Abstract
Using an enzyme-linked immunosorbent assay we demonstrate that, in adult patients with typhoid fever, the sensitivity of a serological test based on the detection of anti-lipopolysaccharide immunoglobulin G is increased when used with paired serum samples taken 1 week apart.
Salmonella enterica subsp. enterica serovar Typhi is the causative agent of typhoid fever, a febrile, systemic illness common in regions of the world where sanitation is poor. Most patients with typhoid fever have a relatively mild disease, with fever, abdominal pain or discomfort, muscle and/or joint pain, and headache being among the most common symptoms. Some typhoid patients develop complications, the most severe of which is gastrointestinal hemorrhage and perforation. A prompt diagnosis of typhoid fever is essential if a patient is to receive appropriate antimicrobial therapy. The definitive laboratory diagnosis of typhoid fever is the isolation of serovar Typhi from a clinical specimen, although the Widal test, which detects raised TO (lipopolysaccharide O antigen [LPS]) and/or TH (flagella H antigen) antibody titers, is commonly used in areas where microbiological culture facilities are not available. In its original form, the Widal test required two serum samples (acute and convalescent), with a fourfold rise in serum anti-LPS (TO) or anti-flagella (TH) agglutinating antibody titer between the two samples being diagnostic of typhoid fever (1, 8, 12). More commonly, the test is used on a single, acute-phase serum sample (14), although several studies have reported that, when used in this way, the test lacks sensitivity and/or specificity in regions where typhoid is endemic (6, 13). This is presumably because not all individuals exposed to serovar Typhi develop a detectable agglutinating antibody response (3) and because elevated Widal TO and TH titers can be common in healthy subjects living in regions where typhoid is endemic (4, 9) and can be raised in patients infected with non-Typhi salmonellae (12, 15). There have been several studies, using enzyme-linked immunosorbent assays (ELISAs), which have attempted to identify an antibody-antigen combination that could form the basis of an alternative serological test to the Widal. These have largely looked for specific classes of anti-LPS antibodies in a single, acute-phase serum sample. Although these assays can have a higher sensitivity and/or specificity than the Widal TO test, none are entirely satisfactory (2, 6, 7, 10, 15). We have reported on the potential use of specific classes of serum antibodies against serovar Typhi LPS and flagella for the diagnosis of typhoid fever in a region where typhoid is endemic (7). In our previous study, we evaluated our assays using single, acute-phase serum samples; here we show that the sensitivity of a test based on the detection of anti-LPS immunoglobulin G (IgG) antibodies can be improved by using paired serum samples taken 6 days apart.
Sequential serum samples were collected from 52 adult patients with bacteriologically confirmed typhoid fever being enrolled into a treatment study at the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam (5). The study was approved by the Scientific and Ethical Committee of the hospital, and informed consent was obtained from all participants. The median (interquartile range; range) age and duration of illness (length of fever prior to admission) were 24 (19 to 32; 15 to 47) years and 10 (8 to 14; 2 to 30) days, respectively. Serum samples were collected before the start of (day 1) and during (days 4 and 7) treatment, and anti-LPS and anti-flagella IgG titers were determined by ELISA as reported previously (7). Sera were assayed in triplicate (two wells with antigen and one well without antigen), and the titer was taken as the highest dilution giving a net optical density (OD) (mean OD of wells with antigen minus OD of well without antigen) of ≥0.3. A standard serum sample with a known titer was included on each plate and the titer of the samples adjusted accordingly. Blank wells with no sera were included on all plates. The sensitivities of the diagnostic tests were calculated using the following formula: sensitivity = ELISA positive/total number of patients.
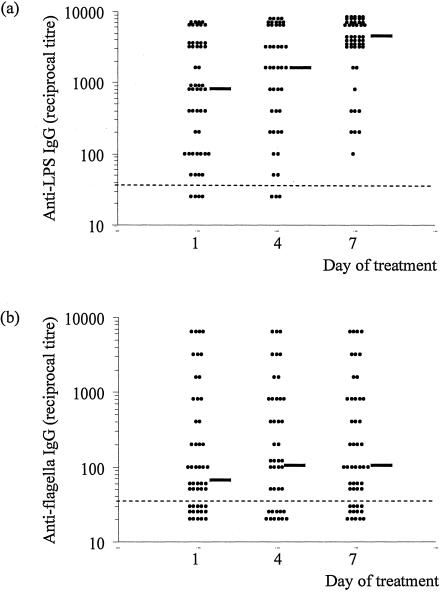
Previously we found that an anti-LPS IgG titer of ≥1/3,200 had a sensitivity (95% confidence interval [95% CI]) of 0.55 (0.43 to 0.67) and a specificity (95% CI) of 0.95 (0.89 to 1.00) (7). In the present study, an anti-LPS IgG titer of ≥1/3,200 was seen in only 19 of 52 sera taken on day 1 before treatment; however, this number had risen to 41 of 51 by day 7 of treatment. Thus, the sensitivity (95% CI) of the LPS IgG ELISA was 0.36 (0.23 to 0.49) on admission and 0.80 (0.69 to 0.91) on day 7. Of the 33 patients with a negative test (i.e., titer of <1/3,200) on day 1, a fourfold rise in titer between the first and the third serum sample was observed for 27 (81%). Thus, the sensitivity of the test would be 0.88 if a fourfold rise in titer was taken as diagnostic in patients who were negative at admission. The sensitivity of the flagella IgG ELISA did not increase over the study period (sensitivity of 0.24 on day 1 and 0.29 on day 7, using a titer of ≥1/800) (Fig. 1b). A fourfold rise in titer between the first and third serum sample was seen in only 5 of 42 patients (12%) with a day 1 titer of <1/3,200.
FIG. 1.
Serum anti-LPS IgG (a) and anti-flagella IgG (b) titers for adult patients with nonsevere typhoid fever. Samples were collected immediately before (day 1), during (day 4), and after (day 7) treatment. Points below the dashed line indicate a titer of <1/50. The median titer for each day is shown by a solid, black horizontal line.
Previous studies (7, 11, 13) have shown that serological tests for typhoid fever lack sensitivity and/or specificity when used on a single, acute-phase serum sample. In this study, we demonstrate that the sensitivity of a diagnostic test based on the detection of serum IgG antibodies against the LPS antigen can be improved when used with two serum samples taken ∼1 week apart. We selected a titer of >1/3,200 as being diagnostic of typhoid fever, as we have previously shown that this gives a test with a high specificity (0.95) (7). Olsen et al. (11) found that the sensitivities of several serological tests for typhoid fever were higher for patients in the second week of illness than for those in the first or the third week. Two of these tests, TUBEX and the Widal, detect anti-LPS (O9) IgM, suggesting that titers of these antibodies decline later in the illness. In our study, the majority of patients (75%) had been ill for at least 8 days on admission to the study, i.e., were in the second week of illness. Even so, we were able to detect a rising anti-LPS IgG titer 1 week later, suggesting that this response may be elicited later in the disease. Taken together, our data and those of Olsen et al. suggest that different antibody-antigen combinations may be required at different stages of the disease for the optimal serodiagnosis of typhoid fever. A test based on paired serum samples may prove useful under certain circumstances, for example, in patients where typhoid fever is strongly suspected but whose admission titer is low and blood culture is either negative or not available, or for epidemiological studies.
Acknowledgments
We thank the staff and directors of the Centre for Tropical Diseases for their help.
This study was funded by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Anonymous. 1978. Typhoid and its serology. Br. Med. J. 1:389-390. [PMC free article] [PubMed] [Google Scholar]
- 2.Beasley, W. J., S. W. Joseph, and E. Weiss. 1981. Improved serodiagnosis of Salmonella enteric fevers by an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 13:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie, J. 1977. Antibodies in the Aberdeen typhoid outbreak of 1964. I. The Widal reaction. J. Hyg. Camb. 79:161-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherian, T., G. Sridharan, V. Mohandas, and T. Jacob John. 1990. Prevalence of Salmonella Typhi O and H antibodies in the serum of infants and pre-school children. Indian Pediatr. 27:293-294. [PubMed] [Google Scholar]
- 5.Chinh, N. T., C. M. Parry, N. T. Ly, H. D. Ha, M. X. Thong, T. S. Diep, J. Wain, N. J. White, and J. J. Farrar. 2000. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob. Agents Chemother. 44:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschl, A., G. Stanek, M. Rotter, A. H. Niemetz, and G. Diridl. 1983. Comparison of ELISA (lipopolysaccharide) and Widal reaction (O antigen) in diagnosis of Salmonella infections. Zentbl. Bakteriol. Mikrobiol. Hyg. A 255:247-257. (In German.) [PubMed] [Google Scholar]
- 7.House, D., J. Wain, V. A. Ho, T. S. Diep, N. T. Chinh, P. V. Bay, H. Vinh, M. Duc, C. M. Parry, G. Dougan, N. J. White, T. T. Hien, and J. J. Farrar. 2001. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J. Clin. Microbiol. 39:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huckstep, R. L. 1962. Typhoid fever and other salmonella infections. E & S Livingstone Ltd., London, United Kingdom.
- 9.Levine, M. M., O. Grandos, R. H. Gilman, W. E. Woodward, R. Solis-Plaza, and W. Waldman. 1978. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am. J. Trop. Med. Hyg. 27:795-800. [DOI] [PubMed] [Google Scholar]
- 10.Nardiello, S., T. Pizzella, M. Russo, and B. Galanti. 1984. Serodiagnosis of typhoid fever by enzyme-linked immunosorbent assay determination of anti-Salmonella typhi lipopolysaccharide antibodies. J. Clin. Microbiol. 20:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen, S. J., J. Pruckler, W. Bibb, N. T. M. Thanh, T. M. Trinh, N. T. Minh, S. Sivapalasingam, A. Gupta, P. T. Phuong, N. T. Chinh, N. V. Chau, P. D. Cam, and E. D. Mintz. 2004. Evaluation of rapid diagnostic tests for typhoid fever. J. Clin. Microbiol. 42:1885-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang, T., and S. D. Puthucheary. 1989. False-positive Widal test in nontyphoid salmonella infections. Southeast Asian J. Trop. Med. Public Health 20:163-164. [PubMed] [Google Scholar]
- 13.Parry, C. M., N. T. T. Hoa, T. S. Diep, J. Wain, N. T. Chinh, H. Vinh, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J. Clin. Microbiol. 37:2882-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder, S. A. 1968. Interpretation of serological tests for typhoid fever. JAMA 206:839-840. [PubMed] [Google Scholar]
- 15.Sippel, J., N. Bukhtiari, M. B. Awan, R. Kreig, J. F. Duncan, K. A. Karamat, I. A. Malik, L. M. Igbal, and L. Legters. 1989. Indirect immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) and IgM capture ELISA for detection of antibodies to lipopolysaccharide in adult typhoid fever patients in Pakistan. J. Clin. Microbiol. 27:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]