats taking AZD7986 orally daily for 8 consecutive days had inhibited NSPs activity down to 10–30%. Subsequently, AZD7986 was renamed brensocatib (INS1007) and successfully entered clinical trials.
ICatCXPZ-01
Korkmaz et al. introduced cyclopropyl in the compound design process to obtain a class of highly effective DPP-1 inhibitors, which had a strong inhibitory effect on DPP-1 in U937 cells (65). ICatCXPZ-01 is a reversible, potent, and selective inhibitor with cellular permeability and its half-maximal inhibitory concentration (IC50) value is ~15 nM. The presence of this DPP-1 inhibitor has rendered the isolated immature bone marrow cells from healthy donors almost totally devoid of elastase (66). The pharmacokinetic studies in mice have demonstrated that ICatCXPZ-01 exhibited bone marrow accumulation at a therapeutically relevant level for effective DPP-1 inhibition. Subcutaneous administration of ICatCXPZ-01 in a mouse model of rheumatoid arthritis induced by monoclonal anti-collagen antibodies resulted in statistically significant antiarthritic activity (65). Rehm et al. found that pre-operative DPP-1 inhibitor (ICatCXPZ-01) therapy improved the function of the transplanted lung in recipient mice at an early stage, resulting in the disappearance of active NSPs in the transplanted lung and decreased NSPs activity within the bone marrow neutrophils (67). Although ICatCXPZ-01 has demonstrated favorable pharmacodynamic and metabolic kinetics properties in various animal experiments, there remains a lack of relevant information on ICatCXPZ-01 as a candidate drug for clinical trials (28).
BI 1291583
BI 1291583 is a novel, highly potent, and selective DPP-1 inhibitor that is being investigated as another treatment option in patients with bronchiectasis. Kreideweiss et al. (68) have demonstrated that BI 1291583 can completely inhibit human recombinant DPP-1, with no inhibitory effect on unrelated proteases from different classes, exhibiting a 6,000-fold higher selectivity. The IC50 of BI 1291583 for inhibiting human recombinant DPP-1 was 0.9 nM, which was significantly lower than the previously reported 4.0 nM for brensocatib. BI 1291583 can suppress the production of active NE in neutrophil progenitor cell lines without affecting cell viability. In the in vivo experiments using LPS challenge mouse model, BI 1291583 demonstrated a 99% inhibition of NE activation in neutrophils derived from bronchoalveolar lavage fluid (BALF), which was higher than the 76% inhibitory rate of NE activation by brensocatib. Compared with brensocatib, BI 1291583 exhibited a significantly lower bone marrow to plasma exposure ratio, with the compound achieving a 100-fold higher exposure in the bone marrow target compartment than in plasma. This may have contributed to the reduced risk of side effects associated with extra-medullary DPP-1 inhibition, and might decrease the incidence of dermatological adverse events in subsequent clinical trials. Indeed, the phase II clinical trial of BI 1281583 has demonstrated its efficacy in the treatment of patients with bronchiectasis (69,70).
Irreversible DPP-1 inhibitor
((S,E)-4-amino-N-(1-(indolin-1-yl)-6-methyl-1-oxohept-2-en-4-yl)tetrahydro-2H-pyran-4-carboxamide hydrochloride), a 4-amino-tetrahydropyranyl-4-carboxylic acid derived dipeptide, is an irreversible covalent α,β-unsaturated amide-based DPP-1 inhibitor. GSK2793660 is the second DPP-1 inhibitor to be evaluated in humans. Miller et al. evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single oral increase of GSK2793660 (from 0.5 to 20 mg) and a repeat oral dose of GSK2793660 (12 mg) for 21 days in healthy male subjects (71). GSK2793660 is a substrate-competitive, potent and selective DPP-1 inhibitor, with an IC50 value between <0.43 and 1 nM against DPP-1 and a kinact/Ki value of 9.0×104 M−1S−1. The effectively irreversible inhibition of DPP-1 by GSK2793660 enables a high level of enzymatic inhibition over an extended period (71). GSK2793660 inhibited DPP-1 activity but not the activity of downstream NSPs. Additionally, following daily administration of 12 mg GSK2793660, NE activity was only partially inhibited and cannot be steadily maintained. Some subjects who received a repeat dose of GSK2793660 experienced skin desquamation (peeling of the skin on the hands, fingers, and/or feet), which the investigators considered to be a drug-related adverse effect (71). These findings have affected the implementation of subsequent clinical trials of the drug as the severe desquamation was thought to be related to the irreversible mechanism of action.
Non-peptidyl non-covalent DPP-1 inhibitor
The design of covalent inhibitors has been a prominent area of focus in DPP-1 inhibitor development over the past decade, with medicinal chemists emphasizing the effective binding of suitable covalent groups to Cys234. The central challenge in DPP-1 inhibitor development rests in achieving a delicate balance between the activity, toxicity, selectivity, metabolic stability, and efficient NSPs inhibition in vivo. Electrophilic “warheads” often enhance the selectivity and efficacy of the target proteins. However, they can also lead to poor selectivity and subsequent off-target effects. To effectively inhibit DPP-1 activity and downstream NSPs activation, while avoiding the poor metabolic stability and safety risks associated with electrophilic “warhead” moiety, non-peptidyl non-covalent DPP-1 inhibitors have recently emerged (28,72).
Results from a previous study demonstrated that a novel non-peptidyl non-covalent DPP-1 inhibitor exhibits favorable pharmacokinetic properties and effectively inhibits NSPs in various tissues. The impact of the drug on DPP-1 inhibition and downstream NSPs activity was assessed through twice-daily administration to mice at doses of 5, 15, and 45 mg/kg for six consecutive days. Remarkable dose-dependent reductions in both DPP-1 activity and downstream NSPs activity have been demonstrated in bone marrow and blood samples. Furthermore, the compound may potently ameliorate the inflammatory storm which is associated with acute and chronic inflammatory diseases within the lung tissues. These findings have provided the impetus to fuel further exploration towards identifying more potent non-peptidyl non-covalent DPP-1 inhibitors in future studies (72).
HSK31858 is a potent non-peptidyl non-covalent DPP-1 inhibitor (with CatC Enz IC50 =57.4 nM). NSPs activity in the mice treated with HSK31858 was reduced by 15–40% compared with the control group. The compound has also showed effective anti-inflammatory activity in a rat model of COPD (22). The phase II clinical trials of HSK31858 have been completed, confirming that HSK31858 can significantly reduce the NSPs concentration, thereby decreasing the exacerbation frequency and prolonging the time to the first acute exacerbation in bronchiectasis (73,74).
Clinical trials of DPP-1 inhibitors in bronchiectasis
GSK2793660
A variety of DPP-1 inhibitors are currently being developed at different stages of clinical trials to explore their potential as therapeutic agents. GSK2793660, an oral DPP-1 inhibitor, not only demonstrated the dose-dependent inhibition of the whole-blood DPP-1 activity in a phase I clinical trial (NCT02058407) but also elicited epidermal exfoliation on the palmar and plantar surfaces, hindering the subsequent clinical application (71).
Brensocatib
AZD7986 (brensocatib) is a non-covalent DPP-1 inhibitor whose clinical trials have been successfully completed in bronchiectasis. The phase I clinical trial of AZD7986 tested its safety and effectiveness following single and multiple oral doses in healthy volunteers. The data showed that AZD7986 can effectively inhibit the activity of NE, measured using a whole-blood assay following zymosan stimulation, with a dose-dependent effect. Although some minor skin issues were observed, they did not affect further initiation of the phase II WILLOW trial (64,75). This phase II trial (NCT03218917) recruited 256 patients with bronchiectasis who were enrolled at 116 sites across 14 countries (21). Participants received either a placebo, 10 mg brensocatib, or 25 mg brensocatib once daily for 24 weeks. Brensocatib treatment markedly prolonged the time to the first exacerbation and decreased the frequency of exacerbations (21), there was a small increase in skin and dental adverse effects. These findings have provided clinicians with sufficient confidence in advancing AZD7986 to phase III trials (21,41). The WILLOW trial findings also showed that brensocatib significantly reduced the levels of NE, PR3, and CatG in the sputum of patients with bronchiectasis, thereby demonstrating its extensive anti-inflammatory effects (41). The phase III ASPEN trial (NCT04594369) have recently been completed (76,77). The trial has enrolled over 1,600 patients across 35 countries, showing that it met the prespecified primary outcome of reducing exacerbation frequency by approximately 20% at both doses (10 and 25 mg) versus placebo. The time to the first exacerbation was prolonged and lung function decline was also slowed, this final outcome only in the 25 mg group. Based on the positive results, brensocatib is being submitted to the regulatory authorities and may become the first licensed therapy for bronchiectasis in the United States, Europe and Japan.
BI 1291583
BI 1291583 is a novel DPP-1 inhibitor, and five phase I trials have shown good safety and tolerability, with a single dose up to 40 mg and multiple doses up to 10 mg (69). For BI 1291583, an international phase II projects among adults with bronchiectasis, the Airleaf™ trial (NCT05238675) has now been completed. The Airleaf™ trial has enrolled 322 adult patients with bronchiectasis who were treated for a minimum of 24 and a maximum of 48 weeks to evaluate the efficacy and safety of BI 1291583 (70). The latest findings suggested that treatment with BI 1291583 reduces the risk of acute exacerbations in adults with bronchiectasis at the 2.5 and 5 mg doses whereas the 1 mg dose was ineffective (20). Both the Clairafly™ (NCT05865886) and Clairleaf™ trials (NCT05846230) are currently underway (69).
HSK31858
A phase II clinical trial of HSK31858 (NCT05601778, SAVE-BE trial) which aims to evaluate the efficacy and safety of 6-month HSK31858 treatment in patients with bronchiectasis has now been completed (73). This trial was conducted exclusively in China. The preliminary findings presented at the European Respiratory Society Congress suggested that, compared with placebo, HSK31858 markedly decreased the exacerbation frequency and prolonged the time to the first exacerbation. Additionally, HSK31858 could decrease the 24-hour sputum volume, an important metric reflecting mucus plugging and inflammation in bronchiectasis. The greater magnitude of therapeutic response reported in the SAVE-BE trial might be associated with a number of factors including potentially the higher baseline sputum NSPs levels identified in the Chinese patients compared with the patients in other existing clinical trials (74). As the trials to date have differences in study design and patient population, and full results of some trials are not yet published, it is premature to speculate on the differences in efficacy or safety between the compounds.
In summary, DPP-1 inhibitors represent a promising therapeutic avenue for bronchiectasis, with several compounds in various stages of clinical development showing the potential to decrease the exacerbation risks and improve clinical outcomes. GSK2793660 demonstrated a dose-dependent inhibition of DPP-1 activity but was hindered by skin-related adverse effects (71). A phase III clinical trial of brensocatib has demonstrated significant benefits in reducing the exacerbation frequency and improving lung function (77), with authority regulatory approval pending. BI 1291583 and HSK31858 have also shown encouraging results in phase II trials (20,74), with HSK31858 demonstrating a notable reduction in exacerbations and 24-hour sputum volume. However, the differences in trial design and patient populations across these studies have made direct comparisons challenging. Overall, these trials highlight the potential of DPP-1 inhibitors to transform the treatment landscape for bronchiectasis, though further research is needed to confirm their long-term efficacy and safety.
Discussion
Current treatments in bronchiectasis focus on ameliorating the airway inflammation and infection, mitigating symptoms, and preventing from disease progression. Antibiotics, low-dose macrolides, and mucolytics are the mainstay therapies for bronchiectasis (78). Pulmonary rehabilitation, particularly airway clearance techniques, is beneficial for patients (79).
There are currently no licensed therapies for bronchiectasis and no existing therapies have been shown to halt the progression of bronchiectasis. Emerging approaches focus on DPP-1 inhibitors to block NSPs activation, thereby alleviating neutrophilic inflammation, achieving breakthroughs in halting disease progression.
The clinical trials of DPP-1 inhibitors in bronchiectasis have shown promising results, but several limitations must be critically assessed. First, the small sample size and regional differences may confine the clinical application of DPP-1 inhibitors. The SAVE-BE trial, conducted in mainland China, reported a greater treatment response (74). This enhanced response might be attributed to the higher baseline levels of sputum NSPs and the higher exacerbation frequency in Chinese patients, suggesting a significant regional heterogeneity in bronchiectasis pathophysiology that may influence the treatment outcomes. These findings underscore the necessity for investigating whether specific patient subpopulations exhibit heightened responsiveness to DPP-1 inhibitors. Notably, post-hoc analyses of HSK31858 across subgroups stratified by baseline percent of predicted forced expiratory volume in one second, geographical factors (e.g., underdeveloped regions in China), and comorbidities (COPD/asthma) revealed differential exacerbation frequencies, emphasizing the critical role of subgroup analyses in guiding personalized DPP-1 inhibitor therapy (74). Second, in phase II clinical trials, the treatment period for efficacy evaluation and long-term safety outcomes is relatively short. The phase III clinical trial of DPP-1 inhibitor brensocatib, which has extended the treatment duration to 52 weeks, showed that DPP-1 inhibitor may decrease the risk of pulmonary exacerbations and improve lung function (77). This indicates the necessity of prolonged treatment and observation to fully unveil the efficacy and safety. Finally, because marketing approval of this emerging treatment is pending, the anticipated costs and regulatory approval processes in different regions will be crucial. Brensocatib’s regulatory submission in the United States, Europe, and Japan is a significant step, but varying regulatory requirements and healthcare economics across different regions could impact on the accessibility and affordability. Addressing these factors will provide an important clinical context for the future application of DPP-1 inhibitors in bronchiectasis management.
Future directions for DPP-1 inhibitors may involve exploring the combination of DPP-1 inhibitor with other therapies (e.g., macrolides) to booster the anti-inflammatory effects. Also, in-depth research on their efficacy in different bronchiectasis subgroups is needed to pinpoint the most likely benefits for precision treatment. Optimizing the dosing regimens to enhance the efficacy and safety, and probing their role in the early intervention to hider disease progression is also crucial.
Conclusions
The development of DPP-1 inhibitors represents a significant breakthrough as they are likely to become the first licensed therapy for bronchiectasis in many countries (Table 2). The efficacy and safety of emerging DPP-1 inhibitors will be tested in ongoing clinical trials of bronchiectasis, where determining the bioequivalence of different DPP-1 inhibitors and best responders will become the next research priority. The triumph of DPP-1 inhibitors in improving the clinical outcomes of bronchiectasis may fuel ongoing research that may benefit patients with other chronic airway diseases which are dominated by the neutrophilic inflammation.
Table 2. Summary of representative clinical trials of DPP-1 inhibitors.
| Authors | Year of publication | Clinical trial registry No. | Study drug | Location | Study stage | Study type | Trial abbreviation | Sample size | Main findings | Main special adverse events | Reference No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Miller et al. | 2017 | NCT02058407 | GSK793660 | UK | Phase 1 | Single center study | – | 33 | GSK2793660 inhibited DPP-1 activity. Conferred no inhibitory effect on downstream NSPs activity. Resulted in palmar-plantar desquamation in some subjects | Skin desquamation (peeling of the skin on the hands, fingers and/or feet) | (71) |
| Palmér et al. | 2018 | NCT02303574 | AZD7986/INS1007 | UK | Phase 1 | Single center study | – | 89 | AZD7986 decreased blood NE activity. Generally well tolerated. Some non-serious adverse skin events possibly related to DPP-1 | Skin desquamation and mild gingival bleeding | (75) |
| Chalmers et al. | 2020 | NCT03218917 | AZD7986/INS1007 | 108 sites | Phase 2 | International multicenter randomized controlled trial | WILLOW | 256 | Markedly prolonged the time to the first exacerbation. Significantly decreased exacerbation frequency. Marked reduction of NSPs activity | Skin and dental event | (21) |
| Chalmers et al. | 2024; 2025 | NCT04594369 | AZD7986/INS1007 | 373 sites | Phase 3 | International multicenter randomized controlled trial | ASPEN | 1,767 | Reduced the frequency of exacerbations. Prolonged time to first exacerbation | Hyperkeratosis | (76,77) |
| Chalmers et al. | 2023; 2025 | NCT05238675 | BI 1291583 | 112 sites | Phase 2 | International multicenter randomized controlled trial | Airleaf | 322 | Decreased the risk of exacerbations. Evidence of a dose-response pattern | Skin exfoliation, hyperkeratosis and erythema | (20,70) |
| Zhong et al. | 2025 | NCT05601778 | HSK31858 | 25 sites | Phase 2 | Chinese multicenter randomized controlled trial | SAVE-BE | 226 | Significantly decreased exacerbation frequency. Markedly prolonged the time to the first exacerbation. Marked reduction of NSPs activity | No increased incidence of hyperkeratosis or gingivitis | (74) |
The completed clinical trials cited in this review and their main findings are highlighted in the table. DPP-1, dipeptidyl peptidase 1; NE, neutrophil elastase; NSP, neutrophil serine protease.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Frontiers in Bronchiectasis Management: Translational Science and Practice”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-289/rc
Funding: This study was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (No. 2024ZD0529600), National Natural Science Foundation - Outstanding Youth Fund (No. 82222001), Major Project of Guangzhou National Laboratory (No. GZNL2024A02003), National Natural Science Foundation (Nos. 82470040 and 81870003), Guangzhou Science and Technology Plans (Nos. 2024A04J6495, 2024A03J1206, 2023B03J0407, and 202102010372), Guangdong Science and Technology Foundation (No. 2019B030316028), and Zhongnanshan Medical Foundation of Guangdong Province (No. ZNSA-2020013).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-289/coif). The series “Frontiers in Bronchiectasis Management: Translational Science and Practice” was commissioned by the editorial office without any funding or sponsorship. J.D.C. and W.J.G. served as the unpaid Guest Editors of the series. W.J.G. serves as an unpaid editorial board member of Journal of Thoracic Disease. J.D.C. reports grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed and Novartis; and consulting fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Grifols, Insmed, Janssen, Novartis, Pfizer and Zambon. W.J.G. reports grants from Haisco Co. Ltd. and consulting fees from Joincare Pharmaceutical Co. Ltd. The authors have no other conflicts of interest to declare.
References
- 1.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018;392:880-90. 10.1016/S0140-6736(18)31767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliberti S, Sotgiu G, Lapi F, et al. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med 2020;20:15. 10.1186/s12890-020-1050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak HJ, Moon JY, Choi YW, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med 2010;222:237-42. 10.1620/tjem.222.237 [DOI] [PubMed] [Google Scholar]
- 4.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629. 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11:519-31. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 6.Poli V, Zanoni I. Neutrophil intrinsic and extrinsic regulation of NETosis in health and disease. Trends Microbiol 2023;31:280-93. 10.1016/j.tim.2022.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am J Respir Crit Care Med 2017;195:1384-93. 10.1164/rccm.201605-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021;9:873-84. 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 9.Korkmaz B, Caughey GH, Chapple I, et al. Therapeutic targeting of cathepsin C: from pathophysiology to treatment. Pharmacol Ther 2018;190:202-36. 10.1016/j.pharmthera.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 10.Polverino E, Rosales-Mayor E, Dale GE, et al. The Role of Neutrophil Elastase Inhibitors in Lung Diseases. Chest 2017;152:249-62. 10.1016/j.chest.2017.03.056 [DOI] [PubMed] [Google Scholar]
- 11.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006;6:541-50. 10.1038/nri1841 [DOI] [PubMed] [Google Scholar]
- 12.Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010;191:677-91. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalmers JD, Mall MA, Chotirmall SH, et al. Targeting neutrophil serine proteases in bronchiectasis. Eur Respir J 2025;65:2401050. 10.1183/13993003.01050-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oriano M, Gramegna A, Terranova L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J 2020;56:2000769. 10.1183/13993003.00769-2020 [DOI] [PubMed] [Google Scholar]
- 15.Janoff A. Elastase in tissue injury. Annu Rev Med 1985;36:207-16. 10.1146/annurev.me.36.020185.001231 [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Huang Y, Ji Q, et al. Interplay between Extracellular Matrix and Neutrophils in Diseases. J Immunol Res 2021;2021:8243378. 10.1155/2021/8243378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strøbech JE, Giuriatti P, Erler JT. Neutrophil granulocytes influence on extracellular matrix in cancer progression. Am J Physiol Cell Physiol 2022;323:C486-93. 10.1152/ajpcell.00122.2022 [DOI] [PubMed] [Google Scholar]
- 18.Pham CT, Ivanovich JL, Raptis SZ, et al. Papillon-Lefèvre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol 2004;173:7277-81. 10.4049/jimmunol.173.12.7277 [DOI] [PubMed] [Google Scholar]
- 19.Adkison AM, Raptis SZ, Kelley DG, et al. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 2002;109:363-71. 10.1172/JCI0213462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalmers JD, Shteinberg M, Mall MA, et al. Cathepsin C (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: AIRLEAF, a phase II randomised, double-blind, placebo-controlled, dose-finding study. Eur Respir J 2025;65:2401551. 10.1183/13993003.01551-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med 2020;383:2127-37. 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
- 22.Chalmers JD, Kettritz R, Korkmaz B. Dipeptidyl peptidase 1 inhibition as a potential therapeutic approach in neutrophil-mediated inflammatory disease. Front Immunol 2023;14:1239151. 10.3389/fimmu.2023.1239151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl SW, Halkier T, Lauritzen C, et al. Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 2001;40:1671-8. 10.1021/bi001693z [DOI] [PubMed] [Google Scholar]
- 24.Hamon Y, Legowska M, Hervé V, et al. Neutrophilic Cathepsin C Is Maturated by a Multistep Proteolytic Process and Secreted by Activated Cells during Inflammatory Lung Diseases. J Biol Chem 2016;291:8486-99. 10.1074/jbc.M115.707109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamort AS, Hamon Y, Czaplewski C, et al. Processing and Maturation of Cathepsin C Zymogen: A Biochemical and Molecular Modeling Analysis. Int J Mol Sci 2019;20:4747. 10.3390/ijms20194747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santilman V, Jadot M, Mainferme F. Importance of the propeptide in the biosynthetic maturation of rat cathepsin C. Eur J Cell Biol 2002;81:654-63. 10.1078/0171-9335-00291 [DOI] [PubMed] [Google Scholar]
- 27.Turk D, Janjić V, Stern I, et al. Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J 2001;20:6570-82. 10.1093/emboj/20.23.6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen XB, Chen X, Zhang ZY, et al. Cathepsin C inhibitors as anti-inflammatory drug discovery: Challenges and opportunities. Eur J Med Chem 2021;225:113818. 10.1016/j.ejmech.2021.113818 [DOI] [PubMed] [Google Scholar]
- 29.Chitsamankhun C, Siritongtaworn N, Fournier BPJ, et al. Cathepsin C in health and disease: from structural insights to therapeutic prospects. J Transl Med 2024;22:777. 10.1186/s12967-024-05589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mølgaard A, Arnau J, Lauritzen C, et al. The crystal structure of human dipeptidyl peptidase I (cathepsin C) in complex with the inhibitor Gly-Phe-CHN2. Biochem J 2007;401:645-50. 10.1042/BJ20061389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneck JL, Villa JP, McDevitt P, et al. Chemical mechanism of a cysteine protease, cathepsin C, as revealed by integration of both steady-state and pre-steady-state solvent kinetic isotope effects. Biochemistry 2008;47:8697-710. 10.1021/bi8007627 [DOI] [PubMed] [Google Scholar]
- 32.Weiss SAI, Rehm SRT, Perera NC, et al. Origin and Expansion of the Serine Protease Repertoire in the Myelomonocyte Lineage. Int J Mol Sci 2021;22:1658. 10.3390/ijms22041658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghdassi AA, Pham C, Zierke L, et al. Cathepsin C role in inflammatory gastroenterological, renal, rheumatic, and pulmonary disorders. Biochimie 2024;216:175-80. 10.1016/j.biochi.2023.09.018 [DOI] [PubMed] [Google Scholar]
- 34.Guay D, Beaulieu C, Percival MD. Therapeutic utility and medicinal chemistry of cathepsin C inhibitors. Curr Top Med Chem 2010;10:708-16. 10.2174/156802610791113469 [DOI] [PubMed] [Google Scholar]
- 35.Wani AA, Devkar N, Patole MS, et al. Description of two new cathepsin C gene mutations in patients with Papillon-Lefèvre syndrome. J Periodontol 2006;77:233-7. 10.1902/jop.2006.050124 [DOI] [PubMed] [Google Scholar]
- 36.Hewitt C, McCormick D, Linden G, et al. The role of cathepsin C in Papillon-Lefèvre syndrome, prepubertal periodontitis, and aggressive periodontitis. Hum Mutat 2004;23:222-8. 10.1002/humu.10314 [DOI] [PubMed] [Google Scholar]
- 37.Toomes C, James J, Wood AJ, et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet 1999;23:421-4. 10.1038/70525 [DOI] [PubMed] [Google Scholar]
- 38.Janjua SA, Iftikhar N, Hussain I, et al. Dermatologic, periodontal, and skeletal manifestations of Haim-Munk syndrome in two siblings. J Am Acad Dermatol 2008;58:339-44. 10.1016/j.jaad.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 39.Hart TC, Hart PS, Michalec MD, et al. Haim-Munk syndrome and Papillon-Lefèvre syndrome are allelic mutations in cathepsin C. J Med Genet 2000;37:88-94. 10.1136/jmg.37.2.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapels DA, Geisbrecht BV, Rooijakkers SH. Neutrophil serine proteases in antibacterial defense. Curr Opin Microbiol 2015;23:42-8. 10.1016/j.mib.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cipolla D, Zhang J, Korkmaz B, et al. Dipeptidyl peptidase-1 inhibition with brensocatib reduces the activity of all major neutrophil serine proteases in patients with bronchiectasis: results from the WILLOW trial. Respir Res 2023;24:133. 10.1186/s12931-023-02444-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JA, He F, Martin LD, et al. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol 2005;167:651-61. 10.1016/S0002-9440(10)62040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers JD, Elborn S, Greene CM. Basic, translational and clinical aspects of bronchiectasis in adults. Eur Respir Rev 2023;32:230015. 10.1183/16000617.0015-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreau T, Baranger K, Dadé S, et al. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie 2008;90:284-95. 10.1016/j.biochi.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Gramegna A, Amati F, Terranova L, et al. Neutrophil elastase in bronchiectasis. Respir Res 2017;18:211. 10.1186/s12931-017-0691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazleen A, Wilkinson T. The emerging role of proteases in α(1)-antitrypsin deficiency and beyond. ERJ Open Res 2021;7:00494-2021. 10.1183/23120541.00494-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oriano M, Amati F, Gramegna A, et al. Protease-Antiprotease Imbalance in Bronchiectasis. Int J Mol Sci 2021;22:5996. 10.3390/ijms22115996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clancy DM, Sullivan GP, Moran HBT, et al. Extracellular Neutrophil Proteases Are Efficient Regulators of IL-1, IL-33, and IL-36 Cytokine Activity but Poor Effectors of Microbial Killing. Cell Rep 2018;22:2937-50. 10.1016/j.celrep.2018.02.062 [DOI] [PubMed] [Google Scholar]
- 49.Mall MA, Davies JC, Donaldson SH, et al. Neutrophil serine proteases in cystic fibrosis: role in disease pathogenesis and rationale as a therapeutic target. Eur Respir Rev 2024;33:240001. 10.1183/16000617.0001-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy C, Reeves EP, McElvaney NG. The Role of Neutrophils in Alpha-1 Antitrypsin Deficiency. Ann Am Thorac Soc 2016;13 Suppl 4:S297-304. 10.1513/AnnalsATS.201509-634KV [DOI] [PubMed] [Google Scholar]
- 51.Greene CM, Marciniak SJ, Teckman J, et al. α1-Antitrypsin deficiency. Nat Rev Dis Primers 2016;2:16051. 10.1038/nrdp.2016.51 [DOI] [PubMed] [Google Scholar]
- 52.Sabbione F, Keitelman IA, Iula L, et al. Neutrophil Extracellular Traps Stimulate Proinflammatory Responses in Human Airway Epithelial Cells. J Innate Immun 2017;9:387-402. 10.1159/000460293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudock KM, Collins MS, Imbrogno M, et al. Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 2020;319:L137-47. 10.1152/ajplung.00144.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perera NC, Wiesmüller KH, Larsen MT, et al. NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J Immunol 2013;191:2700-7. 10.4049/jimmunol.1301293 [DOI] [PubMed] [Google Scholar]
- 55.Seren S, Rashed Abouzaid M, Eulenberg-Gustavus C, et al. Consequences of cathepsin C inactivation for membrane exposure of proteinase 3, the target antigen in autoimmune vasculitis. J Biol Chem 2018;293:12415-28. 10.1074/jbc.RA118.001922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schreiber A, Pham CT, Hu Y, et al. Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 2012;23:470-82. 10.1681/ASN.2010080892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korkmaz B, Lesner A, Marchand-Adam S, et al. Lung Protection by Cathepsin C Inhibition: A New Hope for COVID-19 and ARDS? J Med Chem 2020;63:13258-65. 10.1021/acs.jmedchem.0c00776 [DOI] [PubMed] [Google Scholar]
- 58.Laine DI, Busch-Petersen J. Inhibitors of cathepsin C (dipeptidyl peptidase I). Expert Opin Ther Pat 2010;20:497-506. 10.1517/13543771003657172 [DOI] [PubMed] [Google Scholar]
- 59.Green GD, Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem 1981;256:1923-8. 10.1016/S0021-9258(19)69895-9 [DOI] [PubMed] [Google Scholar]
- 60.Thiele DL, McGuire MJ, Lipsky PE. A selective inhibitor of dipeptidyl peptidase I impairs generation of CD8+ T cell cytotoxic effector function. J Immunol 1997;158:5200-10. 10.4049/jimmunol.158.11.5200 [DOI] [PubMed] [Google Scholar]
- 61.Furber M, Tiden AK, Gardiner P, et al. Cathepsin C inhibitors: property optimization and identification of a clinical candidate. J Med Chem 2014;57:2357-67. 10.1021/jm401705g [DOI] [PubMed] [Google Scholar]
- 62.Gardiner P, Wikell C, Clifton S, et al. Neutrophil maturation rate determines the effects of dipeptidyl peptidase 1 inhibition on neutrophil serine protease activity. Br J Pharmacol 2016;173:2390-401. 10.1111/bph.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bragg RA, Brocklehurst S, Gustafsson F, et al. Aortic Binding of AZD5248: Mechanistic Insight and Reactivity Assays To Support Lead Optimzation. Chem Res Toxicol 2015;28:1991-9. 10.1021/acs.chemrestox.5b00236 [DOI] [PubMed] [Google Scholar]
- 64.Doyle K, Lönn H, Käck H, et al. Discovery of Second Generation Reversible Covalent DPP1 Inhibitors Leading to an Oxazepane Amidoacetonitrile Based Clinical Candidate (AZD7986). J Med Chem 2016;59:9457-72. 10.1021/acs.jmedchem.6b01127 [DOI] [PubMed] [Google Scholar]
- 65.Korkmaz B, Lesner A, Wysocka M, et al. Structure-based design and in vivo anti-arthritic activity evaluation of a potent dipeptidyl cyclopropyl nitrile inhibitor of cathepsin C. Biochem Pharmacol 2019;164:349-67. 10.1016/j.bcp.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 66.Guarino C, Hamon Y, Croix C, et al. Prolonged pharmacological inhibition of cathepsin C results in elimination of neutrophil serine proteases. Biochem Pharmacol 2017;131:52-67. 10.1016/j.bcp.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 67.Rehm SRT, Smirnova NF, Morrone C, et al. Premedication with a cathepsin C inhibitor alleviates early primary graft dysfunction in mouse recipients after lung transplantation. Sci Rep 2019;9:9925. 10.1038/s41598-019-46206-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreideweiss S, Schänzle G, Schnapp G, et al. BI 1291583: a novel selective inhibitor of cathepsin C with superior in vivo profile for the treatment of bronchiectasis. Inflamm Res 2023;72:1709-17. 10.1007/s00011-023-01774-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badorrek P, Diefenbach C, Kögler H, et al. Novel cathepsin C inhibitor, BI 1291583, intended for treatment of bronchiectasis: Phase I characterization in healthy volunteers. Clin Transl Sci 2024;17:e13891. 10.1111/cts.13891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chalmers JD, Gupta A, Chotirmall SH, et al. A Phase 2 randomised study to establish efficacy, safety and dosing of a novel oral cathepsin C inhibitor, BI 1291583, in adults with bronchiectasis: Airleaf. ERJ Open Res 2023;9:00633-2022. 10.1183/23120541.00633-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller BE, Mayer RJ, Goyal N, et al. Epithelial desquamation observed in a phase I study of an oral cathepsin C inhibitor (GSK2793660). Br J Clin Pharmacol 2017;83:2813-20. 10.1111/bcp.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Yan Y, Zhang Z, et al. Discovery and In Vivo Anti-inflammatory Activity Evaluation of a Novel Non-peptidyl Non-covalent Cathepsin C Inhibitor. J Med Chem 2021;64:11857-85. 10.1021/acs.jmedchem.1c00104 [DOI] [PubMed] [Google Scholar]
- 73.National Library of Medicine. A study of HSK31858 in participants with non-cystic fibrosis bronchiectasis. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05601778
- 74.Zhong NS, Qiu R, Cao J, et al. Effects of the DPP-1 inhibitor HSK31858 in adults with bronchiectasis in China (SAVE-BE): a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Respir Med 2025;13:414-24. 10.1016/S2213-2600(25)00019-0 [DOI] [PubMed] [Google Scholar]
- 75.Palmér R, Mäenpää J, Jauhiainen A, et al. Dipeptidyl Peptidase 1 Inhibitor AZD7986 Induces a Sustained, Exposure-Dependent Reduction in Neutrophil Elastase Activity in Healthy Subjects. Clin Pharmacol Ther 2018;104:1155-64. 10.1002/cpt.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chalmers JD, Burgel PR, Daley CL, et al. Brensocatib in non-cystic fibrosis bronchiectasis: ASPEN protocol and baseline characteristics. ERJ Open Res 2024;10:00151-2024. 10.1183/23120541.00151-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chalmers JD, Burgel PR, Daley CL, et al. Phase 3 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med 2025;392:1569-81. 10.1056/NEJMoa2411664 [DOI] [PubMed] [Google Scholar]
- 78.Choi H, McShane PJ, Aliberti S, et al. Bronchiectasis management in adults: state of the art and future directions. Eur Respir J 2024;63:2400518. 10.1183/13993003.00518-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Neill K, O'Donnell AE, Bradley JM. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology 2019;24:227-37. 10.1111/resp.13459 [DOI] [PubMed] [Google Scholar]