Abstract
Background
Acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) is a severe condition with high morbidity and mortality, characterized by increased inflammation, oxidative stress, apoptosis and epithelial barrier damage. However, there are a few drugs available for the treatment of AE-IPF. Although sivelestat sodium (SIV) has been shown to be an effective agent for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) through various mechanisms, including reduction of inflammation and oxidative stress, the therapeutic potential of SIV in managing AE-IPF remains limited. This study aims to investigate the protective potential of SIV in AE-IPF mouse model through multiple mechanisms.
Methods
Male C57BL/6J mice were used to establish the acute exacerbation of pulmonary fibrosis (AE-PF) mouse model through intratracheal instillation of bleomycin (BLM) followed by lipopolysaccharide (LPS). SIV was administered intraperitoneally at a dose of 100 mg/kg daily for 3 or 7 days post-LPS challenge. The therapeutic efficacy was assessed through micro-computed tomographic (Micro-CT), histopathological analysis, immunohistochemistry, immunofluorescence, enzyme-linked immunosorbent assay (ELISA) and Western blotting.
Results
SIV significantly alleviated AE-PF symptoms in mice, as demonstrated by reductions in inflammation, structural damage, and collagen formation. Additionally, SIV reduced neutrophil elastase (NE) activity, highlighting its pharmacological effectiveness. Importantly, SIV reduced oxidative stress in AE-PF mice by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway, leading to increased superoxide dismutase (SOD) activity and decreased reactive oxygen species (ROS) levels. Furthermore, SIV significantly reduced lung inflammation, as evidenced by decreased interleukin-6 (IL-6) and interleukin-1 beta (IL-1β) levels in bronchoalveolar lavage fluid (BALF), possibly mediated by the inhibition of the Toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling. Moreover, SIV exhibited anti-apoptotic properties by modulating the B-cell lymphoma 2 (Bcl-2)/BCL2-associated X protein (Bax)/cleaved caspase-3 pathway. Meanwhile, SIV improved epithelial barrier integrity, as shown by enhanced expression of tight junction proteins and adherens junction protein.
Conclusions
SIV demonstrates significant protective effects in AE-PF mice by mitigating inflammation, oxidative stress, apoptosis, and preserving epithelial barrier integrity. These findings suggest that SIV is a promising therapeutic candidate for the treatment of AE-IPF.
Keywords: Sivelestat sodium (SIV), acute exacerbation pulmonary fibrosis, inflammation, oxidative stress, epithelial barrier
Highlight box.
Key findings
• This study investigated the therapeutic potential of sivelestat sodium (SIV) in mice model of acute exacerbation of pulmonary fibrosis (AE-PF). SIV exerted protective effects in AE-PF mice by targeting oxidative stress, inflammation, apoptosis, and epithelial injury, identifying it as a promising therapeutic candidate for acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF).
What is known and what is new?
• Approximately 30% to 40% of interstitial lung diseases (ILDs) patients, particularly those with advanced disease, experience progressive pulmonary fibrosis. However, effective treatment options for acute exacerbation of ILD are extremely limited.
• This study demonstrated that SIV alleviates AE-PF through multiple mechanisms, including mitigating inflammation, reducing oxidative stress, inhibiting apoptosis, and restoring epithelial barrier integrity.
What is the implication, and what should change now?
• The study has expanded the understanding of SIV’s therapeutic potential and offered a preclinical foundation for clinical trials in AE-IPF.
• Although in vivo findings demonstrate promising therapeutic effects, further in vitro studies and clinical trials are required to validate the efficacy and safety of SIV for AE-PF treatment.
Introduction
Interstitial lung diseases (ILDs) comprise a diverse group of pulmonary disorders characterized by varying degrees of alveolitis and pulmonary fibrosis (PF) (1,2). Idiopathic pulmonary fibrosis (IPF) is the most common form of ILD, marked by chronic and progressive fibrosis of the lung parenchyma with an unclear pathogenesis (3). This condition leads to excessive extracellular matrix deposition (4) and impaired gas exchange (5,6), resulting in a median survival of 2–5 years after diagnosis (7). Patients with ILD often experience a variable clinical course, ranging from gradual to rapid disease progression, including unpredictable acute exacerbations.
Acute exacerbation of interstitial lung disease (AE-ILD) involves a sudden worsening of respiratory symptoms within 30 days, often accompanied by new radiographic findings like bilateral ground-glass opacities or consolidations on high-resolution computed tomography (HRCT), while excluding other identifiable causes such as fluid overload, left heart failure, or pulmonary embolism (8). AE-ILD predominantly occurs in IPF but can also manifest in other ILDs such as connective tissue disease-associated ILD (CTD-ILD) and chronic hypersensitivity pneumonitis (CHP) (9,10). Common triggers of AE-ILD include infections (11,12), environmental pollutants (13-15), medications (16), autoimmune activity (17), and idiopathic factors.
Approximately 30% to 40% of ILD patients experience progressive PF, particularly those with advanced disease (18). Effective treatment options for AE-ILD are extremely limited, typically including respiratory support, high-dose corticosteroids, and broad-spectrum antibiotics (19). Despite these interventions, the in-hospital mortality rate for acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) exceeds 50% (20), highlighting the urgent need for effective therapeutic strategies.
The histopathology of AE-ILD is characterized by diffuse alveolar damage (DAD) superimposed on pre-existing fibrotic ILD, resembling acute respiratory distress syndrome (ARDS) in ILD contexts (21). Inflammation in AE-ILD is driven by neutrophils, macrophages, and lymphocytes releasing pro-inflammatory cytokines and chemokines like interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α), exacerbating lung injury and fibrosis (22,23). Oxidative stress also plays a crucial role in AE-ILD pathogenesis, promoting lung fibrosis and inflammation through reactive oxygen species (ROS) generation (24). Elevated ROS levels enhance the inflammatory response and directly trigger apoptosis and cellular damage (25,26). The imbalance between ROS production and antioxidant defense mechanisms leads to oxidative damage of alveolar epithelial cells (AECs) and further inflammation and fibrosis (27).
The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, which regulates antioxidant proteins, is critical in countering oxidative stress (28). However, insufficient Nrf2 signaling in AE-IPF results in inadequate antioxidant responses. Additionally, apoptosis of AECs significantly contributes to AE-IPF progression, with oxidative stress and inflammation inducing apoptosis through pathways involving pro-apoptotic factors [BCL2-associated X protein (Bax), cleaved caspase-3] and anti-apoptotic factors [B-cell lymphoma 2 (Bcl-2)] (29). This apoptosis disrupts the alveolar-capillary barrier, increasing permeability and exacerbating local inflammation and fibrosis (30,31).
Sivelestat sodium (SIV), initially identified as a selective inhibitor of neutrophil elastase (NE) (32), plays a role in the degradation of extracellular matrix components, contributing to tissue damage and inflammation (33). Consequently, SIV has shown promise in treating various inflammatory and respiratory conditions. In recent years, extensive research has focused on its efficacy in ARDS (34). Clinical trials have demonstrated that it improves lung function and reduces the duration of mechanical ventilation by enhancing oxygenation and reducing inflammation (35). Additionally, during the coronavirus disease 2019 (COVID-19) pandemic, SIV treatment was associated with reduced inflammation, improved oxygenation, and a potential reduction in mortality rates in severe COVID-19-induced ARDS (36).
Preclinical studies have also demonstrated the protective role of SIV in lung injury. For instance, in a rat model of acute lung injury (ALI), treatment with SIV alleviated lung tissue injury and apoptosis, improved lung function, and reduced inflammation and oxidative stress levels (37), illustrating its potential therapeutic benefits in respiratory conditions characterized by severe inflammation and tissue damage. In murine models of bleomycin (BLM)-induced PF, daily intraperitoneal administration of sivelestat for 7 or 14 days post-BLM significantly alleviated fibrotic changes by inhibiting transforming growth factor (TGF)-β1/Smad2 activation and the recruitment of inflammatory cells, highlighting its antifibrotic effects (33,38).
The therapeutic efficacy and underlying mechanisms of SIV in PF have garnered widespread research attention. For instance, Takemasa et al. (39) demonstrated that sivelestat mitigated BLM-induced PF by suppressing TGF-β activation and attenuating pulmonary inflammatory cell recruitment. Similarly, Fujino et al. (40) reported that pharmacological inhibition of NE reduced neutrophil accumulation in bronchoalveolar lavage fluid (BALF) and downstream TGF-β1/SMAD2/3 signaling. In another study, Song et al. (41) investigated the anti-apoptotic effects of SIV on AECs, further supporting its protective role in fibrotic lung injury. In contrast to previous studies that primarily focus on specific mechanistic pathways of SIV in PF, our investigation elucidated its multi-mechanistic protection against AE-PF. AE-IPF is a multifactorial process driven by oxidative stress, dysregulated inflammation, aberrant apoptosis and epithelial barrier disruption. Notably, SIV exhibited therapeutic potential by targeting these pathways, suggesting its applicability in AE-IPF management. Our study evaluated the efficacy of SIV in AE-PF, thereby providing a translational foundation for its clinical use in AE-IPF patients. Given the complex pathophysiology of AE-IPF, our investigation encompassing multiple mechanistic axes is essential to fully characterize SIV’s therapeutic effects and enhance its clinical relevance. Furthermore, diverging from previous studies that primarily utilized BLM alone to induce PF, our experimental approach incorporated a dual challenge with BLM and lipopolysaccharide (LPS) to better mimic the pathophysiological characteristics of AE-IPF. By evaluating the therapeutic effects of SIV at two time points (days 3 and 7 post-LPS challenge), our study provided a protective investigation.
Given that SIV has been shown to be an effective agent for ALI and ARDS through various mechanisms, including reduction of inflammation and oxidative stress, SIV may hold a significant therapeutic potential for AE-IPF due to the pathological overlap between AE-IPF and ARDS (42,43). However, research on its protective effects in AE-IPF remains limited (44). The study aims to investigate the protective potential of SIV in AE-IPF mice, specifically focusing on its anti-inflammatory, anti-oxidative, and anti-apoptotic mechanisms, as well as its effects on epithelial barrier integrity. By investigating these effects, we aim to contribute to the advancement of effective therapies for AE-IPF and improve patient outcomes. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-163/rc).
Methods
Drugs and reagents
BLM was purchased from Hanhui Pharmaceutical (Shanghai, China). LPS (055:B5) was purchased from Sigma-Aldrich (St. Louis, MO, USA). SIV was obtained from Shanghai Huilun Pharmaceutical (Shanghai, China). Fluorescein isothiocyanate (FITC)-dextran [46944] were purchased from Sigma-Aldrich. Antibodies to Nrf2 (12721S), heme oxygenase-1 (HO-1) (43966S), Toll-like receptor 4 (TLR4) (14358S) and myeloid differentiation primary response 88 (MyD88) (4283S), phospho-nuclear factor kappa B (phospho-NF-kappaB) p65 (3033S), NF-κB p65 (8242S), inhibitor of kappa B alpha (IκBα) (4812S), Bax (2772S), Bcl-2 (3498S) and caspase-3 (9662S) were purchased from CST (Danvers, MA, USA). Antibodies to zona occludens-1 (ZO-1) (ab96587), occludin (ab216327) and E-cadherin (ab231303) were purchased from Abcam (Cambridge, UK), β-actin (66009-1-Ig) were purchased from Proteintech (Wuhan, China). Antibodies to CD45 (ZA0122) and Ly6g (ZA0488) were purchased from ZuoChengBio (Shanghai, China). Neutrophil Elastase Activity Assay kit (MAK246) was purchased from Sigma-Aldrich. Mouse IL-6 [431304] enzyme-linked immunosorbent assay (ELISA) kit were purchased from BioLegend (San Diego, CA, USA). IL-1β (LM8K1229M) ELISA kit were purchased from LMAI Bio (Shanghai, China). Superoxide dismutase (SOD) (S0101S) kit were purchased from Beyotime (Shanghai, China). ROS (BB-470538) kit was purchased from Bestbio (Shanghai, China). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) (12156792910) kit were purchased from Roche (Basel, Switzerland).
Establishment of AE-PF model and SIV treatment
Male C57BL/6J mice (6–8 weeks old, 18–22 g) were obtained from the Experimental Animal Center of Xuzhou Medical University. All mice were housed under specific-pathogen-free (SPF) conditions, maintained at a controlled temperature (24±2 ℃) and humidity (55%±5%) with a 12:12 h light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University (approval #202209S071) and in compliance with the institutional guidelines for the care and use of animals. The mice were randomly divided into five groups (n=15 in each group): control group, pulmonary fibrosis group (BLM group), LPS group, acute exacerbation of pulmonary fibrosis (AE-PF) group (BLM + LPS group), and sivelestat treatment group (BLM + LPS + SIV group, BLS group). On day 0, mice in the BLM, BLM + LPS and BLS groups were intratracheally instilled with BLM (100 µL, 5 mg/kg, once) (45). The control and LPS groups received an equivalent volume of saline. Two weeks later, except for the control and BLM groups, mice in the other groups were administered LPS (50 µL, 1 mg/kg, once) (23,46). Three hours later, the BLS group received intraperitoneal injections of SIV (200 µL, 100 mg/kg, daily for 3 or 7 days) (47). The other groups were given equal amounts of sterile saline. After establishing the animal model, the severity and distribution of lung lesions were observed using a micro-computed tomographic (Micro-CT) imaging system. Subsequently, the mice were euthanized, and lung tissues and BALF were collected for further analyses.
Micro-CT imaging
Mice in each group were injected intraperitoneally with 2% sodium pentobarbital and then fixed on a Micro-CT piggyback stage for chest scanning. The Micro-CT imaging acquisition parameters were as follows: X-ray tube voltage of 70 kV, current of 7 A, exposure time of 700 ms, scanning bed 4, scanning length of 120 mm, over a total angle of 360°, detector component mode 4×4, algorithm for reconstructing images: filtered back projection (FBP), average number of frames 1, pixel size of 0.184 mm. Image J software was used to measure grayscale values at the fifth intercostal level for each mouse, and statistical analysis was conducted using GraphPad Prism 8.0 (48).
Histopathology analysis
Hematoxylin and eosin (H&E) staining was performed to evaluate the severity of inflammation, while fibrosis was assessed by Masson Trichrome staining. After sealing the slides, two experienced pathologists analyzed the severity of alveolitis under a light microscope. The grading criteria for alveolitis are as follows (49):
❖ Degree 1: no alveolitis.
❖ Degree 2: mild alveolitis, affecting <20% of the total lung, with infiltration of mononuclear cells into the widened alveolar septa, limited to localized regions involving nearby pleural areas.
❖ Degree 3: moderate alveolitis, influencing an area of 20–50%, with greater pleural involvement.
❖ Degree 4: severe alveolitis, involving an area of >50%, with occasional monocytes in the alveolar space and bleeding caused by consolidation.
The degree of fibrosis was assessed with reference to the Ashcroft score (50):
❖ 0: normal.
❖ 1: mild thickening of alveolar or bronchiolar walls.
❖ 2–3: moderate thickening of alveolar or bronchiolar walls without lung structure damage.
❖ 4–5: marked increase in fibrous tissues, with obvious lung structure damage, formation of fibrous tissue strips or small fibrous tissue clumps.
❖ 6–7: severe lung structural damage, large areas of fibrosis, and honeycomb lungs.
❖ 8: full field of view of fibrous tissue.
Immunohistochemistry (IHC)
Sections were stained following the previous report (51) with antibodies against ZO-1 (1:1,200; Abcam) and occludin (1:50; Abcam). After overnight incubation at 4 ℃ and rinsing with tris-buffered saline (TBS) 0.025% Triton for 3×5 min, the sections were incubated with a secondary antibody diluted in buffer according to the manufacturer’s instructions. Staining for ZO-1 and occludin was analyzed under a light microscope. Positive-staining areas and integrated optical density (IOD) were quantified by ImageJ software. The mean staining intensity was presented as IOD/area.
Immunofluorescence assay
Tissue sections were fixed with 4% paraformaldehyde, rinsed with phosphate-buffered saline (PBS), and incubated in 0.5% Triton X-100 for 20 min. Non-specific antigens were blocked with 10% serum, and the samples were incubated with anti-CD45 (1:100), anti-Ly6G (1:100), anti-Nrf2 (1:100) and anti-E-cadherin (1:100) primary antibody overnight at 4 ℃. Subsequently, the samples were rinsed with PBS and incubated with fluorescence secondary antibody at 37 ℃ for 60 min. Tissue sections were mounted with neutral glycerol and photographed under a confocal laser fluorescence microscope. The average fluorescence intensity was detected using ImageJ software.
NE activity
Add 40 µL of lung homogenate sample per well of a 96-well plate and adjust the volume to 50 µL/well with NE assay buffer. Prepare 50 µL of NE substrate mix for each standard and sample well. Fluorescence was measured at wavelengths of 380 nm for excitation and 500 nm for emission. Plot the NE standard curve and calculate the activity of NE in the sample according to the manufacturer’s protocols.
BALF cell count and ELISA
At specified time intervals, BALF samples were obtained by gently introducing 1 mL of PBS into the lungs three times at room temperature. Lung tissues were then homogenized in radio-immunoprecipitation assay (RIPA) buffer, and the protein content was measured using a BCA kit (Beyotime). BALF cell counts were conducted using a Countess 3 FL cell counter (Thermo, Waltham, MA, USA). In selected experiments, the collected BALF was centrifuged at 1,500 g for 5 min at 4 ℃, and the resulting supernatants were stored at −80 ℃ for cytokine level analysis. The concentrations of IL-6 and IL-1β in the alveolar lavage fluid of mice were measured using ELISA kits according to the manufacturer’s instructions.
Western blotting
A protease inhibitor and a phosphatase inhibitor were added to RIPA lysis buffer, which was added to lung tissue on ice to obtain total proteins. The protein samples were separated on sodium-dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to polyvinylidene fluoride (PVDF) membranes, and then blocked with 5% fat-free milk. The membranes were incubated with antibodies against Nrf2, HO-1, TLR4, MyD88, p-p65, p65, IκBα, ZO-1, E-cadherin, occludin, Bax, Bcl-2, and cleaved-caspase 3 overnight at 4 ℃. All antibodies were diluted at a ratio of 1:1,000. The membranes were then incubated with a secondary antibody at room temperature for 2 h. The blots were visualized using a chemiluminescence kit, and grayscale values were analyzed by ImageJ software.
Detection of SOD and ROS
SOD (S0101S) and ROS (BB-470538) kits were purchased from Beyotime and Bestbio, respectively. Lung tissues were homogenized with tissue homogenizing solution provided by the kits, and SOD activity and ROS levels were detected according to the manufacturer’s protocols.
TUNEL assay
The tissue sections were fixed with 4% paraformaldehyde for 30 min, washed with PBS 3 times for 10 min each, wiped dry with a cotton swab, circled with a fluorescent pen, dried, and incubated with 0.5% Triton X-100 inside the circle for 30 min at room temperature. After incubation, the sections were washed with PBS for 3 times, and 20 µL of TUNEL assay solution was added to each section. The sections were incubated for 60 min at 37 ℃, protected from light. After incubation, the sections were washed 3 times with PBS, sealed with anti-fluorescence quenching sealing solution, and photographed under a laser confocal microscope. The rate of positive cells in each group of mice was counted: 5 high magnification fields (×40) with the highest number of positive cells were selected for each TUNEL-positive section, and the percentage of positive cells among 500 cells was calculated.
Measurements of FITC-dextran flux
To assess pulmonary permeability changes in mice, 50 µL of FITC-dextran (4 kD, 10 mg/kg) in sterile PBS was administered intranasally following the final SIV instillation 24 h later. Mice were euthanized one hour later, blood was collected, and fluorescence intensity was quantified (46).
Statistical analysis
GraphPad Prism 8.0 was used for statistical analysis. ImageJ and Image-Pro Plus 6.0 were used for image analysis. One-way analysis of variance (ANOVA) was employed for comparisons between multiple groups, while a T-test was used for comparisons between two groups. The survival rates were analyzed using Log-rank (Mantel-Cox) test and Kaplan-Meier survival curve analysis. All data were presented as mean ± standard deviation (SD). Statistical significance was considered at P<0.05.
Results
Intratracheal injection of BLM and subsequent LPS challenge induce AE-PF in mice
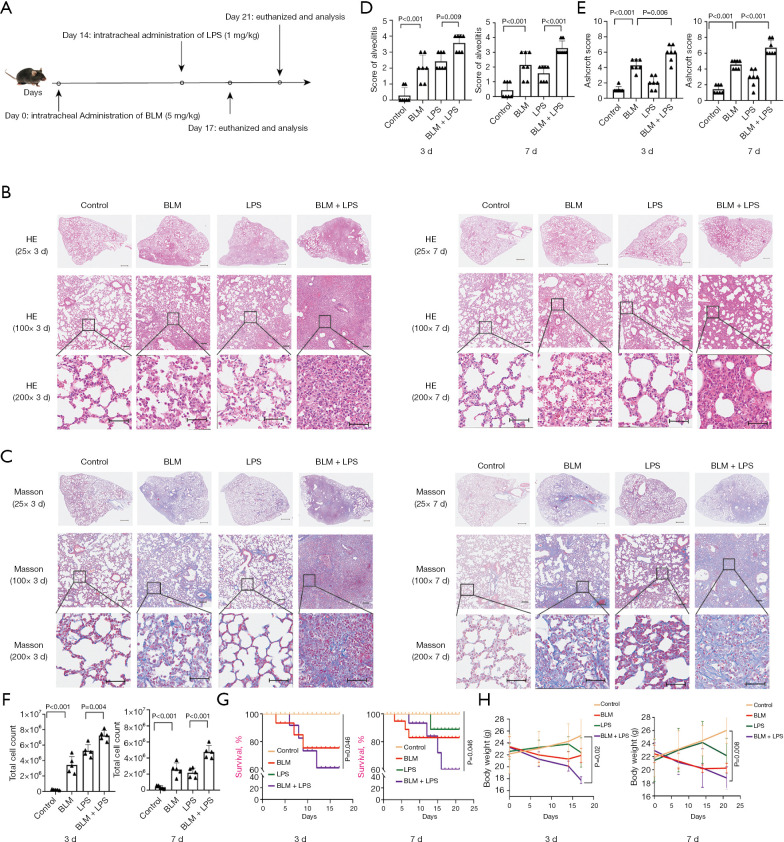
We have previously reported a mouse model of AE-PF induced by the administration of BLM combined with LPS (46). To further investigate the impact of SIV in AE-IPF, the AE-PF mice model was utilized again with minor modification (Figure 1A). In the control group, H&E staining showed intact alveolar structures without inflammation (Figure 1B), and Masson’s trichrome staining showed no collagen deposition (Figure 1C), however, The BLM + LPS group mice exhibited thickened alveolar septa, increased inflammatory cell infiltration and fibrotic changes throughout the lung parenchyma (Figure 1B,1C) post LPS injected 3 days. After LPS administrated 7 days, H&E staining indicated a gradual subsidence of the inflammatory response but significant tissue structure destruction remained visible in the BLM and BLM + LPS groups. Besides, Masson’s trichrome staining also indicated a slight increase in fibrosis (Figure 1B,1C). According to grading criteria previously reported (49), the alveolitis scores of BLM + LPS group mice were significantly elevated (P<0.05, Figure 1D). The Ashcroft scores also confirmed that the degree of fibrosis in BLM + LPS mice is more severe than control and LPS groups (P<0.05, Figure 1E) but similar to the BLM group. Chronic inflammation and inflammatory cells frequently accompany AE-IPF (52). The total cell count in BALF was significantly elevated in AE-PF mice compared to the control (P<0.05) (Figure 1F). The survival rate in BLM group was around 80% and in LPS group could be maintained at approximately 90%. However, the rate of BLM + LPS group mice dramatically decreased to 60% (Figure 1G). Compared to the control group, BLM-treated mice and LPS-treated mice exhibited weight loss. These conditions worsened following LPS administration (Figure 1H). Together, these data confirmed that administering LPS in BLM induced PF mice can effectively simulate the pathological process of AE-IPF.
Figure 1.
Intratracheal injection of BLM and subsequently LPS challenge induce AE-PF in mice. (A) Schematic illustration of the experimental procedure for AE-PF in mice. 14 days following the intratracheal administration of BLM (5 mg/kg), mice were given an intratracheal dose of LPS (1 mg/kg). The results were assessed at day 17 and day 21. (B) The histology of lung tissue was detected by H&E staining after LPS was administrated 3 and 7 days, representative images were obtained from 4 groups (top: magnification: ×25, scale bar =500 µm; middle: magnification: ×100, scale bar =100 µm; bottom: magnification: ×200, scale bar =50 µm). (C) Masson staining was performed to detect collagen deposition in the lung tissue of mice after LPS was administrated 3 and 7 days, representative images were obtained from 4 groups (top: magnification: ×25, scale bar =500 µm; middle: magnification: ×100, scale bar =100 µm; bottom: magnification: ×200, scale bar =50 µm). (D) Pulmonary alveolitis scores for each group. (E) Ashcroft scores for each group. (F) Total cells in BALF for each group after LPS was administrated 3 and 7 days. (G) Survival curve for each group at indicated times. (H) Body weight for each group at indicated times. Data were from one representative experiment of 3 performed and presented as the mean ± SD (n=7 for alveolitis and Ashcroft scores, n=5 for total cells in BALF, n=15 for survival rate, n=6 for body weight). The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; BALF, bronchoalveolar lavage fluid; BLM, bleomycin; H&E, hematoxylin and eosin; LPS, lipopolysaccharide; SD, standard deviation.
Administration of SIV alleviates AE-PF symptoms in mice
SIV, known for its use in treating ARDS and ALI (53), has shown protective effects on PF (33,38,54). To investigate its effects on AE-PF, we administered SIV intraperitoneally at 100 mg/kg on day 14, followed by daily injections for 3 or 7 consecutive days (Figure 2A). In the control group, mice did not exhibit inflammatory responses or collagen deposition in H&E (Figure 2B,2C) and Masson’s trichrome (Figure 2D,2E) staining and no abnormal high-density areas were observed in the lung parenchyma on Micro-CT (Figure 2F,2G). In contrast, histopathological analysis in BLM + LPS mice group revealed inflammatory infiltration (Figure 2B,2C), structural damage, and substantial collagen fiber formation (Figure 2D,2E). Micro-CT scans showed high-density consolidations and bronchial traction (Figure 2F,2G). The alveolitis scores and the Ashcroft scores further verified that mice treated with SIV exhibited significantly lower alveolitis and Ashcroft scores compare to the BLM + LPS mice (P<0.05, Figure 2C,2E). Additionally, following 3 or 7 days of SIV treatment, there were found that significant decreased total BALF cells (Figure 2H), increased survival rate (Figure 2I) and less weight loss (Figure 2J) in BLM + LPS mice. Importantly, a significant decrease in NE activity was found in SIV-treated group compared to the BLM + LPS group (Figure 2K), indicating the pharmacological efficacy of SIV. These findings demonstrated that SIV is able to significantly alleviate AE-PF symptoms in mice.
Figure 2.
Administration of SIV alleviates AE-PF in mice. (A) Schematic illustration of the experimental procedure for SIV treatment in AE-PF mice. After intratracheal administration of BLM (5 mg/kg), LPS (1 mg/kg) was instilled intratracheally into the BLM mice. Subsequently, intraperitoneal injection of SIV at a dose of 100 mg/kg on day 14, followed by daily injections for 3 or 7 consecutive days. (B) The histology of lung tissue was detected by H&E staining after sivelestat was intraperitoneal injected once a day for 3 and 7 days, representative images obtained from 5 groups (top: magnification: ×25, scale bar =500 µm; middle: magnification: ×100, scale bar =100 µm; bottom: magnification: ×200, scale bar =50 µm). (C) Masson staining was performed to detect collagen deposition in the lung tissue of mice after sivelestat was intraperitoneal injected once a day for 3 and 7 days, representative images were obtained from 5 groups (top: magnification: ×25, scale bar =500 µm; middle: magnification: ×100, scale bar =100 µm; bottom: magnification: ×200, scale bar =50 µm). (D) Pulmonary alveolitis scores for each group. (E) Ashcroft scores for each group. (F) Representative images of Micro-CT images for each group after intraperitoneal injected sivelestat once a day for 3 days. (G) The average gray value of the fifth rib for each group. (H) Total cells in BALF for each group after intraperitoneal injected sivelestat once a day for 3 and 7 days. (I) Survival curve of mice for each group at indicated times. (J) Body weight for each group at indicated times. (K) NE activity in lung tissue for each group. Data were from one representative experiment of 3 performed and presented as the mean ± SD (n=7 for alveolitis and Ashcroft scores, n=5 for average gray value of Micro-CT, n=5 for total cells in BALF, n=15 for survival rate, n=6 for body weight, n=5 for NE activity). The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; BALF, bronchoalveolar lavage fluid; BLM, bleomycin; H&E, hematoxylin and eosin; LPS, lipopolysaccharide; Micro-CT, micro-computed tomographic; NE, neutrophil elastase; SD, standard deviation; SIV, sivelestat sodium.
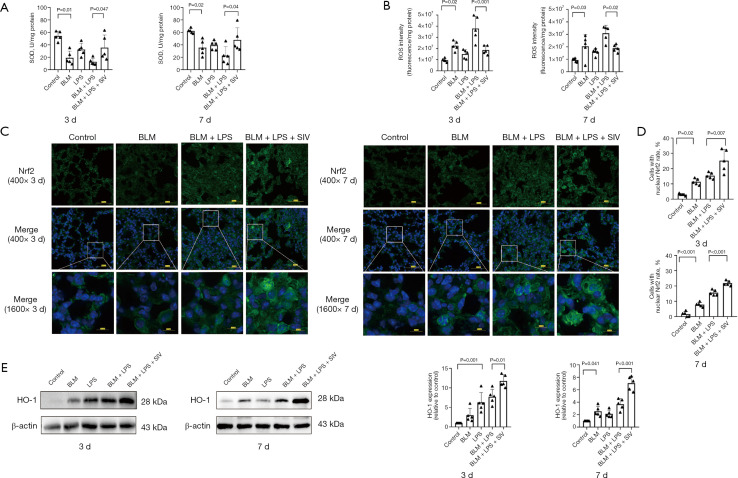
Treatment with SIV prevents oxidative stress via Nrf2/HO-1 signaling in AE-PF mice
Oxidative stress is a key molecular trigger that elevates the secretion of proinflammatory and profibrotic mediators (55,56). SIV is able to inhibit oxidative stress (37), thus, SOD and ROS levels in lung tissues were measured to assess its effects in AE-PF mice. Elevated ROS levels and decreased SOD activity in AE-PF mice indicated oxidative stress, which was mitigated after SIV treatment (Figure 3A,3B). The Nrf2/HO-1 axis plays a crucial role in modulating oxidative stress. The previous study (32) has shown that SIV upregulates Nrf2 expression, exerting an anti-oxidative stress effect. Our investigation revealed that SIV treatment led to nuclear translocation of Nrf2 (Figure 3C,3D) and increased expression of antioxidants, including SOD (Figure 3A) and HO-1 (Figure 3E). Oxidative stress levels were more pronounced on day 3 compared to day 7, with SIV consistently showing therapeutic effects. These results suggest that SIV protects against AE-PF by regulating the Nrf2/HO-1 signaling pathway, thereby reducing oxidative stress.
Figure 3.
Treatment with SIV prevents oxidative stress via Nrf2/HO-1 signal in AE-PF mice. (A) SOD levels in lung tissue after intraperitoneal injected sivelestat once a day for 3 and 7 days (n=5). (B) ROS levels in lung tissue after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5). (C) The representative immunofluorescence images showed Nrf2 in lung tissue after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5) (blue: DAPI; green: Nrf2) (top: magnification: ×400, scale bar =50 µm; middle: magnification: ×400, scale bar =50 µm; bottom: magnification: ×1,600, scale bar =10 µm). (D) The percentage of cells with nuclear Nrf2 in sections for each group (n=5). (E) The representative immunoreactive band intensities and quantification of HO-1 in lung tissue after sivelestat was treated once a day for 3 and 7 days (n=5). Data were from one representative experiment of 3 performed and presented as the mean ± SD. The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; BLM, bleomycin; DAPI, 4’,6-diamidino-2-phenylindole ; HO-1, heme oxygenase-1; LPS, lipopolysaccharide; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; SD, standard deviation; SIV, sivelestat sodium; SOD, superoxide dismutase.
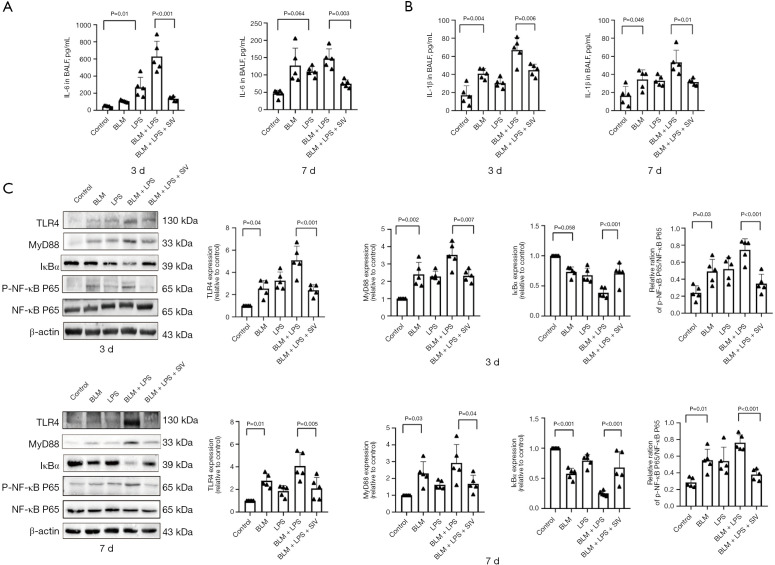
SIV suppresses inflammation by regulating the TLR4/NF-κB signaling pathway in AE-PF mice
Chronic viral infections exacerbate PF (29), and inflammation is the immune system’s response to external stimuli and tissue damage (57). SIV exerts anti-inflammatory effects by inhibiting the NF-κB signaling pathway and reducing inflammatory mediators such as NE and interleukins (58,59). In our study, IL-6 (Figure 4A) and IL-1β (Figure 4B) levels in BALF were significantly increased in the AE-PF group compared to controls, but decreased after sivelestat treatment (P<0.05). TLR4/MyD88/NF-κB inflammation signaling pathway is involved in ARDS/ALI development (60); meanwhile, excessive ROS production can trigger the TLR4/NF-κB pathway, promoting the release of inflammatory cytokines (61). To further investigate the potential mechanism that mediated the inflammatory IL-6 and IL-1β production, TLR4, MyD88, the p-NF-κB P65/NF-κB P65 ratio and IκBα was evaluated by immunoblotting. Our results showed elevated expression of TLR4, MyD88 and p-NF-κB P65/NF-κB P65 ratio, along with reduced IκBα levels in AE-PF mice (Figure 4C). However, SIV inhibited this inflammatory pathway (Figure 4C), suggesting that it suppresses inflammation in AE-PF mice by reversing TLR4/NF-κB activation.
Figure 4.
SIV suppresses inflammation by regulating the TLR4/NF-κB signaling pathway in AE-PF mice. (A) The concentration of pro-inflammatory IL-6 in BALF detected with ELISA after intraperitoneal injected sivelestat once a day for 3 and 7 days (n=5). (B) The concentration of pro-inflammatory IL-1β in BALF detected with ELISA after intraperitoneal injected sivelestat once a day for 3 and 7 days (n=5). (C) The levels and quantifications of TLR4, MyD88, p-NF-κB P65, NF-κB P65 and IκBα in lung tissues after SIV treatment once a day for 3 or 7 days. Data were from one representative experiment of 3 performed and presented as the mean ± SD. The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; BALF, bronchoalveolar lavage fluid; BLM, bleomycin; ELISA, enzyme-linked immunosorbent assay; IκBα, inhibitor of kappa B alpha; IL-1β, interleukin-1 beta; IL-6, interleukin-6; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa B; p, phosphorylated; SD, standard deviation; SIV, sivelestat sodium; TLR4, Toll-like receptor 4.
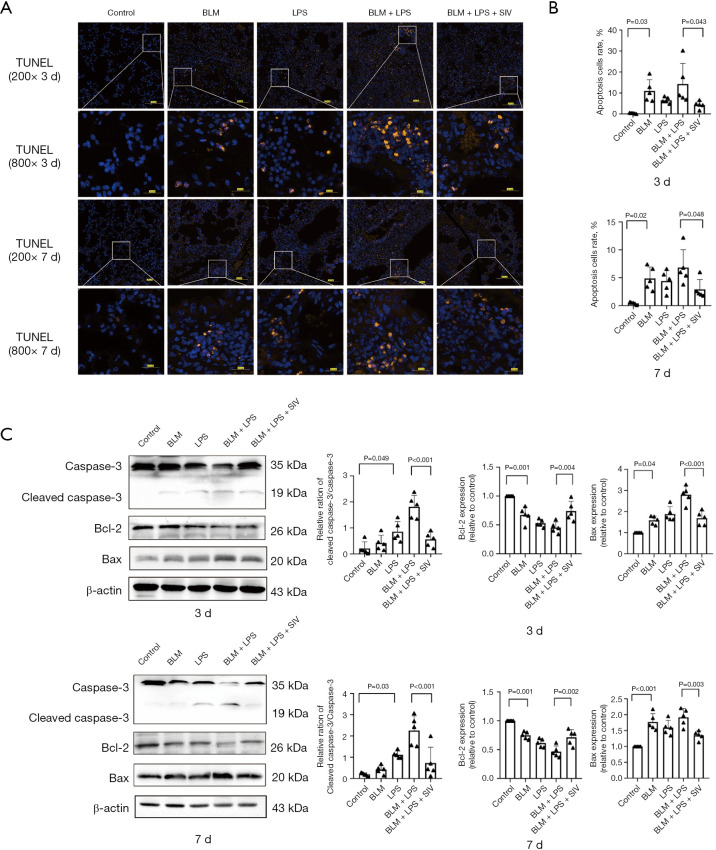
SIV mediates Bcl-2/Bax/cleaved caspase-3 apoptotic pathways to repress apoptosis in AE-PF mice
Oxidative stress and inflammation can trigger apoptosis via the Bcl-2/Bax/cleaved caspase-3 pathways (62). SIV has significant anti-apoptotic functions, as shown in models of acute liver injury (63). Next, our study explored whether SIV exerts a protective effect by intervening in apoptosis. TUNEL staining showed significantly increased apoptosis in AE-PF mice (Figure 5A,5B), with higher rates of positive cells on the 3rd day compared to the 7th day. SIV treatment significantly improved this condition (Figure 5A,5B). A significant increase in active caspase-3 but a significant decrease in full-length caspase-3 protein levels in BLM + LPS-treated mice were observed. Meanwhile, the BLM + LPS groups exhibited upregulation of pro-apoptotic Bax and cleaved caspase-3, alongside downregulation of anti-apoptotic Bcl-2. However, these changes were reversed by SIV treatment (Figure 5C). Collectively, SIV treatment reduced apoptosis in AE-PF mice via the Bcl-2/Bax/cleaved caspase-3 pathways.
Figure 5.
SIV mediates Bcl-2/Bax/cleaved caspase-3 apoptotic pathways to represses apoptosis in AE-PF mice. (A) TUNEL staining of the lung tissues and representative images for each group after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5) (blue: DAPI; orange: TUNEL) (first and third rows: magnification: ×200; scale bar =100 µm; second and fourth rows: magnification: ×800; scale bar =20 µm). (B) Apoptosis cells rate in the lung tissues for each group (n=5). (C) The levels and quantifications of cleaved caspase-3, Bcl-2 and Bax in lung tissues after SIV treatment once a day for 3 or 7 days. Data were from one representative experiment of 3 performed and presented as the mean ± SD. The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; Bax, BCL2-associated X protein; Bcl-2, B-cell lymphoma 2; BLM, bleomycin; DAPI, 4’,6-diamidino-2-phenylindole; LPS, lipopolysaccharide; SD, standard deviation; SIV, sivelestat sodium; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
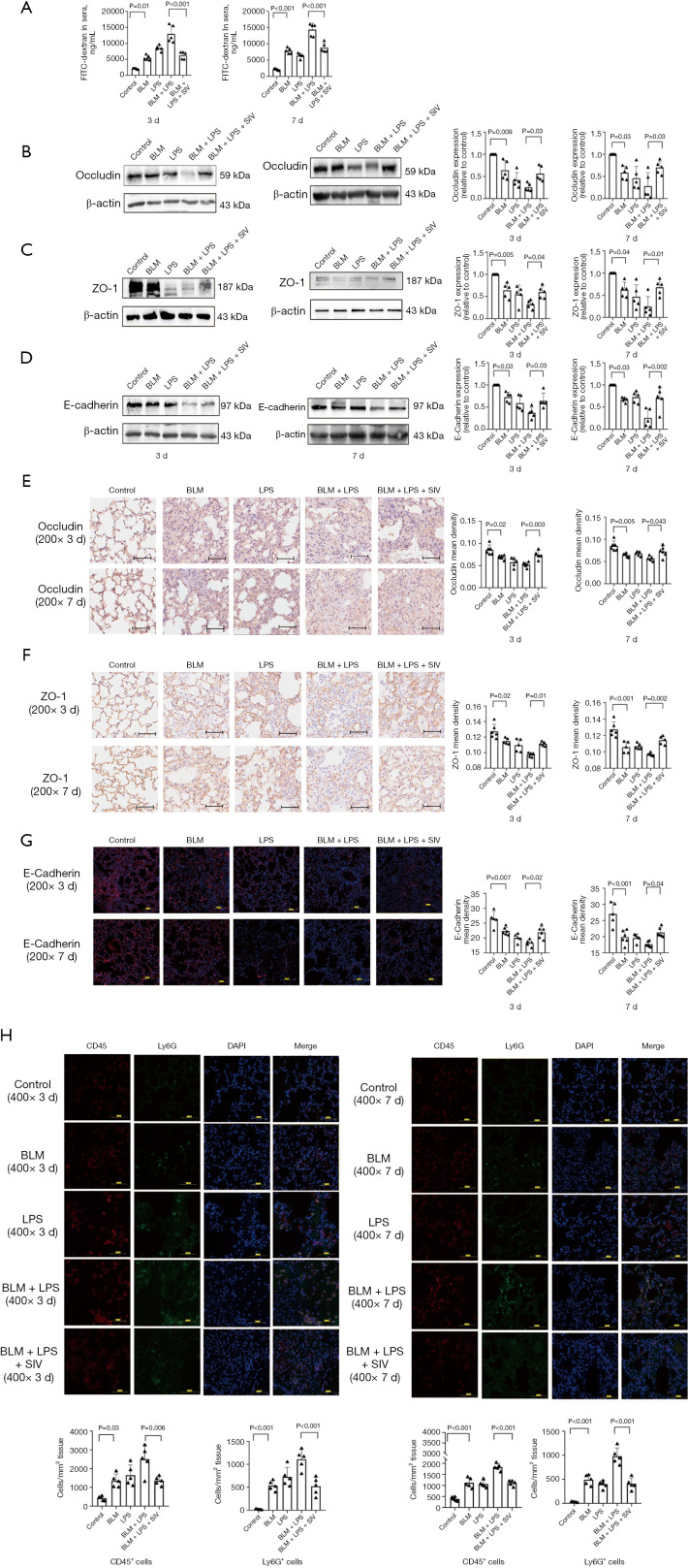
SIV regulates tight junction (TJ) and adherens junction expression to attenuate epithelial barrier damage in AE-PF mice
Oxidative stress and inflammation often lead to apoptosis (64), which is associated with the destruction of the alveolar epithelial barrier (65). Subsequently, the integrity of the alveolar epithelial barrier was evaluated by detecting FITC-dextran flux from lung to blood (Figure 6A). AE-PF mice showed a significant increase in fluorescein, indicating severe epithelial barrier disruption, which was ameliorated by SIV treatment (Figure 6A). Furthermore, in AE-PF mice, reduced expression of ZO-1, occludin, and E-cadherin that are key molecules affecting the alveolar epithelial barrier function was found (Figure 6B-6D). Interestingly, SIV treatment notably restored these molecules expression (Figure 6B-6D). IHC and immunofluorescence results also showed thickened alveolar septa, collapsed alveolar structure, and damaged epithelial tissue barrier were identified, with weak expression of occludin, ZO-1, and E-cadherin in AE-PF mice (Figure 6E-6G). In contrast, SIV treatment resulted in a better alveolar septum, structure and alleviated alveolar epithelial injury, along with restored occludin, ZO-1, and E-cadherin expression, compared with those of AE-PF mice (Figure 6E-6G). In summary, SIV exerts a protective effect on AE-PF by preserving the epithelial barrier and regulating tight and adherens junction expression. Additionally, immunofluorescence staining for CD45+ and Ly6G+ cells (Figure 6H) were performed. The results demonstrated that both 3- and 7-day consecutive intraperitoneal administration of SIV significantly reduced the number of neutrophils per square millimeter in lung tissues, with statistically significant differences (P<0.05). The results indicated that SIV attenuated neutrophil recruitment into the lungs, consistently with previous studies (66,67).
Figure 6.
SIV regulates the tight junction and adherens junction expression to attenuate damage of epithelial barrier through in AE-PF mice. (A) Analysis of pulmonary permeability alterations after sivelestat instillation in mice by measurement of FITC-dextran leakage from the airways into the blood (n=5). (B-D) The levels and quantifications of occludin, ZO-1 and E-cadherin in lung tissues after SIV treatment once a day for 3 or 7 days. (E,F) The representative immunohistochemistry staining of occludin and ZO-1 expression in 5 groups after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5) (magnification: ×200, scale bar =50 µm). (G) The representative immunofluorescence staining of E-cadherin expression in 5 groups after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5) (blue: DAPI; red: E-cadherin) (magnification: ×200, scale bar =100 µm) (n=5). Data were from one representative experiment of 3 performed and presented as the mean ± SD. The exact P values were reported for each statistical comparison. (H) The representative immunofluorescence staining of CD45+ and Ly6G+ cells in 5 groups after sivelestat was intraperitoneal injected once a day for 3 and 7 days (n=5) (blue: DAPI; red: CD45; green: Ly6G) (magnification: ×400, scale bar =50 µm) (n=5). Data were from one representative experiment of 3 performed and presented as the mean ± SD. The exact P values were reported for each statistical comparison. AE-PF, acute exacerbation of pulmonary fibrosis; BLM, bleomycin; DAPI, 4’,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; LPS, lipopolysaccharide; SD, standard deviation; SIV, sivelestat sodium; ZO-1, zona occludens-1.
Discussion
In the current study, we demonstrated the therapeutic potential of SIV in managing AE-IPF using an established AE-PF mouse model. Key findings indicate that SIV markedly reduces inflammation, alleviates oxidative stress, and decreases apoptosis in the AE-PF mice model. Notably, it also preserves the integrity of the alveolar epithelial barrier, which is crucial for maintaining lung function. Therefore, this study underscores SIV as a promising therapeutic agent for AE-IPF, providing new insights into the treatment of the severe disorder.
The AE-PF mice model was established by intratracheal instillation of LPS during the fibrotic phase of the BLM-induced PF. Respiratory infections, predominantly caused by Gram-negative bacteria, are common triggers of AE-IPF, and LPS effectively mimics these clinical scenarios (68). Histopathological analysis and Micro-CT confirmed the success of the model, showing thickened alveolar septa, extensive inflammatory cell infiltration, and significant collagen deposition. Elevated alveolitis and Ashcroft scores, along with increased total cell counts and inflammatory cytokines IL-6 and IL-1β in BALF, indicated a robust inflammatory response. On the third day post-LPS treatment, tissue inflammation was evident, simulating acute infection, while by the seventh day, inflammation had subsided, with noticeable collagen accumulation, mimicking a stable state. These two time points were selected to investigate the protective effects of SIV.
SIV significantly reduced inflammation and lung tissue damage in the AE-PF model. Although no previous studies have examined the use of SIV in an AE-PF animal model, its effects have been demonstrated in BLM-induced PF models. For instance, in the study by Song et al. (41), sivelestat significantly reduced inflammatory cell infiltration in BALF, decreased the release of inflammatory factors such as cytokine-induced neutrophil chemoattractant (CINC)-1, and inhibited collagen deposition. Additionally, it reduced apoptosis of type II epithelial cells by inhibiting caspase-3 and caspase-9 activities. Similarly, another study (39) demonstrated that sivelestat administered intraperitoneally at a dose of 100 mg/kg once a day following BLM instillation alleviated PF by inhibiting inflammatory cell infiltration and reducing levels of active TGF-β1 and phospho-Smad2. These findings align with our results, demonstrating that SIV effectively reduces inflammation, apoptosis, and lung tissue damage in the AE-PF model.
SIV significantly reduced oxidative stress in the AE-PF model, evidenced by decreased ROS level and activation of the Nrf2/HO-1 signaling pathway, upregulating antioxidant proteins such as SOD and HO-1. Several previous studies support these results. Zhang et al. and Wu et al. (32,69) showed that SIV reduced oxidative stress markers and improved lung function in ARDS models and sepsis-induced ALI. Wang et al. (70) also reported that activation of Nrf2 reduced fibrosis in PF, further supporting our findings. Additionally, a recent study (37) has shown that SIV markedly reduces oxidative stress markers such as malondialdehyde (MDA) and myeloperoxidase (MPO) in LPS-induced ALI while improving endoplasmic reticulum stress to maintain cellular homeostasis.
In addition to its antioxidative properties, SIV also demonstrated significant anti-inflammatory effects in the AE-PF model. The previous study (32) has shown that SIV was able to inhibit the NF-κB pathway, thereby reducing inflammation. Another study (40) showed that sivelestat (100 mg/kg) was administered subcutaneously daily for 7 days to C57BL/6 mice following sublethal irradiation and intranasal LPS instillation, significantly mitigating fibrosis by reducing inflammation and TGF-β1 activation. This supports the potential of SIV as a therapeutic strategy for the fibro proliferative phase of ALI and ARDS. Our findings align with these studies, indicating that SIV significantly reduced levels of key inflammatory mediators such as IL-6 and IL-1β in the AE-PF model. Furthermore, our investigation showed that the expression levels of TLR4, MyD88, and the p-NF-κB P65/NF-κB P65 ratio were lower while IκBα levels were higher in comparison with AE-PF mice. This suggests that SIV potentially diminishes inflammation in AE-PF through suppression of the TLR4/NF-κB signaling axis. By effectively controlling these critical inflammatory pathways, SIV mitigates the inflammatory milieu within the lungs, thereby reducing tissue injury and fibrosis.
AEC apoptosis, particularly in AEC2 cells, plays a critical role in the progression of AE-IPF. This apoptosis compromises the alveolar-capillary barrier, leading to inflammation, fibrosis, and disease progression (29). Our study revealed that AE-PF mice exhibited increased TUNEL-positive cells and elevated levels of apoptosis-related proteins such as Bax and cleaved caspase-3, along with a concurrent decrease in the anti-apoptotic protein Bcl-2. These findings highlight the importance of reversing apoptosis for effective AE-IPF treatment. SIV significantly mitigated apoptosis in the AE-PF model. The previous study (71) has demonstrated that SIV inhibits apoptosis-related pathways in various organ dysfunction models, including sepsis-induced myocardial dysfunction. Our results align with these findings, showing that SIV reversed the apoptotic process in AE-PF. Notably, AE-PF mice showed heightened apoptosis on day 3 post-LPS treatment, which was attenuated by day 7, correlating with the severity of tissue oxidative stress and inflammatory reactions. SIV consistently exhibited anti-apoptotic effects, whether administered for 3 or 7 days.
Our results are supported by studies indicating that SIV reduces apoptosis by decreasing oxidative stress and ameliorating endoplasmic reticulum stress (37). The ability of the drug to decrease ROS levels, which are critical in triggering apoptosis via mitochondrial pathways (72), further substantiates its protective role in AECs. By reducing ROS levels and enhancing antioxidative capacity, SIV helps maintain cellular homeostasis and prevents apoptosis-induced tissue damage.
The progression of AE-PF compromises the integrity of bronchial epithelial cells, increasing alveolar-capillary permeability and disrupting the epithelial barrier formed by TJs and adherence junctions (AJs) (46). In our study, AE-PF mice exhibited significant disruption of alveolar structures, epithelial tissue damage, and irregular distribution of ZO-1, occludin, and E-cadherin. These detrimental effects were markedly reversed by SIV treatment. The ability of SIV to maintain the integrity of the epithelial barrier is linked to its capacity to reduce oxidative damage and inhibit the NF-κB signaling pathway. Zhang et al. demonstrated that SIV reduces oxidative damage and inflammation by inhibiting NF-κB activation (32). Additionally, Yao et al. showed that SIV prevents the degradation of TJ proteins, thereby maintaining barrier function in lung tissue during liver transplantation (73). By preserving the expression and distribution of key junctional proteins, SIV inhibits alveolar-capillary permeability and subsequently reduces inflammation and fibrosis. Analysis of lung tissue homogenates in our study found that SIV inhibited NE activity (Figure 2K), indicating that the drug reduced lung damage by inhibiting elastase activity. Other reports also indicated that SIV directly inhibited the activity of NE to protected alveolar epithelium from injury through degradation of ZO-1 and E-cadherin (74,75). Based on the CD45 and Ly6G immunofluorescence results (Figure 6H), we proposed that early intervention with SIV suppressed neutrophil infiltration, reduced pro-inflammatory cytokine release and consequently mitigated alveolar epithelial barrier injury. Together, our results demonstrated that SIV not only directly inhibited elastase activity (Figure 2K) but also reduced polymorphonuclear neutrophil (PMN) recruitment (Figure 6H), thereby alleviating lung damage in AE-PF mice. This protective action underscores its potential as a therapeutic strategy for improving lung function and outcomes in AE-IPF patients.
Despite these encouraging findings, there is limited research on the use of SIV for AE-PF treatment. Our study is limited to in vivo experiments, further in vitro studies are necessary to validate these conclusions. Additionally, although we explored the possibility of SIV for AE-PF treatment in various aspects such as oxidative stress, inflammation, apoptosis, and epithelial injury, the precise molecular mechanisms underlying the protective effects of SIV should be further investigated. Additionally, the physiological differences between preclinical models and humans imply that these findings do not always translate directly; therefore, further clinical trials are essential to confirm both efficacy and safety.
Conclusions
In conclusion, SIV shows significant therapeutic potential in AE-PF mouse model by reducing inflammation, oxidative stress, and apoptosis while preserving epithelial barrier integrity. Future clinical trials are warranted to ensure its effectiveness in treating AE-IPF patients and improving patient outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University (approval #202209S071) and in compliance with the institutional guidelines for the care and use of animals.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-163/rc
Funding: This work was financially supported by the Scientific Research Project of Jiangsu Provincial Health Commission (No. H2023005 to B.C.), the Xuzhou Medical Key Talents Program (No. XWRCHT20220063 to B.C.), the Construction Project of High Level Hospital of Jiangsu Province (No. LCZX202411 to B.C., Nos. GSPJS202419 and GSPJS202425 to X.Y.). Xuzhou National Clinical Key Specialty Cultivation Project (No. 2018ZK004 to X.Y.), Natural Science Foundation of Jiangsu Province (No. BK20231162 to X.Y.).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-163/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-163/dss
References
- 1.Moui A, Dirou S, Sagan C, et al. Immune alveolitis in interstitial lung disease: an attractive cytological profile in immunocompromised patients. BMC Pulm Med 2022;22:79. 10.1186/s12890-022-01871-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasheed AZ, Metersky ML, Ghazal F. Mechanisms and management of cough in interstitial lung disease. Expert Rev Respir Med 2023;17:1177-90. 10.1080/17476348.2023.2299751 [DOI] [PubMed] [Google Scholar]
- 3.Maher TM. Interstitial Lung Disease: A Review. JAMA 2024;331:1655-65. 10.1001/jama.2024.3669 [DOI] [PubMed] [Google Scholar]
- 4.Mei Q, Liu Z, Zuo H, et al. Idiopathic Pulmonary Fibrosis: An Update on Pathogenesis. Front Pharmacol 2021;12:797292. 10.3389/fphar.2021.797292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal AK, Huda N. Interstitial Pulmonary Fibrosis. Treasure Island, FL, USA: StatPearls Publishing; 2025. [PubMed] [Google Scholar]
- 6.Zhang Y, Wang J. Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis. Adv Respir Med 2023;91:26-48. 10.3390/arm91010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang M, Marts L, Kempker JA, et al. Minimal clinically important difference in idiopathic pulmonary fibrosis. Breathe (Sheff) 2021;17:200345. 10.1183/20734735.0345-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drakopanagiotakis F, Markart P, Steiropoulos P. Acute Exacerbations of Interstitial Lung Diseases: Focus on Biomarkers. Int J Mol Sci 2023;24:10196. 10.3390/ijms241210196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyashita K, Kono M, Saito G, et al. Prognosis after acute exacerbation in patients with interstitial lung disease other than idiopathic pulmonary fibrosis. Clin Respir J 2021;15:336-44. 10.1111/crj.13304 [DOI] [PubMed] [Google Scholar]
- 10.Ba C, Jiang C, Wang H, et al. Prognostic value of serum oncomarkers for patients hospitalized with acute exacerbation of interstitial lung disease. Ther Adv Respir Dis 2024;18:17534666241250332. 10.1177/17534666241250332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhan S, Li S, Cao Y, et al. Value of bronchoalveolar lavage fluid metagenomic next-generation sequencing in acute exacerbation of fibrosing interstitial lung disease: an individualized treatment protocol based on microbiological evidence. BMC Pulm Med 2024;24:400. 10.1186/s12890-024-03216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntolios P, Tzilas V, Bouros E, et al. The Role of Microbiome and Virome in Idiopathic Pulmonary Fibrosis. Biomedicines 2021;9:442. 10.3390/biomedicines9040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang L, Cai Y, Lyu B, et al. Air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in Beijing: a time-series study. Respir Res 2022;23:81. 10.1186/s12931-022-01998-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomos I, Dimakopoulou K, Manali ED, et al. Long-term personal air pollution exposure and risk for acute exacerbation of idiopathic pulmonary fibrosis. Environ Health 2021;20:99. 10.1186/s12940-021-00786-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi S, Tonelli R, Murray M, et al. Environmental Causes of Idiopathic Pulmonary Fibrosis. Int J Mol Sci 2023;24:16481. 10.3390/ijms242216481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi R, Takoi H, Tsuji T, et al. Glasgow Prognostic Score predicts chemotherapy-triggered acute exacerbation-interstitial lung disease in patients with non-small cell lung cancer. Thorac Cancer 2021;12:667-75. 10.1111/1759-7714.13792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J, Cao H, Ke Y, et al. Acute Exacerbation of Interstitial Lung Disease in Adult Patients With Idiopathic Inflammatory Myopathies: A Retrospective Case-Control Study. Front Med (Lausanne) 2020;7:12. 10.3389/fmed.2020.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charokopos A, Moua T, Ryu JH, et al. Acute exacerbation of interstitial lung disease in the intensive care unit. World J Crit Care Med 2022;11:22-32. 10.5492/wjccm.v11.i1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer N, Bendstrup E, Davidsen JR, et al. Acute exacerbation of fibrotic interstitial lung diseases. Ugeskr Laeger 2023;185:V04230261. [PubMed] [Google Scholar]
- 20.Ba C, Wang H, Jiang C, et al. Clinical manifestations and prognostic factors analysis of patients hospitalised with acute exacerbation of idiopathic pulmonary fibrosis and other interstitial lung diseases. BMJ Open Respir Res 2024;11:e001997. 10.1136/bmjresp-2023-001997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luppi F, Manfredi A, Faverio P, et al. Treatment of acute exacerbation in interstitial lung disease secondary to autoimmune rheumatic diseases: More questions than answers. Autoimmun Rev 2024;23:103668. 10.1016/j.autrev.2024.103668 [DOI] [PubMed] [Google Scholar]
- 22.Jia K, Wu J, Li Y, et al. A novel pulmonary fibrosis murine model with immune-related liver injury. Animal Model Exp Med 2023;6:274-82. 10.1002/ame2.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koike A, Hayashi K, Fujimori K. Involvement of necroptotic cell death in macrophages in progression of bleomycin and LPS-induced acute exacerbation of idiopathic pulmonary fibrosis. Eur J Pharmacol 2024;972:176572. 10.1016/j.ejphar.2024.176572 [DOI] [PubMed] [Google Scholar]
- 24.Estornut C, Milara J, Bayarri MA, et al. Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis. Front Pharmacol 2021;12:794997. 10.3389/fphar.2021.794997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng D, Liu J, Piao H, et al. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol 2022;13:1039241. 10.3389/fimmu.2022.1039241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YY, Wang M, Zuo CY, et al. Nrf-2 as a novel target in radiation induced lung injury. Heliyon 2024;10:e29492. 10.1016/j.heliyon.2024.e29492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Wu Z, Liu Y, et al. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J Nanobiotechnology 2022;20:213. 10.1186/s12951-022-01435-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Shen C, Zhou X, et al. Local Treatment of Hydrogen-Rich Saline Promotes Wound Healing In Vivo by Inhibiting Oxidative Stress via Nrf-2/HO-1 Pathway. Oxid Med Cell Longev 2022;2022:2949824. 10.1155/2022/2949824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye X, Zhang M, Gu H, et al. Animal models of acute exacerbation of pulmonary fibrosis. Respir Res 2023;24:296. 10.1186/s12931-023-02595-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Xu GX, Tai QH, et al. Lipoxin A4 Reduces Ventilator-Induced Lung Injury in Rats with Large-Volume Mechanical Ventilation. Mediators Inflamm 2020;2020:6705985. 10.1155/2020/6705985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Wu Y, Meng X, et al. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ 2022;29:1395-408. 10.1038/s41418-022-00928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Zeng J, Li J, et al. Sivelestat sodium attenuates acute lung injury by inhibiting JNK/NF-κB and activating Nrf2/HO-1 signaling pathways. Biomol Biomed 2023;23:457-70. 10.17305/bb.2022.8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng W, Song Y, Wang R, et al. Neutrophil elastase: From mechanisms to therapeutic potential. J Pharm Anal 2023;13:355-66. 10.1016/j.jpha.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Q, Wang Y, Yang C, et al. Clinical Utility of the Sivelestat for the Treatment of ALI/ARDS: Moving on in the Controversy? Intensive Care Res 2023;3:12-7. 10.1007/s44231-022-00012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding Q, Wang Y, Yang C, et al. Effect of Sivelestat in the Treatment of Acute Lung Injury and Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Intensive Care Res 2023. [Epub ahead of print]. doi: . 10.1007/s44231-023-00032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahebnasagh A, Saghafi F, Safdari M, et al. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J Clin Pharm Ther 2020;45:1515-9. 10.1111/jcpt.13251 [DOI] [PubMed] [Google Scholar]
- 37.Weng J, Liu D, Shi B, et al. Sivelestat sodium alleviated lipopolysaccharide-induced acute lung injury by improving endoplasmic reticulum stress. Gene 2023;884:147702. 10.1016/j.gene.2023.147702 [DOI] [PubMed] [Google Scholar]
- 38.King PT, Dousha L. Neutrophil Extracellular Traps and Respiratory Disease. J Clin Med 2024;13:2390. 10.3390/jcm13082390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J 2012;40:1475-82. 10.1183/09031936.00127011 [DOI] [PubMed] [Google Scholar]
- 40.Fujino N, Kubo H, Suzuki T, et al. Administration of a specific inhibitor of neutrophil elastase attenuates pulmonary fibrosis after acute lung injury in mice. Exp Lung Res 2012;38:28-36. 10.3109/01902148.2011.633306 [DOI] [PubMed] [Google Scholar]
- 41.Song JS, Kang CM, Rhee CK, et al. Effects of elastase inhibitor on the epithelial cell apoptosis in bleomycin-induced pulmonary fibrosis. Exp Lung Res 2009;35:817-29. 10.3109/01902140902912527 [DOI] [PubMed] [Google Scholar]
- 42.Enomoto N. Pathological Roles of Pulmonary Cells in Acute Lung Injury: Lessons from Clinical Practice. Int J Mol Sci 2022;23:15027. 10.3390/ijms232315027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batah SS, Fabro AT. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir Med 2021;176:106239. 10.1016/j.rmed.2020.106239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268-91. 10.1016/j.resinv.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 45.Li S, Shi J, Tang H. Animal models of drug-induced pulmonary fibrosis: an overview of molecular mechanisms and characteristics. Cell Biol Toxicol 2022;38:699-723. 10.1007/s10565-021-09676-z [DOI] [PubMed] [Google Scholar]
- 46.Guo B, Liu W, Ji X, et al. CSF3 aggravates acute exacerbation of pulmonary fibrosis by disrupting alveolar epithelial barrier integrity. Int Immunopharmacol 2024;135:112322. 10.1016/j.intimp.2024.112322 [DOI] [PubMed] [Google Scholar]
- 47.Tian W, Dong T. Comparative study of the protective effect of Xuebijing injection and Sivelestat sodium on acute lung injury/acute respiratory distress syndrome rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2022;34:837-41. 10.3760/cma.j.cn121430-20220401-00334 [DOI] [PubMed] [Google Scholar]
- 48.Yang X, Huang XJ, Chen Z, et al. A novel quantification method of lung fibrosis based on Micro-CT images developed with the optimized pulmonary fibrosis mice model induced by bleomycin. Heliyon 2023;9:e13598. 10.1016/j.heliyon.2023.e13598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Wang C, Chen H, et al. The profibrotic effects of chronic microaspiration of bile acids on lungs of rats at different stages. Int Immunopharmacol 2020;84:106545. 10.1016/j.intimp.2020.106545 [DOI] [PubMed] [Google Scholar]
- 50.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41:467-70. 10.1136/jcp.41.4.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B, Jiang Q, Chen R, et al. Tacrolimus ameliorates bleomycin-induced pulmonary fibrosis by inhibiting M2 macrophage polarization via JAK2/STAT3 signaling. Int Immunopharmacol 2022;113:109424. 10.1016/j.intimp.2022.109424 [DOI] [PubMed] [Google Scholar]
- 52.Nie YJ, Wu SH, Xuan YH, et al. Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Mil Med Res 2022;9:21. 10.1186/s40779-022-00382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matera MG, Rogliani P, Ora J, et al. A comprehensive overview of investigational elastase inhibitors for the treatment of acute respiratory distress syndrome. Expert Opin Investig Drugs 2023;32:793-802. 10.1080/13543784.2023.2263366 [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Gao R, Hong H, et al. Emodin inhibiting neutrophil elastase-induced epithelial-mesenchymal transition through Notch1 signalling in alveolar epithelial cells. J Cell Mol Med 2020;24:11998-2007. 10.1111/jcmm.15827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makena P, Kikalova T, Prasad GL, et al. Oxidative Stress and Lung Fibrosis: Towards an Adverse Outcome Pathway. Int J Mol Sci 2023;24:12490. 10.3390/ijms241512490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan YW, Chen YC, Yen CC, et al. Kefir peptides mitigate bleomycin-induced pulmonary fibrosis in mice through modulating oxidative stress, inflammation and gut microbiota. Biomed Pharmacother 2024;174:116431. 10.1016/j.biopha.2024.116431 [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Yang L, Li Y, et al. Autophagy and Inflammation: Regulatory Roles in Viral Infections. Biomolecules 2023;13:1454. 10.3390/biom13101454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Wu Y, Mao M, et al. Sivelestat Sodium Alleviates Ischemia-Reperfusion-Induced Acute Kidney Injury via Suppressing TLR4/Myd88/NF-κB Signaling Pathway in Mice. Drug Des Devel Ther 2024;18:4449-58. 10.2147/DDDT.S480148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen L, Li M, Lin Y, et al. Neutrophil elastase in dexmedetomidine alleviating sepsis-related renal injury in rats. Int Immunopharmacol 2023;122:110441. 10.1016/j.intimp.2023.110441 [DOI] [PubMed] [Google Scholar]
- 60.Sun ZC, Liao R, Xian C, et al. Natural pachypodol integrated, lung targeted and inhaled lipid nanomedicine ameliorates acute lung injury via anti-inflammation and repairing lung barrier. J Control Release 2024;375:300-15. 10.1016/j.jconrel.2024.09.013 [DOI] [PubMed] [Google Scholar]
- 61.Liu M, Gao M, Shi X, et al. Quercetin attenuates SiO(2)-induced ZBP-1-mediated PANoptosis in mouse neuronal cells via the ROS/TLR4/NF-κb pathway. J Environ Manage 2024;370:122948. 10.1016/j.jenvman.2024.122948 [DOI] [PubMed] [Google Scholar]
- 62.Sapian S, Budin SB, Taib IS, et al. Role of Polyphenol in Regulating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis in Diabetic Nephropathy. Endocr Metab Immune Disord Drug Targets 2022;22:453-70. 10.2174/1871530321666211119144309 [DOI] [PubMed] [Google Scholar]
- 63.Uchida Y, Freitas MC, Zhao D, et al. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 2010;89:1050-6. 10.1097/TP.0b013e3181d45a98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan WJT, Vlajkovic SM. Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int J Mol Sci 2023;24:16545. 10.3390/ijms242216545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Xiong Q, Yang Q, et al. PTPRO inhibits LPS-induced apoptosis in alveolar epithelial cells. Biochem Biophys Res Commun 2024;718:150083. 10.1016/j.bbrc.2024.150083 [DOI] [PubMed] [Google Scholar]
- 66.Hu Y, Zhan F, Wang Y, et al. The Ninj1/Dusp1 Axis Contributes to Liver Ischemia Reperfusion Injury by Regulating Macrophage Activation and Neutrophil Infiltration. Cell Mol Gastroenterol Hepatol 2023;15:1071-84. 10.1016/j.jcmgh.2023.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishida Y, Zhang S, Kuninaka Y, et al. Essential Involvement of Neutrophil Elastase in Acute Acetaminophen Hepatotoxicity Using BALB/c Mice. Int J Mol Sci 2023;24:7845. 10.3390/ijms24097845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen S, Zhang X, Yang C, et al. Essential role of IL-17 in acute exacerbation of pulmonary fibrosis induced by non-typeable Haemophilus influenzae. Theranostics 2022;12:5125-37. 10.7150/thno.74809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu T, Wang T, Jiang J, et al. Effect of Neutrophil Elastase Inhibitor (Sivelestat Sodium) on Oxygenation in Patients with Sepsis-Induced Acute Respiratory Distress Syndrome. J Inflamm Res 2025;18:4449-58. 10.2147/JIR.S506549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Wei J, Deng H, et al. The Role of Nrf2 in Pulmonary Fibrosis: Molecular Mechanisms and Treatment Approaches. Antioxidants (Basel) 2022;11:1685. 10.3390/antiox11091685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng H, Zhang H, Cheng L, et al. Sivelestat ameliorates sepsis-induced myocardial dysfunction by activating the PI3K/AKT/mTOR signaling pathway. Int Immunopharmacol 2024;128:111466. 10.1016/j.intimp.2023.111466 [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi M, Nishisho T, Toki S, et al. Blue light induces apoptosis and autophagy by promoting ROS-mediated mitochondrial dysfunction in synovial sarcoma. Cancer Med 2023;12:9668-83. 10.1002/cam4.5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao W, Han X, Guan Y, et al. Neutrophil Elastase Inhibitors Suppress Oxidative Stress in Lung during Liver Transplantation. Oxid Med Cell Longev 2019;2019:7323986. 10.1155/2019/7323986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voynow JA, Shinbashi M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021;11:1065. 10.3390/biom11081065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gehrig S, Duerr J, Weitnauer M, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am J Respir Crit Care Med 2014;189:1082-92. 10.1164/rccm.201311-1932OC [DOI] [PubMed] [Google Scholar]