Abstract
The clinical significance and prevalence of Mycobacterium avium and Mycobacterium intracellulare were analyzed in a cohort of 7,472 patients who, from 1999 to 2003, sought care at the University of Texas M.D. Anderson Cancer Center, Houston, and had cultures performed for mycobacteria. Patients were stratified for age, sex, and underlying diseases, and bacteria were identified by 16S rRNA gene sequencing. M. avium was isolated in 62 (0.83%) of 7,472 patients and M. intracellulare in 65 (0.87%). Clinically, only 10 of the 62 (16.2%) patients with M. avium had probable to definite evidence of infection, whereas the majority (83.8%) had weak evidence of infection. Sex and age did not affect the isolation or infection of M. avium. Hematological tumors predisposed to M. avium colonization but not infection. In contrast, 41 of the 65 (63.1%) patients with M. intracellulare had probable to definite infection, a level much higher than those with M. avium (P < 0.001). M. intracellulare was more prevalent in women (1.33% of 3,311) than in men (0.50% of 4,161) (P < 0.001), and underlying diseases had no effect in women. Men with lung cancer had a higher prevalence (1.37%) than men without (0.34%) (4.0-fold; P < 0.001), but it was similar to that in women. A marked age trend for the isolation of M. intracellulare among women was noted: 0.27% (1-fold) for ages of <50 years, 0.85% (3.1-fold) for ages 50 to 59 years, 1.50% (5.6-fold) for ages 60 to 69 years, and 3.74% (13.9-fold) for ages ≥70 years (trend, P < 0.001). The combined rate for women ≥50 was 1.86% (95% confidence interval [1.30 to 2.42%]) (6.9-fold). Together, these results suggest that, among non-AIDS patients, M. intracellulare is more pathogenic and tends to infect women increasingly beyond menopause (age ≥50 years) regardless of underlying disease. The prevalence rate of 1.86% in postmenopausal women suggests the need to further investigate the public health significance of M. intracellulare.
Mycobacterium avium and Mycobacterium intracellulare are slow-growing, nontuberculous mycobacterial (NTM) species that, due to similar biochemical characteristics, are usually grouped in the Mycobacterium avium-M. intracellulare complex (MAIC). The organisms are probably the most significant NTM associated with human diseases, causing disseminated infection in patients with AIDS, nodular bronchiectasis and other pulmonary infections, lymphadenitis, and skin infection (10).
Although infrequently differentiated in the literature, M. avium and M. intracellulare exhibit significantly different pathogenicity and biology, as suggested by the following. First, the vast majority (>95%) of AIDS-related MAIC infections are due to M. avium, not M. intracellulare, and the M. avium infection typically occurs when the CD4 cell count falls to less than 0.05 × 109/liter (7, 10). Thus, deficiency in cellular immunity is an infection risk for M. avium but not for M. intracellulare. From a microbiology standpoint, the term MAIC in AIDS is obsolete (9). Second, nodular bronchiectasis caused by MAIC is relatively common among older women (historically so-called Lady Windermere syndrome), and many of them do not have preexisting risk factors (9, 13, 16). Two case-based studies suggest that M. intracellulare causes 72 to 91% of these infections (20, 21). Third, although both mycobacteria are aquatic organisms, their niches are different: M. avium tends to grow in water suspension, whereas M. intracellulare forms biofilm (4, 12). The environmental origin of MAIC also implies that isolation of these organisms from nonsterile sources in a patient may represent mere colonization or water contamination and, to diagnose true infection, a correlation with clinical manifestations, culture results, and radiologic findings is needed. Accordingly, the American Thoracic Society has established criteria to assess the significance of NTM isolates (1, 2).
In the present study, we analyzed the isolation and infection rates of M. avium and M. intracellulare in a cohort of 7472 hospital patients, mostly with cancer, who had mycobacteriologic cultures. Patients were stratified for age, sex, and underlying diseases, and the mycobacteria were identified to species by nucleotide sequencing. From this cohort, 127 patients with either organism were further analyzed for clinical significance. Our results suggest different epidemiology, risk factors, and clinical significance for the two mycobacteria.
MATERIALS AND METHODS
Study setting and data acquisition.
This cohort study was conducted at The University of Texas M.D. Anderson Cancer Center in Houston, Texas, a 500-bed tertiary hospital, from January 1999 to December 2003. Most patients cared at the institution carried a primary diagnosis of cancer; some were referred for suspected cancer or surveillance.
All patients with mycobacteriologic culture(s) were study subjects. For stratified analyses, the age, sex, and primary diagnosis of each patient were retrieved through the Texas Tumor Registry located at the institution. The medical records of patients with M. avium or M. intracellulare were reviewed for clinical significance.
Culture and identification of mycobacteria.
Cultures for mycobacteria were used liberally whenever such infections were in the differential diagnoses. Standard culture techniques were used (14). Nonsterile specimens were decontaminated before inoculation. During 1999 and 2000, only solid media (Lowenstein-Jensen tube and Middlebrook 7H10 agar) were used. Since January 2001, both the Lowenstein-Jensen tube and liquid medium (BacT/Alert MB; BioMerieux, Durham, NC) were used for each specimen. Once the cultures turned positive and the presence of mycobacteria confirmed by special stains, identification of MAIC was achieved through nucleic acid hybridization (Gen-Probe, San Diego, CA).
Further species identification of MAIC was achieved through sequencing analysis of the 16S rRNA gene as described previously (8). In the 665-bp sequenced region, M. avium and M. intracellulare differ by six nucleotides, which well differentiate these two species. When a patient had two or more MAIC isolated, at least two isolates were sequenced to ensure individual identification.
Definition of infection.
The criteria established by the American Thoracic Society (1, 2), preferentially the earlier version for definite infection, were used to categorize the significance of MAIC infections as definite, probable, possible, or doubtful (colonization/contamination). Briefly, a definite infection means isolation of MAIC from a sterile site or tissue with compatible tissue reactions (granulomas) or repeated (≥3 times) isolations from a nonsterile source with compatible radiological features (airspace opacities, branching nodular opacities, and bronchiectasis) (23), clinical manifestations, and exclusion of other possible etiologies. Single or double isolations from nonsterile sources with compatible radiological and/or clinical findings suggest a probable infection. Single isolation from a nonsterile source with a significant coisolated microorganism and some radiological evidence with or without clinical manifestations constitutes a possible infection. Single isolation from a nonsterile source without radiological or clinical evidence suggests doubtful infection. Because the clinical manifestations of most patients who also had underlying diseases and other comorbidities were frequently overlapping, nonspecific, or vague, emphasis was placed on more objective findings, such as radiological evidence and the source and times of MAIC isolation. Infections in our patients were usually worked up rigorously through the use of fine-needle biopsy and/or close radiologic follow-up to differentiate them from the cancer.
Grouping of underlying diseases.
In order to analyze their effect on MAIC infection, the underlying diseases were broadly grouped into lung cancer, other solid tumors, hematologic tumors, and no tumors. These diagnoses, generally made confidently in the institution, served as estimation of previous lung injury or immune defect. The preexisting chronic obstructive pulmonary disease that predisposes to MAIC infection, was not uniformly diagnosed or documented in the institution and thus not used. For the purpose of the present study, lung cancer represented squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and carcinoid tumors arising from the lung, bronchi, and trachea. Other solid tumors were tumors not related to the lung, bronchi, or trachea, with or without secondary metastasis. Hematologic tumors included leukemias, lymphomas, allogeneic (but not autologous) stem cell transplantation, severe aplastic anemia, multiple myeloma, and other rare entities. For the no tumor group, patients were either referred for suspected tumors but found to be otherwise later or had surveillance cultures.
Data analysis.
When appropriate, statistical analyses were performed by using χ2 or weighted-trend χ2 methods.
RESULTS
During the 5-year period, 15,435 various clinical specimens were cultured for mycobacteria. Of these, 1,717 specimens (11.1%) were excluded from the study because of very low culture yield, such as urine and cerebrospinal fluid, or specimens prone to or resulting from fecal contamination, such as abdominal or pelvic abscesses. As a result, 13,718 specimens (88.9%), mostly from respiratory sources (sputum, bronchial washing, and bronchoalveolar lavage), and sterile tissue or other fluids were included in the study. These specimens came from 7,472 patients (mean, 1.84 cultures per patient), who comprised the study cohort. Of these patients, 4,590 of them (61.4%) had single culture performed without follow-up culture, and the rest (38.6%) had 2 to 27 cultures per patient for up to 5 years. Among all of the stratified groups, patients with hematologic tumors had most mean number of cultures (2.31 per patient), paralleling their frequent infection complications, whereas the other groups had a mean of 1.44 to 1.61 cultures.
From the 7,472 patients, 133 of them had at least one MAIC isolated initially. Of the 133 patients, 6 were excluded from analysis: 1 with AIDS, 2 with M. intracellulare in the urine only, and 3 due to other NTM after sequencing analysis. The remaining 127 (1.70%) of 7,472 patients, including 62 (0.83%) with M. avium and 65 (0.87%) with M. intracellulare, were subjected to review and analyses. Chest computed tomographs taken within a month before or after the culture were available for 109 (85.9%) patients.
Clinical significance.
The clinical significance of the 127 patients is shown in Table 1. For the 62 patients with M. avium, 10 (16.2%) had probable or definite infections, whereas most had weak evidence of infection, i.e., either possible infections (40.3% [25 of 62]) or colonization/contamination (43.5% [27 of 62]). In contrast, 41 (63.1%) of the 65 patients with M. intracellulare had probable or definite infections compared to 16 (24.6%) with possible infections and 8 (12.3%) with colonization. In addition, 15 (23.1%) of 65 patients had the organism isolated twice or more, significantly more than those with M. avium (4.8% [3 of 62]). These significant differences between M. avium and M. intracellulare suggest that, despite similar isolation frequencies among our patients, M. intracellulare had higher pathogenicity and more clinical significance than M. avium. Furthermore, it is possible that patients with weak evidence of M. intracellulare infection might have been in the early stage.
TABLE 1.
Clinical significance of 127 patients with isolation of M. avium or M. intracellulare
| Infection status | No. of patients (%) with isolation of:
|
||
|---|---|---|---|
| M. avium | M. intracellulare | Both | |
| Infection wasa: | |||
| Definite | 5 (8.1) | 11 (16.9) | 16 (12.6) |
| Sterile sources | 4 (6.5) | 4 (6.1) | 8 (6.3) |
| Serial (≥3) isolates | 1 (1.6) | 7 (10.8) | 8 (6.3) |
| Probable | 5 (8.1) | 30 (46.2) | 35 (27.6) |
| Possible | 25 (40.3) | 16 (24.6) | 41 (32.3) |
| Doubtful | 27 (43.5) | 8 (12.3) | 35 (27.6) |
| Total | 62 (100.0) | 65 (100.0) | 127 (100.1) |
| Infected byb: | |||
| Single isolate | 59 (95.2) | 50 (76.9) | |
| Multiple (≥2) isolates | 3 (4.8) | 15 (23.1) | |
χ2 = 32.34, df = 3, P < 0.001.
χ2 = 8.68, df = 1, P = 0.003.
For all 51 infected (probable or definite) patients, M. intracellulare caused 41 (80.4%), whereas M. avium caused only 10 (19.6%), and there were 37 (72.5%) women compared to 14 (27.5%) men. The mean age of the 51 patients was 66.6 (range, 49 to 83) years.
Analyses of M. intracellulare.
Stratified analyses were performed. All of the 65 patients with M. intracellulare, irrespective of clinical judgment or potentially at different stages of infection, were analyzed first, followed by analyses of the 41 infected patients (Table 2).
TABLE 2.
Isolation and infection of M. intracellulare by underlying disease, sex, and agea
| Category | Isolation
|
Infection
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients with isolate/no. who had culture performed (per 100)
|
OR (P) | No. of patients with infection (per 100)d
|
OR (P) | |||||
| Men | Women | Both | Men | Women | Both | |||
| All | 21/4,161 (0.50) | 44/3,311 (1.33) | 65/7,472 (0.87) | 2.66 (< 0.001) | 11 (0.26) | 30 (0.91) | 41 (0.55) | 3.43 (<0.001) |
| Cancer statusb | ||||||||
| Lung cancer | 9/656 (1.37) | 6/454 (1.32) | 15/1,110 (1.35) | 0.96 (NS) | 5 (0.76) | 4 (0.88) | 9 (0.81) | 1.16 (NS) |
| Not lung cancer | 12/3,505 (0.34) | 38/2,857 (1.33) | 50/6,362 (0.79) | 3.91 (<0.001) | 6 (0.17) | 26 (0.91) | 32 (0.50) | 5.32 (<0.001) |
| Other solid tumors | 4/1,607 (0.25) | 19/1,529 (1.24) | 23/3,136 (0.73) | 4.96 (0.001) | 1 (0.06) | 14 (0.91) | 15 (0.48) | 14.7 (<0.001) |
| Hematologic tumors | 7/1,706 (0.41) | 11/1,099 (1.00) | 18/2,805 (0.64) | 2.44 (0.056) | 4 (0.23) | 4 (0.36) | 8 (0.29) | 1.55 (NS) |
| No tumor | 1/192 (0.52) | 8/229 (3.49) | 9/421 (2.14) | 6.71 (0.036) | 1 (0.52) | 8 (3.49) | 9 (2.14) | 6.71 (0.036) |
| Age (yr)c | ||||||||
| ≤49 | 3/1,228 (0.24) | 3/1,102 (0.27) | 6/2,330 (0.26) | 1.13 (NS) | 1 (0.08) | 1 (0.09) | 2 (0.09) | 1.11 (NS) |
| 50-59 | 3/977 (0.31) | 7/821 (0.85) | 10/1,798 (0.56) | 2.74 (NS) | 3 (0.31) | 5 (0.61) | 8 (0.44) | 1.98 (NS) |
| 60-69 | 8/1,120 (0.71) | 12/801 (1.50) | 20/1,921 (1.04) | 2.11 (NS) | 4 (0.36) | 6 (0.75) | 10 (0.52) | 2.10 (NS) |
| ≥70 | 7/836 (0.84) | 22/587 (3.74) | 29/1,423 (2.04) | 4.45 (<0.001) | 3 (0.36) | 18 (3.07) | 21 (1.48) | 8.56 (<0.001) |
OR, odds ratio; NS, not significant.
Odds ratios were determined for lung cancer patients versus patients without lung cancer and were as follows for the isolation group: for men, 4.03(P < 0.001); for women, 0.99 (P was not significant); for both, 1.71 (P was not significant). For the infection group, odds ratios were as follows: for men, 4.45 (P = 0.007); for women, 0.97 (P was not significant); for both, 1.61 (P was not significant).
In the isolation group, the mean ages of men, women, and both combined were 63.9, 67.5, and 66.3 years, respectively. In the infection group, the mean ages of men, women, and both combined were 63.3, 69.4, and 67.8 years, respectively. For all patients who had culture performed, the mean ages of men, women, and both combined were 56.3, 55.4, and 55.9 years, respectively. Age trends were examined, and P values were determined. In the isolation group, for men, women, and both combined, P values were 0.072 (not significant), 0.001, and 0.001, respectively. In the infection group, for men, women, and both combined, P values were not significant, 0.001, and 0.001, respectively.
To determine rates (%), values were compared to numbers of persons who had culture performed.
The isolation rate among women (1.33% [44 of 3,311]) was significantly higher than that among men (0.50% [21 of 4,161]) (odds ratio = 2.66, χ2 = 14.53, P < 0.001). Among women with primary lung cancer, other solid tumors, or hematological tumors, the rates were similar, 1.32, 1.24, and 1.00%, respectively, or combined (1.17% [36 of 3,082]). Among women without tumor, the rate was higher (3.49%) (χ2 = 8.79, P = 0.003). This was likely due to referral effect because these women presented with lung nodules initially suspected of lung cancer. Therefore, underlying cancers do not increase the rate for M. intracellulare.
Men with lung cancer had a significantly higher rate than men in other groups combined (1.37% versus 0.34%, odds ratio = 4.03, χ2 = 11.67, P < 0.001), but it was similar to those among women with (1.32%) or without (1.33%) lung cancer. History of heavy smoking (at least 20 cigarettes per day for 10 years or total cigarettes approximating 73,000) was a feature for men with M. intracellulare (66.7% [14 of 21]) but not for women (9.1% [4 of 44]) (χ2 = 23.5, P < 0.001).
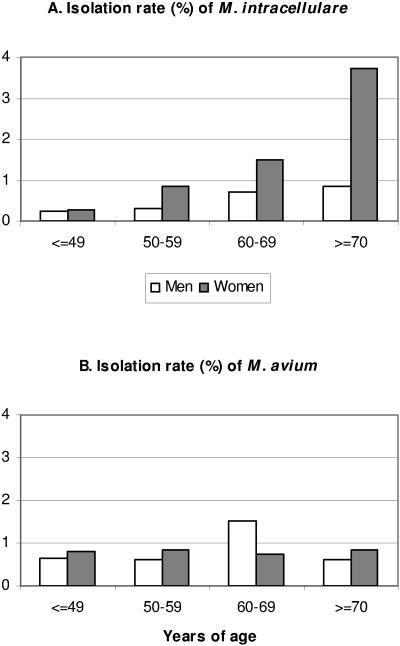
An age trend for the isolation of M. intracellulare in women was also noted (Table 2 and Fig. 1). The rates were 0.27% (1-fold) for women <50 years old, 0.85% (3.1-fold) for women 50 to 59 years old, 1.50% (5.6-fold) for women 60 to 69 years old, and 3.74% (13.9-fold) for women ≥70 years old (weighted trend χ2 = 32.51, df = 2, P < 0.001). The combined rate for women ≥ 50 was 1.86% (6.9-fold). Conversely, there was no significant age trend for men (χ2 = 5.26, df = 2, P = 0.072). Patients with M. intracellulare were older (mean, 66.3 years old) than the study cohort (55.9 years) and those with M. avium (57.2 years).
FIG. 1.
Effects of age and sex on the isolation rates of M. intracellulare (A) and M. avium (B). The data are from Tables 2 and 3.
The infection rates of M. intracellulare followed the patterns of isolation rates for the various groups (Table 2), but the difference between men and women with hematologic tumors became insignificant. Overall, these results suggest that M. intracellulare preferentially infects older, postmenopausal women, irrespective of underlying diseases, and men with lung cancer.
Analyses of M. avium.
The isolation rates of M. avium for men and women were similar, combined or categorized (all P > 0.05) (Table 3). Men with different underlying diseases also had similar rates. Women with hematological tumors had significantly higher rate than the combined rate among women of other groups (16 of 1,099 versus 10 of 2,212, χ2 = 9.50, P = 0.002). For both men and women with hematologic tumors, the combined rate was also significantly higher than that among men and women of other groups (35 of 2,805 versus 27 of 4,667, χ2 = 9.54, P = 0.002). Therefore, hematologic tumors predisposed to M. avium colonization or contamination but not infection (see below). More cultures in these patients (mean, 2.31/patient) than those in other groups (mean, 1.55) were also a contributing factor (as contaminants). No age trend was noted (Table 3 and Fig. 1), and the mean patient age (57.2 years old) was similar to that of study cohort (55.9 years old).
TABLE 3.
Isolation and infection of M. avium by underlying disease, sex, and agea
| Category | No. of patients with isolateb
|
ORc | No. of patients with infectionb
|
ORd | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Both | Men (5) | Women (6) | Both | |||
| All | 36 (0.87) | 26 (0.79) | 62 (0.83) | 0.91 | 3 (0.07) | 7 (0.21) | 10 (0.13) | 2.93 |
| Cancer statuse | ||||||||
| Lung cancer | 8 (1.22) | 2 (0.44) | 10 (0.90) | 0.36 | 0 (0) | 0 (0) | 0 (0) | - |
| Not lung cancer | 28 (0.80) | 24 (0.84) | 52 (0.82) | 1.05 | 3 (0.09) | 7 (0.25) | 10 (0.16) | 2.86 |
| Other solid tumors | 8 (0.50) | 7 (0.46) | 15 (0.47) | 0.92 | 2 (0.12) | 3 (0.20) | 5 (0.16) | 1.58 |
| Hematologic tumors | 19 (1.11) | 16 (1.46) | 35 (1.25) | 1.32 | 1 (0.06) | 3 (0.27) | 4 (0.14) | 4.66 |
| No tumor | 1 (0.52) | 1 (0.44) | 2 (0.48) | 0.85 | 0 (0) | 1 (0.44) | 1 (0.24) | - |
| Age (yr)f | ||||||||
| ≤49 | 8 (0.65) | 9 (0.82) | 17 (0.73) | 1.26 | 0 (0) | 1 (0.09) | 1 (0.04) | - |
| 50-59 | 6 (0.61) | 7 (0.85) | 13 (0.72) | 1.39 | 1 (0.10) | 1 (0.12) | 2 (0.11) | 1.19 |
| 60-69 | 17 (1.52) | 6 (0.75) | 23 (1.20) | 0.49 | 1 (0.09) | 3 (0.37) | 4 (0.21) | 4.19 |
| ≥70 | 5 (0.60) | 4 (0.85) | 9 (0.63) | 1.42 | 1 (0.12) | 2 (0.34) | 3 (0.21) | 2.85 |
OR, odds ratio.
Values in parentheses are rates (%) determined in comparison to number of patients who had culture performed (for details, see footnote c of Table 2).
Women versus men in isolate group. P values were not significant.
Women versus men in infection group. P values were not significant.
Lung cancer patients were compared to patients without lung cancer, and the odds ratios were 1.53, 0.52, and 1.10 for men, women, and both combined, respectively, in the isolate group. In no case was P significant.
Age trends were examined but not found to be significant. In the isolate group, the mean ages of men, women, and both combined were 58.5, 55.4, and 57.2 years, respectively. In the infection group, the mean ages of men, women, and both combined were 65.3, 62.3, and 63.2 years, respectively.
For the 10 infected cases, the underlying diseases were hematological tumor (4 cases, 0.14% of 2,805), solid tumor (5 cases, 0.16% of 3,136), and no tumor (1 case, 0.24% of 421). The similar infection rates among these groups (total 6,362 patients) suggest that underlying cancer does not appreciably predispose to M. avium infection. The slight differences between women and men and between ages were insignificant after stratification (numbers too small).
DISCUSSION
Medical practice from office settings to large academic centers hardly differentiates between M. avium and M. intracellulare because of technical difficulty, as well as a paucity of definitive data to show the significance of such differentiation, particularly early in the course. At present, MAIC (and almost all 100 or so mycobacterial species) can be differentiated confidently by the increasingly popular 16S rRNA gene sequencing (8, 17, 19). By such a method, we have presented strong evidence here that, among a cohort of non-AIDS hospital patients, M. avium and M. intracellulare differed in pathogenicity, epidemiology, and predisposing factors of the infections. Although both organisms were isolated at similar frequencies, only 16.1% of patients with M. avium had strong evidence of infection, in contrast to the 63.1% of patients with M. intracellulare in this category (Table 1), suggesting higher pathogenicity of M. intracellulare. Among these infected patients, M. intracellulare accounted for far more (80.4%) than M. avium did (19.6%), a finding similar to those derived from case series by Wallace et al. (20, 21). Therefore, M. intracellulare appears more important than M. avium in the non-AIDS population.
Many patients with hematologic tumors, such as lymphocytic leukemia, lymphoma, and hematopoietic stem cell transplantation, had immune derangements or suppression as a result of disease and/or treatment. Our results also showed that hematologic tumors predisposed patients to M. avium colonization, but not infection (Table 3). Compared to the disseminated M. avium infection in AIDS patients, the colonization in our patients rarely progressed to disseminated or overwhelming infection as seen in AIDS.
The diagnosis and prevalence of MAIC infection, like other diseases, is influenced by diagnostic techniques and frequencies (culture and chest radiography) and length of follow-up of denominator population. The scale of our study and innate severity of underlying disease, as well as the reference nature of many of our patients, made follow-up culture and other studies impossible or practically unfeasible in many of them. Consequently, some of our 127 patients with M. avium or M. intracellulare who had little or weak evidence of infection might have been in the early stage of infection and, had they been monitored long enough, probable or definite infections could have emerged. This scenario was more likely for the more pathogenic M. intracellulare. Therefore, the present study depicted a more cross-sectional than longitudinal picture of MAIC infections. Despite this limitation, our study, being based on a well-defined denominator instead of case-based, described prevalence rates and a spectrum of clinical significance for both organisms. In addition, most (85.8%) of the 127 patients had chest computed tomography scans, making clinical assessment more reliable at the time of a positive culture. The scale, accuracy of speciation, and stratified analyses strengthened the study.
Report of MAIC infection or isolation to health authority is not mandatory, and nationwide data of its prevalence in the general population have been sparse and probably underestimated (3, 5). For instance, of the NTM reported to the public health information system from 1993 to 1996, there were yearly 7,687 to 9,281 isolates of M. avium compared to 184 to 409 M. intracellulare (3). Our data showed that the isolation rates for M. avium and M. intracellulare were similar. i.e., 0.83 and 0.87%, respectively. The rate for M. avium was probably overestimated because of possible contamination/colonization and more cultures performed for our hospital patient population. However, the rate for women with M. intracellulare (1.33%) might be accurate in view of the higher pathogenicity of the organism and the lack of effect of underlying diseases (even higher rate among individuals without cancer, Table 2). If our isolation rate of 1.86% (95% confidence interval of 1.30 to 2.42%) for women aged 50 and above were used for the 42 million women of these ages (2000 U.S. census), it would predict 781,000 (range, 546,000 to 1,016,000) of them with M. intracellulare. Considering the geographic variations for MAIC (10), i.e., being less common in northern states by a factor of 2 to 3, the estimated prevalence would still be around 500,000 cases. Therefore, the public health significance of M. intracellulare in these women needs to be investigated.
It is known that MAIC causes infection in elderly women who do not have underlying diseases or obvious risk factors (10, 16). The data from Wallace et al. (20, 21) and the present study show consistently that M. intracellulare causes ∼80% of such infections. In addition, the present study further showed that M. intracellulare was rare in women younger than 50 and, as the number of postmenopausal years increased, so went up the prevalence rates (Table 2 and Fig. 1). This remarkable age trend is unique and intriguing; to our knowledge, it has not been seen with other mycobacteria (including M. avium found in the present study) or other microorganisms in general. Plausible explanations may include a combination of protective factors in premenopausal women, increased susceptibility after menopause due to changing physiology and anatomy (along the respiratory tract), chronicity and indolence of the colonization and infection that makes the prevalence accumulate with age, and repeated infections by different strains over years, as previously demonstrated (21). By correlating our results with the literature, we speculate that the protection versus susceptibility centers surrounding estrogen. The proinflammatory effect of estrogen is well known. This hormone regulates the functions of macrophage through its receptors (15, 22, 24). During the early years of menopause (within 10 years), the serum level of macrophage colony-stimulating factor decreases significantly, but hormone replacement therapy restores it (11). In an experimental model, estrogen protects mice from M. avium infection, mainly through augmenting macrophage's functions (18). Currently, we are examining estrogen's effect on macrophages against M. intracellulare. Microbiologically, in its natural aquatic environment, M. intracellulare forms biofilm for attachment and growth to a greater degree than M. avium (4). The similar high prevalence of M. intracellulare in men with lung cancer (1.37%) implies that prior lung injury by cancer risk factors, such as smoking and others, predisposes to attachment by the organism, leading to colonization and infection. Perhaps, M. intracellulare takes advantage of some common features between severely injured male lung and postmenopausal female lung and sets up a niche. A few common changes known in cancerous lung are squamous metaplasia, bronchiectasis, and ciliary dysfunction along the tracheobronchial tree, and various degrees of hyperplasia to dysplasia. These pathological changes also predispose to colonization and infection by Pseudomonas aeruginosa, another well-known biofilm-forming bacterium (6). M. intracellulare infection and bronchiectasis are inter-related, as seen in the present study and others (20, 21). It remains to be elucidated whether the tracheobronchial tree in susceptible postmenopausal women also contains squamous metaplasia and/or ciliary dysfunction and whether such changes intensify or become more prevalent with aging.
Finally, our findings also have practical significance for the diagnosis and management of patients who might be in the early stages of MAIC infection without radiographic stigmata. Speciation may predict progression of infection in those with M. intracellulare but far less with M. avium. Thus, M. intracellulare may call for antimycobacterial therapy.
Acknowledgments
We thank all of the staff in our clinical microbiology laboratory for assistance with the cultures, Sary Joudah and Carla Guillot for assistance with the culture database, Suzy Wallace and Danna Kurtin for assistance with searches of the Texas Tumor Registry, and Audrey Pham for technical assistance.
This study was approved by an Institutional Review Board protocol RCR03-019 and supported by a University Cancer Foundation grant (to X.Y.H.) from The University of Texas M.D. Anderson Cancer Center and in part by National Institutes of Health grant (CA16672) for the institutional core DNA sequencing facility.
REFERENCES
- 1.American Thoracic Society. 1990. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. Rev. Respir. Dis. 142:940-953. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. Rev. Respir. Dis. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1999. Nontuberculous mycobacteria reported to the public health information system by the state public health laboratories, United States, 1993-1996. [Online.] www.cdc.gov/ncidod/dastlr/mycobacteriology.htm.
- 4.Falkinham, J. O., III, C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Good, R. C., and D. E. Snider. 1982. Isolation of nontuberculous mycobacteria in the United States, 1980. J. Infect. Dis. 146:829-833. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg, E. P. 2003. Bacterial communication: tiny teamwork. Nature 424:134. [DOI] [PubMed] [Google Scholar]
- 7.Guthertz, L. S., B. Damsker, E. J. Bottone, E. G. Ford, T. F. Midura, and J. M. Janda. 1989. Mycobacterium avium and Mycobacterium intracellulare infections in patients with or without AIDS. J. Infect. Dis. 160:1037-1041. [DOI] [PubMed] [Google Scholar]
- 8.Han, X. Y., A. S. Pham, J. J. Tarrand, P. K. Sood, and R. Luthra. 2002. Rapid and accurate identification of mycobacteria by sequencing hypervariable regions of the 16S ribosomal RNA gene. Am. J. Clin. Pathol. 118:796-801. [DOI] [PubMed] [Google Scholar]
- 9.Hirschel, B. 2001. Infections due to nontuberculous mycobacteria, p. 1040-1044. In E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison's principles of internal medicine, 15th ed. McGraw-Hill Book Co., Inc., New York, N.Y.
- 10.Inderlied, C. B., C. A. Kemper, and L. M. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamada, M., M. Irahara, M. Maegawa, Y. Ohmoto, T. Takeji, T. Yasui, and T. Aono. 2001. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am. J. Obstet. Gynecol. 184:309-314. [DOI] [PubMed] [Google Scholar]
- 12.Kirschner, R. A., Jr., B. C. Parker, and J. O. Falkinham III. 1992. Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am. Rev. Respir. Dis. 145:271-275. [DOI] [PubMed] [Google Scholar]
- 13.Kubo, K., Y. Yamazaki, T. Hachiya, M. Hayasaka, T. Honda, M. Hasegawa, and S. Sone. 1998. Mycobacterium avium-intracellulare pulmonary infection in patients without known predisposing lung disease. Lung 176:381-391. [DOI] [PubMed] [Google Scholar]
- 14.Metchock, B. G., F. S. Nolte, and R. J. Wallace. 1997. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 15.Mor, G., E. Sapi, V. M. Abrahams, T. Rutherford, J. Song, X. Y. Hao, S. Musaffar, and F. Kohen. 2003. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J. Immunol. 170:114-122. [DOI] [PubMed] [Google Scholar]
- 16.Prince, D. S., D. D. Peterson, R. M. Steiner, J. E. Gottlieb, R. Scott, H. L. Israel, W. G. Figueroa, and J. E. Fish. 1989. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 321:863-868. [DOI] [PubMed] [Google Scholar]
- 17.Rogall, T., T. Flohr, and E. C. Bottger. 1990. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J. Gen. Microbiol. 136:1915-1920. [DOI] [PubMed] [Google Scholar]
- 18.Tsuyuguchi, K., K. Suzuki, H. Matsumoto, E. Tanaka, R. Amitani, and F. Kuze. 2001. Effect of estrogen on Mycobacterium avium complex pulmonary infection in mice. Clin. Exp. Immunol. 123:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turenne, C. Y., L. Tschetter, J. Wolfe, and A. Kabani. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J. Clin. Microbiol. 39:3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace, R. J., Jr., Y. S. Zhang, B. A. Brown, D. Dawson, D. T. Murphy, R. Wilson, and D. E. Griffith. 1998. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am. J. Respir. Crit. Care. Med. 158:1235-1244. [DOI] [PubMed] [Google Scholar]
- 21.Wallace, R. J., Jr., Y. S. Zhang, B. A. Brown-Elliott, M. A. Yakrus, R. W. Wilson, L. Mann, L. Couch, W. M. Girard, and D. E. Griffith. 2002. Repeat positive cultures in Mycobacterium intracellulare lung disease after macrolide therapy represent new infections in patients with nodular bronchiectasis. J. Infect. Dis. 186:266-273. [DOI] [PubMed] [Google Scholar]
- 22.Weusten, J. J., M. A. Blankenstein, F. H. Gmelig-Meyling, H. J. Schuurman, L. Kater, and J. H. Thijssen. 1986. Presence of estrogen receptors in human blood mononuclear cells and thymocytes. Acta Endocrinol. 112:409-414. [DOI] [PubMed] [Google Scholar]
- 23.Wittram, C., and G. L. Weisbrod. 2002. Mycobacterium avium complex lung disease in immunocompetent patients: radiography-CT correlation. Br. J. Radiol. 75:340-344. [DOI] [PubMed] [Google Scholar]
- 24.You, H. J., J. Y. Kim, and H. G. Jeong. 2003. 17β-Estradiol increases inducible nitric oxide synthase expression in macrophages. Biochem. Biophys. Res. Commun. 303:1129-1134. [DOI] [PubMed] [Google Scholar]