This cohort study examines the occurrence of diabetic retinopathy, ischemic optic nerve disease, or other ocular complications within 2 years of glucagon-like peptide-1 receptor agonist (GLP-1 RA) use among patients with type 2 diabetes.
Key Points
Question
Is the use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in patients with type 2 diabetes (T2D) associated with an increased risk of sight-threatening diabetic retinopathy (DR) or nonarteritic anterior ischemic optic neuropathy (NAION)?
Findings
This cohort study of 185 066 individuals prescribed treatment with GLP-1 RAs revealed an association with slightly higher risk of developing incident DR but a similar incidence of NAION and fewer serious DR-associated complications, including new-onset blindness.
Meaning
These findings suggest that all patients with T2D treated with GLP-1 RAs, regardless of preexisting DR, should receive regular screening and monitoring for potential complications of T2D.
Abstract
Importance
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are associated with increased risk of diabetic retinopathy (DR) and nonarteritic anterior ischemic optic neuropathy (NAION). The risk of sight-threatening complications associated with GLP-1 RAs is underexamined.
Objective
To investigate whether the use of GLP-1 RAs in patients with T2D is associated with the development of DR, NAION, or DR complications.
Design, Setting, and Participants
This retrospective cohort study of adults (aged ≥18 years) with T2D and a recent hemoglobin A1c level of 6.5% or higher was conducted between January 1, 2015, and September 30, 2022, using the TriNetX database. The cohort was divided into 2 groups, adjusted for baseline characteristics through propensity score matching (PSM), based on whether the individuals received prescriptions for a GLP-1 RA. The statistical analysis was conducted on October 10, 2024.
Exposures
At least 2 prescriptions of a GLP-1 RA given 6 months apart.
Main Outcomes and Measures
Cox proportional hazard regression models were used to evaluate the primary outcome: association between GLP-1 RAs and the risk of incident DR, NAION, or sight-threatening complications over a 2-year follow-up period.
Results
After PSM, 185 066 individuals (mean [SD] age, 59.0 [12.5] years; 93 389 females [50.5%]) were prescribed GLP-1 receptor agonists. Use of GLP-1 RAs was associated with an increased incidence of DR (hazard ratio [HR], 1.07; 95% CI, 1.03-1.11), while no statistically significant difference was observed in the risk of NAION (HR, 1.26; 95% CI, 0.94-1.70). In a subgroup analysis of 32 695 patients with preexisting DR, GLP-1 RAs were not associated with progression to proliferative DR (HR, 1.06; 95% CI, 0.97-1.15) or diabetic macular edema (HR, 0.98; 95% CI, 0.95-1.01) but were associated with a lower occurrence of vitreous hemorrhages (HR, 0.74; 95% CI, 0.68-0.80), neovascular glaucoma (HR, 0.78; 95% CI, 0.68-0.88), or blindness (HR, 0.77; 95% CI, 0.73-0.82).
Conclusions and Relevance
In this cohort study of individuals with T2D, GLP-1 RA use was associated with a modestly increased risk of incident DR; however, fewer patients experienced sight-threatening DR complications, including blindness, even among those with preexisting DR. These findings suggest that all patients with T2D treated with GLP-1 RAs, regardless of preexisting DR, should be regularly screened and monitored for potential complications of T2D.
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are an important class of antihyperglycemic medications approved by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D). They have been highly effective in helping patients with T2D meet glycemic targets,1 achieve substantial weight loss,2 and improve cardiometabolic and kidney outcomes in high-risk subgroups.3,4,5,6,7 As a result, the American Diabetes Association recommends GLP-1 RAs as 1 of 2 possible first-line therapies for patients with T2D who have, or are at high risk for, atherosclerotic cardiovascular disease.8
Despite the effectiveness of GLP-1 RAs in reducing glycemic levels and improving hemoglobin A1c (HbA1c) levels, some concerning adverse effects have been reported.1,2,3,4 Randomized clinical trials and observational studies have linked GLP-1 RAs with the development of diabetic retinopathy (DR)5,9 and an increased risk of nonarteritic anterior ischemic optic neuropathy (NAION) within the first year of use.10 However, a recent meta-analysis of randomized clinical trials suggests that GLP-1 RAs may not increase the risk of DR.11 Furthermore, a large cohort study of patients with T2D, with and without obesity, did not find an increased risk of NAION associated with these agents.12 A major limitation of prior studies of GLP-1 RAs in patients with T2D is that they did not specifically assess for sight-threatening complications from DR, which account for most of the long-term ocular morbidity associated with T2D. This retrospective cohort study aimed to investigate whether GLP-1 RA use is associated with the development of DR, NAION, or DR complications in patients with T2D.
Methods
The Lahey Hospital and Medical Center Institutional Review Board deemed this cohort study exempt from ethics review and the informed consent requirement since the analysis used aggregate, anonymized data from a research network database. The study findings are reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source
We used the TriNetX database, a multicenter federated health research network that aggregates anonymized data from electronic health records (EHRs) drawn from more than 120 participating health care organizations, including academic medical centers, specialty physician practices, and community hospitals, covering approximately 275 million patients. While the data are in aggregate and deidentified, the built-in analytics of the database enable cohort selection and matching, analysis of the incidence and prevalence of events within a cohort, and comparison of characteristics and outcomes between matched cohorts. More information can be found on the TriNetX website.13,14
Race and ethnicity were self-identified in the EHRs. The categories we used in matching cohorts were Asian, Black or African American, Hispanic or Latino, not Hispanic or Latino, White, and unknown. Race and ethnicity data were collected because of their potential implications for the rate of GLP-1 RA prescriptions or study outcomes.
Population and Design
Between January 1, 2015, and September 30, 2022, we conducted a retrospective cohort study of patients aged 18 years or older with T2D and a recent HbA1c of 6.5% or higher identified from the TriNetX database (to convert HbA1c to proportion of total hemoglobin, multiply by 0.01). Patients were sorted into 2 cohorts based on whether they had received a prescription for a GLP-1 RA (semaglutide, lixisenatide, tirzepatide, dulaglutide, liraglutide, or exenatide): those with GLP-1 RA prescriptions were considered the treatment group, whereas those without GLP-1 RA prescriptions were considered the control group. We included only patients with at least 2 GLP-1 RA prescriptions given at least 6 months apart. The index event was defined as the date of the second GLP-1 RA prescription for the treatment group and as the date of meeting the inclusion criteria for the control group. Individuals with more than 2 prescriptions were included in the treatment group based on the query criteria. Those with only 1 prescription or 2 prescriptions given less than 6 months apart were excluded. Cohorts were matched using propensity scores for clinically relevant variables (eAppendix and eTable 1 in Supplement 1). Outcomes were assessed within a 2-year window after the index event.
End Points
The primary end points were the association between GLP-1 RAs and the risk of incident DR, NAION, or sight-threatening complications over a 2-year follow-up period. The study quantified the incidence of diabetic eye disease and other ischemic retinal vascular disorders, including incident DR, neovascular glaucoma, and NAION, by using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) or Current Procedural Terminology codes in the 2 cohorts (eTable 2 in Supplement 1). A subgroup analysis was conducted on individuals with a prior diagnosis of DR to assess the risk of disease progression to proliferative diabetic retinopathy (PDR), the development of a vitreous hemorrhage, or new-onset diabetic macular edema (DME). We also assessed whether patients with preexisting DR received treatment with agents targeting vascular endothelial growth factor (VEGF) or, more broadly, with intravitreal injections, laser panretinal photocoagulation, or retinal surgery via vitrectomy either individually or as a composite treatment outcome. Additionally, we examined the incidence of new-onset blindness, including cases coded as low vision or legal blindness, in both the entire cohort and the subset of patients with prior DR.
Statistical Analysis
Continuous variables are presented as mean (SD), and categorical variables are presented as number (%). One-to-one propensity score matching (PSM) was performed using greedy nearest-neighbor matching with a caliper of 0.1 times the pooled SD of the linear propensity scores to control for baseline differences between the study groups. The standard mean difference is a quantitative method used to represent the difference between the means of 2 groups in terms of SD units; it assesses the balance in measured variables in a sample weighted by the inverse probability of treatment. The variables were chosen according to their potential association with the study outcomes selected.
After PSM, outcomes were compared between the 2 cohorts using absolute and relative risk difference. Kaplan-Meier curves and Cox proportional hazards regression models were used for survival analysis. Statistical significance was set at a 2-sided P < .05. Statistical analyses were performed on October 10, 2024, using an integrated R, version 4.0.3 (R Project for Statistical Computing) on the TriNetX platform.
To strengthen the reliability of the observational data, we evaluated falsification outcomes, such as the incidence of appendicitis, within the same 2-year follow-up time frame for the GLP-1 RA and comparator groups. We also calculated E-values for primary and secondary outcomes to assess potential confounding from unmeasured factors. Higher E-values indicate that unmeasured confounders with a greater influence on the outcome of interest would be required to negate the observed association between exposure and outcome.
Recognizing that a longer duration of known diabetes was associated with an increased risk of incident DR, we accounted for the duration of T2D by comparing the incidence of diabetic eye disease in individuals with GLP-1 RA prescriptions who had a diagnosis of T2D within less than 10 years vs greater than 10 years after the index event.15 A duration of 10 years was selected because previous studies have shown a direct association between HbA1c levels and 10-year incidence of DR.16 To ensure that the severity of DR did not confound the study results for the preexisting DR subgroup, we did a look-back on individuals with vs without GLP-1 RA prescriptions to assess the number of individuals with varying severity of DR at baseline.
Results
Baseline Characteristics
After PSM, 185 066 individuals were prescribed GLP-1 RAs. These patients had a mean (SD) age of 59.0 (12.5) years and included 93 389 females (50.5%) and 91 677 males (49.5%), with 6070 individuals who identified as Asian (3.3%), 33 306 as Black or African American (18.0%), 17 891 as Hispanic or Latino (9.7%), 128 515 as not Hispanic or Latino (69.4%), and 113 256 as White (61.2%) (Table 1). The matched control group of individuals without GLP-1 RA prescriptions had a mean (SD) age of 59.3 (13.9) years and included 93 757 females (50.7%) and 91 309 males (49.3%), with 5716 individuals who identified as Asian (3.1%), 33 209 as Black or African American (17.9%), 17 693 as Hispanic (9.6%), 128 769 as not Hispanic or Latino (69.6%), and as 114 315 White (61.8%). The ICD-10 and Veterans Affairs health care–specific codes for these baseline characteristics are presented in eTable 1 in Supplement 1.
Table 1. Baseline Characteristics of Patients With Type 2 Diabetes Before and After Propensity Score Matching.
| Characteristic | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| With GLP-1 RA prescriptions, No. (%) (n = 280 593) | Without GLP-1 RA prescriptions, No. (%) (n = 1 399 684) | Standardized difference | With GLP-1 RA prescriptions, No. (%) (n = 185 066) | Without GLP-1 RA prescriptions, No. (%) (n = 185 066) | Standardized difference | |
| Demographics | ||||||
| Age, mean (SD), y | 58.0 (12.3) | 61.9 (15.1) | 0.277 | 59.0 (12.5) | 59.3 (13.9) | 0.028 |
| Sex | ||||||
| Female | 145 019 (51.7) | 613 628 (43.8) | 0.157 | 93 389 (50.5) | 93 757 (50.7) | 0.004 |
| Male | 135 574 (48.3) | 786 056 (56.2) | 0.139 | 91 677 (49.5) | 91 309 (49.3) | 0.008 |
| Race and ethnicitya | ||||||
| Asian | 8088 (2.9) | 83 142 (5.9) | 0.149 | 6070 (3.3) | 5716 (3.1) | 0.011 |
| Black or African American | 50 182 (17.9) | 231 167 (16.5) | 0.036 | 33 306 (18.0) | 33 209 (17.9) | 0.001 |
| Hispanic or Latino | 27 154 (9.7) | 139 875 (10.0) | 0.011 | 17 891 (9.7) | 17 693 (9.6) | 0.004 |
| Not Hispanic or Latino | 197 797 (70.5) | 922 855 (65.9) | 0.098 | 128 515 (69.4) | 128 769 (69.6) | 0.003 |
| White | 174 381 (62.1) | 787 430 (56.3) | 0.120 | 113 256 (61.2) | 114 315 (61.8) | 0.012 |
| Comorbidities | ||||||
| Dyslipidemia | 240 406 (85.7) | 687 772 (49.1) | 0.847 | 151 500 (81.9) | 153 450 (82.9) | 0.028 |
| Hypertension | 236 233 (84.2) | 822 105 (58.7) | 0.588 | 151 086 (81.6) | 153 047 (82.7) | 0.028 |
| Ischemic heart diseases | 70 524 (25.1) | 299 960 (21.4) | 0.088 | 46 973 (25.4) | 47 746 (25.8) | 0.010 |
| Atrial fibrillation or flutter | 23 156 (8.3) | 125 265 (8.9) | 0.025 | 16 313 (8.8) | 16 582 (9.0) | 0.005 |
| Stroke | 14 784 (5.3) | 73 803 (5.3) | 0.001 | 10 337 (5.6) | 10 384 (5.6) | 0.001 |
| Peripheral vascular diseases | 8501 (3.0) | 29 879 (2.1) | 0.056 | 5735 (3.1) | 5972 (3.2) | 0.007 |
| CKD | 55 081 (19.6) | 199 192 (14.2) | 0.144 | 35 718 (19.3) | 36 617 (19.8) | 0.012 |
| COPD | 82 728 (29.5) | 230 628 (16.5) | 0.313 | 50 525 (27.3) | 50 917 (27.5) | 0.005 |
| Benign neoplasms | 84 306 (30.0) | 167 466 (12.0) | 0.455 | 48 855 (26.4) | 48 782 (26.4) | 0.001 |
| Malignant neoplasms | 109 702 (39.1) | 310 278 (22.2) | 0.374 | 66 903 (36.2) | 66 823 (36.1) | 0.001 |
| Personal history of nicotine dependence | 46 852 (16.7) | 125 620 (9.0) | 0.232 | 26 969 (14.6) | 26 916 (14.5) | 0.001 |
| Tobacco use | 18 287 (6.5) | 38 178 (2.7) | 0.181 | 9711 (5.2) | 9543 (5.2) | 0.004 |
| Medication use | ||||||
| Insulin | 178 341 (63.6) | 501 520 (35.8) | 0.577 | 108 236 (58.5) | 109 517 (59.2) | 0.014 |
| Metformin | 234 768 (83.7) | 444 171 (31.7) | 1.236 | 143 999 (77.8) | 146 838 (79.3) | 0.037 |
| Glipizide | 73 495 (26.2) | 121 824 (8.7) | 0.474 | 40 933 (22.1) | 41 613 (22.5) | 0.009 |
| Sitagliptin | 65 279 (23.3) | 84 857 (6.1) | 0.501 | 32 739 (17.7) | 32 426 (17.5) | 0.004 |
| Empagliflozin | 60 058 (21.4) | 20 251 (1.4) | 0.661 | 14 558 (7.9) | 13 168 (7.1) | 0.029 |
| Canagliflozin | 25 510 (9.1) | 9942 (0.7) | 0.396 | 6808 (3.7) | 6117 (3.3) | 0.020 |
| Glyburide | 19 498 (6.9) | 41 677 (3.0) | 0.184 | 12 361 (6.7) | 12 817 (6.9) | 0.010 |
| Dapagliflozin | 25 635 (9.1) | 11 995 (0.9) | 0.387 | 7311 (4.0) | 6673 (3.6) | 0.018 |
| Tirzepatide | 4387 (1.6) | 0 | 0.178 | 359 (0.2) | 0 | 0.062 |
| Antilipemic agents | 231 637 (82.6) | 597 510 (42.7) | 0.904 | 145 314 (78.5) | 147 711 (79.8) | 0.032 |
| Loop diuretics | 64 241 (22.9) | 222 373 (15.9) | 0.178 | 42 316 (22.9) | 43 257 (23.4) | 0.012 |
| Spironolactone | 24 483 (8.7) | 54 878 (3.9) | 0.198 | 14 223 (7.7) | 14 156 (7.6) | 0.001 |
| ACE inhibitors | 149 701 (53.4) | 380 045 (27.2) | 0.554 | 93 915 (50.7) | 96 051 (51.9) | 0.023 |
| Angiotensin II inhibitors | 97 349 (34.7) | 226 471 (16.2) | 0.435 | 58 249 (31.5) | 58 529 (31.6) | 0.003 |
| Sacubitril | 3859 (1.4) | 6857 (0.5) | 0.092 | 1868 (1.0) | 1797 (1.0) | 0.004 |
| Antineoplastics | 24 701 (8.8) | 64 302 (4.6) | 0.169 | 14 966 (8.1) | 14 984 (8.1) | 0.001 |
| Laboratory values | ||||||
| LDL cholesterol, mean (SD), mg/dL | 85.6 (36.7) | 94.0 (39.2) | 0.221 | 85.7 (36.4) | 93.2 (39.7) | 0.198 |
| BNP ≥150 pg/mL | 37 469 (13.4) | 138 826 (9.9) | 0.045 | 23 307 (12.6) | 26 144 (14.1) | 0.045 |
| NT-proBNP ≥450 pg/mL | 22 626 (8.1) | 76 539 (5.5) | 0.103 | 13 925 (7.5) | 14 930 (8.1) | 0.020 |
| HbA1c ≥6.5% | 278 326 (99.2) | 534 952 (38.2) | 1.745 | 183 229 (99.0) | 145 874 (78.8) | 0.185 |
| HbA1c, mean (SD), % | 8.0 (1.9) | 7.7 (2.0) | 0.180 | 7.9 (1.9) | 7.6 (1.9) | 0.128 |
| Iron, mean (SD), μg/dL | 67.0 (35.4) | 64.6 (41.7) | 0.062 | 67.4 (35.4) | 65.1 (39.1) | 0.060 |
| CRP, mean (SD), mg/L | 23.5 (47.5) | 37.7 (65.5) | 0.248 | 23.7 (47.7) | 29.4 (55.6) | 0.109 |
| LVEF, mean (SD), % | 59.2 (12.1) | 56.3 (14.7) | 0.222 | 59.6 (11.7) | 56.1 (14.7) | 0.258 |
| BMI ≥30 | 141 429 (50.4) | 363 942 (26.0) | 0.519 | 86 360 (46.7) | 87 834 (47.5) | 0.016 |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–brain natriuretic peptide; PSM, propensity score matching.
SI conversion factor: To convert BNP to nanogram per liter, multiply by 1.0; CRP to milligram per liter, multiply by 10; HbA1c to proportion of total hemoglobin, multiply by 0.01; iron to micromole per liter, multiply by 0.179; LDL cholesterol to millimoles per liter, multiply by 0.0259.
Race and ethnicity were self-reported in the organizational electronic health records and obtained from TriNetX database.
Before PSM, individuals with GLP-1 RA prescriptions were younger; female; identified as not Hispanic or Latino or White; had higher rates of hypertension, dyslipidemia, and ischemic heart disease; and greater use of insulin, metformin, glipizide, sodium-glucose cotransporter 2 inhibitors, and renin-angiotensin-aldosterone system inhibitors compared with individuals without GLP-1 RA prescriptions. Following PSM, the 2 cohorts were well-matched for demographics, comorbidities, baseline medication use, and laboratory values, as indicated by a standardized difference of less than 0.1 for most baseline characteristics included in the matching process (Table 1; eMethods in Supplement 1).
Incidence of DR and NAION
During the 2-year follow-up period, 5037 (2.7%) individuals in the GLP-1 RA group developed incident DR compared with 4938 (2.7%) individuals in the group without GLP-1 RA prescriptions (n = 185 066 per group; HR, 1.07; 95% CI, 1.03-1.11; P = .001) (Table 2). The risk of NAION was modestly higher among the treatment group than the control group (96 [0.1%] vs 79 [<0.1%] events; HR, 1.26 [95% CI, 0.94-1.70]; P = .12), as was the risk of a broader set of ischemic optic nerve conditions (243 [0.1%] vs 230 [0.1%] events; HR, 1.10 [95% CI, 0.92-1.32]; P = .31). However, these differences were not statistically significant, and the wide CIs suggested imprecision due to limited event counts. The incidence of all-cause blindness was less often coded for individuals with GLP-1 RA prescriptions compared with those without GLP-1 RA prescriptions (2313 [1.2%] vs 3051 [1.6%] events; HR, 0.77 [95% CI, 0.73-0.82]; P < .001), a 24.2% relative reduction in the risk of blindness.
Table 2. Comparison of Ocular Complications Among Patients With T2D With vs Without GLP-1 RA Prescriptions.
| Outcomea | T2D with GLP-1 RA prescriptions, No. (%) (n = 185 066) | T2D without GLP-1 RA prescriptions, No. (%) (n = 185 066) | RD (95% CI) | RRR, % | HR (95% CI) | P value | E-value for HR | E-value for lower CI of HR |
|---|---|---|---|---|---|---|---|---|
| DR | 5037 (2.7) | 4938 (2.7) | 0.001 (−0.000 to 0.002) | −2.00 | 1.07 (1.03 to 1.11) | .001 | 1.35 | 1.21 |
| NAION | 96 (0.1) | 79 (0.0) | 0.000 (−0.000 to 0.000) | −21.50 | 1.26 (0.94 to 1.70) | .12 | 1.84 | 1.33 |
| Neovascular glaucoma | 446 (0.2) | 559 (0.3) | −0.001 (−0.001 to −0.000) | 20.21 | 0.82 (0.73 to 0.93) | .002 | 1.72 | 2.09 |
| Blindness | 2313 (1.2) | 3051 (1.6) | −0.004 (−0.005 to −0.003) | 24.19 | 0.77 (0.73 to 0.82) | <.001 | 1.91 | 2.07 |
Abbreviations: DR, diabetic retinopathy; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; NAION, nonarteritic anterior ischemic optic neuropathy; RD, risk difference; RRR, relative risk reduction; T2D, type 2 diabetes.
After propensity matching.
Risk of DR Progression and Sight-Threatening Complications
We evaluated the risk of retinopathy progression, sight-threatening complications, and DR treatments in a subgroup of patients with T2D and preexisting DR at the time of GLP-1 RA prescription. Initially, 44 241 individuals with DR received GLP-1 RA prescriptions, while 124 760 individuals did not. After PSM, 32 695 individuals remained in each group and were well-matched for demographics, comorbidities, baseline medication use, and laboratory values (Table 3).
Table 3. Comparison of Ocular Complications and Other Outcomes Among Patients With Preexisting Diabetic Retinopathy With vs Without GLP-1 RA Prescriptions .
| Outcomea | Preexisting DR with GLP-1 RA prescriptions (n = 32 695), No. (%) | Preexisting DR without GLP-1 RA prescriptions (n = 32 695), No. (%) | RD (95% CI) | RRR, % | HR (95% CI) | P value | E-value for HR | E-value for lower CI of HR |
|---|---|---|---|---|---|---|---|---|
| PDR | 1121 (3.4) | 1039 (3.2) | 0.002 (−0.001 to 0.005) | −7.89 | 1.06 (0.97 to 1.15) | .18 | 1.31 | 1.19 |
| DME | 7390 (22.6) | 7425 (22.7) | −0.001 (−0.007 to 0.005) | 0.47 | 0.98 (0.95 to 1.01) | .25 | 1.12 | 1.05 |
| Neovascular glaucoma | 434 (1.3) | 562 (1.7) | −0.004 (−0.006 to −0.002) | 22.78 | 0.78 (0.68 to 0.88) | <.001 | 1.90 | 2.28 |
| NAION | 34 (0.1) | 38 (0.1) | −0.000 (−0.001 to 0.000) | 10.53 | 0.91 (0.57 to 1.45) | .69 | 1.43 | 2.89 |
| Vitreous hemorrhage | 1135 (3.5) | 1542 (4.7) | −0.012 (−0.015 to −0.009) | 26.39 | 0.74 (0.68 to 0.80) | <.001 | 2.05 | 2.28 |
| Panretinal laser photocoagulation | 698 (2.1) | 894 (2.7) | −0.006 (−0.008 to −0.004) | 21.92 | 0.79 (0.71 to 0.87) | <.001 | 1.86 | 2.16 |
| Vitrectomy | 445 (1.4) | 722 (2.2) | −0.008 (−0.010 to −0.006) | 38.40 | 0.62 (0.55 to 0.70) | <.001 | 2.61 | 3.03 |
| Treatment with anti-VEGF medications | 296 (0.9) | 355 (1.1) | −0.002 (−0.003 to −0.000) | 16.62 | 0.84 (0.72 to 0.98) | .03 | 1.66 | 2.12 |
| Composite treatmentb | 2341 (7.2) | 2547 (7.8) | −0.006 (−0.010 to −0.002) | 8.10 | 0.92 (0.87 to 0.97) | .003 | 1.40 | 1.57 |
| Blindness | 1280 (3.9) | 1833 (5.6) | −0.016 (−0.020 to −0.013) | 30.17 | 0.70 (0.65 to 0.75) | <.001 | 2.22 | 2.45 |
Abbreviations: DME, diabetic macular edema; DR, diabetic retinopathy; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; NAION, nonarteritic anterior ischemic optic neuropathy; PDR, proliferative diabetic retinopathy; RD, risk difference; RRR, relative risk reduction; VEGF, vascular endothelial growth factor.
After propensity matching.
Composite treatment consists of treatment of DR with anti-VEGF agents, panretinal laser photocoagulation, or vitrectomy.
Treatment with GLP-1 RAs was not associated with progression to PDR (1121 of 32 695 [3.4%] vs 1039 of 32 695 [3.2%] events; HR, 1.06 [95% CI, 0.97-1.15]; P = .18) or new-onset DME (7390 [22.6%] vs 7425 [22.7%] events; HR, 0.98 [95% CI, 0.95-1.01]; P = .25) (Table 3). There was also a reduced occurrence of vitreous hemorrhage (1135 [3.5%] vs 1542 [4.7%] events; HR, 0.74 [95% CI, 0.68-0.80]; P < .001) and neovascular glaucoma (434 [1.3%] vs 562 [1.7%] events; HR, 0.78 [95% CI, 0.68-0.88]; P < .001) as well as a reduced need for treatment with anti-VEGF inhibitors (296 [0.9%] vs 355 [1.1%] events; HR, 0.84 [95% CI, 0.72-0.98]; P = .03), panretinal laser photocoagulation (698 [2.1%] vs 894 [2.7%] events; HR, 0.79 [95% CI, 0.71-0.87]; P < .001), a vitreoretinal procedure for DR (445 [1.4%] vs 722 [2.2%] events; HR, 0.62 [95% CI, 0.55-0.70]; P < .001), or composite treatment involving any of these procedures (2341 [7.2%] vs 2547 [7.8%] events; HR, 0.92 [95% CI, 0.87-0.97]; P = .003).
In this subgroup (Figure), there was no difference in the rate of ischemic optic neuropathy between groups (34 [0.1%] vs 38 [0.1%] events; HR, 0.91 [95% CI, 0.57-1.45]; P = .69), even when more broadly defined (78 [0.2%] vs 96 [0.3%] events; HR, 0.82 [95% CI, 0.61-1.11]; P = .21). The incidence of all-cause blindness was even less often coded in those patients who had preexisting DR and received GLP-1 RA prescriptions compared with those without GLP-1 RA prescriptions (1280 [3.9%] vs 1833 [5.6%] events; HR, 0.70 [95% CI, 0.65-0.75], P < .001; Z = −2.166, P = .02), a 30.2% relative risk reduction in blindness.
Figure. Ophthalmic End Points and Development of Sight-Threatening Complications in Patients With Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) Prescriptions.
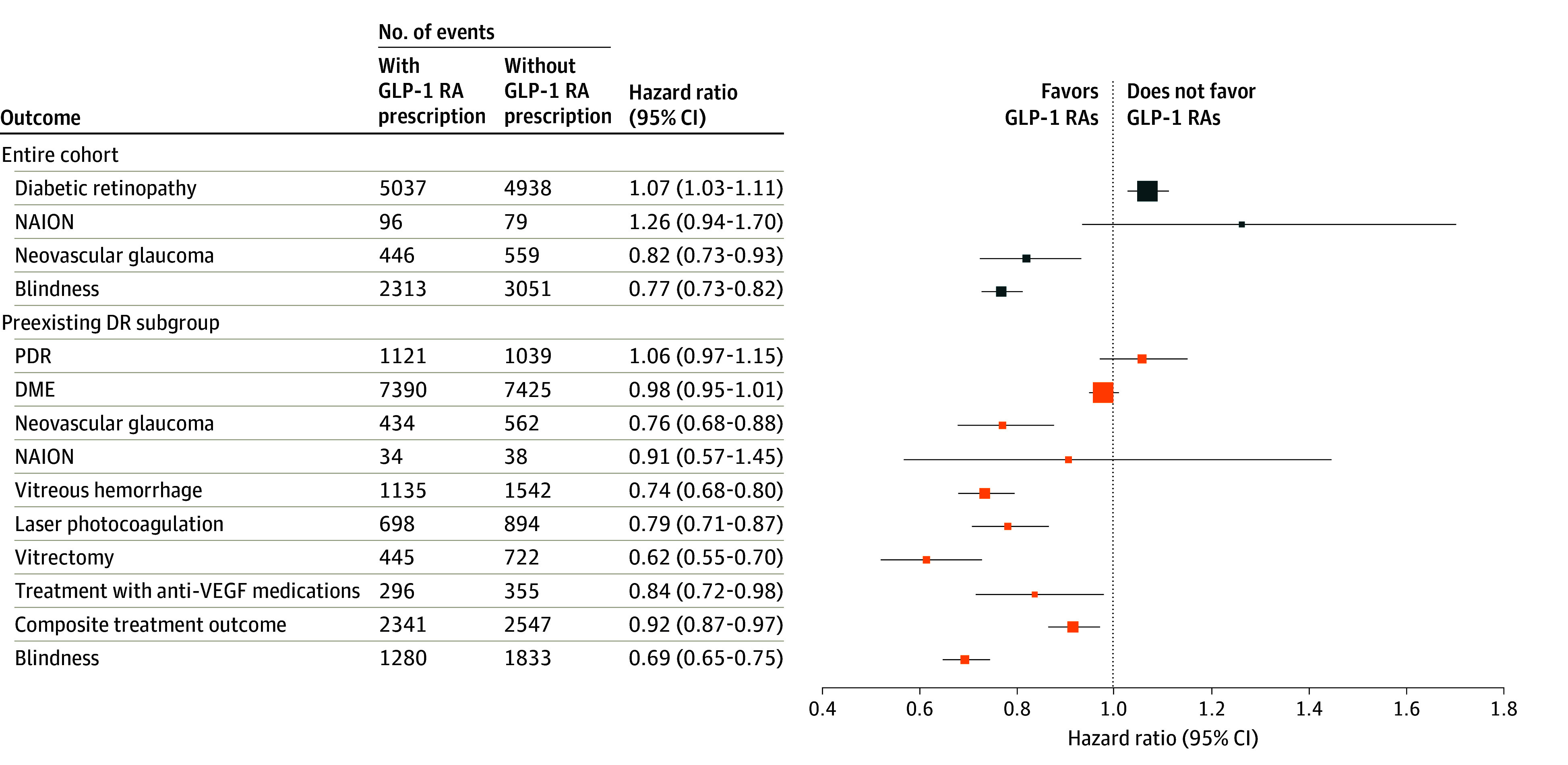
Outcomes in the entire cohort (n = 185 066 individuals per group) are shown in blue, while outcomes for the subgroup with preexisting diabetic retinopathy (DR) (n = 32 695 individuals per group) are shown in orange. Marker size is proportional to the mean total number of events in each category. DME indicates diabetic macular edema; NAION, nonarteritic anterior ischemic optic neuropathy; PDR, proliferative diabetic retinopathy; and VEGF, vascular endothelial growth factor.
Sensitivity Analyses
The falsification outcome of appendicitis during the follow-up period showed no difference between the treatment and control cohorts, regardless of preexisting DR (Table 4). By contrast, the higher E-values for the study outcomes, shown in Table 2 and Table 3, suggest that substantial unmeasured confounding would be required to fully explain the observed associations. In our additional analyses of ophthalmologic end points among the treatment group, both the well-matched cohorts (n = 68 424 per group) with a T2D diagnosis less than 10 years vs greater than 10 years after the index event showed similar incidence of diabetic eye disease over a 2-year follow-up (eg, DME: 5127 [7.5%] vs 5185 [7.6%] events; HR, 0.99 [95% CI, 0.95-1.03]; P = .59) (eTable 3 in Supplement 1), indicating that the role of the duration of diabetes in our study outcomes was relatively negligible. In the preexisting DR subgroup, the severity of diabetic retinopathy was similar between individuals with and without GLP-1 RA prescriptions at baseline after PSM (severe: 1537 [4.7%] vs 1569 [4.8%]; standard mean difference, 0.001) (eTable 4 in Supplement 1).
Table 4. Comparison of Falsification Outcome Among Patients With Type 2 Diabetes and With or Without Preexisting Diabetic Retinopathy With vs Without GLP-1 RA Prescriptions .
| Outcomea | With GLP-1 RA prescriptions, No. (%) (n = 185 066) | Without GLP-1 RA prescriptions, No. (%) (n = 185 066) | RD (95% CI) | RRR, % | HR (95% CI) | P value | E-value for HR |
|---|---|---|---|---|---|---|---|
| Appendicitis in entire cohort | 389 (0.2) | 400 (0.2) | −0.000 (−0.000 to 0.000) | 2.75 | 1.00 (0.87 to 1.15) | .99 | 1.03 |
| Appendicitis in a subgroup with preexisting DR | 108 (0.1) | 112 (0.1) | −0.000 (−0.001 to 0.001) | 3.57 | 0.98 (0.75 to 1.28) | .88 | 1.17 |
Abbreviations: DR, diabetic retinopathy; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; RD, risk difference; RRR, relative risk reduction.
After propensity matching.
Discussion
To our knowledge, the findings of this study provide some of the first evidence that, among individuals with T2D, the use of GLP-1 RAs was associated with a lower risk of sight-threatening complications, including PDR, DME, and neovascular glaucoma, despite a marginally increased incidence of any DR. Among individuals with preexisting DR, GLP-1 RAs were not associated with progression to PDR and new-onset DME but associated with reduced incidence of vitreous hemorrhage and neovascular glaucoma. The risk of ischemic optic nerve disease, including NAION, was similar for individuals with and without GLP-1 RA prescriptions and with or without preexisting DR. We also found that those with GLP-1 RA prescriptions had a reduced need for medical, surgical, or laser-based interventions and a lower incidence of all-cause blindness regardless of preexisting DR.
Our clinical analysis revealed a modest increase in the incidence of DR but a lower risk of progression to sight-threatening disease in patients with preexisting DR. Prior studies of incretin mimetics have produced mixed results regarding their role in diabetic eye disease complications. No safety signals related to DR were reported in the EXSCEL (Exenatide Study of Cardiovascular Event Lowering) and the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trials.17,18 In contrast, the SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) series of phase 3 trials found a small increased risk of DR as well as the need for retinal laser treatment or intravitreal injections, vitreous hemorrhage, or diabetes-related blindness.7 Notably, the PIONEER-6 (A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes)1 and AWARD-11 (Efficacy and Safety of Dulaglutide 3.0 mg and 4.5 mg Versus Dulaglutide 1.5 mg in Metformin-Treated Patients With Type 2 Diabetes)19 studies did not show a worsening of DR or other retinal complications. A post hoc analysis of SUSTAIN-6 linked worsening of DR to the rate of glycemic improvement, with the semaglutide group experiencing HbA1c reductions of 1.9% to 2.5% by week 16, compared with slower reductions in the placebo group.20 Additionally, a trial in patients with T2D and a body mass index (calculated as weight in kilograms divided by height in meters squared) of 27 reported worsening of DR in 4.0% of patients who were treated with semaglutide vs 2.7% of patients who received placebo.21 This difference was attributed to baseline DR history and insulin use. However, patients with uncontrolled or unstable DR were excluded from this trial. While our study balanced cohorts by insulin use and preexisting DR, we could not evaluate the rate of glycemic improvement or adjust for baseline DR stability or severity.
The potential protective properties of GLP-1 RAs against sight-threatening DR complications are likely multifactorial. While the association between HbA1c and progression of DR may resemble that seen in type 1 diabetes after initiating intensive insulin treatment or in T2D with tight blood glucose control,22,23 the reduction of risk in sight-threatening DR complications also occurs even in the shorter term using these agents.22,23 GLP-1 RAs may exert direct and indirect benefits on diabetic complications, primarily through improved glycemic control, which is reflected by decreased HbA1c levels. Secondary benefits of GLP-1 RAs include weight loss and prevention of cardiovascular and chronic kidney disease,2,3,4,5,6 which may provide additional vascular benefits in patients with diabetic eye disease. Experimental studies have shown that GLP-1 RAs can reverse early retinal changes associated with DR, restoring blood-retinal barrier integrity and preventing retinal cell death.24,25,26
Patients with diabetes are at an increased risk of NAION.27 A recent retrospective study raised concerns about a potential association between semaglutide use and NAION in the first year, suggesting that these agents might affect glucose metabolism or availability, risking damage to the highly metabolically active anterior optic nerve.10 However, a larger cohort study did not find any increased risk of NAION associated with GLP-1 RA use in patients with T2D, with or without obesity.12 We did not observe a statistically significant difference in the incidence of NAION between groups, likely due to the limited number of events and resulting imprecision in effect estimates.
In our study, although the use of GLP-1 RAs was associated with a modest increase in the rate of incident DR (0.2% increase) over 2 years, the absolute number of new DR diagnoses was low at 2.7%, likely below the expected annual incidence in US patients with T2D.28 Furthermore, GLP-1 RA use was associated with a 24.2% reduction in the risk of vision loss progressing to blindness, with 30.2% reduction in patients with preexisting DR. Diabetes is a leading cause of blindness in the US, and more advanced DR at diagnosis substantially increases the risk of blindness.29,30,31 Untreated PDR or DME also poses higher risks.32,33 Improving HbA1c is the key factor in delaying DR progression and preventing sight-threatening complications.23,34 With advances in DR screening reducing visual impairment, our findings suggest that GLP-1 RAs could further contribute to these positive patterns.35
The cost of GLP-1 RAs must be weighed against the potential of these agents to lower the costs associated with the treatment of diabetes and its complications.36 Our study found that many interventions for DR, including intravitreal injections, panretinal laser photocoagulation, and vitrectomy for DR, were less frequently needed by patients with GLP-1 RA prescriptions compared with those without GLP-1 RA prescriptions. We also found a lower rate of initiation of treatment with anti-VEGF inhibitors, a class of agents commonly used to treat DR and DME that costs Medicare Part D nearly $1 billion per year.37 The compliance rate, considering the adverse effect profile of GLP-1 RAs, should be factored in when prescribing them. Studies that used administrative claims data from the US, UK, and Europe reported that approximately 20% to 50% of patients receiving GLP-1 RAs discontinued treatment within 12 months of initiation.38 Future studies should examine how compliance and adherence affect effectiveness end points to improve understanding of the clinical effectiveness outcomes of GLP-1 RAs, particularly considering gastrointestinal adverse effects. Our study adds to the growing body of evidence that GLP-1 RAs show promise in reducing the cost of treating diabetes.
Limitations
Our study is limited by the anonymized data available from the electronic health record systems of participating health care organizations, which may have varying coding practices and data completeness, potentially affecting data quality. While the TriNetX database allows for the analysis of large T2D cohorts treated with GLP-1 RAs in clinical settings, it may not capture intraclass and interclass differences between medications and prescribing patterns. Although we used PSM to control for metabolic and medical factors, it is unclear if our findings apply to individuals with different metabolic phenotypes or to those using GLP-1 RAs for off-label indications. The study is also limited by the lack of longitudinal HbA1c measurements at fixed time intervals that would allow assessment of glycemic changes over the follow-up period. As a result, we could not directly evaluate the potential implications of rapid glucose lowering for the observed outcomes, a mechanism hypothesized to contribute to the initial worsening of DR with GLP-1 RA use. Due to the aggregate nature of our data, we were unable to determine the precise number of individuals excluded due to inadequate follow-up. Our analysis did not capture data on the switching or discontinuation of GLP-1 RAs, which may affect treatment patterns and outcomes. Additionally, certain demographic groups, such as Asian, Black, and Hispanic individuals or patients with lower income, are less likely to receive GLP-1 RA prescriptions, while patients using these medications may have greater health care access or diabetes self-efficacy.36,39 We were unable to account for all factors in social determinants of health, including access to eye specialists, which could have changed our results. Our analysis also did not control for other retinal conditions unrelated to diabetes that may have required treatment. Lastly, the findings on the association between GLP-1 RA use and lower rates of new-onset blindness were based on administrative coding of visual impairment, not eye examination data. Future studies are needed to quantify both the extent and types of blindness that may be prevented through the use of GLP-1 RAs.
Conclusions
This retrospective cohort study showed that while GLP-1 RA use was associated with a slight increase in incident DR, fewer patients experienced progression to sight-threatening stages of DR, developed DR complications, or required invasive treatments. These findings suggest that GLP-1 RAs may be a factor in reduced rate of vision loss leading to blindness, even among individuals with preexisting DR. It is crucial that all patients with T2D treated with GLP-1 RAs, regardless of preexisting DR, receive regular screening and monitoring for potential complications of T2D.
eAppendix. Cohort Definition
eMethods
eTable 1. Standardized Codes, and Data Types for Baseline Covariates Used in the TriNetX Database
eTable 2. Outcome Definitions and Corresponding ICD/CPT Codes
eTable 3. Comparison of Ophthalmologic Endpoints Among GLP-1 RA Users With <10 Years Versus >10 Years of Type 2 Diabetes
eTable 4. Baseline Adjustment of Diabetic Retinopathy Severity Among GLP-1 RA Users and Non-users in the Pre-existing DR Subgroup
Data Sharing Statement
References
- 1.Husain M, Birkenfeld AL, Donsmark M, et al. ; PIONEER 6 Investigators . Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-851. doi: 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 2.Wilding JPH, Batterham RL, Calanna S, et al. ; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 3.Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. doi: 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 4.Tuttle KR, Bain SC, Bosch-Traberg H, et al. Effects of once-weekly semaglutide on kidney disease outcomes by KDIGO risk category in the SUSTAIN 6 trial. Kidney Int Rep. 2024;9(7):2006-2015. doi: 10.1016/j.ekir.2024.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R, Wadid M, Makwana B, et al. GLP-1 receptor agonists among patients with overweight or obesity, diabetes, and HFpEF on SGLT2 inhibitors. JACC Heart Fail. 2024;12(11):1814-1826. doi: 10.1016/j.jchf.2024.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Khadke S, Kumar A, Bhatti A, et al. GLP-1 receptor agonist in nonobese patients with type 2 diabetes mellitus and heart failure with preserved ejection fraction. J Card Fail. 2024;S1071-9164(24)00962-X. doi: 10.1016/j.cardfail.2024.10.448 [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Professional Practice Committee . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 9.Barkmeier AJ, Herrin J, Swarna KS, et al. Comparative effectiveness of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase-4 inhibitors, and sulfonylureas for sight-threatening diabetic retinopathy. Ophthalmol Retina. 2024;8(10):943-952. doi: 10.1016/j.oret.2024.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hathaway JT, Shah MP, Hathaway DB, et al. Risk of nonarteritic anterior ischemic optic neuropathy in patients prescribed semaglutide. JAMA Ophthalmol. 2024;142(8):732-739. doi: 10.1001/jamaophthalmol.2024.2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Małyszczak A, Przeździecka-Dołyk J, Szydełko-Paśko U, Misiuk-Hojło M. Novel antidiabetic drugs and the risk of diabetic retinopathy: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2024;13(6):1797. doi: 10.3390/jcm13061797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou CC, Pan SY, Sheen YJ, et al. Association between semaglutide and nonarteritic anterior ischemic optic neuropathy: a multinational population-based study. Ophthalmology. 2025;132(4):381-388. doi: 10.1016/j.ophtha.2024.10.030 [DOI] [PubMed] [Google Scholar]
- 13.TriNetX. Real-world data for the life sciences and healthcare. Accessed January 1, 2025. https://trinetx.com/
- 14.Palchuk MB, London JW, Perez-Rey D, et al. A global federated real-world data and analytics platform for research. JAMIA Open. 2023;6(2):ooad035. doi: 10.1093/jamiaopen/ooad035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma R, Macias GL, Torres M, Klein R, Peña FY, Azen SP; Los Angeles Latino Eye Study Group . Biologic risk factors associated with diabetic retinopathy: the Los Angeles Latino Eye Study. Ophthalmology. 2007;114(7):1332-1340. doi: 10.1016/j.ophtha.2006.10.023 [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Xu L, Wang YX, You QS, Jonas JB, Wei WB. Ten-year cumulative incidence of diabetic retinopathy: the Beijing Eye Study 2001/2011. PLoS One. 2014;9(10):e111320. doi: 10.1371/journal.pone.0111320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman RR, Bethel MA, Mentz RJ, et al. ; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buse JB, Rosenstock J, Sesti G, et al. ; LEAD-6 Study Group . Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47. doi: 10.1016/S0140-6736(09)60659-0 [DOI] [PubMed] [Google Scholar]
- 19.Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care. 2021;44(3):765-773. doi: 10.2337/dc20-1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889-897. doi: 10.1111/dom.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies M, Færch L, Jeppesen OK, et al. ; STEP 2 Study Group . Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 23.Buehler AM, Cavalcanti AB, Berwanger O, et al. Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther. 2013;31(3):147-160. doi: 10.1111/j.1755-5922.2011.00308.x [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Wang Q, Zhang J, Lei X, Xu GT, Ye W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):699-706. doi: 10.1007/s00417-008-1004-3 [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res. 2014;127:104-116. doi: 10.1016/j.exer.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 26.Wei L, Mo W, Lan S, et al. GLP-1 RA improves diabetic retinopathy by protecting the blood-retinal barrier through GLP-1R-ROCK-p-MLC signaling pathway. J Diabetes Res. 2022;2022:1861940. doi: 10.1155/2022/1861940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayreh SS. Ischaemic optic neuropathy. Indian J Ophthalmol. 2000;48(3):171-194. [PubMed] [Google Scholar]
- 28.Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140-149. doi: 10.1016/S2213-8587(18)30128-1 [DOI] [PubMed] [Google Scholar]
- 29.Lundeen EA, Andes LJ, Rein DB, et al. Trends in prevalence and treatment of diabetic macular edema and vision-threatening diabetic retinopathy among Medicare Part B fee-for-service beneficiaries. JAMA Ophthalmol. 2022;140(4):345-353. doi: 10.1001/jamaophthalmol.2022.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention . About common eye disorders and diseases. Vision and Eye Health. May 21, 2024. Accessed January 1, 2025. https://www.cdc.gov/vision-health/about-eye-disorders/index.html
- 31.Wykoff CC, Khurana RN, Nguyen QD, et al. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care. 2021;44(3):748-756. doi: 10.2337/dc20-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferris FL III, Davis MD, Aiello LM. Treatment of diabetic retinopathy. N Engl J Med. 1999;341(9):667-678. doi: 10.1056/NEJM199908263410907 [DOI] [PubMed] [Google Scholar]
- 33.Brown DM, Nguyen QD, Marcus DM, et al. ; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 34.Tapp RJ, Shaw JE, Harper CA, et al. ; AusDiab Study Group . The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26(6):1731-1737. doi: 10.2337/diacare.26.6.1731 [DOI] [PubMed] [Google Scholar]
- 35.Arun CS, Al-Bermani A, Stannard K, Taylor R. Long-term impact of retinal screening on significant diabetes-related visual impairment in the working age population. Diabet Med. 2009;26(5):489-492. doi: 10.1111/j.1464-5491.2009.02718.x [DOI] [PubMed] [Google Scholar]
- 36.Eberly LA, Yang L, Essien UR, et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12):e214182. doi: 10.1001/jamahealthforum.2021.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai S, Sekimitsu S, Rossin EJ, Zebardast N. Trends in anti-vascular endothelial growth factor original Medicare Part B claims in the United States, 2014-2019. Ophthalmic Epidemiol. 2024;31(5):468-477. doi: 10.1080/09286586.2024.2310854 [DOI] [PubMed] [Google Scholar]
- 38.Weiss T, Carr RD, Pal S, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337-2345. doi: 10.2147/PPA.S277676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCoy RG, Van Houten HK, Deng Y, et al. Comparison of diabetes medications used by adults with commercial insurance vs Medicare advantage, 2016 to 2019. JAMA Netw Open. 2021;4(2):e2035792. doi: 10.1001/jamanetworkopen.2020.35792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Cohort Definition
eMethods
eTable 1. Standardized Codes, and Data Types for Baseline Covariates Used in the TriNetX Database
eTable 2. Outcome Definitions and Corresponding ICD/CPT Codes
eTable 3. Comparison of Ophthalmologic Endpoints Among GLP-1 RA Users With <10 Years Versus >10 Years of Type 2 Diabetes
eTable 4. Baseline Adjustment of Diabetic Retinopathy Severity Among GLP-1 RA Users and Non-users in the Pre-existing DR Subgroup
Data Sharing Statement


