Abstract
The cultivation of Neisseria gonorrhoeae by use of fastidious broth (FB) was evaluated. FB was found to be able to support the growth of all N. gonorrhoeae strains tested in this study without a rapid decrease in the viable count after exponential growth. After 24 h of incubation at 35°C with 5% CO2, viable counts of all strains reached over 108 CFU/ml in FB. Similar growth of the wild-type strain and its target-altered quinolone-resistant derivatives was observed. The susceptibilities of laboratory-adapted strains and clinical isolates to quinolones were tested by the microdilution method using FB. The MICs determined by microdilution were not significantly different from those determined by the agar dilution method recommended by the CLSI (formerly National Committee for Clinical Laboratory Standards). Moreover, the concentration-dependent time-kill of quinolones such as gatifloxacin and ciprofloxacin was observed in FB. At 2 to 4 times the MIC, gatifloxacin and ciprofloxacin were predominantly bactericidal against N. gonorrhoeae WHO A. At the MIC, the activities of both quinolones ranged from bactericidal to bacteriostatic. At 0.25 to 0.5 times the MIC, gonococcal growth was comparable to that of the growth control. These results suggest that the cultivation of N. gonorrhoeae by use of FB may be useful for evaluation of the antibacterial effects of quinolones.
Neisseria gonorrhoeae is one of the causatives of sexually transmitted diseases, and it is a fastidious organism. This organism is typically cultured using an agar medium such as chocolate agar plate (GCII agar base with 1% IsoVitaleX [BBL] and purified hemoglobin). The CLSI (formerly the National Committee for Clinical Laboratory Standards) recommends only the agar dilution procedure for antibacterial susceptibility testing of N. gonorrhoeae (20). Before the 1980s, a number of studies were published regarding the liquid culture methodology for N. gonorrhoeae (9, 13, 21, 23, 25). However, these reports demonstrated that the number of bacteria decreased rapidly after bacterial exponential growth, and broth microdilution methods for susceptibility testing of N. gonorrhoeae were reported to give higher MICs of β-lactam antibiotics against penicillinase-producing N. gonorrhoeae than agar dilution methods (7, 22).
Recently, the antibacterial activity of antigonococcal agents against clinical isolates of N. gonorrhoeae has been declining (1, 11, 24). Quinolones initially appeared to be promising agents for the treatment of N. gonorrhoeae infections; however, the extensive clinical use of quinolones carries the risk of the development of resistance, and indeed, there have been increasing numbers of recent isolates of N. gonorrhoeae that are highly resistant to quinolones, especially in Asia (11, 24, 27). Therefore, the evaluation of the antibacterial susceptibility of N. gonorrhoeae is important in the clinical setting. According to the CLSI (formerly NCCLS) procedure, the drug susceptibility of N. gonorrhoeae should be measured by the agar dilution method, and it is recommended that direct colony suspension be used for the inoculum preparation for the susceptibility test of N. gonorrhoeae (20). However, these approaches are tedious, time-consuming, and inconvenient, especially in the investigation of the antimicrobial susceptibility of large numbers of isolates and of the activities of many antimicrobial agents. The broth microdilution method and the growth method using liquid culture for the inoculum preparation are both simple methods to test the susceptibilities of clinical isolates; however, they have not yet been established for testing the susceptibility of N. gonorrhoeae. To date, few studies have been reported regarding the susceptibilities of antibacterial-resistant N. gonorrhoeae to antigonococcal agents by use of the microdilution method (7, 22). Therefore, a more convenient procedure than the agar dilution procedure recommended by the CLSI (formerly NCCLS) is still needed.
In addition to its potential usefulness for susceptibility testing, a modified liquid culture methodology is necessary for conducting bactericidal studies of antibiotics, such as in vitro pharmacokinetic/pharmacodynamic (PK/PD) analysis. In vitro PK/PD analysis of antimicrobial agents offers an alternative method for the determination of an adequate clinical regimen and is useful for predicting the clinical efficacy of a given antibiotic against various bacteria (2, 6, 8, 14-16). Therefore, the in vitro PK model, which can simulate human PK, is rapidly becoming one of the most important examinations in the development of new antimicrobial agents. In order to simulate human PK in vitro, it is essential to use a liquid culture. In vitro PK/PD analysis for N. gonorrhoeae has not yet been performed, because no liquid culture methodology has yet been established. Therefore, the development of such an approach remains necessary for in vitro PK/PD studies, as well as for testing the susceptibility of N. gonorrhoeae.
Cartwright et al. reported developing a fastidious broth (FB) that is capable of growing fastidious organisms (3). FB is used primarily for the recovery of clinically significant organisms from specimens. It is known that the viability of fastidious organisms, including N. gonorrhoeae, does not decline for several days in FB (3). However, thus far there has been no detailed report on the growth of N. gonorrhoeae in FB. In the present study, we examined the growth of N. gonorrhoeae in FB and considered whether or not FB could be used for the biological evaluation of N. gonorrhoeae, such as in vitro susceptibility testing and bactericidal activity of quinolones.
MATERIALS AND METHODS
Quinolones, bacterial strains, and media.
The quinolones tested here were synthesized at Kyorin Pharmaceutical Co., Ltd. (Tokyo, Japan) or were purchased from commercial sources. Data on the bacterial strains used in this study are shown in Table 1. N. gonorrhoeae WHO A and its quinolone-resistant derivatives have been described previously (26). N. gonorrhoeae ATCC49226 was a quality control strain of the CLSI (formerly NCCLS), and its gyrA mutant (N4-2) and gyrA parC double mutant (N4-2-4-2) were obtained by selection with norfloxacin in the present study. Mutations of the quinolone resistance-determining region of gyrA and parC were determined following a previously described method (4). N. gonorrhoeae TK106 and TK109 are known to be quinolone-resistant clinical isolates, as reported previously (5). The clinical isolates used in this study were isolated from patients with gonococcal urethritis in Japan.
TABLE 1.
Strains used in this study
| Strain | Mutation(s) | Source or reference |
|---|---|---|
| WHO A | Wild-type (parent) | 26 |
| R-4/5 | gyrA (Ser91Phe) | 26 |
| R-8/1 | gyrA (Asp95Tyr) | 26 |
| R-8/4 | gyrA (Ser91Tyr) | 26 |
| R-8/5 | gyrA (Asp95Asn) | 26 |
| R-4/5/1 | gyrA (Ser91Phe), parC (Glu91Lys) | 26 |
| R-4/5/2 | gyrA (Ser91Phe), parC (Asp86Asn) | 26 |
| R-4/5/3 | gyrA (Ser91Phe) | 26 |
| ATCC49226 | Wild-type (parent) | American Type Culture Collection |
| N-4-2 | gyrA (Ser91Phe) | This study |
| N-4-2-4-2 | gyrA (Ser91Phe), parC (Glu91Lys) | This study |
| TK106 | gyrA (Ser91Phe), parC (Ser87Ile) | 5 |
| TK109 | gyrA (Ser91Phe), parC (Ser87Ile) | 5 |
GCII agar base medium (Becton Dickinson, Cockeysville, MD) supplemented with 1% IsoVitaleX (Becton Dickinson) and chocolate agar were used to prepare the inocula, determine the visible colony count, and confirm the presence of N. gonorrhoeae by the oxidase test. FB was prepared as described previously by Cartwright et al. (3), and consisted of 35 g of Columbia broth base, 5 g of glucose, 5 g of yeast extract, 2 g of neopeptone, and 0.75 g of agarose dissolved in 960 ml of distilled water. A total of 30 ml of hematin solution (0.05% [wt/vol] in 0.1 M NaOH) and 5 ml of Tween 80 (10% [vol/vol]) was then added, and the resultant broth was sterilized by autoclaving, after which 6 ml of pyridoxal solution (0.1% [wt/vol]) and 1.5 ml of NAD solution (1% [wt/vol[) were added.
Growth studies using FB.
Colonies were removed from GCII agar after 24 h of incubation at 35°C in a moist atmosphere containing 5% CO2. The colonies were dispersed in sterile saline, and the inoculum turbidity was adjusted to approximately 1.0 at an optical density of 520 nm (ca. 108 to 109 CFU/ml). A total of 100 μl of this suspension was transferred to test tubes containing 10 ml of FB. The final inoculum was approximately 106 CFU/ml. Test tubes were incubated at 37°C in a water bath in the absence of CO2 and were shaken at 100 rpm. Growth was monitored by determination of the viable count. Portions (0.1 ml) of the cultures were removed at the indicated time points and were plated onto drug-free GCII agar supplemented with 1% IsoVitaleX, after dilution as necessary. The numbers of colonies were counted after 24 h of incubation at 35°C in a moist atmosphere containing 5% CO2.
Determination of MICs.
The MICs were determined by agar dilution with GCII agar supplemented with 1% IsoVitaleX and by microdilution with FB.
The agar dilution procedure was identical to that recommended by the CLSI (formerly NCCLS) (20). The MIC was defined as the lowest concentration of an antibacterial agent that inhibited visible growth after incubation for 24 h at 35°C in a moist atmosphere containing 5% CO2.
Microdilution plates were prepared using serial twofold dilutions of antimicrobial agents in FB. To prepare the inoculum, the turbidity of the actively growing FB culture was adjusted with sterile saline to obtain turbidity optically comparable to that of the 0.5 McFarland standard. The suspension was diluted 10-fold in sterile saline, and 5 μl of diluent was inoculated into 100 μl of FB. The final inoculum was approximately 5 × 105 CFU/ml (ca. 5 × 104 CFU/well). The plates were incubated for 24 h at 35°C in a moist atmosphere containing 5% CO2. The MIC was defined as the lowest concentration of an antibacterial agent that prevented macroscopically visible growth under the test conditions.
Killing curve study.
The bactericidal activities of gatifloxacin and ciprofloxacin were measured by a previously described method (10, 19) with minor modifications. In brief, representative strains incubated in FB for 24 h at 35°C in a moist atmosphere containing 5% CO2 were diluted with fresh broth to approximately 106 CFU/ml, and the diluted cultures were incubated for 2 h at 37°C in the absence of CO2 with shaking. After 2 h of preincubation, gatifloxacin and ciprofloxacin were added to the culture at various concentrations around the MIC. Portions (0.1 ml) of the cultures were removed at the indicated time points and were plated onto drug-free GCII agar supplemented with 1% IsoVitaleX, after dilution as necessary. The numbers of colonies was counted after 24 h of incubation at 35°C in a moist atmosphere containing 5% CO2.
In vitro PK model.
The in vitro PK model has been described previously (6). A dilutional in vitro PK model (PASS-400; Dainipponseiki, Kyoto, Japan) was used to simulate serum concentrations of ciprofloxacin on the basis of the PK parameters reported previously (17). N. gonorrhoeae R-4/5/1, which possessed single-point mutations in the quinolone resistance-determining region of both GyrA (Ser91→Phe) and ParC (Glu91→Lys), were used. Representative strains incubated in FB for 24 h at 37°C with shaking were diluted with fresh broth to approximately 106 CFU/ml, and the diluted cultures were incubated for 2 h at 37°C in the absence of CO2 with shaking. After 2 h of preincubation, ciprofloxacin was then added to the culture chamber according to the dosing regimen. Aliquots of the cultures were collected via an outflow tube at the indicated time points and were plated onto drug-free GCII agar supplemented with 1% IsoVitaleX, after dilution as necessary. The number of colonies was counted after 24 h of incubation at 35°C in a moist atmosphere containing 5% CO2.
RESULTS
Culture of N. gonorrhoeae by FB.
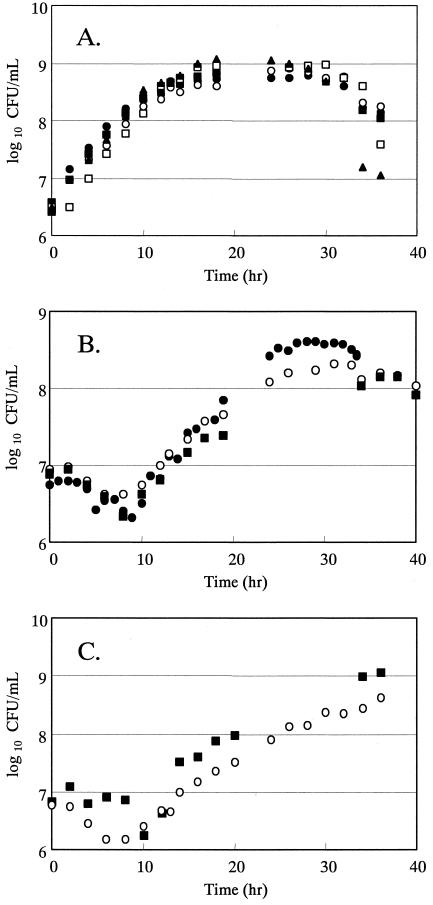
The cultivation of N. gonorrhoeae was examined using FB. All of the tested strains of N. gonorrhoeae were found to be able to grow in FB (Fig. 1). N. gonorrhoeae WHO A (wild type) and its quinolone-resistant derivatives grew well in FB without any rapid decrease in the viable count after exponential growth. No significant changes in the growth rate were observed for the wild-type strain and its target-altered quinolone-resistant derivatives. The generation times for WHO A (wild type), R-4/5 (gyrA, Ser91→Phe), R-4/5/1 (gyrA, Ser91→Phe; parC, Glu91→Lys), R-4/5/2 (gyrA, Ser91→Phe; parC, Asp86→Asn), and R-4/5/3 (gyrA, Ser91→Phe; parC, unknown) were 83, 105, 94, 90, and 94 min, respectively. At 24 h after inoculation, the viable counts of the bacteria in culture had reached their maximum numbers, which ranged from 6 × 108 to 9 × 108 CFU/ml.
FIG. 1.
Growth of N. gonorrhoeae strains, including quinolone-resistant strains, in FB. Symbols: A. •, WHO A (wild type); ○, R-4/5 (gyrA); ▪, R-4/5/1 (gyrA parC); □, R-4/5/2 (gyrA parC); ▴, R-4/5/3 (gyrA); B. •, ATCC49226 (wild type); ○, N4-2 (gyrA); ▪, N4-2-4-2 (gyrA parC); C. ▪, TK106; ○, TK109 (quinolone-resistant clinical isolate).
For N. gonorrhoeae ATCC49226 (wild type), which was the quality control strain recommended by the CLSI (formerly NCCLS), and its target-altered quinolone-resistant derivatives, the exponential growth phase was observed for 15 h after a lag phase of 10 h. Maximal growth was observed 24 h after incubation with viable counts of approximately 4 × 108 CFU/ml. The generation time of the exponential phase of ATCC49226 (wild type), N4-2 (gyrA, Ser91→Phe), and N4-2-4-2 (gyrA, Ser91→Phe; parC, Asp86→Asn) were 127, 173, and 197 min, respectively.
Viable cells could be counted at 10 h after incubation for the clinical isolates of N. gonorrhoeae TK106 and TK109. The growth rates of these strains were the lowest among the strains tested, since their generation times were 222 min (TK106) and 215 min (TK109). The viable counts under the present culture conditions ranged from 4 × 108 (TK109) to 1 × 109 CFU/ml (TK106) at 36 h after inoculation.
Bacterial growth in FB was evaluated for the remaining 20 strains of N. gonorrhoeae, including laboratory-adapted strains and clinical isolates. One colony of each strain was transferred into a tube containing 0.5 ml of FB and was then cultivated at 35°C in a moist atmosphere containing 5% CO2. All tested strains were found to have grown to over 108 CFU/ml at 24 h after inoculation. Additionally, N. gonorrhoeae ATCC49226 was found to have grown to over 108 CFU/ml at a stationary phase after 104, 105, and 106 CFU/ml inoculation (data not shown).
Comparison of the MICs of quinolones determined by microdilution and agar dilution procedures.
No difference in the MICs of quinolones was observed by use of an agar dilution procedure using both direct colony suspension as recommended by the CLSI (formerly NCCLS) and FB culture for inoculum preparation (data not shown).
The MICs of quinolones used in this study are summarized in Table 2 to Table 4. The MICs of quinolones, as measured by the microdilution procedure using FB, were within a fourfold deviation, compared with those obtained by the agar dilution procedure. Additionally, the MICs of quinolones measured by the microdilution procedure for N. gonorrhoeae ATCC49226 were within the ranges permitted by the CLSI (formerly NCCLS) using the agar dilution procedure. Furthermore, the susceptibility to quinolones of target-altered quinolone-resistant strains was found to have decreased compared with that of the parent strains. Against clinical isolates, the MIC50s and the MIC90s obtained by the microdilution procedure were comparable to those observed with the agar dilution procedure. The percentage of agreement between the agar dilution procedure and microdilution procedure within a single doubling dilution exceeded 90% for all quinolones tested (Table 5).
TABLE 2.
Comparison of MICs by microdilution versus agar dilution for type strains of N. gonorrhoeae
| N. gonorrhoeae strain | MIC (μg/ml) ofa:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norfloxacin
|
Ciprofloxacin
|
Ofloxacin
|
Levofloxacin
|
Gatifloxacin
|
|||||||||||
| AD | MD | AD | MD | AD | MD | AD | MD | AD | MD | ||||||
| ATCC49226 | 0.016 | 0.031 | 0.002 | 0.004 | 0.008 | 0.016 | 0.004 | 0.008 | 0.002 | 0.004 | |||||
| WHO A | 0.008 | 0.031 | 0.002 | 0.004 | 0.008 | 0.016 | 0.004 | 0.008 | 0.002 | 0.004 | |||||
| Type | 0.008 | 0.016 | 0.002 | 0.004 | 0.008 | 0.008 | 0.004 | 0.004 | 0.002 | 0.004 | |||||
| IID835 | 0.008 | 0.016 | 0.002 | 0.004 | 0.008 | 0.016 | 0.008 | 0.008 | 0.008 | 0.004 | |||||
| Moxifloxacin
|
Sparfloxacin
|
Trovafloxacin
|
Garenoxacin
|
Gemifloxacin
|
|||||||||||
|
|
AD | MD | AD | MD | AD | MD | AD | MD | AD | MD | |||||
| ATCC49226 | 0.008 | 0.008 | 0.004 | 0.004 | 0.008 | 0.008 | 0.008 | 0.004 | 0.002 | 0.002 | |||||
| WHO A | 0.004 | 0.008 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | |||||
| Type | 0.004 | 0.004 | 0.001 | 0.002 | 0.002 | 0.004 | 0.002 | 0.002 | 0.001 | 0.002 | |||||
| IID835 | 0.008 | 0.008 | 0.002 | 0.004 | 0.004 | 0.008 | 0.008 | 0.008 | 0.004 | 0.004 | |||||
AD, agar dilution; MD, microdilution.
TABLE 4.
Comparative antimicrobial susceptibilities of N. gonorrhoeae clinical isolates (71 strains)
| Antimicrobial agent | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Agar dilution
|
Microdilution
|
|||||
| 50% | 90% | Range | 50% | 90% | Range | |
| Norfloxacin | 8 | 32 | 0.008-64 | 8 | 32 | 0.016-64 |
| Ciprofloxacin | 2 | 16 | 0.001-32 | 4 | 16 | 0.002-32 |
| Ofloxacin | 4 | 16 | 0.008-32 | 4 | 16 | 0.008-32 |
| Levofloxacin | 2 | 8 | 0.004-8 | 2 | 8 | 0.004-16 |
| Gatifloxacin | 0.5 | 2 | 0.002-2 | 0.5 | 2 | 0.002-2 |
| Moxifloxacin | 1 | 4 | 0.004-4 | 2 | 4 | 0.004-4 |
| Sparfloxacin | 1 | 4 | 0.001-8 | 2 | 8 | 0.001-16 |
| Trovafloxacin | 0.5 | 4 | 0.001-8 | 1 | 8 | 0.002-16 |
| Garenoxacin | 0.5 | 2 | 0.001-4 | 0.5 | 2 | 0.001-4 |
| Gemifloxacin | 0.5 | 2 | 0.001-4 | 0.5 | 4 | 0.001-8 |
TABLE 5.
Agreement of microdilution with agar dilution for susceptibility testing of N. gonorrhoeae
| Antimicrobial agent | % Deviation between MICs at dilution step:
|
|||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | +3 | |
| Norfloxacin | 5.6 | 53.5 | 40.8 | |||
| Ciprofloxacin | 2.8 | 54.9 | 40.8 | 1.4 | ||
| Ofloxacin | 4.2 | 53.5 | 42.3 | |||
| Levofloxacin | 7.0 | 47.9 | 43.7 | 1.4 | ||
| Gatifloxacin | 14.1 | 54.9 | 31.0 | |||
| Moxifloxacin | 9.9 | 50.7 | 35.2 | 4.2 | ||
| Sparfloxacin | 1.4 | 28.2 | 60.6 | 9.9 | ||
| Trovafloxacin | 1.4 | 9.9 | 29.6 | 54.9 | 4.2 | |
| Garenoxacin | 5.6 | 16.9 | 42.3 | 35.2 | ||
| Gemifloxacin | 1.4 | 9.9 | 28.2 | 57.7 | 2.8 | |
Bactericidal activity of quinolones against N. gonorrhoeae.
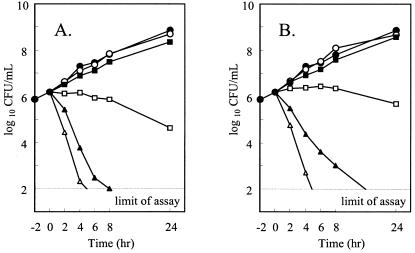
Killing curve studies were performed with gatifloxacin and ciprofloxacin against N. gonorrhoeae WHO A, and the bactericidal activities were found to increase with the concentrations of both gatifloxacin and ciprofloxacin (Fig. 2). More than 99% of the initial viable N. gonorrhoeae were killed within 2 to 4 h of incubation with quinolones at concentrations in excess of 2 × MIC. Bacteriostatic activity was observed at a concentration equal to the MIC.
FIG. 2.
Time-kill curves of gatifloxacin (panel A; MIC, 0.004 μg/ml) and ciprofloxacin (panel B; MIC, 0.004 μg/ml) against N. gonorrhoeae WHO A in FB. Symbols: •, control; ○, 0.25 × MIC; ▪, 0.5 × MIC; □, 1 × MIC; ▴, 2 × MIC; Δ, 4 × MIC.
Bacterial time-kill study using an in vitro simulation model.
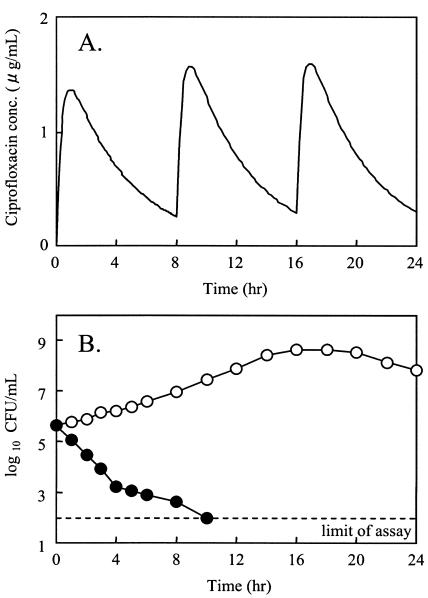
The time-kill curve for ciprofloxacin at the simulated serum concentration after oral dosing of 200 mg three times a day (t.i.d.), which is the Japanese clinical regimen, is shown in Fig. 3. The growth control curve demonstrated logarithmic growth to approximately 109 CFU/ml. At a dose of 200 mg t.i.d., ciprofloxacin reduced the number of quinolone-resistant N. gonorrhoeae R-4/5/1 bacteria to below the limit of detection (<102 CFU/ml) within 8 h following the initial exposure. No regrowth was observed until 24 h after exposure.
FIG. 3.
Bactericidal effect of ciprofloxacin with a simulation concentration in serum at a dose of 200 mg t.i.d. on quinolone-resistant N. gonorrhoeae R-4/5/1 (A); MIC, 0.125 μg/ml (B). Symbols: ○, control; •, 200 mg t.i.d. simulation.
DISCUSSION
It was observed that FB was capable of supporting the growth of all of the N. gonorrhoeae strains tested in the present study. In some strains, a lag growth phase atypical for N. gonorrhoeae was observed prior to the log growth phase. The duration of the lag phase differed according to the strain tested. A long lag phase was observed for the growth of N. gonorrhoeae ATCC49226, N4-2, N4-2-4-2, TK106, and TK109 (10 h). However, after 24 h of incubation, the viable counts of all strains reached levels of more than 108 CFU/ml in FB. Therefore, it is possible that FB can be used to culture N. gonorrhoeae for various types of biological evaluation.
The evaluation of antibacterial agents, e.g., by in vitro PK/PD analysis, is performed based on the MICs. Therefore, the susceptibility of N. gonorrhoeae in FB would not be expected to differ substantially from that generally reported in the literature. Thus, the antibacterial susceptibilities determined by these microdilution procedures were compared with those determined by the agar dilution procedures recommended by the CLSI (formerly NCCLS). As regards the quality control strain of the CLSI (formerly NCCLS) (ATCC49226) and the laboratory-adapted strains used in this study, no significant differences were observed between the MICs obtained by either of the procedures. Additionally, the MIC90s of quinolones against clinical isolates determined by microdilution were almost equal to or even twofold higher than those determined by agar dilution; moreover, the MICs associated with more than 90% of the clinical isolates tested were observed to be within only 1 doubling difference when the agar dilution and microdilution procedures were compared. As regards various types of bacteria, including both laboratory-adapted strains and clinical isolates, the MICs of quinolones determined by both procedures were found differ slightly. It appears that such slight differences between the MICs are reflective of the standard deviation in this study. Therefore, the microdilution method may prove to be useful for the determination of the antibacterial activities of quinolones against N. gonorrhoeae, and the present culture method using FB could be applied for various types of evaluation of quinolone.
In Asia, the number of gonococcal infections caused by antibacterial-resistant strains has gradually increased since the mid-1990s. The rapid emergence of clinical isolates of N. gonorrhoeae with decreased susceptibility to quinolones has been reported previously (11, 24). In Hawaii, it was reported that a total of 10.4% of gonococcal isolates in 2000 were ciprofloxacin resistant, compared with <1.5% per year from 1990 to 1997 (12). In order to investigate this emerging quinolone resistance among clinical isolates of N. gonorrhoeae, it will be necessary to develop more expedient studies such as sequential resistance development and in vitro PK/PD analysis as well as time-kill studies, etc. Growth methods using a liquid medium offer several advantages over the more commonly used agar methods. Here, we performed time-kill kinetic studies of gatifloxacin and ciprofloxacin. These results indicated that both quinolones produced concentration-dependent killing effects over a 24-h period. At 2 to 4 × MIC, both compounds were predominantly bactericidal, and at the MIC, the activity ranged from bactericidal to bacteriostatic. At 0.25 to 0.5 × MIC, gonococcal growth was comparable to that of the growth control. Concentration-dependent time-kill was observed among the quinolones. These results suggest that the cultivation of N. gonorrhoeae in FB is useful for PK/PD analysis using an in vitro simulation model.
Unlike the United States regimen, that frequently used as the primary treatment for gonorrhea in Japan involves multiple and lower doses of quinolones, which results in the exposure of N. gonorrhoeae clinical strains to low concentrations of the agents. It has been suggested that the administration of large amounts of quinolones for clinical use will enhance the prevalence of quinolone resistance (11). However, the viable CFU counts of a genetically characterized quinolone-resistant strain (R-4/5/1) were reduced below the limit of detection by a simulated serum concentration after oral dosing of ciprofloxacin according to the Japanese clinical regimen (200 mg t.i.d.). These results contradict the present situation observed in the clinical setting in Japan.
Moran and Levine proposed “therapeutic time” as a pharmacodynamic parameter for the treatment of gonorrhea, whereby the therapeutic time was defined as the interval between the time the peak plasma concentration was reached and the time the plasma concentration dropped to less than four times the MIC90 (18). According to that report, an effective gonococcal cure was expected when the value of the therapeutic time was 10 h or more according to all antimicrobial regimens, including the use of quinolones. The therapeutic time of the Japanese clinical regimen of ciprofloxacin against R-4/5/1 (MIC, 0.125 μg/ml) was 14.4 h. No discrepancy was observed between the prediction of the pharmacodynamic parameter (therapeutic time) and the results of the in vitro simulation model in this study. Therefore, in order to predict a clinical outcome, further detailed PK/PD analysis using an in vitro simulation model is still needed to determine the impact of the PK/PD parameters on bactericidal activity and resistance selectivity.
The use of in vitro PK/PD dynamic models using FB is likely to give the optimum pharmacodynamic parameters for the application of quinolone in the treatment of gonorrhea. It is hoped that such an approach will render it possible to propose an effective regimen of quinolones without risking increased microbial resistance.
TABLE 3.
Comparison of MICs by microdilution versus agar dilution for quinolone-resistant strains of N. gonorrhoeae
| N. gonorrhoeae strain | MIC (μg/ml) ofa:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norfloxacin
|
Ciprofloxacin
|
Ofloxacin
|
Levofloxacin
|
Gatifloxacin
|
|||||||||||
| AD | MD | AD | MD | AD | MD | AD | MD | AD | MD | ||||||
| WHO A | 0.008 | 0.031 | 0.002 | 0.004 | 0.008 | 0.016 | 0.004 | 0.008 | 0.002 | 0.004 | |||||
| R-4/5 | 0.25 | 0.5 | 0.031 | 0.063 | 0.125 | 0.25 | 0.063 | 0.125 | 0.016 | 0.016 | |||||
| R-8/1 | 0.125 | 0.5 | 0.031 | 0.063 | 0.063 | 0.125 | 0.031 | 0.063 | 0.008 | 0.016 | |||||
| R-8/4 | 0.25 | 0.5 | 0.031 | 0.063 | 0.125 | 0.25 | 0.063 | 0.125 | 0.016 | 0.031 | |||||
| R-8/5 | 0.25 | 0.5 | 0.016 | 0.063 | 0.063 | 0.125 | 0.031 | 0.063 | 0.016 | 0.016 | |||||
| R-4/5/1 | 0.5 | 2 | 0.063 | 0.125 | 0.125 | 0.25 | 0.063 | 0.25 | 0.016 | 0.031 | |||||
| R-4/5/2 | 0.5 | 2 | 0.063 | 0.125 | 0.125 | 0.5 | 0.063 | 0.25 | 0.016 | 0.031 | |||||
| R-4/5/3 | 0.5 | 2 | 0.063 | 0.125 | 0.125 | 0.25 | 0.063 | 0.125 | 0.016 | 0.031 | |||||
| ATCC49226 | 0.016 | 0.031 | 0.002 | 0.004 | 0.008 | 0.016 | 0.004 | 0.008 | 0.002 | 0.004 | |||||
| N-4-2 | 0.25 | 0.5 | 0.063 | 0.125 | 0.125 | 0.25 | 0.063 | 0.125 | 0.016 | 0.031 | |||||
| N-4-2-4-2 | 1 | 2 | 0.125 | 0.25 | 0.25 | 0.25 | 0.125 | 0.125 | 0.031 | 0.031 | |||||
| TK106 | 4 | 2 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.125 | 0.125 | |||||
| TK109 | 16 | 16 | 2 | 2 | 4 | 8 | 4 | 4 | 0.5 | 0.5 | |||||
| Moxifloxacin
|
Sparfloxacin
|
Trovafloxacin
|
Garenoxacin
|
Gemifloxacin
|
|||||||||||
|
|
AD | MD | AD | MD | AD | MD | AD | MD | AD | MD | |||||
| WHO A | 0.004 | 0.008 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | |||||
| R-4/5 | 0.016 | 0.031 | 0.008 | 0.031 | 0.008 | 0.016 | 0.008 | 0.016 | 0.008 | 0.008 | |||||
| R-8/1 | 0.016 | 0.031 | 0.008 | 0.016 | 0.008 | 0.016 | 0.008 | 0.008 | 0.008 | 0.008 | |||||
| R-8/4 | 0.016 | 0.031 | 0.016 | 0.031 | 0.008 | 0.016 | 0.008 | 0.008 | 0.008 | 0.008 | |||||
| R-8/5 | 0.016 | 0.031 | 0.008 | 0.016 | 0.008 | 0.016 | 0.008 | 0.008 | 0.008 | 0.008 | |||||
| R-4/5/1 | 0.031 | 0.063 | 0.031 | 0.063 | 0.063 | 0.25 | 0.063 | 0.063 | 0.031 | 0.063 | |||||
| R-4/5/2 | 0.031 | 0.063 | 0.031 | 0.063 | 0.063 | 0.125 | 0.031 | 0.063 | 0.031 | 0.063 | |||||
| R-4/5/3 | 0.031 | 0.063 | 0.016 | 0.063 | 0.031 | 0.063 | 0.016 | 0.031 | 0.008 | 0.016 | |||||
| ATCC49226 | 0.008 | 0.008 | 0.004 | 0.004 | 0.008 | 0.008 | 0.008 | 0.004 | 0.002 | 0.002 | |||||
| N-4-2 | 0.031 | 0.031 | 0.063 | 0.063 | 0.031 | 0.063 | 0.031 | 0.031 | 0.016 | 0.016 | |||||
| N-4-2-4-2 | 0.063 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 0.25 | 0.25 | 0.125 | 0.125 | |||||
| TK106 | 0.125 | 0.125 | 0.25 | 0.25 | 0.5 | 1 | 0.25 | 0.25 | 0.25 | 0.5 | |||||
| TK109 | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 1 | |||||
AD, agar dilution; MD, microdilution.
REFERENCES
- 1.Berrón, S., J. A. Vázquez, M. J. Giménez, L. de la Fuente, and L. Aguilar. 2000. In vitro susceptibilities of 400 Spanish isolates of Neisseria gonorrhoeae to gemifloxacin and 11 other antimicrobial agents. Antimicrob. Agents Chemother. 44:2543-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowker, K. E., M. Wootton, C. A. Rogers, R. Lewis, H. A. Holt, and A. P. MacGowan. 1999. Comparison of in-vitro pharmacodynamics of once and twice daily ciprofloxacin. J. Antimicrob. Chemother. 44:661-667. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright, C. P., F. Stock, and V. J. Gill. 1994. Improved enrichment broth for cultivation of fastidious organisms. J. Clin. Microbiol. 32:1825-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deguchi, T., M. Yasuda, M. Nakano, S. Ozeki, T. Ezaki, I. Saito, and Y. Kawada. 1996. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob. Agents Chemother. 40:1020-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi, T., I. Saito, M. Tanaka, K. Sato, K. Deguchi, M. Yasuda, M. Nakano, Y. Nishino, E. Kanematsu, S. Ozeki, and Y. Kawada. 1997. Fluoroquinolone treatment failure in gonorrhea. Emergence of a Neisseria gonorrhoeae strain with enhanced resistance to fluoroquinolones. Sex. Transm. Dis. 24:247-250. [DOI] [PubMed] [Google Scholar]
- 6.Ebisu, H., R. Kishii, M. Takei, and H. Fukuda. 2003. The effect of pharmacokinetic/ pharmacodynamic (PK/PD) parameters of gatifloxacin on its bactericidal activity and resistance selectivity against clinical isolates of Streptococcus pneumoniae. J. Infect. Chemother. 9:210-214. [DOI] [PubMed] [Google Scholar]
- 7.Geers, T. A., and A. M. Donabedian. 1989. Comparison of broth microdilution and agar dilution for susceptibility testing of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 33:233-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafsson, I., E. Lowdin, I. Odenholt, and O. Cars. 2001. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45:2436-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendry, A. T. 1983. Growth responses of Neisseria gonorrhoeae auxotypes to required amino acids and bases in liquid medium. Can. J. Microbiol. 29:1309-1313. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka, M., T. Yasue, H. Fukuda, H. Tomizawa, H. Aoyama, and K. Hirai. 1992. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob. Agents Chemother. 36:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito, M., M. Yasuda, S. Yokoi, S. Ito, Y. Takahashi, S. Ishihara, S. Maeda, and T. Deguchi. 2004. Remarkable increase in central Japan in 2001-2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob. Agents Chemother. 48:3185-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iverson, C. J., S. A. Wang, M. V. Lee, R. G. Ohye, D. L. Trees, J. S. Knapp, P. V. Effler, N. P. O'Connor, and W. C. Levine. 2004. Fluoroquinolone resistance among Neisseria gonorrhoeae isolates in Hawaii, 1990-2000: role of foreign importation and increasing endemic spread. Sex. Transm. Dis. 31:702-708. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. T., and R. S. Talley. 1977. Simplified complete medium for the growth of Neisseria gonorrhoeae. J. Clin. Microbiol. 5:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacGowan, A., C. Rogers, H. A. Holt, M. Wootton, and K. Bowker. 2000. Assessment of different antibacterial effect measures used in in vitro models of infection and subsequent use in pharmacodynamic correlations for moxifloxacin. J. Antimicrob. Chemother. 46:73-78. [DOI] [PubMed] [Google Scholar]
- 15.MacGowan, A., C. Rogers, and K. Bowker. 2000. The use of in vitro pharmacodynamic models of infection to optimize fluoroquinolone dosing regimens. J. Antimicrob. Chemother. 46:163-170. [DOI] [PubMed] [Google Scholar]
- 16.Madaras-Kelly, K. J., B. E. Ostergaard, L. B. Hovde, and J. C. Rotschafer. 1996. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 40:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, F., H. Kobayashi, K. Takamura, and H. Takeda. 1985. Pharmacokinetics with BAY O 9867 (ciprofloxacin). Jpn. J. Chemother. 33:140-188. [Google Scholar]
- 18.Moran, J. S., and W. C. Levine. 1995. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin. Infect. Dis. 20:S47-S65. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline. NCCLS M26-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard NCCLS M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Norrod, E. P., and S. A. Morse. 1982. Presence of hydrogen peroxide in media used for cultivation of Neisseria gonorrhoeae. J. Clin. Microbiol. 15:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro, M. A., C. L. Heifetz, and J. C. Sesnie. 1984. Comparison of microdilution and agar dilution procedures for testing antibiotic susceptibility of Neisseria gonorrhoeae. J. Clin. Microbiol. 20:828-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shockley, R. K., E. E. Coffee, and K. H. Johnston. 1980. SJ-GC, a modified complete medium for growth of Neisseria gonorrhoeae. J. Clin. Microbiol. 12:35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka, M., H. Nakayama, T. Notomi, S. Irie, Y. Tsunoda, A. Okadome, T. Saika, and I. Kobayashi. 2004. Antimicrobial resistance of Neisseria gonorrhoeae in Japan, 1993-2002: continuous increasing of ciprofloxacin-resistant isolates. Int. J. Antimicrob. Agents 24:15-22. [DOI] [PubMed] [Google Scholar]
- 25.Wong, T. P., R. K. Shockley, and K. H. Johnston. 1980. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J. Clin. Microbiol. 11:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasuda, M., H. Fukuda, S. Yokoi, S. Ishihara, Y. Kawada, and T. Deguchi. 2000. In vitro selection of fluoroquinolone-resistant Neisseria gonorrhoeae harboring alterations in DNA gyrase and topoisomerase IV. J. Urol. 164:847-851. [DOI] [PubMed] [Google Scholar]
- 27.Yong, D., T. S. Kim, J. R. Choi, J. H. Yum, K. Lee, Y. Chong, H. B. Oh, T. Shultz, and J. W. Tapsall. 2004. Epidemiological characteristics and molecular basis of fluoroquinolone-resistant Neisseria gonorrhoeae strains isolated in Korea and nearby countries. J. Antimicrob. Chemother. 54:451-455. [DOI] [PubMed] [Google Scholar]