Abstract
In this study a multilocus sequence typing (MLST) scheme for Acinetobacter baumannii was developed and evaluated by using 40 clinical A. baumannii isolates recovered from outbreaks in Spanish and German hospitals during the years 1990 to 2001, as well as isolates from other European hospitals and two DSMZ reference strains of A. baumannii. For comparison, two isolates of Acinetobacter species 13 (sensu Tjernberg and Ursing), two clinical isolates, and three DSMZ strains of A. calcoaceticus (both belonging to the A. calcoaceticus-A. baumannii complex) were also investigated. Primers were designed for conserved regions of housekeeping genes, and 305- to 513-bp internal fragments of seven such genes—gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD—were sequenced for all strains. The number of alleles at individual loci ranged from 6 to 12, and a total of 20 allelic profiles or sequence types were distinguished among the investigated A. baumannii strains. The MLST data were in high concordance with the epidemiologic typing results generated by pulsed-field gel electrophoresis and amplified fragment length polymorphism fingerprinting. The MLST scheme provides a high level of resolution and an excellent tool for studying the population structure and long-term epidemiology of A. baumannii.
Bacteria of the genus Acinetobacter are ubiquitously distributed in nature and often involved in the colonization and infection of hospitalized patients, particularly those in intensive care units (4, 10). The taxonomy of the genus Acinetobacter has a long and complicated history. In 1986, Bouvet and Grimont divided the genus into 12 genomic species based on DNA-DNA hybridization and proposed a variety of biochemical tests for phenotypic species identification (5). Three years later, Bouvet and Jeanjean reported new proteolytic Acinetobacter strains designated genomic species 13, 14, 15, and 17 (6). At the same time, three additional genomic species were proposed by Tjernberg and Ursing and designated Acinetobacter genomic species 13TU, 14TU, and 15TU (37). Currently, at least 32 genomic species are recognized among the genus Acinetobacter. This classification opened the possibility to analyze the epidemiology of this diverse genus. However, conventional or commercially available biochemical test systems are not able to identify unambiguously most genomic species (4, 23). In particular, isolates belonging to genomic species 1 (Acinetobacter calcoaceticus), 2 (A. baumannii), 3, and 13TU are almost indistinguishable by biochemical tests (17). In 1992, the designation A. calcoaceticus-A. baumannii complex was therefore suggested for these genotypically distinct but phenotypically very similar bacterial species (16).
Numerous studies have supported the observation that A. baumannii is the species most commonly involved in nosocomial infections such as pneumonia, wound infection, and bloodstream infection (4, 7, 15). The implication of A. baumannii in hospital outbreaks of nosocomial infection (8, 10, 29) has been attributed to their increasing antimicrobial resistance (21, 39), and their ability to survive on inanimate and dry surfaces (2, 24), both contributing to an increased survival time in the hospital environment.
To better understand the epidemiology and in particular the mode of spread of A. baumannii, a number of molecular typing systems have been developed, including PCR-based methods such as random(ly) amplified polymorphic DNA analysis (20), integrase gene PCR (25), infrequent-restriction-site PCR (42), ribotyping (16, 35), amplified fragment length polymorphism (AFLP) analysis (9), and pulsed-field gel electrophoresis (PFGE) (18, 35). PFGE restriction analysis of chromosomal bacterial DNA has been used with excellent results in epidemiologic studies of numerous A. baumannii outbreaks and is currently regarded as the gold standard for epidemiologic typing (8, 18).
Some of the genomic methods, in particular ribotyping and AFLP, are able to resolve bacteria of the A. calcoaceticus-A. baumannii complex at the species level with a high degree of discrimination (9, 16). All of these methods rely on the generation of a distinct pattern or DNA “fingerprint” that is usually visualized by ethidium bromide staining or nucleic acid hybridization. So-called comparative typing systems, i.e., methods that depend on comparisons of DNA fragment patterns on gels, such as PFGE and random(ly) amplified polymorphic DNA analysis, are well suited for local outbreak investigation. For a global epidemiologic analysis, however, comparison of the results obtained at different laboratories would be required, but poor interlaboratory reproducibility remains a critical and unresolved issue.
Multilocus sequence typing (MLST) is a highly discriminative method of typing microorganisms (28) and has been applied successfully for the epidemiologic characterization of a variety of clinically important bacterial pathogens including Neisseria meningitidis (28), Streptococcus pneumoniae (14), Streptococcus pyogenes (13), Staphylococcus aureus (12, 19), Campylobacter jejuni (11), Enterococcus faecium (22), Vibrio cholerae (26), and Haemophilus influenzae (30).
MLST is based on the same principles as multilocus enzyme electrophoresis but relies directly on DNA sequence comparison of internal fragments of protein encoding housekeeping genes. These housekeeping genes are chosen for analysis because their products play vital function, being present in all isolates of a given species, and mutations within them are assumed to be neutral. For each gene fragment, the different sequences are assigned as distinct alleles, and each isolate is defined by the alleles at each of the housekeeping loci (the allelic profile or sequence type [ST]). MLST offers the possibility to transfer typing data from laboratory to laboratory or compare results via the internet (http://mlst.zoo.ox.ac.uk), thus providing a powerful tool for global epidemiologic studies, as well as for studies of the population biology of bacterial species. We describe here an MLST scheme for A. baumannii based on the nucleotide sequences of seven housekeeping loci.
MATERIALS AND METHODS
Bacterial isolates.
A total of 49 Acinetobacter isolates were used in the present study: 42 A. baumannii isolates, including reference strains DSM 30008 and DSM 30007 (A. baumannii type strain ATCC 19606) from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ); 2 clinical Acinetobacter genomic species 13TU isolates; and 5 A. calcoaceticus isolates, including reference strains DSM 1139, DSM 7324, and DSM 30006 (Table 1). A. baumannii isolates were selected to represent the major strain types involved in hospital outbreaks that were observed in different Spanish and German hospitals between 1990 and 2002; also included were three isolates from The Netherlands and from Sweden to represent other geographic locations. All isolates had been identified previously by routine phenotypic tests and assigned to the A. calcoaceticus-A. baumannii complex. Species identification was confirmed by 5′-end sequencing of the 16S rRNA gene of all Spanish, Dutch, and Swedish isolates (data not shown) (31) and by ribotyping of the German isolates (35).
TABLE 1.
Epidemiologic characteristics of Acinetobacter isolates used in the present studya
| Strain no. | Designation | Origin or hospitalb | City of isolation | Yr of isolation | Species | Source | PFGE type | AFLP typec | STd | Allele no.
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gtlA | gyrB | gdhB | recA | cpn60 | gpi | rpoD | ||||||||||
| 1 | 62791 | HB | Barcelona | 1993 | A. baumannii | Blood | A | a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| 2 | 52400 | HB | Barcelona | 2000 | A. baumannii | Urine | A | a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| 3 | 151101 | HB | Barcelona | 1994 | A. baumannii | Blood | B | b | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 7 |
| 4 | 288611 | HB | Barcelona | 1999 | A. baumannii | Blood | B | b | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 7 |
| 5 | 218011 | HB | Barcelona | 2000 | A. baumannii | Urine | A | a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| 6 | 228620 | HB | Barcelona | 2000 | A. baumannii | Urine | A | a | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 4 |
| 7 | 220383 | HB | Barcelona | 2000 | A. baumannii | Bronchial aspirate | B | b | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 7 |
| 8 | 213278 | HB | Barcelona | 1999 | A. baumannii | Blood | B | b | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 7 |
| 9 | 60127 | HB | Barcelona | 1997 | A. baumannii | Wound swab | D | c | 5 | 1 | 3 | 3 | 2 | 2 | 3 | 1 |
| 10 | 113402 | HB | Barcelona | 1999 | A. baumannii | Blood | D | c | 5 | 1 | 3 | 3 | 2 | 2 | 3 | 1 |
| 11 | 118151 | HB | Barcelona | 1997 | A. baumannii | Blood | E | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 |
| 12 | 224220 | HB | Barcelona | 2000 | A. baumannii | Bronchial aspirate | E | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 |
| 13 | 135172 | HB | Barcelona | 2001 | A. baumannii | Blood | D | c | 5 | 1 | 3 | 3 | 2 | 2 | 3 | 1 |
| 14 | 135323 | HB | Barcelona | 2001 | A. baumannii | Bronchial aspirate | D | c | 5 | 1 | 3 | 3 | 2 | 2 | 3 | 1 |
| 15 | 135467 | HB | Barcelona | 2001 | A. baumannii | Wound swab | D | c | 5 | 1 | 3 | 3 | 2 | 2 | 3 | 1 |
| 16 | 61588 | UC | Santander | 2000 | A. baumannii | Unknown | c | 7 | 3 | 3 | 3 | 2 | 3 | 3 | 8 | |
| 17 | 61812 | UC | Santander | 2000 | A. baumannii | Unknown | a | 8 | 4 | 4 | 4 | 4 | 4 | 5 | 9 | |
| 18 | 62309 | UC | Santander | 2000 | A. baumannii | Unknown | c | 9 | 1 | 3 | 3 | 2 | 2 | 6 | 8 | |
| 19 | 2002-34100 | HE | Elche | 2002 | A. baumannii | Wound swab | c | 10 | 1 | 4 | 3 | 2 | 2 | 7 | 3 | |
| 20 | 2002-9198 | HE | Elche | 2002 | A. baumannii | Wound swab | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | |
| 21 | 2002-26724 | HE | Elche | 2002 | A. calcoaceticus | Bronchial aspirate | 8 | 5 | ND | 8 | 5 | ND | 10 | |||
| 22 | 2001-30645 | HE | Elche | 2001 | A. baumannii | Bronchial aspirate | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | |
| 23 | 2001-12583 | HE | Elche | 2001 | A. baumannii | Sputum | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | |
| 24 | 2001-11094 | HE | Elche | 2001 | A. baumannii | Bronchial aspirate | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | |
| 25 | 2001-16313 | HE | Elche | 2001 | A. calcoaceticus | Wound swab | 9 | 6 | 5 | 7 | 6 | 1 | 11 | |||
| 26 | RUH2207 | LUMC | Malmö | 1980 | A. baumannii | Sputum | c | 11 | 4 | 11 | 6 | 1 | 7 | 8 | 6 | |
| 27 | RUH134 | LUMC | Rotterdam | 1982 | A. baumannii | Urine | c | 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | |
| 28 | RUH875 | LUMC | Dordrecht | 1984 | A. baumannii | Urine | a | 12 | 8 | 4 | 4 | 4 | 4 | 5 | 5 | |
| 29 | DSM 1139 | DSMZ | A. calcoaceticus | 5 | 8 | ND | 4 | 8 | 1 | ND | ||||||
| 30 | DSM 7324 | DSMZ | A. calcoaceticus | 6 | 9 | 7 | 5 | 9 | 1 | 12 | ||||||
| 31 | DSM 30008 | DSMZ | A. baumannii | c | 13 | 1 | 7 | 8 | 9 | 1 | 4 | 14 | ||||
| 32 | DSM 30006 | DSMZ | A. calcoaceticus | 7 | ND | 9 | 3 | 10 | 4 | 13 | ||||||
| 33 | DSM 30007 | DSMZ | A. baumannii | c | 14 | 1 | 10 | 10 | 6 | 1 | 4 | 14 | ||||
| 34 | W 5420 | CUH | Cologne | 1991 | A. baumannii | Tracheal aspirate | F | f | 15 | 1 | 12 | 11 | 10 | 1 | 9 | 4 |
| 35 | U 1901 | CUH | Cologne | 1991 | A. baumannii | Tracheal aspirate | F | f | 15 | 1 | 12 | 11 | 10 | 1 | 9 | 4 |
| 36 | U 10247 | CUH | Cologne | 1991 | A. baumannii | Urine | G | g | 16 | 10 | 12 | 4 | 11 | 11 | 9 | 5 |
| 37 | U 11177 | CUH | Cologne | 1991 | A. baumannii | Urine | G | g | 16 | 10 | 12 | 4 | 11 | 11 | 9 | 5 |
| 38 | St 284 | CCH | Cologne | 1991 | A. baumannii | Blood | H | h | 17 | 1 | 12 | 12 | 11 | 4 | 10 | 3 |
| 39 | St 14733 | CCH | Cologne | 1990 | A. baumannii | Blood | H | h | 17 | 1 | 12 | 12 | 11 | 4 | 10 | 3 |
| 40 | St 20820 | CMH | Cologne | 1991 | A. baumannii | Blood | I | i | 18 | 10 | 13 | 4 | 11 | 12 | 11 | 5 |
| 41 | St 21359 | CMH | Cologne | 1991 | A. baumannii | Catheter | I | i | 18 | 10 | 13 | 4 | 11 | 12 | 11 | 5 |
| 42 | St 15598 | CMH | Cologne | 1991 | A. baumannii | Catheter | K | k | 19 | 1 | 14 | 3 | 2 | 2 | 9 | 3 |
| 43 | St 14970 | CMH | Cologne | 1991 | A. baumannii | Catheter | K | k | 19 | 1 | 14 | 3 | 2 | 2 | 9 | 3 |
| 44 | St 17093 | CMH | Cologne | 1991 | A. baumannii | Blood | L | k | 20 | 1 | 15 | 13 | 12 | 4 | 12 | 2 |
| 45 | V 7459 | CMH | Cologne | 1991 | A. baumannii | Tracheal aspirate | L | k | 20 | 1 | 15 | 13 | 12 | 4 | 12 | 2 |
| 46 | St 11681 | CMH | Cologne | 1991 | Acinetobacter 13TUe | Blood | M | 11 | 16 | 14 | 13 | 13 | 13 | 15 | ||
| 47 | St 7961 | CMH | Cologne | 1991 | Acinetobacter 13TUe | Blood | M | 11 | 16 | 14 | 13 | 13 | 13 | 15 | ||
| 48 | St 1650 | CMH | Cologne | 1992 | A. baumannii | Blood | N | k | 21 | 1 | 12 | 15 | 2 | 2 | 9 | 3 |
| 49 | St 1954 | CMH | Cologne | 1992 | A. baumannii | Blood | N | k | 21 | 1 | 12 | 15 | 2 | 2 | 9 | 3 |
ND, the sequence could not be determined.
HB, Hospital Bellvitge; UC, Universidad de Cantabría; HE, Hospital General de Elche; LUMC, Leids Universitair Medisch Centrum; CUH, Cologne University Hospital; CCH, Cologne Childrens Hospital; CMH, Cologne Community Hospital.
AFLP type, determined for A. baumannii isolates only.
ST, determined for A. baumannii isolates only.
Acinetobacter genomic species 13TU.
DNA extraction.
Strains were maintained at −70°C in 20% (vol/vol) glycerol in LB medium to preserve genetic variation during storage and were grown overnight on MacConkey agar at 37°C. A loopful from a colony was suspended in 500 μl of distilled water. DNA extraction was carried out by using InstaGene Matrix (Bio-Rad Laboratories, Hercules, CA) in accordance with the manufacturer's instructions. DNA was stored at −20°C until further use.
MLST.
The housekeeping genes for the MLST scheme were selected on the basis of their sequence availability in GenBank and prior studies of the phylogenetic relationships for the genus Acinetobacter and their presence in other MLST schemes available for other bacterial species (22, 26, 28, 30). PCR primers were chosen from previous studies or were designed for amplification of the 10 selected genes: citrate synthase (gltA), DNA gyrase subunit B (gyrB), glucose dehydrogenase B (gdhB), homologous recombination factor (recA), 60-kDa chaperonin (cpn60), glucose-6-phosphate isomerase (gpi), RNA polymerase σ70 factor (rpoD), phospho-glucomutase (pgm), quinate shikimate dehydrogenase (quiA), and coenzyme A thiolase (pcaf) (Table 2).
TABLE 2.
Details of loci and oligonucletide primers used in the present study
| Locus | Gene product | Primer | Sequences (5′→3′) | Amplicon size (bp) | Usage |
|---|---|---|---|---|---|
| gltA | Citrate synthasea | Citrato F1 | AATTTACAGTGGCACATTAGGTCCC | 722 | Amplification/sequencing |
| Citrato R12 | GCAGAGATACCAGCAGAGATACACG | Amplification/sequencing | |||
| gyrB | DNA gyrase subunit Bb | APRU F | TGTAAAACGACGGCCAGTGCNGGRTCYTTYTCYTGRCA | 909 | Amplification |
| M13 [−21] | TGTAAAACGACGGCCAGT | Sequencing | |||
| UP1E R | CAGGAAACAGCTATGACCAYGSNGGNGGNAARTTYRA | Amplification | |||
| M13 F | CAGGAAACAGCTATGACC | Sequencing | |||
| gdhB | Glucose dehydrogenase Ba | GDHB 1F | GCTACTTTTATGCAACAGAGCC | 775 | Amplification |
| GDH SEC F | ACCACATGCTTTGTTATG | Sequencing | |||
| GDHB 775R | GTTGAGTTGGCGTATGTTGTGC | Amplification | |||
| GDH SEC R | GTTGGCGTATGTTGTGC | Sequencing | |||
| recA | Homologous recombination factorc | RA1 | CCTGAATCTTCYGGTAAAAC | 425 | Amplification/sequencing |
| RA2 | GTTTCTGGGCTGCCAAACATTAC | Amplification/sequencing | |||
| cpn60 | 60-kDa chaperonina | CPN 3F2 | ACTGTACTTGCTCAAGC | 479 | Amplification/sequencing |
| CPN R2 | TTCAGCGATGATAAGAAGTGG | Amplification/sequencing | |||
| gpi | Glucose-6-phosphate isomerasea | GPI F1 | AATACCGTGGTGCTACGGG | 508 | Amplification/sequencing |
| GPI R1 | AACTTGATTTTCAGGAGC | Amplification/sequencing | |||
| rpoD | RNA polymerase sigma factor rpoD (Sigma-70)b | 70F RPOD | ACGACTGACCCGGTACGCATGTAYATGMGNGARATCGCNACNCT | 492 | Amplification |
| 70FS | ACGACTGACCCGGTACGCATGTA | Sequencing | |||
| 70R RPOD | ATAGAAATAACCAGACGTAAGTTNGCYTCNACCATYTGYTTYTT | Amplification | |||
| 70RS | ATAGAAATAACCAGACGTAAGTT | Sequencing |
Primers for citrate synthase (accession no. M33037), glucose dehydrogenase B (gdhB; accession no. X15871), 60-kDa chaperonin (cpn60; accession no. AY123669), and glucose-6-phosphate isomerase (gpi; accession no. X89900) were constructed by using available Acinetobacter sequences from conserved regions of each gene.
Primers for DNA gyrase subunit B (gyrB) and RNA polymerase sigma factor rpoD (Sigma-70) (rpoB) were selected and used as described previously (41).
Homologous recombination factor (recA) primers were selected and used as described previously (33).
All PCR amplifications were carried out in a MasterCycler gradient (Eppendorf, Hamburg, Germany) under the following conditions: 30 cycles (denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min) preceded by a 2-min denaturation at 94°C and followed by a 2-min extension at 72°C. PCR products were directly purified from the reaction mixture with the QIAquick PCR purification kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's recommendations. Sequencing of internal fragments of about 450 bp of the selected housekeeping genes was performed in an ABI Prism 377 sequencer using the ABI Prism BigDye terminator cycle sequencing ready reaction kit V2 (PE Applied Biosystems, Foster City, CA) according to the manufacturer's recommendations. Specific sequencing primers and amplification lengths for each gene are listed in Table 2. Sequence data were aligned by CLUSTALX and manually corrected by using the BioEdit program version 5.09 (www.mbio.ncsu.edu/BioEdit/bioedit.html).
For each locus, distinct allele sequences were assigned an arbitrary allele number for identification; these were in-frame internal fragments of the gene which contained an exact number of codons. Each isolate was characterized by a pattern of numbers defining its allelic profile or ST. The similarities between the allelic profiles were obtained by the UPGMA (unweighted pair-group method with arithmetic averages) method available in the MEGA suite programs (version 2.1 [http://www.megasoftware.net]). This set of programs was also used to calculate the maximum percent nucleotide divergence between pairs of alleles at a given locus. The dN/dS ratios, dN being the proportion of nonsynonymous substitutions and dS being the proportion of synonymous substitutions per site, were calculated by using ST analysis and recombinational tests (START; version 1.05 http://outbreak.ceid.ox.ac.uk/software.htm). We also used this program to obtain the index of association (Ia) and the number of polymorphic nucleotide sites. The Ia is defined as the observed variance in the distribution of allelic mismatches in all pairwise comparisons of the allelic profiles divided by the expected variance in a freely recombining population, minus 1. When the alleles are in linkage disequilibrium the Ia is expected not to deviate significantly from zero.
AFLP fingerprinting.
The AFLP fingerprinting was performed according to the method of Nemec et al. (32). DNA digestion was carried out with 5 U of EcoRI and 1 U of MseI, while ligation of EcoRI and MseI adaptors was performed simulteously with 1 U of T4 ligase (Invitrogen, Carlsbad, CA) in a final volume of 20 μl at 37°C overnight. Selective PCRs with fluorescently labeled primer EcoRI-C (NED) and unlabeled primer MseI-T were performed in a MasterCycler gradient with the reagents supplied with the AFLP Microbial Fingerprinting Kit (PE Applied Biosystems) according to the manufacturer's recommendations. The resulting products were denaturalized and charged on to an ABI Prism 377 sequencer for fragment separation. Normalization and fragment sizing was carried out by using GeneScan software (PE Applied Biosystems) in combination with Genographer software (version 1.6.0 [http://hordeum.oscs.montana.edu/genographer]). A similarity matrix for the presence or absence of bands was obtained by using the program Restdist in the PHYLIP 3.63 package. UPGMA was used for grouping and the Pearson product-moment coefficient (r) was used as the measure of similarity.
PFGE.
Twenty-nine of the forty A. baumannii clinical isolates included in the present study, as well as the two Acinetobacter genomic species 13TU isolates, were also characterized by PFGE as described previously (18). For this analysis, two low-frequency cutting restriction enzymes, SmaI and ApaI, were used separately according to the manufacturer's specifications (New England Biolabs, Beverly, MA). DNA restriction fragments were separated in a CHEF-DR III unit (Bio-Rad Laboratories) for 20 h at 14°C and 6 V/cm, with pulse times ranging from 0.5 to 15 s when SmaI was used for restriction and from 1 to 30 s after ApaI restriction. Strain relatedness was assigned in accordance with published criteria (36).
Genome location.
To locate the selected A. baumannii genes in the recently annotated sequence of Acinetobacter ADP1, a local protein database was created with the published open reading frame (ftp://ftp.ncbi.nih.gov/genomes/Bacteria/Acinetobacter_sp_ADP1). The amino acid sequences from A. baumannii amplified PCR products were launched onto this database using BlastP to obtain the genome position of the homologous ADP1 genes (1).
Nucleotide sequence accession numbers.
GenBank accession number ranges for the sequences used in this study are as follows: for cpn60, D156565 to DQ156613; for gdh, from DQ156614 to DQ156661; for gpi, from DQ156662 to DQ156709; for gt1A, from DQ156710 to DQ156758; for gyrB from Q156759 to DQ156806; for recA, from DQ156807 to DQ156855; and for rpoD, from DQ156856 to DQ156904.
RESULTS
Sequencing of housekeeping gene fragments.
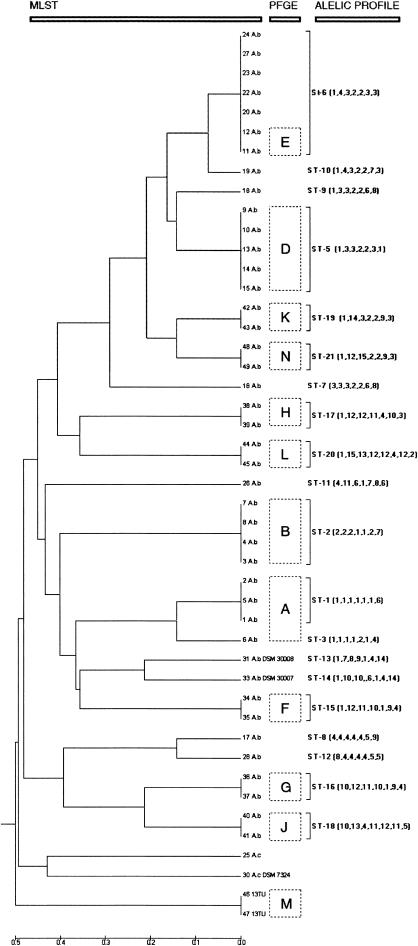
Ten metabolic genes were initially selected to develop the MLST scheme. For two of the selected genes, phospho-glucomutase (pgm) and quinate shikimate dehydrogenase (quiA), the primers failed to amplify the fragment in some of the A. baumannii isolates. Examination of the sequence traces of the beta-ketoadipyl CoA thiolase gene (pcaf) showed that there were two overlapping peaks, suggesting two different nucleotides at a few sites. For the remaining seven genes, the results were satisfactory, i.e., all strains produced one single amplicon and the number of different alleles appeared to be sufficiently discriminative to be used for typing (Table 3). The selected gene fragments were sequenced from each of the 40 clinical A. baumannii isolates and the two reference strains, DSM 30007 (ATCC 19606) and DSM 30008. All sequences had the same size for each gene ranging in length from 305 bp (gpi) to 513 bp (rpoD) (Table 3). Obtained alleles were defined as distinct if they differed at a single nucleotide site and were numbered consecutively for identification. Homologous genes in the Acinetobacter genomic species 13TU and A. calcoaceticus isolates were also sequenced to be used as outgroups in the dendrogram construction. A. calcoaceticus strains 21, 29, and 32 were not used in the MLST scheme because sequences could not be obtained for all loci (Table 1). In A. baumannii isolates, the number of alleles at each locus ranged from 6 alleles for gltA to 12 alleles for gpi. The overall mean value for A. baumannii isolates was 10 alleles per locus, and the percent variable nucleotide sites for these housekeeping genes ranged from 2.13% (cpn60) to 18.03% (gpi) (Table 3). The dN/dS ratio was calculated for all loci and ranged from 0.0318 for gtlA to 0.2570 for cpnd60 (Table 3). The index of association (Ia) was used to test for linkage disequilibrium between alleles at the seven housekeeping genes. For the entire A. baumannii population the Ia value was 2.592. Using only one representative strain for each ST this value was reduced to 1.393 but was still greater than 1. A concatenated loci alignment for each of the strains was used to construct the dendrogram with the UPGMA method (Fig. 1).
TABLE 3.
Variation in loci used in the present A. baumannii MLST scheme
| Locus | Fragment size (bp) | No. of alleles | No. of polymorphic nucleotide sites | % Variable sites | % Nucleotide divergence between pair of alleles
|
dN/dSa | |
|---|---|---|---|---|---|---|---|
| Max | Avg | ||||||
| gltA | 484 | 6 | 45 | 9.29752 | 0.093 | 0.033 | 0.0318 (0.0273) |
| gyrB | 457 | 11 | 22 | 4.81400 | 0.031 | 0.017 | 0.0786 (0.0275) |
| gdhB | 396 | 11 | 23 | 5.80808 | 0.038 | 0.019 | 0.0594 (0.0520) |
| recA | 371 | 8 | 9 | 2.42588 | 0.016 | 0.009 | 0.0561 (0.0094) |
| cpn60 | 421 | 7 | 9 | 2.13777 | 0.083 | 0.025 | 0.2570 (0.0483) |
| gpi | 305 | 12 | 55 | 18.03280 | 0.141 | 0.060 | 0.0415 (0.0327) |
| rpoD | 513 | 10 | 8 | 1.55945 | 0.022 | 0.009 | 0.1232 (0.0826) |
The dN/dS values for all Acinetobacter gene sequences obtained in this study are given in parentheses. Max, maximum.
FIG. 1.
Dendrogram constructed by UPGMA cluster analysis based on the nucleotide differences obtained by sequencing of gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD gene fragments. From left to right are indicated isolate number, species identification (A.b, A. baumannii; 13TU, Acinetobacter genomic species 13TU; A.c, A. calcoaceticus), PFGE type (boxed capital letters), ST, and allelic profile.
Mapping of the selected genes.
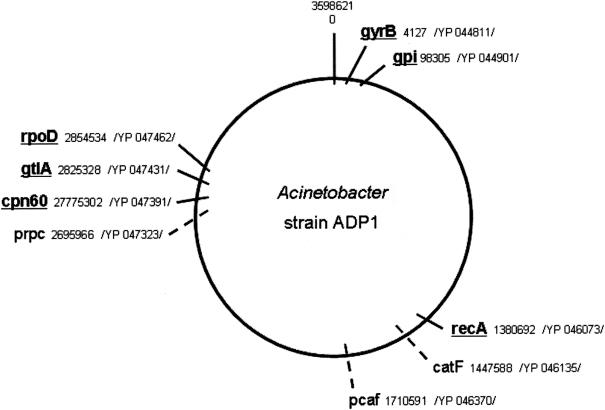
The only available genome sequence of the genus Acinetobacter, Acinetobacter sp. strain ADP1 (3), was used to estimate the genome position of the seven selected A. baumannii genes (Fig. 2). Five of these genes (gyrB, recA, gpi, cpn60, and rpoD) were found to have one copy within the ADP1 genome. The citrate synthase gene (gtlA) seems to have a paralogous gene (prpC) with homologous function. Although both genes are present within the ADP1 genome, the nucleotide differences between the gtlA and prpC genes (sequence identity 47%) are sufficient to be differentiated by standard PCR. No homologous nucleotide or protein sequence with the gdhB gene was detected in the ADP1 genome. As had been expected from our sequence data the genome analysis also revealed the presence of two highly conserved copies of the beta-ketoadipyl CoA thiolase gene (98% nucleotide sequence identity) within the ADP1 genome (pcaf and catF), precluding its use for further analysis.
FIG. 2.
Relative location of the selected housekeeping genes used in the present study on a schematic map of the genome of Acinetobacter strain ADP1 (NC_005966.1). The A. baumannii cpn60 gene is homologous to chaperone Hsp60 from ADP1. Genes used in the present MLST scheme are underlined. The coordinates are given in bases, and the protein accession numbers are indicated.
Evaluation of the A. baumannii MLST scheme.
Table 1 shows the 20 different allelic profiles or STs identified among the 42 A. baumannii isolates. The MLST scheme was evaluated by comparing allelic profiles with restriction fragment patterns generated by AFLP and PFGE.
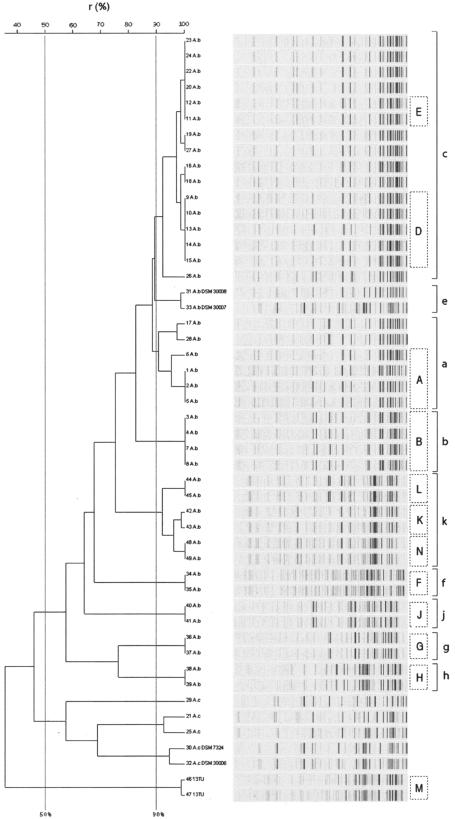
All A. baumannii isolates, including 13 isolates that were not characterized by PFGE, the two Acinetobacter genomic species 13TU isolates, and also the five A. calcoaceticus isolates, were analyzed by AFLP (Fig. 3). Using a similarity cutoff value of 50% for species delineation (9), the strains were grouped into three distinct clusters, one including the reference strain A. baumannii DSM 30007 and all A. baumannii clinical isolates, a second cluster contained all A. calcoaceticus isolates, and the third cluster comprised the two Acinetobacter genomic species 13TU isolates. Within the A. baumannii cluster, using a clustering level of 90% (9), analysis of AFLP fingerprints showed nine different clusters. The largest cluster contained strain 27 (RUH134), a representative of the so-called European clone II, and 14 Spanish isolates from Barcelona, Elche, and the University Hospital of Cantabria. Among these isolates, six different STs (ST5, ST6, ST7, ST9, ST10, and ST11) were identified that differed from each other at one to four loci with one exception; strain 26 (ST 11) differed from strain 27 (RUH134) at all seven loci. Strain 28 (RUH875), the reference strain for European clone I, clustered with outbreak strains from Barcelona and the University Hospital of Cantabria. Among these strains, four different STs were identified (ST1, ST3, ST8, and ST12), with ST1 and ST3, as well as ST8 and ST12, being double-locus variants each. In contrast, most of the German outbreak isolates, the two A. baumannii reference strains (both environtmental isolates), and one group of outbreak-related isolates from Barcelona exhibited different STs and also clustered distinct from each other by AFLP. Three German epidemic strains exhibiting three STs (ST19, ST20, and ST21, two of them being double-locus variants) clustered together by AFLP.
FIG. 3.
Fluorescently labeled AFLP patterns and UPGMA/product-moment cluster analysis of the 49 Acinetobacter strains. Twenty-seven different patterns were obtained after PCR with EcoC and MseT templates among all isolates. Levels of correlation are expressed as percentages of similarity. The known PFGE types are indicated inside boxes in capital letters for comparison. AFLP types are indicated at the far right side.
PFGE analysis of 29 of the A. baumannii clinical isolates generated 11 different PFGE patterns (Table 1). Fifteen of these isolates were representative of the main clonal types (PFGE types A, B, D, and E) involved in a sustained outbreak in Hospital Universitari de Bellvitge (Barcelona) that took place between 1993 and 2001. Another 14 A. baumannii isolates (PFGE types F to L and N), as well as two Acinetobacter genomic species 13TU isolates (PFGE type M), were from eight major outbreaks in various hospitals in Cologne, Germany, that occurred between 1990 and 1992. Epidemiologic details of these strains have been described previously (35). The PFGE clusters were in high concordance with the dendrogram constructed by using MLST data for these strains (Fig. 1). The Barcelona isolates exhibited four different PFGE types and clustered into five different STs, while seven different PFGE types and seven STs were identified among the Cologne isolates. One of the Barcelona epidemic strains (ST6) was also responsible for the outbreak observed in Elche General Hospital in 2001 and 2002. The two Acinetobacter genomic species 13TU isolates, as well as the A. calcoaceticus isolates, clustered distantly from the A. baumannii isolates by MLST.
DISCUSSION
A. baumannii has emerged as an important nosocomial pathogen in various countries in Europe, Asia, the United States, and Latin America (27, 39). This organism is known for its involvement in hospital outbreaks and has sometimes caused interinstitutional spread (27). Whereas several researchers emphasized the genetic heterogeneity among epidemic A. baumannii strains (34, 35, 40), recent data indicate that a few successful epidemic A. baumannii strains (clones) circulate in Europe, including the so-called EU clones I and II that have been isolated in northern Europe (9, 32), and the geographically more widespread EU clone III (38). The contribution of these widespread clones to the overall burden of epidemic A. baumannii strains remains to be determined. In view of the worldwide expansion of multidrug and even panresistant epidemic A. baumannii strains, a better understanding of the global epidemiology of this the species is urgently needed. For early recognition of epidemic A. baumannii strains a typing system is required that is characterized by excellent discriminatory power but even more importantly by high interlaboratory reproducibility that permits the development of a central databank of strain patterns. After local analysis, typing data can then be submitted to the central databank and compared to other patterns of known epidemic strains, thus alerting the investigator if an epidemic strain or a strain with particular virulence properties has been detected.
The main objective of the present study was to develop a new typing scheme for A. baumannii that allows unambiguous comparison of typing data generated at different laboratories. A major advantage of MLST compared to other typing methods results from the use of nucleotide sequence data that offer the possibility of data exchange via an electronic platform. This feature makes this technique ideally suited for national or international surveillance programs involving multiple laboratories and for monitoring the spread of drug-resistant clones.
The A. baumannii MLST scheme described here is based on the allelic variations in seven housekeeping genes of A. baumannii by using a sample of 40 clinical isolates and 2 reference strains of A. baumannii and 7 other isolates belonging to the A. calcoaceticus-A. baumannii complex. Several candidate loci were eliminated, since the chosen gene fragments could not be readily amplified from all initial test isolates. The internal fragments of the seven loci that were selected for the final MLST scheme—gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD—could be amplified from all isolates that were investigated. The dN/dS ratio was less than 1 for all of the selected housekeeping genes, indicating a very low contribution of environmental selection to variation in these genes. The allele diversity ranged from 2.1 to 18% and was relatively large. For comparison, in N. meningitidis the allelic diversity ranged from 5.6% (gdh) to 33.9% (aroE) (28). Considering the smaller number of strains investigated, our data are comparable and consistent with a species of average genetic diversity. Therefore, the selected housekeeping genes are probably adequate for population-genetic studies of A. baumannii (11).
The clustering of A. baumannii isolates achieved by MLST was in good agreement with strain grouping obtained by AFLP and PFGE; both techniques have proven their discriminatory power for A. baumannii in previous studies. Although our strain collection comprised 20 STs, 9 AFLP types were identified. Similarly, among the 29 isolates subjected to PFGE, 11 PFGE types were discernible, corresponding to 12 STs. The MLST scheme was validated by showing that pairs of isolates with the same allelic profile produced identical or very similar fragment patterns by PFGE. The collection of isolates that we examined included the type strains for EU clone I (RUH 875; ST12) and EU clone II (RUH 134; ST6) (9). Although ST12 was not identified among the remaining isolates, ST6 was the strain type involved in hospital outbreaks in Barcelona and in Elche, confirming the widespread occurrence of this successful epidemic A. baumannii clone over a period as long as two decades. Thus, the A. baumannii MLST scheme provides a promising method for unambiguous epidemiologic strain characterization.
Our study is based on only a limited number of A. baumannii isolates from different hospitals distributed throughout Spain, as well as from Germany and from a few other European locations. Overall, these isolates should represent a significant fraction of the European variability within this species. To broaden our understanding of the molecular epidemiology of A. baumannii, the present study should be expanded to larger strain collections from diverse locations around the world. It would also be warranted to extend MLST typing of Acinetobacter species 13TU. Since conventional identification of acinetobacters does not permit one to discern this species from A. baumannii, it would considerably add to the usefulness of our MLST scheme if Acinetobacter species 13TU isolates could be unambiguously characterized and separated from A. baumannii by MLST without prior species identification. The only Acinetobacter species 13TU strain investigated in the present study differed from all A. baumannii isolates at all loci, thus holding promise that our MLST scheme might also be applicable for this species.
In summary, the A. baumannii MLST scheme developed in the present study provides a powerful tool for molecular epidemiologic studies of clinical strains of A. baumannii and offers a new way to study the population biology of this pathogen. The discriminatory power of the MLST typing system is comparable to both PFGE and AFLP. It provides a portable method that is suitable for global epidemiologic study and allows the recognition of epidemic, multiresistant, and virulent clones and monitoring of their international spread. Investigations with more strains from widespread origins will allow a better understanding of the population genetics of this important nosocomial pathogen.
Acknowledgments
This study was funded by grant PM99-0078 of the Spanish Ministry of Science and Technology.
We are grateful to Lenie Dijkshoorn, Leids Universitair Medisch Centrum (The Netherlands) and to Monserrat Ruiz and Juan Carlos Rodríguez, Servicio de Microbiología, Hospital General Universitari d'Elx (Spain), for providing some of the clinical isolates of A. baumannii and A. calcoaceticus.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Aygun, G., O. Demirkiran, T. Utku, B. Mete, S. Urkmez, M. Yilmaz, H. Yasar, Y. Dikmen, and R. Ozturk. 2002. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J. Hosp. Infect. 52:259-262. [DOI] [PubMed] [Google Scholar]
- 3.Barbe, V., D. Vallenet, N. Fonknechten, A. Kreimeyer, S. Oztas, L. Labarre, S. Cruveiller, C. Robert, S. Duprat, P. Wincker, L. N. Ornston, J. Weissenbach, P. Marliere, G. N. Cohen, and C. Medigue. 2004. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 32:5766-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvet, P. J., and P. A. Grimont. 1986. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov., and emended description of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Bacteriol. 36:228-240. [Google Scholar]
- 6.Bouvet, P. J., and S. Jeanjean. 1989. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res. Microbiol. 140:291-299. [DOI] [PubMed] [Google Scholar]
- 7.Cisneros, J. M., and J. Rodriguez-Bano. 2002. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features, and treatment. Clin. Microbiol. Infect. 8:687-693. [DOI] [PubMed] [Google Scholar]
- 8.Corbella, X., A. Montero, M. Pujol, M. A. Dominguez, J. Ayats, M. J. Argerich, F. Garrigosa, J. Ariza, and F. Gudiol. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkshoorn, L., H. Aucken, P. Gerner-Smidt, P. Janssen, M. E. Kaufmann, J. Garaizar, J. Ursing, and T. L. Pitt. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkshoorn, L., R. van Dalen, A. van Ooyen, D. Bijl, I. Tjernberg, M. F. Michel, and A. M. Horrevorts. 1993. Endemic Acinetobacter in intensive care units: epidemiology and clinical impact. J. Clin. Pathol. 46:533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingle, K. E., F. M. Colles, D. R. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feil, E. J., J. M. Smith, M. C. Enright, and B. G. Spratt. 2000. Estimating recombinational parameters in Streptococcus pneumoniae from multilocus sequence typing data. Genetics 154:1439-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnacho, J., J. Sole-Violan, M. Sa-Borges, E. Diaz, and J. Rello. 2003. Clinical impact of pneumonia caused by Acinetobacter baumannii in intubated patients: a matched cohort study. Crit. Care Med. 31:2478-2482. [DOI] [PubMed] [Google Scholar]
- 16.Gerner-Smidt, P. 1992. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Clin. Microbiol. 30:2680-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerner-Smidt, P., I. Tjernberg, and J. Ursing. 1991. Reliability of phenotypic tests for identification of Acinetobacter species. J. Clin. Microbiol. 29:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouby, A., M. J. Carles-Nurit, N. Bouziges, G. Bourg, R. Mesnard, and P. J. Bouvet. 1992. Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J. Clin. Microbiol. 30:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundmann, H., S. Hori, M. C. Enright, C. Webster, A. Tami, E. J. Feil, and T. Pitt. 2002. Determining the genetic structure of the natural population of Staphylococcus aureus: a comparison of multilocus sequence typing with pulsed-field gel electrophoresis, randomly amplified polymorphic DNA analysis, and phage typing. J. Clin. Microbiol. 40:4544-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundmann, H. J., K. J. Towner, L. Dijkshoorn, P. Gerner-Smidt, M. Maher, H. Seifert, and M. Vaneechoutte. 1997. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J. Clin. Microbiol. 35:3071-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpression of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. J. Antimicrob. Chemother. 54:821-823. [DOI] [PubMed] [Google Scholar]
- 22.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. Van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim, A., P. Gerner-Smidt, and W. Liesack. 1997. Phylogenetic relationship of the twenty-one DNA groups of the genus Acinetobacter as revealed by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:837-841. [DOI] [PubMed] [Google Scholar]
- 24.Jawad, A., H. Seifert, A. M. Snelling, J. Heritage, and P. M. Hawkey. 1998. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 36:1938-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeleman, J. G., J. Stoof, M. W. Van Der Bijl, C. M. Vandenbroucke-Grauls, and P. H. Savelkoul. 2001. Identification of epidemic strains of Acinetobacter baumannii by integrase gene PCR. J. Clin. Microbiol. 39:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotetishvili, M., O. C. Stine, Y. Chen, A. Kreger, A. Sulakvelidze, S. Sozhamannan, and J. G. Morris, Jr. 2003. Multilocus sequence typing has better discriminatory ability for typing Vibrio cholerae than does pulsed-field gel electrophoresis and provides a measure of phylogenetic relatedness. J. Clin. Microbiol. 41:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landman, D., J. M. Quale, D. Mayorga, A. Adedeji, K. Vangala, J. Ravishankar, C. Flores, and S. Brooks. 2002. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, N.Y.: the preantibiotic era has returned. Arch. Intern. Med. 162:1515-1520. [DOI] [PubMed] [Google Scholar]
- 28.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maragakis, L. L., S. E. Cosgrove, X. Song, D. Kim, P. Rosenbaum, N. Ciesla, A. Srinivasan, T. Ross, K. Carroll, and T. M. Perl. 2004. An outbreak of multidrug-resistant Acinetobacter baumannii associated with pulsatile lavage wound treatment. JAMA 292:3006-3011. [DOI] [PubMed] [Google Scholar]
- 30.Meats, E., E. J. Feil, S. Stringer, A. J. Cody, R. Goldstein, J. S. Kroll, T. Popovic, and B. G. Spratt. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 41:1623-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellmann, A., J. L. Cloud, S. Andrees, K. Blackwood, K. C. Carroll, A. Kabani, A. Roth, and D. Harmsen. 2003. Evaluation of RIDOM, MicroSeq, and GenBank services in the molecular identification of Nocardia species. Int. J. Med. Microbiol. 293:359-370. [DOI] [PubMed] [Google Scholar]
- 32.Nemec, A., L. Dijkshoorn, and T. J. van der Reijden. 2004. Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J. Med. Microbiol. 53:147-153. [DOI] [PubMed] [Google Scholar]
- 33.Nowak, A., and J. Kur. 1995. Genomic species typing of acinetobacters by polymerase chain reaction amplification of the recA gene. FEMS Microbiol. Lett. 130:327-332. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Bano, J., J. M. Cisneros, F. Fernandez-Cuenca, A. Ribera, J. Vila, A. Pascual, L. Martinez-Martinez, G. Bou, and J. Pachon. 2004. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect. Control Hosp. Epidemiol. 25:819-824. [DOI] [PubMed] [Google Scholar]
- 35.Seifert, H., and P. Gerner-Smidt. 1995. Comparison of ribotyping and pulsed-field gel electrophoresis for molecular typing of Acinetobacter isolates. J. Clin. Microbiol. 33:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tjernberg, I., and J. Ursing. 1989. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. APMIS 97:595-605. [DOI] [PubMed] [Google Scholar]
- 38.van Dessel, H., L. Dijkshoorn, T. van der Reijden, N. Bakker, A. Paauw, P. van den Broek, J. Verhoef, and S. Brisse. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 155:105-112. [DOI] [PubMed] [Google Scholar]
- 39.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]
- 40.Wisplinghoff, H., M. B. Edmond, M. A. Pfaller, R. N. Jones, R. P. Wenzel, and H. Seifert. 2000. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin. Infect. Dis. 31:690-697. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto, S., P. J. Bouvet, and S. Harayama. 1999. Phylogenetic structures of the genus Acinetobacter based on gyrB sequences: comparison with the grouping by DNA-DNA hybridization. Int. J. Syst. Bacteriol. 49(Pt. 1):87-95. [DOI] [PubMed] [Google Scholar]
- 42.Yoo, J. H., J. H. Choi, W. S. Shin, D. H. Huh, Y. K. Cho, K. M. Kim, M. Y. Kim, and M. W. Kang. 1999. Application of infrequent-restriction-site PCR to clinical isolates of Acinetobacter baumannii and Serratia marcescens. J. Clin. Microbiol. 37:3108-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]