Abstract
Traditional lung-protective ventilation [low tidal volume (VT) with fixed positive end-expiratory pressure (PEEP)] reduces intraoperative lung injury but exhibits limited efficacy in preventing postoperative pulmonary complications (PPCs) following thoracic surgery requiring one-lung ventilation (OLV). This review systematically examines the multifactorial mechanisms of OLV-associated lung injury, encompassing hypoxemia [device malposition, atelectasis, ventilation/perfusion (V/Q) mismatch, impaired hypoxic pulmonary vasoconstriction (HPV)], oxidative stress, ischemia-reperfusion injury (IRI) (glycocalyx degradation, mechanical stress, inflammation), and ventilator-induced trauma (volutrauma, biotrauma). To address these limitations, we propose an open-lung protective ventilation strategy integrating alveolar recruitment maneuvers (RMs) with individualized PEEP (iPEEP) titration based on optimal respiratory compliance. Furthermore, we innovatively introduce oxygen reserve index (ORI)-guided titration of fraction of inspired oxygen (FiO2), enabling dynamic determination of the minimum effective FiO2 to mitigate hyperoxia-related toxicity. This synergistic “RM-iPEEP-FiO2 triad” facilitates personalized intraoperative respiratory management by stabilizing alveoli, optimizing V/Q matching, and minimizing oxidative stress, thereby significantly reducing PPCs risk compared to conventional fixed-parameter approaches. Current limitations include insufficient multicenter validation, technical dependency on advanced monitoring/ventilators, and lack of subgroup analyses for high-risk populations. Future research should prioritize multicenter randomized controlled trials to establish universal thresholds for tailored parameters. Integration of artificial intelligence (AI) for real-time respiratory mechanics analysis and multimodal imaging is essential to refine precision thresholds. Ultimately, this strategy aims to establish an evidence-based, precision perioperative ventilation framework that optimizes clinical outcomes in thoracic surgical patients by overcoming the constraints of standardized ventilation protocols.
Keywords: One-lung ventilation (OLV), postoperative pulmonary complications (PPCs), tailored lung protective ventilation strategies, mechanical ventilation, lung injury
Introduction
Thoracotomy has long been the standard approach for removing lung cancer tissue in thoracic surgery. However, with the growing maturity of video-assisted thoracoscopic surgery (VATS), its minimally invasive advantages have led to the widespread adoption of VATS in place of traditional thoracotomy. One-lung ventilation (OLV) has become the preferred method to collapse the operative lung while ensuring ventilation and oxygenation of the healthy lung, preventing contamination between the lungs, and providing clear surgical visibility and space (1). The most commonly used OLV techniques include double-lumen endotracheal tubes (DLTs), bronchial blockers (BBs), and single-lumen tubes (SLTs) are positioned directly into the main bronchus of the healthy lung (2-5). Among these, SLTs are primarily limited to rigid bronchoscopy rather than being routine choices for thoracic surgery due to their shortcomings such as difficult tube positioning, delayed ventilation mode switching, and poor anatomical adaptability (6). DLT has become the clinical preferred choice due to its advantages of straightforward operation, reliable lung isolation efficacy, and high intraoperative stability (7). During OLV, factors such as postural changes and surgical pneumothorax may lead to hypoxic pulmonary vasoconstriction (HPV), ventilation/perfusion (V/Q) mismatch, pulmonary blood flow redistribution, ischemia-reperfusion injury (IRI), alveolar trauma, and biological trauma from high oxygen concentrations during re-expansion. These mechanisms can trigger systemic inflammatory responses and cause varying degrees of lung injury (8-10). Inappropriate ventilation strategies during OLV can contribute to acute lung injury (ALI), hypoxemia, and respiratory failure (8).
Postoperative pulmonary complications (PPCs), including respiratory infection, pleural effusion, respiratory failure, atelectasis, bronchospasm, pneumothorax, and aspiration pneumonia, are frequent after pulmonary resection in thoracic surgery (11). The causes and mechanisms underlying these complications are complex and may involve multiple factors, including the patient’s pre-existing conditions, surgical trauma, and anesthesia (12). Given their significant impact on patient prognosis, these complications have been extensively investigated. A study has reported an overall PPCs incidence rate of 19–59% in thoracic surgery (13). The study found that compared with traditional open thoracic surgery, VATS significantly reduces the incidence of PPCs (14,15). Due to the characteristics of thoracic surgical procedures and the application of OLV techniques, the incidence of PPCs after VATS remains as high as 16–30% (16,17). The occurrence of PPCs is closely associated with increased hospital stays and elevated in-hospital mortality, potentially shortening the average overall survival period (18). OLV, as an independent risk factor for the occurrence of PPCs, can directly induce lung injury through alveolar overdistension, thereby exacerbating the incidence of PPCs (19).
The traditional lung-protective ventilation strategy comprises low tidal volume (VT) [6–8 mL/kg ideal body weight (IBW) during double-lung ventilation and 4–6 mL/kg IBW during OLV], moderate positive end-expiratory pressure (PEEP) levels (6–8 cmH2O), and periodic recruitment maneuvers (RMs) (20,21). Although these approaches have been successful in reducing intraoperative lung injury, they have certain limitations. Given the high incidence of PPCs, the currently prevalent traditional lung-protective ventilation strategies demonstrate insufficient efficacy in mitigating PPCs occurrence, urgently requiring modifications to existing ventilation modalities to reduce PPCs incidence. A recent study has demonstrated that the tailored open-lung ventilation strategy, combining RMs with tailored PEEP, provides superior outcomes in improving respiratory mechanics, reducing driving pressure, and enhancing ventilation efficiency. However, no unified standard exists for determining the optimal intraoperative inspired oxygen concentration (22).
This paper systematically analyzes the pathological mechanisms of OLV-associated lung injury and discusses the impact of key ventilation parameters on PPCs. Furthermore, it innovatively introduces a third tailored dimension: individualized precise titration of fraction of inspired oxygen (FiO2) based on the oxygen reserve index (ORI). By individually titrating the minimum effective FiO2 and implementing a tailored lung-protective ventilation strategy incorporating RM-individualized PEEP (iPEEP)-FiO2, this approach systematically optimizes intraoperative respiratory management to minimize the risk of PPCs. To provide reference evidence for selecting ventilation strategies that reduce PPCs in thoracic surgical procedures.
Mechanism of one-lung ventilator-related lung injury
The mechanism of lung injury during single-lung ventilation is complex, and no definitive conclusions have been made. This section analyzes three key factors: hypoxemia, oxidative stress, IRI, and mechanical ventilation-related injury.
Hypoxemia during OLV
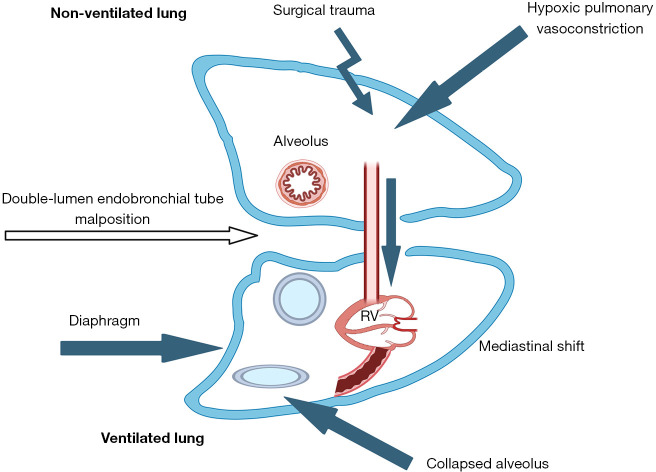
Hypoxemia is traditionally defined as peripheral oxygen saturation (SpO2) <90% or partial pressure of arterial oxygen (PaO2) <60 mmHg, representing reduced oxygen content in arterial blood—a multifactorial outcome (23). Notably, during OLV, issues such as collapse of the non-ventilated lung, malpositioning of ventilation devices, and restricted ventilation lead to increased atelectasis in the ventilated lung. Mandatory intrapulmonary shunting may consequently induce systemic hypoxemia (24) (see Figure 1).
Figure 1.
Factors contributing to hypoxemia during OLV. (I) Ventilator malposition causing hypoventilation. (II) Collapse of non-ventilated lung and partial atelectasis in ventilated lung. (III) V/Q mismatch due to redistribution of ventilation and perfusion caused by body position and other factors. (IV) HPV regulated by various factors. HPV, hypoxic pulmonary vasoconstriction; OLV, one-lung ventilation; RV, right ventricle; V/Q, ventilation/perfusion.
Improper device positioning
DLT and BBs are commonly used airway devices for achieving OLV, capable of achieving proper lung isolation, ventilation, and suction. Malpositioning of ventilation devices constitutes the most common correctable cause of overt hypoxemia during OLV (25). Data indicates that among anesthesiologists with limited experience in thoracic anesthesia, the incidence of ventilator misalignment exceeds 32%. Utilizing fiberoptic bronchoscopy for precise device positioning can effectively reduce the occurrence of hypoxemia (26).
Reduced oxygen reserve and ventilation limitations due to atelectasis
Under OLV conditions, surgical trauma-induced collapse of the non-ventilated lung significantly diminishes functional residual capacity (FRC) and oxygen reserves, particularly pronounced in patients with poor preoperative pulmonary function. Moreover, as lateral decubitus positioning is typically required during OLV procedures, ventilation is not only compromised by cephalad displacement of the diaphragm but also subjected to gravitational compression from mediastinal shift. This dual mechanism reduces lung compliance in the perfused lower lung regions and exacerbates atelectasis in dependent pulmonary areas (27). Furthermore, general anesthesia and neuromuscular blockade reduce respiratory muscle tone and alter chest wall mechanics, thereby decreasing FRC. The reduction in FRC may alter the lung volume at which small airway closure occurs, leading to partial airway closure in the ventilated lung during inspiration. This creates intrapulmonary shunts with low V/Q ratios, exacerbating hypoxemia (28).
V/Q mismatch
During OLV, V/Q mismatch serves as the central mechanism underlying hypoxemia development. During OLV in the lateral decubitus position, the complete collapse of the non-ventilated lung creates an anatomical right-to-left shunt, while compressive atelectasis in the ventilated lung contributes to physiological shunting. The synergistic interaction between these two factors significantly reduces mixed venous oxygen content, constituting the core mechanism underlying hypoxemia (29). Research has found that in the lateral decubitus position, gravitational effects influence perfusion in both ventilated and non-ventilated lungs, with gravity causing better perfusion in the dependent ventilated lung compared to the non-dependent ventilated lung. The transition from supine to lateral decubitus position alters pulmonary blood flow distribution, increasing perfusion to the dependent lung by 5% while decreasing non-dependent lung perfusion by 5%. Under these conditions, dependent lung blood flow may increase up to 10%, though the effects of one-lung positive pressure ventilation mitigate a portion of this increased perfusion (30).
Easily adjustable airway pressure parameters (such as PEEP and inspiratory pressure levels) and high circulatory dynamics hold valuable potential in modulating this ratio. Applying PEEP to the dependent lung can reduce intrapulmonary shunt. The application of PEEP combined with high inspiratory pressure increases FRC, helps reopen collapsed or closed small airways, potentially improves ventilation in dependent lung regions, and mitigates V/Q mismatch (28). For mismatch caused by low blood perfusion, adjusting systemic circulatory status to reduce shunt flow ensures adequate perfusion to the dependent lung, thereby maintaining optimal alveolar oxygenation (31).
Compensatory suppression of HPV
HPV refers to the reflexive constriction of vascular smooth muscle within the pulmonary circulation, triggered by low local oxygen partial pressure (32). This mechanism is regarded as an automatic regulatory reflex. The HPV response comprises two distinct phases: the initial phase is activated within seconds and peaks at 15 minutes. If hypoxia persists for 30 to 60 minutes, the response enters a second phase, during which pulmonary vascular resistance further increases, reaching its peak within 2 hours (33). During OLV, factors influencing HPV are multifactorial, including hypotension, hypocapnia, hypothermia, presence and severity of chronic obstructive pulmonary disease (COPD), use of vasodilators, vasoconstrictors and anesthetics, positive pressure ventilation-induced increases in pulmonary vascular resistance, sympathetic nervous system-mediated hyperdynamic circulation, and anemia (25,32). Studies indicate that excessive airway pressure or PEEP may collectively restrict blood flow redistribution to ventilated areas by increasing vascular resistance in the ventilated lung (via alveolar overdistension) and in the non-ventilated lung (through HPV superimposed with mechanical pressure), impairing the compensatory effect of HPV, ultimately leading to persistent shunting in non-ventilated regions, elevated pulmonary artery pressure, and systemic oxygenation reduction (32). Additionally, sympathetic nervous system activation induced by surgical stress or inadequate anesthetic depth may trigger a hyperdynamic circulatory state, further exacerbating V/Q mismatch (34). Anemia directly impairs the hypoxic sensing mechanism of HPV by reducing the oxygen-carrying capacity of blood, a phenomenon particularly pronounced in thoracic surgery patients (35). Although respiratory alkalosis induced by hyperventilation theoretically interferes with HPV through calcium ion channel inhibition, however, the actual clinical impact of this mechanism remains limited when non-extreme ventilation parameters are applied (e.g., VT <10 mL/kg, PEEP <15 cmH2O) (36,37). This underscores the necessity for comprehensive perioperative management integrating mechanical ventilation strategy optimization, circulatory status monitoring, and oxygen-carrying capacity maintenance during OLV, rather than relying solely on ventilatory parameter adjustments to address hypoxemia.
Xidative stress and IRI
During OLV, the non-ventilated lung undergoes a dynamic pathological process of “ischemia-collapse-reperfusion-recruitment” influenced by surgical trauma. The synergistic damaging effect arises from the accumulation of anaerobic metabolites during the ischemic phase of lung tissue and the subsequent oxidative stress burst post-recruitment, with core mechanisms involving mitochondrial dysfunction, endothelial barrier disruption, and mechanical stress imbalance. A study has confirmed that pulmonary IRI serves as a critical trigger for postoperative ALI (38).
Oxidative stress cascade
The hypoxic state in the non-ventilated lung forces cellular metabolism to shift from oxidative phosphorylation to glycolysis, resulting in NADH/NAD+ ratio imbalance and lactic acid accumulation (39). The rapid elevation of oxygen partial pressure during recruitment promotes mitochondrial complexes to generate superoxide anion (O2−) through reduction reactions, initiating explosive production of reactive oxygen species (ROS) (40). Notably, the ventilated lung also faces oxidative stress risks due to excessive FiO2, where high oxygen environments impair antioxidant defenses while simultaneously activating nuclear factor-κB (NF-κB)-mediated pro-inflammatory responses (41).
Triple mechanism of IRI
The essence of pulmonary IRI lies in bidirectional destruction of the alveolar-capillary barrier, and its pathological process can be summarized as the following mechanisms.
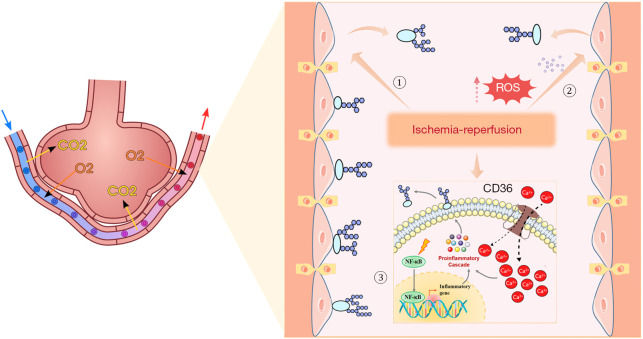
Degradation of endothelial glycocalyx and barrier dysfunction.The vascular endothelial glycocalyx, composed of heparan sulfate, hyaluronic acid, and other components, serves as a dynamic molecular sieve that maintains vascular permeability (42). During the ischemic phase, hypoperfusion induces matrix metalloproteinase activation, leading to shedding of glycocalyx core proteoglycans. Reperfusion injury disrupts the glycocalyx through a quadruple mechanism involving mechanical stress, ROS storm, calcium overload, and NF-κB activation. Specifically, fluid shear stress and Ca2+ influx directly damage endothelial architecture, while the ROS storm and tumor necrosis factor (TNF)-α upregulation synergistically exacerbate glycocalyx shedding via oxidative stress and inflammatory responses. These mechanisms intertwine to form a mechano-chemical signaling network, ultimately leading to endothelial barrier dysfunction (43-46) (see Figure 2).
Shear injury mediated by mechanical stress.The pulmonary capillary network exhibits high sensitivity to hemodynamic changes. During ischemia, low shear stress downregulates the expression of integrin proteins in endothelial cells and induces cytoskeletal remodeling; During reperfusion, abrupt increases in blood flow velocity trigger calcium overload through mechanosensitive ion channels under high shear stress, while simultaneously expanding endothelial gaps. This mechanism is particularly prominent in pulmonary basal segments with lower hemodynamic reserve capacity and in ischemic collapsed lung regions (47). This injury process involves complex inflammatory responses, where the mechano-molecular cascade significantly increases risks of vascular leakage and edema, further exacerbating pulmonary tissue damage.
Inflammatory cascade and immune cell infiltration.Within 2 hours post-reperfusion, complement C5a and damage-associated molecular patterns (DAMPs) activate pulmonary macrophages to release pro-inflammatory cytokines such as interleukin (IL)-6 and TNF-α (48). Concurrently, ICAM-1-mediated neutrophil adhesion creates a “plugging effect”, intensifying tissue oxidative damage through myeloperoxidase (MPO) release and neutrophil extracellular traps (NETs) (49,50). This process establishes a ‘ROS-inflammation-ROS’ positive feedback loop (51).
Figure 2.
IRI in pulmonary vascular endothelial cells. ① Reperfusion increases fluid shear stress to directly damage endothelial cells, leading to glycocalyx shedding. ② Reperfusion induces ROS storm generation in endothelial cells and leukocytes, causing glycocalyx shedding. ③ Reperfusion activates Ca2+ channels in endothelial cells, increases Ca2+ influx, and triggers cytokine storm, resulting in glycocalyx shedding. Reperfusion activates the NF-κB signaling pathway, enhances TNF-α transcription, and consequently leads to glycocalyx shedding. IRI, ischemia-reperfusion injury; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Microstructural pathological features of lung collapse
Lung collapse not only causes the closure of alveolar spaces but also induces apoptosis of type II epithelial cells and reduces the secretion of surfactant protein D (SP-D). The basement membranes of collapsed alveoli exhibit exposed collagen fibers, facilitating neutrophil transendothelial migration during reperfusion. Furthermore, sustained hypoxia-inducible factor (HIF)-1α activation in collapsed lung tissue promotes vascular endothelial growth factor (VEGF) overexpression, increasing the risk of capillary leak syndrome (CLS) following RM (52).
Mechanical ventilator-related injury
Mechanical ventilation may induce lung injury, especially during single-lung ventilation. The main mechanisms include the following.
VT and high inhaled oxygen fraction
Mechanical ventilation puts patients at risk of ventilator-induced lung injury, especially during single-lung ventilation. High VTs can cause excessive lung expansion (volume-induced injury), while high airway pressure and elevated oxygen fractions can lead to oxygen toxicity and inflammation. Research has demonstrated that the synergistic effects of these factors significantly enhance the release of inflammatory mediators, such as TNF-α and IL-6, thereby worsening lung injury (37,53,54).
Inflammatory factors and immune response
Imbalance of inflammatory mediators plays a critical role in OLV-associated lung injury. Surgical trauma-induced and mechanical ventilation-triggered macrophage activation collectively lead to elevated levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and decreased levels of anti-inflammatory cytokines (e.g., IL-10), resulting in inflammatory cytokine-mediated lung injury (55). Recent studies have revealed that the inflammatory response induced by OLV not only manifests as a generalized elevation of cytokine levels, but also mediates specific molecular interactions through dysregulation of the nitric oxide (NO) signaling pathway. Elevated TNF-α activates inducible NO synthase (iNOS), leading to excessive NO production that combines with superoxide anion to form peroxynitrite—a potent oxidant that directly damages the alveolar epithelium (56). Concurrently, IL-6 overexpression shows significant correlation with decreased arginase-1 activity, disrupting the arginine-NO metabolic axis and promoting NETs formation (49,57). This specific cytokine-enzyme interaction explains the elevated levels of nitrotyrosine (a biomarker of protein nitration damage) observed during OLV (58). These findings elucidate specific clinical correlations between cytokine shift, altered NO metabolism, and pulmonary injury.
Core parameters in tailored OLV strategy
VT
VT refers to the amount of gas inhaled or exhaled during each respiratory cycle. During mechanical ventilation, excessively high VT can induce alveolar overdistension, disrupt cellular junctions, and trigger the release of pro-inflammatory cytokines. Excessively low VT may be insufficient to maintain alveolar patency, leading to cyclical collapse and recruitment that generates shear forces capable of damaging the alveolar epithelium (59). The 2019 international expert consensus recommends using low VT (6–8 mL/kg IBW) as the foundation for lung-protective ventilation during double-lung ventilation (60). The optimal VT settings for lung-protective ventilation during OLV have not yet reached a definitive conclusion. Low VT during OLV combined with appropriate PEEP maintains oxygenation while providing lower airway pressure, which can effectively prevent or reduce ALI (61). The definition of low VT remains highly inconsistent, primarily ranging from 4 to 6 mL/kg IBW, while higher VT is considered 8–15 mL/kg (62). Multiple studies demonstrate that low VT protocols improve postoperative outcomes. However, without sufficient PEEP support, low VT may elevate the risk of atelectasis (63). A randomized trial involving 147 patients examined the impact of fixed VT vs. variable VT on the occurrence of PPCs. The findings indicated that variable VT did not significantly enhance oxygenation or decrease the incidence of PPCs during OLV (64) (see Table 1).
Table 1. Meta-summary of low VT’s impact on PPCs during OLV.
| Authors | Key findings | Study design | Results | Evidence gaps |
|---|---|---|---|---|
| Peel et al. (65) | Low VT (5.6±0.9 mL/kg) during OLV does not impair oxygenation or compliance but significantly reduces PPCs | Included 18 studies (3,693 patients), including RCTs and observational studies | (I) No significant differences in oxygenation or compliance; (II) reduced pulmonary complications (OR =0.40; P<0.001) | (I) High heterogeneity among studies; (II) lack of systematic analysis on PEEP and driving pressure effects |
| El Tahan et al. (66) | Lower VTs (4–7 mL/kg) improve intra/postoperative PaO2/FiO2 ratios and reduce postoperative complications and arrhythmia risk | Analyzed 17 RCTs (1,463 patients), focusing on precise timing during OLV | (I) Higher PaO2/FiO2 ratio (MD =18.59 mmHg; P<0.001); (II) reduced complications (OR =0.50) and arrhythmia (OR =0.58) | (I) Heterogeneity in some outcomes; (II) unclear synergy with other protective strategies (e.g., driving pressure) |
| El Tahan et al. (67) | Low VTs may worsen intraoperative gas exchange but preserve postoperative oxygenation, with no impact on complications or hospital stay | Reviewed 14 RCTs (820 patients), analyzing gas exchange and outcomes during OLV | (I) Reduced intraoperative PaO2 and higher PaCO2; (II) postoperative infiltrates/ARDS reduced, but no difference in complications | (I) Small sample sizes; (II) variability across surgery types and patient subgroups not addressed |
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; MD, mean difference; OLV, one-lung ventilation; OR, odds ratio; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PaO2/FiO2, oxygenation index; PEEP, positive end-expiratory pressure; PPCs, postoperative pulmonary complications; RCTs, randomized controlled trials; VT, tidal volume.
PEEP
Zero PEEP (ZPEEP) has been shown to cause perioperative atelectasis and increase the risk of PPCs (68). Recent studies demonstrate that RM combined with tailored PEEP titration can significantly improve the oxygenation index (PaO2/FiO2) during OLV. This mechanism works through phased incremental increases in airway pressure to reopen collapsed alveoli while maintaining end-expiratory alveolar stability (69). The physiological effects of PEEP are influenced by multiple factors, including: (I) lung parenchyma characteristics: such as lung compliance and alveolar surfactant content; (II) thoracic mechanics: elastic resistance of the chest wall and diaphragmatic mobility; and (III) pathological factors: interstitial fluid leakage, accumulation of inflammatory exudate in alveoli, etc. (70).
Methods to determine optimal PEEP levels include computed tomography, electrical impedance tomography, ultrasound, power-voltage (P-V) curve method, optimal oxygenation titration, and optimal lung compliance titration. PEEP settings should adhere to the “lowest effective pressure” principle. In clinical practice, a stepwise titration strategy is recommended: first implement a RM, followed by determining tailored PEEP using either the best compliance method or the optimal oxygenation method (71). Multiple meta-analyses demonstrate that tailored PEEP exhibits positive effects in reducing PPCs compared to fixed PEEP (see Table 2).
Table 2. Meta-summary of the impact of tailored PEEP on PPCs during OLV.
| Authors | Main findings | Experimental design | Results | Evidence gaps |
|---|---|---|---|---|
| Gu et al. (72) |
iPEEP (based on lung compliance) during OLV reduces PPCs, improves respiratory mechanics and oxygenation, without significant hemodynamic impact | Included 10 RCTs (3,426 patients) comparing iPEEP (dynamic compliance or driving pressure-based titration) vs. fixed PEEP in thoracic surgery | (I) Reduced PPC risk (RR =0.55), pneumonia (RR =0.71), and atelectasis (RR =0.63); (II) improved dynamic compliance, PaO2, and reduced driving pressure. No differences in ARDS, mortality, or hemodynamic variables | (I) Long-term hemodynamic effects not assessed; (II) limited evaluation of titration methods (e.g., EIT-guided PEEP) |
| Li et al. (73) |
iPEEP during OLV reduces PPCs and improves oxygenation (PaO2 and PaO2/FiO2) | Analyzed 8 RCTs (849 patients) comparing individualized vs. fixed PEEP in thoracic surgery | (I) Lower PPC risk (RR =0.52); (II) higher PaO2 (MD =34.2 mmHg) and PaO2/FiO2 (MD =49.07 mmHg) |
(I) Small sample size. Methodological biases (e.g., lack of blinding); (II) no long-term outcomes (e.g., mortality) analyzed |
| Peel et al. (10) | RMs and PEEP improve physiological metrics (e.g., PaO2, compliance) during OLV, but clinical outcome evidence is lacking | Included 16 studies (11 RCTs) evaluating PEEP/recruitment effects on oxygenation and ventilation during OLV | (I) Recruitment increased PaO2 (+82 mmHg) and reduced dead space; (II) PEEP improved PaO2 (+30.3 mmHg) and compliance (+4.33 mL/cmH2O); (III) only 2 studies reported clinical outcomes (inconclusive) | (I) Focus on surrogate outcomes (e.g., PaO2) over patient-centered outcomes (e.g., mortality, hospital stay); (II) high heterogeneity in PEEP definitions and study designs |
ARDS, acute respiratory distress syndrome; EIT, electric impedance tomography; iPEEP, individualized PEEP; MD, mean difference; OLV, one-lung ventilation; PaO2, partial pressure of arterial oxygen; PaO2/FiO2, oxygenation index; PEEP, positive end-expiratory pressure; PPCs, postoperative pulmonary complications; RCTs, randomized controlled trials; RM, recruitment maneuver; RR, risk ratio.
FiO2 and ORI
FiO2 is identified as a risk factor for pulmonary complications. Excessive oxygen administration may increase absorption atelectasis, reduce mucociliary transport, and damage lung tissue through the generation of ROS (74). The conventional strategy of maintaining adequate oxygenation by elevating the FiO2 during OLV is now facing emerging evidence-based challenges. Animal experiments have confirmed that reducing FiO2 from 1.0 to 0.6 under normoxemic conditions decreases alveolar injury markers such as IL-6 and TNF-α (75). Multiple clinical studies have found that higher inspired oxygen fractions during OLV increase the incidence of PPCs (41,76,77).
The ORI is a novel non-invasive monitoring parameter based on multi-wavelength pulse oximetry (Masimo Rainbow SET, Irvine, CA, USA). It reflects dynamic changes in oxygen reserves under moderate hyperoxia (PaO2 100–200mmHg) by analyzing differences in light absorption between arterial and venous pulsatile blood flow. Its quantification principle integrates Fick’s law with the oxygen content equation. In oxygen-supplemented patients, when PaO2 >100 mmHg, SpO2 reaches a plateau phase (approximately 100%), while venous oxygen saturation (SvO2) continues to increase with rising PaO2 levels until stabilizing at approximately 80% when PaO2 reaches around 200 mmHg. ORI is generated through the rate of optical absorption signal changes during this phase, serving as a unitless index scaled between 0.00 and 1.00 that specifically evaluates PaO2 fluctuations within the moderate hyperoxia range (78). Multiple studies have demonstrated that during OLV, oxygen titration guided by the ORI can reduce hyperoxia-related risks and decrease PPCs (79-81).
RM
As a core strategy in lung-protective ventilation, the RM derives its theoretical foundation from the transient elevation of transpulmonary pressure to reopen collapsed alveoli. This mechanism achieves lung protection by reducing shear injury, optimizing V/Q ratios, and suppressing inflammatory cytokine release. It is particularly applicable in Open Lung Approach strategies to delay acute respiratory distress syndrome (ARDS) progression and mitigate ventilator-induced lung injury (82). Clinical data indicate that during OLV, the application of RM maintains improved intraoperative oxygenation until surgery completion, while effectively suppressing the release of local inflammatory mediators by reducing repetitive alveolar opening and closing. Furthermore, combining RM with a tailored PEEP strategy can further stabilize the V/Q ratio and reduce the risk of PPCs (83). Several meta-analyses demonstrate that RM improves intraoperative oxygenation and postoperative outcomes (see Table 3).
Table 3. Meta-summary of RM’s impact on PPCs during OLV.
| Authors | Main perspectives on RM | Experimental design | Key results | Evidence gaps |
|---|---|---|---|---|
| Hu et al. (84) | RM reduces intrapulmonary shunt, improves lung compliance and oxygenation (PaO2/FiO2 ratio) without affecting hemodynamics, but no statistically significant impact on the incidence of atelectasis | Included 6 RCTs involving 526 thoracic surgery patients, comparing RM with conventional mechanical ventilation | (I) Significant reduction in intrapulmonary shunt (WMD =−0.02), improved lung compliance (WMD =2.16), increased PaO2/FiO2 (WMD =31.31); (II) no significant difference in atelectasis incidence (RR =0.55) | (I) Insufficient evidence on atelectasis incidence; (II) small sample size; (III) high heterogeneity among studies (e.g., variations in PEEP settings) |
| Peel et al. (10) | RM improves oxygenation (PaO2 +82 mmHg) and reduces dead-space ventilation (Vd/Vt −5.9%) during OLV, but lacks evidence on clinical outcomes | Analyzed 16 studies (11 RCTs) evaluating RM and PEEP during OLV | (I) RM significantly increased PaO2 (P<0.05); (II) PEEP improved oxygenation (+30.3 mmHg) and compliance (+4.33 mL/cmH2O) |
(I) Lack of patient-centered clinical outcomes (e.g., mortality, complication rates); (II) high heterogeneity (I2=82–92%) |
| Zhu et al. (85) | Protective ventilation (including low VT, PEEP, and possibly RM) reduces PPCs (e.g., pneumonia, hospital stay) | Included 8 retrospective studies (736 patients) comparing protective vs. conventional ventilation in thoracic surgery | Protective ventilation reduced lung injury (P=0.006), pneumonia (P=0.01), and hospital stay (P=0.002) | (I) RM’s role not isolated; (II) high heterogeneity; (III) limited sample size and retrospective design |
FiO2, fraction of inspired oxygen; OLV, one-lung ventilation; PaO2, partial pressure of arterial oxygen; PaO2/FiO2, oxygenation index; PEEP, positive end-expiratory pressure; PPCs, postoperative pulmonary complications; RCTs, randomized controlled trials; RM, recruitment maneuver; RR, risk ratio; Vd/Vt, the ratio of dead space ventilation to tidal volume; VT, tidal volume; WMD, weighted mean difference.
Notably, the RM is not universally applicable to all patients. The clinical application of RM requires strict adherence to the principles of contraindication evaluation and dynamic monitoring. The main contraindications comprise four categories: (I) thoracic structural abnormalities (e.g., untreated pneumothorax, pulmonary bullae, or bronchopleural fistula), as high-pressure ventilation may exacerbate tissue damage or fistula enlargement; (II) circulatory dysfunction (e.g., hypovolemic shock, severe cardiac insufficiency), where thoracic pressure variations may aggravate hemodynamic instability; (III) craniocerebral injury-related conditions (e.g., intracranial hypertension, acute cerebral edema), as impaired jugular venous return may induce intracranial pressure fluctuations; and (IV) severe parenchymal lung diseases (such as extensive pulmonary fibrosis, emphysema, or active hemorrhage) due to poor lung tissue compliance and risk of barotrauma (60). Prior to clinical implementation, clinicians should perform tailored assessments by comprehensively evaluating the patient’s pathophysiological status, weighing the risks between improving oxygenation and inducing complications. Particular attention should be paid to excluding potential fragile lung tissue lesions through imaging examinations. Continuous monitoring of hemodynamic parameters and oxygenation indices during procedures is essential to prevent severe consequences from indiscriminate application.
Application of tailored lung-protective ventilation
Studies on pressure-volume curve demonstrate that implementing lung-protective ventilation strategies can simultaneously prevent barotrauma, volutrauma, and atelectasis formation. For the implementation of OLV, comprehensive pre-OLV optimization and tailored precision regulation during OLV are required to balance multiple physiological demands and clinical constraints, thereby reducing PPCs incidence and improving postoperative outcomes (86).
Preoperative optimization for OLV
Device selection and positioning
The DLT remains the preferred choice for OLV. Its precise positioning requires standardized protocols: During fiberoptic bronchoscopy verification, left DLT placement must ensure complete blue cuff entry into the left main bronchus with direct visualization of the left upper lobe orifice, while right DLT positioning requires precise alignment of its ventilation slot with the right upper lobe bronchus. After positioning, auscultation (unilateral disappearance of breath sounds) and verification of single-peak waveform on end-tidal carbon dioxide monitoring should be combined to confirm isolation effectiveness. Intraoperatively, repeat fiberoptic bronchoscopy examinations should be performed after position fixation, at initiation of OLV, and every 30 minutes thereafter. Particular attention should be paid to signs of displacement such as sudden airway pressure elevation (>5 cmH2O) or oxygenation decline (SpO2 reduction >5%) to maintain the stability of OLV (87).
Implementation of tailored lung-protective ventilation strategy
During OLV, the core components of current tailored lung-protective ventilation strategy comprise two critical elements: low VT based on IBW (4–6 mL/kg IBW) and synergistic application of RM with tailored PEEP (88). Recent clinical evidence demonstrates that implementing an individualized intraoperative open-lung approach during OLV shows significant clinical value in reducing the incidence of PPCs (22). This strategy is implemented in stages: first through alveolar recruitment procedures (reversing alveolar collapse via controlled elevation of airway pressure), followed by tailored PEEP titration based on respiratory system mechanics parameters such as driving pressure or lung compliance.
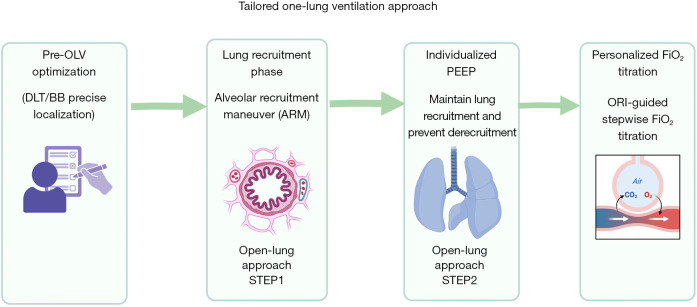
Building upon this core technology, the implementation of tailored FiO2 titration guided by the ORI enables determination of the minimum effective FiO2, systematically optimizing intraoperative respiratory management and reducing PPCs risk (see Figure 3).
Figure 3.
Implementation steps of tailored OLV. (I) Optimize ventilator positioning prior to OLV. (II) The RM serves as the first step in tailored intraoperative lung opening strategy, facilitating re-expansion of atelectatic lungs. (III) Titration of tailored PEEP constitutes the second step in tailored intraoperative lung opening strategy, maintaining alveolar stability. (IV) Implement individualized inspired oxygen concentration titration based on ORI, establishing the minimum effective FiO2 to mitigate hyperoxic injury. BB, bronchial blocker; DLT, double-lumen endotracheal tube; FiO2, fraction of inspired oxygen; OLV, one-lung ventilation; ORI, oxygen reserve index; PEEP, positive end-expiratory pressure; RM, recruitment maneuver.
RM
The alveolar recruitment procedure achieves re-expansion of collapsed lung tissue by applying effective opening pressure. Its core principle lies in: When transpulmonary inspiratory pressure reaches a critical threshold, it can immediately induce physiological-structural-functional changes in lung tissue. Due to technical limitations in monitoring transpulmonary inspiratory pressure, airway pressure is commonly used as a surrogate indicator in clinical practice, with a positive correlation existing between the two parameters (89). The critical opening pressure required for alveolar recruitment in healthy lung tissue is approximately 40 cmH2O airway pressure. However, in specific clinical scenarios such as obesity, where reduced chest wall compliance is present, airway pressure needs to be increased to 45–50 cmH2O to achieve equivalent opening pressure (84).
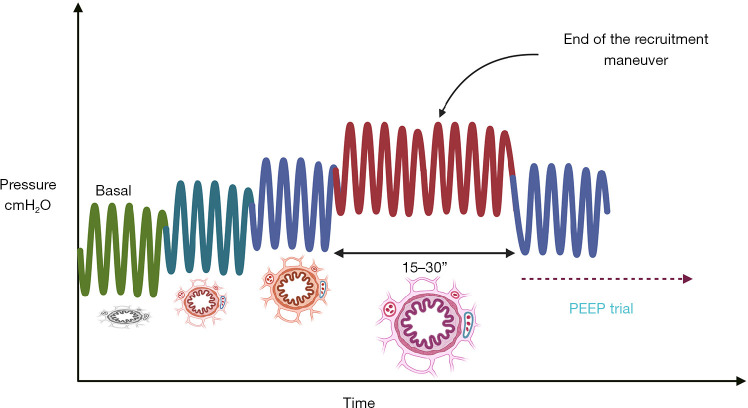
The progressive PEEP adjustment method under pressure-controlled ventilation (PCV) mode currently represents the most widely accepted operational protocol. This modality employs a fixed driving pressure of 20 cmH2O with a ventilation frequency of 10–20 breaths/minute. The inspiration-to-expiration ratio is typically set at 1:1 (extended expiration time is required for patients with emphysema or obstructive pulmonary disease). During implementation, the baseline PEEP should be incrementally elevated from 5 cmH2O (or current adjustment value) in 5 cmH2O increments to 10, 15, and 20 cmH2O (with potential increases for special cases including obesity, Trendelenburg positioning, or robotic surgery). Each increment should be maintained for 3–5 respiratory cycles. Ultimately, an opening pressure of 40 cmH2O must be maintained for 30 seconds to achieve complete lung recruitment (84). Effective RM can induce the following physiological effects: increased end-expiratory lung volume (EELV), improved lung compliance, and enhanced oxygenation resulting from expanded gas exchange surface area due to alveolar recruitment. In clinical assessment, the effectiveness of lung recruitment is typically judged by changes in oxygenation indices. Specifically, when FiO2 is 1.0, a FiO2/SpO2 ratio >400 must be satisfied; or when FiO2 is 0.21, SpO2 ≥97%, with a shunt fraction <10%, the “open lung” state can be confirmed (82) (see Figure 4).
Figure 4.
Implementation process of the alveolar RM. Starting with a baseline PEEP of 5 cmH2O (or current adjusted value), incrementally increase in 5 cmH2O steps to 10, 15, and 20 cmH2O (may be appropriately elevated in special cases such as obesity, Trendelenburg position, or robotic surgery), maintaining each level for 3–5 respiratory cycles. Ultimately, maintain an opening pressure of 40 cmH2O for 30 seconds to complete lung recruitment. PEEP, positive end-expiratory pressure; RM, recruitment maneuver.
iPEEP
iPEEP demonstrates significant clinical value in lung-protective ventilation strategies. Its core advantage lies in maintaining alveolar stability through anesthesiologists’ precise adjustment, effectively minimizing the risk of alveolar collapse. The ideal tailored parameter configuration should aim for optimal lung compliance, establishing a dynamic equilibrium between alveolar overdistension and collapse. This equilibrium ensures the lowest driving pressure under specific VT conditions, significantly reducing the risk of PPCs occurrence (90).
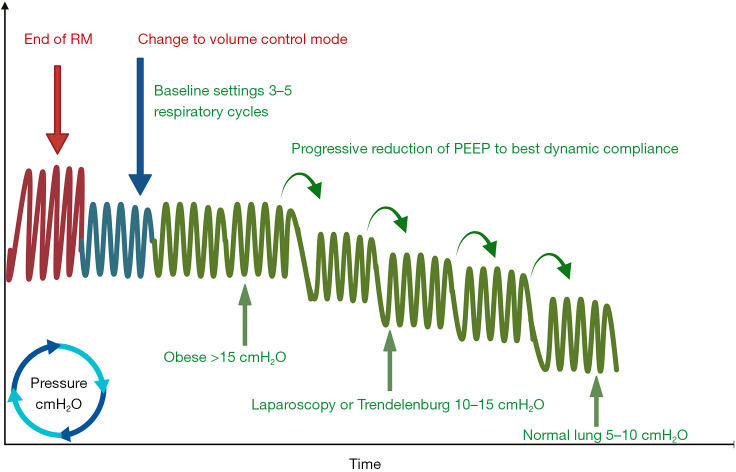
Currently, the commonly used decremental PEEP titration method involves dynamically monitoring lung mechanics parameters (such as the airway pressure-volume curve) to identify the critical alveolar closing pressure (the minimal maintenance pressure at which recruited alveoli begin to collapse), thereby achieving tailored PEEP setting. The standardized protocol includes: selecting volume control (or pressure control mode) after RM while maintaining VT and inspiration-expiration ratio parameters consistent with baseline settings; initial PEEP is determined based on patient characteristics such as lung compliance and BMI (16 cmH2O for healthy individuals, 18–20 cmH2O for patients with obesity or undergoing laparoscopic surgery); gradual stepwise reduction in 2 cmH2O increments sustained over 3–5 respiratory cycles, with closure pressure determined by monitoring compliance decreases >10% or changes in oxygenation indices (91).
Detection of lung closing pressure can be comprehensively assessed through respiratory mechanics parameters, oxygenation indices, pulmonary efficiency evaluations, and imaging techniques. Among respiratory mechanics parameters [including dynamic lung compliance (Cdyn), driving pressure, and transpulmonary pressure], Cdyn is the most clinically prevalent and representative indicator. This parameter’s prominent advantage lies in its device versatility—applicable across various ventilator models. Through continuous monitoring during PEEP titration, it enables precise identification of lung closing pressure corresponding to compliance peaks (88) (see Figure 5).
Figure 5.
Tailored PEEP titration. After lung RMs, select volume control mode while maintaining parameters including VT and inspiratory-to-expiratory ratio consistent with baseline settings. The initial PEEP is set based on patient characteristics such as lung compliance and BMI (16 cmH2O for healthy individuals, 18–20 cmH2O for obese patients or those undergoing laparoscopic surgery). Gradually decrease PEEP in 2 cmH2O increments while maintaining for 3–5 respiratory cycles. Monitor compliance reductions >10% or changes in oxygenation indices to determine closing pressure, ultimately identifying the optimal PEEP value. Obese populations require PEEP >15 cmH2O, while laparoscopic surgeries or Trendelenburg positioning necessitate 10–15 cmH2O, and healthy populations typically require approximately 5–10 cmH2O. BMI, body mass index; PEEP, positive end-expiratory pressure; RM, recruitment maneuver; VT, tidal volume.
Tailored titration of FiO2
In clinical surgery, pulse oximetry is routinely employed to monitor oxygen saturation (SpO2). However, SpO2 monitoring has limitations in sensitivity to oxygenation status changes: unless there is a significant decrease in PaO2 (typically below 80 mmHg), SpO2 readings may remain within normal ranges, making it difficult to detect early deterioration of oxygenation status in a timely manner. The novel multi-wavelength pulse carbon monoxide (CO)-oximetry (Masimo) combined with ORI monitoring technology provides a non-invasive solution for real-time assessment of moderate hyperoxia (PaO2 between 100 and 200 mmHg). Particularly when SpO2 exceeds 98%, the ORI can provide precise quantitative data on hyperoxemia, which holds significant guidance value for optimizing perioperative oxygen therapy protocols (80).
Tailored titration of the FiO2 should be dynamically adjusted through continuous monitoring of the ORI combined with SpO2 and clinical signs, following the implementation of Open Lung Approach ventilation in patients. The operational procedure involves first establishing tailored oxygenation targets (recommended SpO2 92–96%). After initially adjusting FiO2 to reach the lower target limit of SpO2, gradually decrease FiO2 in 0.05–0.1 increments while simultaneously monitoring ORI. Oxygen reduction should be terminated when ORI drops to 0.3–0.5 (indicating oxygen reserves approaching critical levels) or when SpO2 approaches the lower target limit. The FiO2 at this stage represents the minimum required concentration for maintaining adequate oxygenation. If SpO2 fluctuations or respiratory deterioration occur, FiO2 should be incrementally increased in reverse at 0.02–0.05 gradients until the ORI recovers above 0.5. Throughout the process, multi-parameter validation should be performed every 5–10 minutes in conjunction with blood gas analysis, with particular attention to ORI response differences in COPD and ARDS patients and motion interference factors. By dynamically balancing FiO2 adjustment magnitudes and monitoring frequency (it is recommended to stabilize for 3 minutes after each adjustment), the dual objectives of precisely avoiding hyperoxic injury and hypoxemia risks can ultimately be achieved (80,92).
Limitations
Recent studies have confirmed that mechanical ventilation-associated lung injury during OLV is a core contributor to PPCs (12,19). The implementation of perioperative lung-protective ventilation strategies (low VT, tailored PEEP combined with RM) has gained consensus. Within the enhanced recovery after surgery (ERAS) framework, tailored treatment can improve postoperative outcomes. Current strategies effectively reduce PPCs risk by integrating RM with tailored PEEP titration based on optimal lung compliance, while dynamically regulating FiO2 through the ORI monitoring system. However, the study faces limitations including insufficient multicenter collaboration, small sample sizes, high technical dependency (e.g., reliance on artificial intelligence (AI)-assisted monitoring and advanced ventilator functionalities), inadequate integration of postoperative coordinated care elements (analgesia and fluid management), lack of subgroup analyses for special populations (COPD, obesity, airway structural anomalies), and significant variations in ventilation strategy selection among different lung isolation techniques (e.g., DLT and BB). These constraints present dual challenges for clinical implementation—standardizing equipment requirements and ensuring operational feasibility.
Future efforts should establish standardized databases through multicenter collaboration to validate tailored parameter thresholds by incorporating dynamic imaging technologies; develop lightweight AI-assisted systems to reduce technical barriers and construct perioperative multifactorial collaborative management models. Concurrently, focus should be directed toward targeted research on high-risk subgroups, implement long-term prognostic tracking, and strengthen ethical risk assessment (such as balancing intraoperative emergency adjustments with risks of high PEEP cycling), ultimately driving the establishment of tailored lung-protective ventilation strategies as the core pillar of perioperative management in thoracic surgery.
Conclusions
Tailored lung ventilation strategies can significantly reduce the risk of OLV-associated PPCs through RM, dynamic titration of PEEP, and precise adjustment of FiO2 based on the ORI, outperforming conventional fixed-parameter strategies. Future multicenter randomized controlled trials and AI-assisted monitoring technologies are required to further validate its universal applicability, while integrating multimodal imaging to optimize tailored thresholds, thereby advancing precision perioperative management.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-314/coif). The authors have no conflicts of interest to declare.
References
- 1.Falzon D, Alston RP, Coley E, et al. Lung Isolation for Thoracic Surgery: From Inception to Evidence-Based. J Cardiothorac Vasc Anesth 2017;31:678-93. 10.1053/j.jvca.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 2.Smith S, Arthur ME. Lung Isolation Anesthesia. 2025. [PubMed]
- 3.Morris BN, Fernando RJ, Garner CR, et al. A Randomized Comparison of Positional Stability: The EZ-Blocker Versus Left-Sided Double-Lumen Endobronchial Tubes in Adult Patients Undergoing Thoracic Surgery. J Cardiothorac Vasc Anesth 2021;35:2319-25. 10.1053/j.jvca.2020.11.056 [DOI] [PubMed] [Google Scholar]
- 4.Moreault O, Couture EJ, Provencher S, et al. Double-lumen endotracheal tubes and bronchial blockers exhibit similar lung collapse physiology during lung isolation. Can J Anaesth 2021;68:791-800. 10.1007/s12630-021-01938-y [DOI] [PubMed] [Google Scholar]
- 5.Xiang YY, Chen Q, Tang XX, et al. Comparison of the effect of double-lumen endotracheal tubes and bronchial blockers on lung collapse in video-assisted thoracoscopic surgery: a systematic review and meta-analysis. BMC Anesthesiol 2022;22:330. 10.1186/s12871-022-01876-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinack C, Balmer H, Ulrich S, et al. One-Lung Ventilation during Rigid Bronchoscopy Using a Single-Lumen Endotracheal Tube: A Descriptive, Retrospective Single-Center Study. J Clin Med 2023;12:2426. 10.3390/jcm12062426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao ZR, Lau RWH, Ng CSH. Anaesthesiology for uniportal VATS: double lumen, single lumen and tubeless. J Vis Surg 2017;3:108. 10.21037/jovs.2017.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender SP, Anderson EP, Hieronimus RI, et al. One-Lung Ventilation and Acute Lung Injury. Int Anesthesiol Clin 2018;56:88-106. 10.1097/AIA.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 9.Kidane B, Palma DC, Badner NH, et al. The Potential Dangers of Recruitment Maneuvers During One Lung Ventilation Surgery. J Surg Res 2019;234:178-83. 10.1016/j.jss.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 10.Peel JK, Funk DJ, Slinger P, et al. Positive end-expiratory pressure and recruitment maneuvers during one-lung ventilation: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2020;160:1112-1122.e3. 10.1016/j.jtcvs.2020.02.077 [DOI] [PubMed] [Google Scholar]
- 11.Uhlig C, Neto AS, van der Woude M, et al. Intraoperative mechanical ventilation practice in thoracic surgery patients and its association with postoperative pulmonary complications: results of a multicenter prospective observational study. BMC Anesthesiol 2020;20:179. 10.1186/s12871-020-01098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler D, Mosieri C, Kallurkar A, et al. Perioperative strategies for the reduction of postoperative pulmonary complications. Best Pract Res Clin Anaesthesiol 2020;34:153-66. 10.1016/j.bpa.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Chen Z, Zhao R, et al. Development and validation of a nomogram to predict postoperative pulmonary complications following thoracoscopic surgery. PeerJ 2021;9:e12366. 10.7717/peerj.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong MKH, Sit AKY, Au TWK. Minimally invasive thoracic surgery: beyond surgical access. J Thorac Dis 2018;10:S1884-91. 10.21037/jtd.2018.05.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cypel M, Yasufuku K. Complications during minimal invasive thoracic surgery: are new surgeons prepared? Lancet Oncol 2018;19:17-9. 10.1016/S1470-2045(17)30915-4 [DOI] [PubMed] [Google Scholar]
- 16.Nozaki I, Mizusawa J, Kato K, et al. Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg Endosc 2018;32:651-9. 10.1007/s00464-017-5716-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann KB, Loop T, Heinrich S, et al. Risk factors for post-operative pulmonary complications in lung cancer patients after video-assisted thoracoscopic lung resection: Results of the German Thorax Registry. Acta Anaesthesiol Scand 2019;63:1009-18. 10.1111/aas.13388 [DOI] [PubMed] [Google Scholar]
- 18.Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171-6. 10.1136/thoraxjnl-2015-207697 [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Xia F, Lu Z, et al. Effects of tidal volume on physiology and clinical outcomes in patients with one‑lung ventilation undergoing surgery: A meta‑analysis of randomized controlled trials. Biomed Rep 2024;20:73. 10.3892/br.2024.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Gara B, Talmor D. Perioperative lung protective ventilation. BMJ 2018;362:k3030. 10.1136/bmj.k3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spadaro S, Grasso S, Karbing DS, et al. Physiologic Evaluation of Ventilation Perfusion Mismatch and Respiratory Mechanics at Different Positive End-expiratory Pressure in Patients Undergoing Protective One-lung Ventilation. Anesthesiology 2018;128:531-8. 10.1097/ALN.0000000000002011 [DOI] [PubMed] [Google Scholar]
- 22.Ferrando C, Carramiñana A, Piñeiro P, et al. Individualised, perioperative open-lung ventilation strategy during one-lung ventilation (iPROVE-OLV): a multicentre, randomised, controlled clinical trial. Lancet Respir Med 2024;12:195-206. 10.1016/S2213-2600(23)00346-6 [DOI] [PubMed] [Google Scholar]
- 23.Purohit A, Bhargava S, Mangal V, et al. Lung isolation, one-lung ventilation and hypoxaemia during lung isolation. Indian J Anaesth 2015;59:606-17. 10.4103/0019-5049.165855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos JH, Sharma A. Predictors of Hypoxemia During One-Lung Ventilation in Thoracic Surgery: Is Oxygen Reserve Index (ORi) the Answer? J Cardiothorac Vasc Anesth 2020;34:423-5. 10.1053/j.jvca.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 25.Campos JH, Feider A. Hypoxia During One-Lung Ventilation-A Review and Update. J Cardiothorac Vasc Anesth 2018;32:2330-8. 10.1053/j.jvca.2017.12.026 [DOI] [PubMed] [Google Scholar]
- 26.Ng A, Swanevelder J. Hypoxaemia associated with one-lung anaesthesia: new discoveries in ventilation and perfusion. Br J Anaesth 2011;106:761-3. 10.1093/bja/aer113 [DOI] [PubMed] [Google Scholar]
- 27.Hedenstierna G, Edmark L. Effects of anesthesia on the respiratory system. Best Pract Res Clin Anaesthesiol 2015;29:273-84. 10.1016/j.bpa.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 28.Shum S, Huang A, Slinger P. Hypoxaemia during one lung ventilation. BJA Educ 2023;23:328-36. 10.1016/j.bjae.2023.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozian A, Schilling T, Fredén F, et al. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: an experimental study. Br J Anaesth 2008;100:549-59. 10.1093/bja/aen021 [DOI] [PubMed] [Google Scholar]
- 30.Szegedi LL, D'Hollander AA, Vermassen FE, et al. Gravity is an important determinant of oxygenation during one-lung ventilation. Acta Anaesthesiol Scand 2010;54:744-50. 10.1111/j.1399-6576.2010.02238.x [DOI] [PubMed] [Google Scholar]
- 31.Roth JV. Complications of One-lung Ventilation: Is It the Blood Flow or the Ventilation? Anesthesiology 2016;125:1253-4. 10.1097/ALN.0000000000001349 [DOI] [PubMed] [Google Scholar]
- 32.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 2015;122:932-46. 10.1097/ALN.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 33.Licker M, Hagerman A, Jeleff A, et al. The hypoxic pulmonary vasoconstriction: From physiology to clinical application in thoracic surgery. Saudi J Anaesth 2021;15:250-63. 10.4103/sja.sja_1216_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XQ, Tan WF, Wang J, et al. The effects of thoracic epidural analgesia on oxygenation and pulmonary shunt fraction during one-lung ventilation: an meta-analysis. BMC Anesthesiol 2015;15:166. 10.1186/s12871-015-0142-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neto AS, da Costa LGV, Hemmes SNT, et al. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: An observational study. Eur J Anaesthesiol 2018;35:691-701. 10.1097/EJA.0000000000000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017;151:181-92. 10.1016/j.chest.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. 10.1213/ANE.0000000000000808 [DOI] [PubMed] [Google Scholar]
- 38.Wu MY, Yiang GT, Liao WT, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem 2018;46:1650-67. 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 39.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 2010;40:294-309. 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126-67. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi A, Deng H, Fuller M, et al. Intraoperative FiO(2) and risk of impaired postoperative oxygenation in lung resection: A propensity score-weighted analysis. J Clin Anesth 2025;101:111739. 10.1016/j.jclinane.2024.111739 [DOI] [PubMed] [Google Scholar]
- 42.Brettner F, von Dossow V, Chappell D. The endothelial glycocalyx and perioperative lung injury. Curr Opin Anaesthesiol 2017;30:36-41. 10.1097/ACO.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 43.Ferrari RS, Andrade CF. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid Med Cell Longev 2015;2015:590987. 10.1155/2015/590987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ou D, Xu W, Feng Z, et al. Vascular endothelial glycocalyx shedding in ventilator-induced lung injury in rats. Microvasc Res 2024;153:104658. 10.1016/j.mvr.2024.104658 [DOI] [PubMed] [Google Scholar]
- 45.Ta HQ, Kuppusamy M, Sonkusare SK, et al. The endothelium: gatekeeper to lung ischemia-reperfusion injury. Respir Res 2024;25:172. 10.1186/s12931-024-02776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abassi Z, Armaly Z, Heyman SN. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am J Pathol 2020;190:752-67. 10.1016/j.ajpath.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 47.Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc 2015;12 Suppl 1:S3-8. 10.1513/AnnalsATS.201407-340MG [DOI] [PubMed] [Google Scholar]
- 48.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 2016;12:383-401. 10.1038/nrneph.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134-47. 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Jin J, Lv T, et al. A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury. Int J Mol Sci 2024;25:1464. 10.3390/ijms25031464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 2013;13:349-61. 10.1038/nri3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mo L, Xu G, Wu C, et al. Key Regulatory Effect of Activated HIF-1α/VEGFA Signaling Pathway in Systemic Capillary Leak Syndrome Confirmed by Bioinformatics Analysis. J Comput Biol 2020;27:914-22. 10.1089/cmb.2019.0222 [DOI] [PubMed] [Google Scholar]
- 53.Kimura S, Stoicea N, Rosero Britton BR, et al. Preventing Ventilator-Associated Lung Injury: A Perioperative Perspective. Front Med (Lausanne) 2016;3:25. 10.3389/fmed.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced Lung Injury. Clin Chest Med 2016;37:633-46. 10.1016/j.ccm.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruinooge AJG, Mao R, Gottschalk TH, et al. Identifying biomarkers of ventilator induced lung injury during one-lung ventilation surgery: a scoping review. J Thorac Dis 2022;14:4506-20. 10.21037/jtd-20-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang K, Liu Y, Deng M, et al. Imaging peroxynitrite in endoplasmic reticulum stress and acute lung injury with a near-infrared fluorescent probe. Anal Chim Acta 2024;1286:342050. 10.1016/j.aca.2023.342050 [DOI] [PubMed] [Google Scholar]
- 57.Chepurnova DA, Samoilova EV, Anisimov AA, et al. Compounds of IL-6 Receptor Complex during Acute Lung Injury. Bull Exp Biol Med 2018;164:609-11. 10.1007/s10517-018-4042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martínez-Caro L, Nin N, Sánchez-Rodríguez C, et al. Inhibition of Nitro-Oxidative Stress Attenuates Pulmonary and Systemic Injury Induced by High-Tidal Volume Mechanical Ventilation. Shock 2015;44:36-43. 10.1097/SHK.0000000000000381 [DOI] [PubMed] [Google Scholar]
- 59.Silva PL, Ball L, Rocco PRM, et al. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin Respir Crit Care Med 2022;43:321-34. 10.1055/s-0042-1744447 [DOI] [PubMed] [Google Scholar]
- 60.Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth 2019;123:898-913. 10.1016/j.bja.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 61.Slinger PD. Optimizing One-Lung Ventilation: Moving Beyond Tidal Volume. J Cardiothorac Vasc Anesth 2018;32:2673-5. 10.1053/j.jvca.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 62.Campos JH, Sharma A. What is the Ideal Tidal Volume During One-Lung Ventilation? J Cardiothorac Vasc Anesth 2023;37:1993-5. 10.1053/j.jvca.2023.06.021 [DOI] [PubMed] [Google Scholar]
- 63.Yang D, Grant MC, Stone A, et al. A Meta-analysis of Intraoperative Ventilation Strategies to Prevent Pulmonary Complications: Is Low Tidal Volume Alone Sufficient to Protect Healthy Lungs? Ann Surg 2016;263:881-7. 10.1097/SLA.0000000000001443 [DOI] [PubMed] [Google Scholar]
- 64.Szamos K, Balla B, Pálóczi B, et al. One-lung ventilation with fixed and variable tidal volumes on oxygenation and pulmonary outcomes: A randomized trial. J Clin Anesth 2024;95:111465. 10.1016/j.jclinane.2024.111465 [DOI] [PubMed] [Google Scholar]
- 65.Peel JK, Funk DJ, Slinger P, et al. Tidal volume during 1-lung ventilation: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2022;163:1573-1585.e1. 10.1016/j.jtcvs.2020.12.054 [DOI] [PubMed] [Google Scholar]
- 66.El Tahan MR, Samara E, Marczin N, et al. Impact of Lower Tidal Volumes During One-Lung Ventilation: A 2022 Update of the Meta-analysis of Randomized Controlled Trials. J Cardiothorac Vasc Anesth 2023;37:1983-92. 10.1053/j.jvca.2023.04.018 [DOI] [PubMed] [Google Scholar]
- 67.El Tahan MR, Pasin L, Marczin N, et al. Impact of Low Tidal Volumes During One-Lung Ventilation. A Meta-Analysis of Randomized Controlled Trials. J Cardiothorac Vasc Anesth 2017;31:1767-73. 10.1053/j.jvca.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 68.Xu D, Wei W, Chen L, et al. Effects of different positive end-expiratory pressure titrating strategies on oxygenation and respiratory mechanics during one- lung ventilation: a randomized controlled trial. Ann Palliat Med 2021;10:1133-44. 10.21037/apm-19-441 [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Zhang M, Wang X, et al. Individualized positive end-expiratory pressure in patients undergoing thoracoscopic lobectomy: a randomized controlled trial. Braz J Anesthesiol 2021;71:565-71. 10.1016/j.bjane.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millington SJ, Cardinal P, Brochard L. Setting and Titrating Positive End-Expiratory Pressure. Chest 2022;161:1566-75. 10.1016/j.chest.2022.01.052 [DOI] [PubMed] [Google Scholar]
- 71.Yao W, Yang B, Wang W, et al. Effect of Positive End-Expiratory Pressure (PEEP) Titration in Elderly Patients Undergoing Lobectomy. Med Sci Monit 2022;28:e938225. 10.12659/MSM.938225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu WJ, Zhao FZ, Piccioni F, et al. Individualized PEEP titration by lung compliance during one-lung ventilation: a meta-analysis. Crit Care 2025;29:27. 10.1186/s13054-024-05237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li P, Kang X, Miao M, et al. Individualized positive end-expiratory pressure (PEEP) during one-lung ventilation for prevention of postoperative pulmonary complications in patients undergoing thoracic surgery: A meta-analysis. Medicine (Baltimore) 2021;100:e26638. 10.1097/MD.0000000000026638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douville NJ, Smolkin ME, Naik BI, et al. Association between inspired oxygen fraction and development of postoperative pulmonary complications in thoracic surgery: a multicentre retrospective cohort study. Br J Anaesth 2024;133:1073-84. 10.1016/j.bja.2024.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Z, Gu L, Bian Q, et al. Oxygenation, inflammatory response and lung injury during one lung ventilation in rabbits using inspired oxygen fraction of 0.6 vs. 1.0. J Biomed Res 2016;31:56-64. 10.7555/JBR.31.20160108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okahara S, Shimizu K, Suzuki S, et al. Associations between intraoperative ventilator settings during one-lung ventilation and postoperative pulmonary complications: a prospective observational study. BMC Anesthesiol 2018;18:13. 10.1186/s12871-018-0476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Q, Li P. Association between inspired oxygen fraction and development of postoperative pulmonary complications in thoracic surgery. Comment on Br J Anaesth 2024; 133: 1073-84. Br J Anaesth 2025;134:1571-2. 10.1016/j.bja.2025.02.021 [DOI] [PubMed] [Google Scholar]
- 78.Alday E, Nieves JM, Planas A. Oxygen Reserve Index Predicts Hypoxemia During One-Lung Ventilation: An Observational Diagnostic Study. J Cardiothorac Vasc Anesth 2020;34:417-22. 10.1053/j.jvca.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 79.Sagiroglu G, Baysal A, Karamustafaoglu YA. The use of oxygen reserve index in one-lung ventilation and its impact on peripheral oxygen saturation, perfusion index and, pleth variability index. BMC Anesthesiol 2021;21:319. 10.1186/s12871-021-01539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saraçoğlu A, Yamansavcı Şirzai E, Yıldızeli B, et al. Oxygen reserve index guided oxygen titration in one lung ventilation with low fresh gas flow. Turk J Med Sci 2021;51:2413-9. 10.3906/sag-2009-149 [DOI] [PubMed] [Google Scholar]
- 81.Koishi W, Kumagai M, Ogawa S, et al. Monitoring the Oxygen Reserve Index can contribute to the early detection of deterioration in blood oxygenation during one-lung ventilation. Minerva Anestesiol 2018;84:1063-9. 10.23736/S0375-9393.18.12622-8 [DOI] [PubMed] [Google Scholar]
- 82.Choi YS, Bae MK, Kim SH, et al. Effects of Alveolar Recruitment and Positive End-Expiratory Pressure on Oxygenation during One-Lung Ventilation in the Supine Position. Yonsei Med J 2015;56:1421-7. 10.3349/ymj.2015.56.5.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meleiro H, Correia I, Charco Mora P. New evidence in one-lung ventilation. Rev Esp Anestesiol Reanim (Engl Ed) 2018;65:149-53. 10.1016/j.redar.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 84.Hu MC, Yang YL, Chen TT, et al. Recruitment maneuvers in patients undergoing thoracic surgery: a meta-analysis. Gen Thorac Cardiovasc Surg 2021;69:1553-9. 10.1007/s11748-021-01673-7 [DOI] [PubMed] [Google Scholar]
- 85.Zhu Y, Chen X, Wang F, et al. Effect of perioperative mechanical ventilation strategies on postoperative pulmonary complications in patients undergoing thoracic surgery:a Meta-analysis. Asian J Surg 2021;44:776-7. 10.1016/j.asjsur.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gentili A. Lung-protective strategy during one-lung ventilation: current and future approaches to quantify the role of positive end-expiratory pressure. Minerva Anestesiol 2024;90:3-5. 10.23736/S0375-9393.23.17841-2 [DOI] [PubMed] [Google Scholar]
- 87.Cohen E. Current Practice Issues in Thoracic Anesthesia. Anesth Analg 2021;133:1520-31. 10.1213/ANE.0000000000005707 [DOI] [PubMed] [Google Scholar]
- 88.Ferrando C, Vallverdú J, Zattera L, et al. Improving lung protective mechanical ventilation: the individualised intraoperative open-lung approach. Br J Anaesth 2025;134:281-7. 10.1016/j.bja.2024.10.007 [DOI] [PubMed] [Google Scholar]
- 89.Suzumura EA, Amato MBP, Cavalcanti AB. Understanding recruitment maneuvers. Intensive Care Med 2016;42:908-11. 10.1007/s00134-015-4025-5 [DOI] [PubMed] [Google Scholar]
- 90.Neto AS, Hemmes SN, Barbas CS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. 10.1016/S2213-2600(16)00057-6 [DOI] [PubMed] [Google Scholar]
- 91.Ferrando C, Mugarra A, Gutierrez A, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 2014;118:657-65. 10.1213/ANE.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 92.Yang M, Kim JA, Ahn HJ, et al. Continuous Titration of Inspired Oxygen Using Oxygen Reserve Index to Decrease Oxygen Exposure During One-Lung Ventilation: A Randomized Controlled Trial. Anesth Analg 2022;135:91-9. 10.1213/ANE.0000000000005967 [DOI] [PubMed] [Google Scholar]