Abstract
Menstrual toxic shock syndrome (mTSS) is thought to be associated with colonization with toxic shock syndrome toxin 1 (TSST-1)-producing Staphylococcus aureus in women with insufficient antibody titers. mTSS has been associated with menstruation and tampon use, and although it is rare, the effects can be life threatening. It remains of interest because of the widespread use of tampons, reported to be about 70% of women in the United States, Canada, and much of Western Europe. This comprehensive study was designed to determine S. aureus colonization and TSST-1 serum antibody titers in 3,012 menstruating women in North America between the ages of 13 and 40, particularly among age and racial groups that could not be assessed reliably in previous small studies. One out of every four subjects was found to be colonized with S. aureus in at least one of three body sites (nose, vagina, or anus), with approximately 9% colonized vaginally. Eighty-five percent of subjects had antibody titers (≥1:32) to TSST-1, and the vast majority (81%) of teenaged subjects (13 to 18 years) had already developed antibody titers. Among carriers of toxigenic S. aureus, a significantly lower percentage of black women than of white or Hispanic women were found to have antibody titers (≥1:32) to TSST-1 (89% versus 98% and 100%). These findings demonstrate that the majority of teenagers have antibody titers (≥1:32) to TSST-1 and are presumed to be protected from mTSS. These findings also suggest that black women may be more susceptible to mTSS than previously thought.
Toxic shock syndrome (TSS) is a systemic disease of acute onset characterized by fever, hypotension, myalgia, rash, multiple-organ failure, and late desquamation of hands and feet (6). It is associated with colonization with toxin-producing Staphylococcus aureus in the vagina during menstruation, at other sites due to complications of a staphylococcal infection (especially skin or respiratory tract), or as a complication of a surgical procedure or other medical condition (10, 29).
Menstrual TSS (mTSS) has been associated with menstruation and tampon use. Despite the very low incidence of mTSS, the disease remains of interest, because tampons are widely used. Czerwicnski (8) reported in a recent descriptive research study that approximately 80% of the study participants (women under the age of 41 from California) used tampons at some point during menstruation. It has also been reported recently that about 70% of women in the United States, Canada, and much of Western Europe use tampons at some point during menstruation (17).
In the early 1980s, the yearly incidence of mTSS in the United States was reported to be approximately 10 cases/100,000 women of menstrual age (27). In 1986, the incidence of mTSS was estimated to be approximately 1 case/100,000 women aged 15 to 44 years (10). The reported incidence rates of mTSS in the United States have varied depending upon the geographical region, with the highest rates in the Mountain and North Central states and the lowest in the Southeast (5, 16). Epidemiology studies found that women who developed mTSS were more likely to be white and were users of barrier contraceptives or tampons (5, 10, 11, 20, 21, 24). It has been also reported, based on epidemiology studies, that younger women are at an increased risk of developing mTSS (10).
Although the association between tampon use and mTSS has been documented, little is known about how tampons are involved in the pathogenesis of mTSS. Tampons are not the source of the toxigenic (toxic shock syndrome toxin 1 [TSST-1]-producing) strains of S. aureus and do not appear to increase the cell density of S. aureus in the vagina (15, 18, 19).
Menstrual TSS is generally thought to be caused by S. aureus producing TSST-1 in a susceptible host (2, 26). Four factors are thought to be required for the development of the syndrome: (i) vaginal colonization with a toxigenic strain of S. aureus, (ii) production of TSST-1, (iii) penetration of a sufficient concentration of TSST-1 across the epithelium to cause the disease, and (iv) absence or insufficient titers of neutralizing antibody to the toxin. Vaginal colonization by toxigenic S. aureus has been reported in 1% to 4% of the populations studied (1, 7, 14, 22; J. Parsonnet, A. Tosteson, P. Modern, and K. Wissemann, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1327, 1993). In vitro studies have shown that the production of TSST-1 by toxigenic S. aureus is dependent on environmental factors such as the partial pressure of O2 and CO2 gases (34), as well as other factors such as iron, pH, and temperature (25, 28, 33). Once produced, TSST-1 must then penetrate the vaginal mucosal surface. It has been shown that topical exposure to this superantigen can alter the permeability of vaginal mucosa in a non-dose-dependent manner (9). If TSST-1 is capable of penetrating the epithelial surfaces, it can be neutralized effectively by anti-TSST-1 antibodies (4). Bergdoll et al. have shown that individuals who have developed TSS tend to have lower levels of serum antibody to TSST-1 than healthy individuals (3). Studies have also shown that the presence of serum antibody increases with age (32) and that the vast majority (87 to 100%) of the adult population has developed serum antibody to TSST-1 (12, 22, 32; J. M. Vergeront, L. E. Blouse, B. A. Crass, S. J. Stolz, M. S. Bergdoll, and J. P. Davis, Program Abstr. 24th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 610, 1984).
We hypothesized that the low incidence of mTSS is influenced by a combination of two factors: (i) the majority of women colonized with S. aureus are colonized by nontoxigenic strains, and (ii) most women are protected by the presence of positive antibody titers to TSST-1. We tested this hypothesis by determining S. aureus colonization and TSST-1 antibody titers in a large, diverse population of healthy, menstruating women reflective of the 1990 U.S. census. To our knowledge, there have been no studies of this kind among a large group of menstruating women published in the literature. We are aware of two smaller studies that investigated both vaginal colonization and serum antibody within the same population. The first evaluated 509 adolescent females aged 10 to 19 years from three cities in the United States (Parsonnet et al., 33rd ICAAC). The second evaluated 610 women aged 18 to 45 years in London, United Kingdom; Barcelona, Spain; and Moscow, Russia (22). The present study, consisting of over 3,000 menstruating women aged 13 to 40 years, was designed to evaluate trends in colonization and antibody prevalence among a population reflective of the 1990 United States census and within subgroups by geographic region, race and/or ethnicity, and age.
MATERIALS AND METHODS
Subjects.
Healthy, menstruating women between the ages of 13 and 40 years of age were recruited from five geographically separate sites in North America in order to match the racial profile found in the 1990 U.S. census (white, 80%; black, 12%; Hispanic, 5%; and Asian, 3%). Subjects were recruited by Hill Top Research Inc. in Cincinnati, OH; East Brunswick, NJ; St. Petersburg, FL; Scottsdale, AZ; and Winnipeg, Manitoba, Canada.
Subjects were eligible for enrollment if they had regular menstrual cycles (minimum of 21 days and maximum of 35 days); used tampons at least occasionally; were able to read, write, and understand English; did not bathe or shower within the 2 h prior to their scheduled visit; refrained from using douching substances, vaginal medications, suppositories, feminine sprays, genital wipes, or contraceptive spermacides for 48 h prior to their scheduled visit; and were willing to comply with all other protocol requirements. Subjects were not eligible if they were participating in another clinical study; were pregnant, were actively trying to get pregnant, or suspected they were pregnant; had a gynecological abnormality as judged by the study medical personnel; had an infection of the genitals within the past 6 weeks; had been medically diagnosed as having diabetes, kidney failure, hepatitis, AIDS (human immunodeficiency virus positive), or toxic shock syndrome; or were currently taking (within the last 30 days) immunosuppressive drugs, chemotherapy, systemic antimicrobial drugs, or antifungals or antimicrobials to treat a vaginal infection.
Subjects read and signed informed-consent forms prior to the collection of any information or clinical sampling. Subjects were removed from the study if they failed to meet the inclusion criteria and satisfied any of the exclusion criteria at any time during the study.
Study conduct.
Study protocol and informed-consent document were reviewed and approved by Hill Top's Institutional Review Board. Informed consent was obtained from all subjects participating in this study. Subjects were recruited to sites in each of the five cities and underwent inclusion/exclusion screening. The study was conducted from August 1998 through May 1999.
Sample collection.
Immediately after acceptance of each subject into the study, trained personnel at each recruitment site collected several samples from the subject for analysis. Both anterior nares were swabbed at a penetration of 0.5 to 1.0 in., using a standard Culturette (Becton Dickinson Microbiology Systems, Sparks, Maryland). The anus was sampled using a second Culturette swab (approximately 0.5 to 1 in. past the anal sphincter). The swabs were returned to the Culturettes and the culture medium broken. A midpoint vaginal swab (approximately 3 to 4 in. into the vagina) was taken using a speculum to minimize entry and withdrawal contamination of the swab samples by the flora of the labia. The vaginal swab was placed in a labeled vial containing Amies transport medium with agar (PML Microbiologicals, Mississauga, Ontario, Canada). Blood samples (5 to 10 ml) were drawn from each subject at this visit, using a red/gray top (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) tube. Blood was allowed to clot at room temperature, and the serum was prepared by centrifugation.
Sample handling.
Swabs (nasal, anal, and vaginal) that had been placed in transport medium were refrigerated, packed in frozen cold packs, and shipped overnight to Harvard Medical School, Channing Laboratory (Boston, MA) for isolation and phenotypic identification of S. aureus and TSST-1 production. Collected serum was refrigerated, packed in frozen cold packs, and shipped overnight to Dartmouth-Hitchcock Medical Center (Lebanon, NH) for analysis of TSST-1 antibodies. Clinical laboratories were supplied with subject number, subject's initials, and area of the body sampled.
Swab analyses.
The nasal and anal Culturette swabs from each subject were streaked for isolation onto a mannitol salt agar plate (PML Microbiologicals, Mississauga, Ontario, Canada). The vaginal swab was agitated on a Vortex mixer until the samples were dispersed into the Amies medium with agar. An aliquot (0.1 ml) of undiluted sample and one 10-fold dilution were streaked onto a mannitol salt agar plate. All plates were incubated at 36°C for 48 h in air. After incubation, colony types were visualized for characteristic morphology of S. aureus. Colonies were enumerated, isolated on plates containing tryptic soy agar with 5% sheep blood (PML Microbiologicals), and incubated for 24 h. Gram staining, a catalase test, and a rapid S. aureus-specific latex agglutination test (Staphaurex; Murex Biotech, Kent, England) were performed to phenotypically identify S. aureus.
Determination of TSST-1 production.
After being positively identified as S. aureus, isolates were placed in sterile (16- by 150-mm) glass tubes containing 5 ml of brain heart infusion broth (Becton Dickinson & Company, Sparks, Maryland). Tubes were mixed end over end for 24 h at 36°C. Samples were pelleted by centrifugation (10 min at 3,000 rpm) and filtered into microcentrifuge tubes. Historical samples of S. aureus that were known not to produce TSST-1 and others that produced TSST-1 served as negative and positive controls, respectively. A competitive enzyme-linked immunosorbent assay (ELISA) was used to quantify TSST-1 production (23). All S. aureus strains isolated at the Channing Laboratory were shipped to Dartmouth-Hitchcock Medical Center for confirmatory analyses using ELISA and/or PCR techniques (13).
Serum analyses.
A sandwich ELISA was used to measure human TSST-1 immunoglobulin G (IgG) antibodies. Nonimmune human sera from healthy volunteers (1:4 dilution) and Human Immune Globulin Intravenous IgG (Sandoglobulin; Sandoz Pharmaceutical Corp, East Hanover, NJ) at a 1:64 dilution served as negative and positive controls, respectively. Microtiter plates were coated with TSST-1 (0.5 μg/ml), and sera were diluted serially in phosphate-buffered saline solution and added to the coated wells. Plates were then incubated overnight at room temperature and washed. Goat anti-human IgG-alkaline phosphatase (ICN Biomedicals, Aurora, Ohio) was then added to the wells. After a 3-h incubation at 37°C, wells were washed and enzyme substrate (1 mg/ml p-nitrophenyl phosphate in 10% diethanolamine buffer; Sigma-Aldrich, St. Louis, Missouri) was added. Plates were incubated at 24°C until positive control wells reached an optical density (OD) of 1.0 (405 nm). The ODs of sample solutions were determined, and dilution curves were plotted for each sample. The threshold OD was determined previously by testing sera from patients with TSS caused by TSST-1. All such sera (diluted 1:4) yielded an OD of less than 0.2 times that of the positive control. The antibody titer of each sample was calculated as the highest dilution yielding an OD greater than that of the positive control. Samples with titers of ≤1:4 were classified as being “negative” for antibody, and those with titers of ≥1:32 were classified as “positive.” These classifications are based on previous clinical experience with several hundred patients with either mTSS or TSST-1-induced nonmenstrual TSS. Of these patients, greater than 90% had a titer of ≤1:4 and 100% had a titer of <1:32 (J. Parsonnet, unpublished observations). Titers of 1:8 and 1:16 were classified as “intermediate” (i.e., likely to contain antibodies to TSST-1 but may not be associated with protection from mTSS).
Statistical analyses.
Logistic regression and Fisher's exact tests were used to examine the relationship between being a carrier of S. aureus (both toxigenic and nontoxigenic [nonproducing] separately and combined) or antibody and the following explanatory variables: age category, ethnicity, geographical site, and day of menstrual cycle that the swab sample was taken. For comparisons of the presence/absence of S. aureus, analyses were conducted across (at any) colonization areas and within each specific colonization area.
McNemar's test was used to test for an association between the presence/absence of S. aureus (both toxigenic and nontoxigenic separately and combined) and colonization area (vaginal versus nasal, vaginal versus rectal, and nasal versus rectal).
Logistic regression was used to examine the relationship between the titer of antibody in the blood and the following explanatory variables: presence of toxigenic S. aureus bacteria in each colonization area, age category, ethnicity, and geographical site. Each model was fitted both on the actual antibody value and on the categorized antibody value (≤1:4, 1:8, 1:16, and ≥1:32).
RESULTS
Demographics of subjects.
The ethnic distribution of the 3,012 women enrolled in the study was determined to be as follows: white, 79%; black, 12%; Hispanic, 5%; and Asian, 4%. This distribution generally met the target, except that the percentage of white subjects was slightly lower than targeted (79% versus 80%) and that of Asian subjects was slightly higher (4% versus 3%). The age distribution was the following: ages 13 to 18, 16% (n = 475); ages 19 to 35, 68% (n = 2,036); and ages 36 to 40, 17% (n = 501). The geographic distribution of subjects enrolled in the study was as follows: Arizona, 20% (n = 605); Florida, 21% (n = 642); New Jersey, 20% (n = 602); and Ohio, 21%; (n = 626) (United States) and Manitoba, 18% (n = 537) (Canada).
S. aureus colonization and antibody prevalence for study population.
Table 1 summarizes S. aureus colonization and antibody prevalence results for the overall study population. Evidence of S. aureus colonization at any body site was found in 26% of subjects by using culture methods. Colonization by S. aureus was found to be highest in the nares. Of those subjects colonized by S. aureus, 75% were found to be carriers of nontoxigenic strains (TSST-1 negative) and 25% were carriers of toxigenic strains (TSST-1 positive). Colonization of toxigenic S. aureus was found to be highest in the nares and lowest in the vagina. Fifty-three subjects were colonized with S. aureus at all three body sites, 38 were culture positive in both the vagina and nares, 79 were colonized in the both the vagina and anus, and 42 were culture positive in both the anus and nares. The prevalence of positive titers of antibody to TSST-1 (titer of ≥1:32) in the study population was high, with an overall rate of 85%. This was the case whether or not subjects were found to be colonized with S. aureus. The highest prevalence of antibody (97 to 100%) was found for subjects colonized with toxigenic TSST-1-positive strains.
TABLE 1.
S. aureus colonization and antibody prevalence for general study population
| Subject description | No. of subjects | % of total | % with positive antibody (≥1:32) |
|---|---|---|---|
| All | 3,012 | 100 | 85 |
| Not colonized with S. aureus | 2,222 | 74 | 84 |
| Colonized with S. aureusa | 790 | 26 | 87 |
| Nasal colonization | 548 | 69d | 88 |
| Vaginal colonization | 268 | 34d | 87 |
| Anal colonization | 239 | 30d | 84 |
| Colonized with TSST-1-negativeb strain (any site) | 589 | 20 | 84 |
| Colonized with TSST-1-positivec strain (any site) | 201 | 7 | 98 |
| Nasal colonization | 177 | 88e | 98 |
| Vaginal colonization | 32 | 16e | 97 |
| Anal colonization | 36 | 18e | 100 |
TSST-1-positive and -negative strains.
Nontoxigenic strains.
Toxigenic strains.
Percentage of the those colonized with S. aureus; numbers do not add to 100% due to colonization in multiple body sites.
Percentage of the those colonized with toxigenic S. aureus; numbers do not add to 100% due to colonization in multiple body sites.
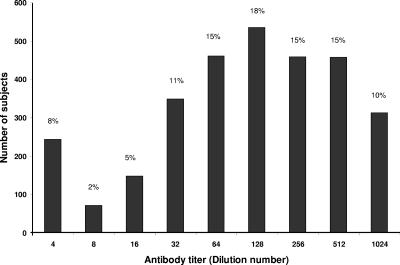
As shown in Fig. 1, the distribution of antibody titers of >1:4 in the study population was found to follow a nearly bell-shaped curve, peaking with 18% of the study population having a 1:128 antibody titer. Only 8 percent (n = 240) of the study population was found to have little or no (titer of ≤1:4) detectable antibody to TSST-1 (Fig. 1). Of these, only four out of 240 were colonized with toxigenic strains, three of whom were colonized in the nose and one in the vagina. The remaining subjects were found to have higher antibody titers to TSST-1.
FIG. 1.
Distribution of antibody titers to TSST-1 in all subjects (n = 3,012). Dilutions above 1:1,024 were not measured individually and are included in the 1:1,024 class.
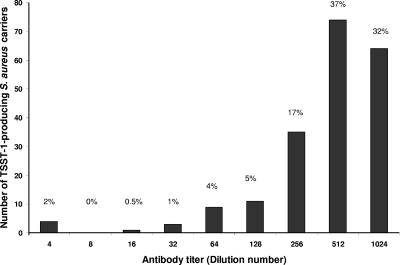
The distribution of antibody titers among subjects colonized with toxigenic S. aureus at any body site is shown in Fig. 2. In this case, the distribution is skewed to higher antibody titers. Most (69%) of these subjects had high (≥1:512) serum antibody titers. In contrast, only 22% of subjects with no detectable colonization with toxigenic S. aureus had high (≥1:512) serum antibody titers.
FIG. 2.
Distribution of antibody titers to TSST-1 among those colonized with toxigenic strains of S. aureus (n = 201). Dilutions above 1:1,024 were not measured individually and are included in the 1:1,024 class.
It is interesting that across all geographic sites, S. aureus was isolated more often from vaginal swabs taken during the first 10 days of the menstrual cycle (P < 0.05) than would be expected on the basis of the distribution of subject visits.
S. aureus colonization and antibody prevalence by geographic location.
Across age groups, no significant differences were found in the rates of S. aureus colonization or toxigenic S. aureus colonization according to the geographic area from which the subjects were recruited (Table 2). The prevalence of positive antibody to TSST-1 (titer of ≥1:32) found to be high in all geographic sites (82 to 89%). Subjects from Manitoba were significantly (P < 0.05) more likely to have a positive antibody titer than were subjects from the other locations except Arizona.
TABLE 2.
S aureus colonization and antibody prevalence according to geographic region
| Location | No. of subjects | No. (%) with S. aureus | No. of S. aureus carriers with TSST-1 | % of S. aureus carriers with TSST-1 | No. (%) with:
|
|
|---|---|---|---|---|---|---|
| Absent antibodya | Positive antibodyb | |||||
| New Jersey | 602 | 140 (23) | 37 | 26 | 45 (7) | 511 (85) |
| Florida | 642 | 188 (29) | 41 | 22 | 53 (8) | 526 (82) |
| Ohio | 626 | 145 (23) | 37 | 26 | 55 (9) | 519 (83) |
| Manitoba | 537 | 153 (28) | 40 | 26 | 32 (6) | 478 (89)c |
| Arizona | 605 | 164 (27) | 46 | 28 | 55 (9) | 518 (86) |
| All subjects | 3,012 | 790 (26) | 201 | 25 | 240 (8) | 2,552 (85) |
Titer, ≤1:4.
Titer, ≥1:32; intermediate values add to 100%.
P ≤ 0.05 versus all other sites except Arizona.
S. aureus colonization and antibody prevalence by racial/ethnic group.
No significant differences were found in the colonization rates of S. aureus across all body sites for the four racial/ethnic groups evaluated (Table 3). Toxigenic S. aureus was more commonly identified in samples from white subjects (28%) than in those from black, Hispanic, or Asian subjects, but these differences were not found to be significant. A significantly (P < 0.05) higher percentage of black subjects than white subjects were found to be colonized vaginally with S. aureus (14% versus 8%). Among those with S. aureus, proportionately greater than twice as many white subjects than black subjects were found to be colonized vaginally with toxigenic strains of S. aureus (15% of white subjects versus 6% of black subjects; P = 0.105). Subjects from all four groups were found to have a high prevalence of positive antibody titer, but the percentage of black subjects with positive antibody titer was found to be significantly (P < 0.05) lower than that of white subjects. In addition, the percentage of black TSST-1 carriers with positive antibody titer was found to be significantly (P < 0.05) lower than that found for white or Hispanic subjects.
TABLE 3.
S. aureus colonization and antibody prevalence according to racial/ethnic group
| Group | No. of subjects | No. (%) with S. aureus | No. of S. aureus carriers with TSST-1 | % of S. aureus carriers with TSST-1 | No. (%) with:
|
% with positive antibodyc among TSST-1 carriers | |
|---|---|---|---|---|---|---|---|
| Absent antibodya | Positive antibodyb | ||||||
| White | 2,375 | 628 (26) | 173 | 28 | 173 (7) | 2038 (86) | 98 |
| Black | 369 | 98 (27) | 19 | 19 | 43 (12) | 289 (78)d | 89e |
| Hispanic | 159 | 40 (25) | 6 | 15 | 15 (9) | 133 (84) | 100 |
| Asian | 109 | 24 (22) | 3 | 13 | 9 (8) | 92 (84) | 100 |
| All subjects | 3,012 | 790 (26) | 201 | 25 | 240 (8) | 2552 (85) | 98 |
Titer, ≤1:4.
Titer, ≥1:32; intermediate values add to 100%.
Titer, ≥1:32.
P < 0.01 versus white subjects.
P < 0.05 versus white and Hispanic subjects.
S. aureus colonization and antibody prevalence according to age.
No significant differences were found in the rates of S. aureus colonization (25 to 30%) across all body sites for the age groups evaluated (Table 4). Similarly, no significant differences were noted in rate of toxigenic carriage (24 to 29%) between age groups. Subjects from all age groups were found to have a high prevalence of positive antibody titer. Eighty-one percent of subjects between the ages of 13 and 18 were found to have already developed positive antibody titers to TSST-1. Among this group of teenagers, 79% of subjects 13 to 15 years of age (n = 121) and 81% of subjects 16 to 18 years of age (n = 354) were found to have already developed positive antibody titers to TSST-1. Compared to older women in this study, the teenaged subjects (13 to 18 years of age) were found to have a significantly (P < 0.05) lower level of positive antibody, but 100% of teenaged carriers of toxigenic S. aureus were found to have already developed positive antibodies to TSST-1.
TABLE 4.
S. aureus colonization and antibody prevalence according to age
| Age range | No. of subjects | % with S. aureus | % with positive antibodya | % of S. aureus carriers with TSST-1 | % with positive antibodya among TSST-1 carriers |
|---|---|---|---|---|---|
| 13-18 | 475 | 30 | 81b | 27 | 100 |
| 19-35 | 2,036 | 26 | 85 | 24 | 97 |
| 36-40 | 501 | 25 | 86 | 29 | 97 |
| All subjects | 3,012 | 26 | 85 | 25 | 98c |
Titer of ≥1:32.
P < 0.05 versus the other two age groups.
Ages of the five S. aureus TSST-1 carriers without positive antibody titer were 19, 20, 23, 34, and 40.
DISCUSSION
The results of this study support the hypothesis that the low incidence of mTSS is influenced by a combination of two factors: (i) the majority of women colonized with S. aureus are colonized by nontoxigenic strains, and (ii) most women are protected by the presence of positive antibody titers to TSST-1. We found that approximately one out of every four healthy subjects (26%) of reproductive age was colonized with S. aureus in either the nose, vagina, or anus. Of those, 25% were carriers of toxigenic strains. About 1/10 of the study population (∼9%) was colonized vaginally with S. aureus, and only 1% of the study population was colonized vaginally with toxigenic strains. Seventy-five percent of S. aureus carriers (or 20% of the population) were colonized with nontoxigenic S. aureus. Positive antibody prevalence, defined in this study as a titer of ≥1:32, was high in the general study population (85%) and even higher among subjects colonized by toxigenic S. aureus in the nose (98%), vagina (97%), or anus (100%). Only 8% of the study population was classified as antibody “negative,” or having an antibody titer of ≤1:4. Of the 460 (15%) subjects found to have antibody titers of ≤1:4, 1:8, or 1:16, only 5 subjects (1.1%) were colonized with TSST-1-producing toxigenic strains at any body site. Of the 32 subjects who were found to have vaginal colonization with toxigenic S. aureus, only one subject (3.1%) was antibody negative (titer of ≤1:4). Based on these findings, it appears that the low incidence of mTSS may be attributed to the facts that the majority of women colonized with vaginal S. aureus are colonized with nontoxigenic strains and that the vast majority of women may be protected by serum antibodies to TSST-1.
The vaginal colonization rates reported here (9% total and 1% toxigenic) are within the range (5% to 12% total and 1% to 2.6% toxigenic) of values reported previously (7, 14, 15, 22; Parsonnet et al., 33rd ICAAC), excluding those reported by Adesium et al. (1) for women in Trinidad, West Indies (28.5% total and 4% toxigenic).
Of the three sites evaluated in this study, the nasal cavity was found to be the major colonization site, with twice the rate of colonization (18.2%) of the other two body cavities evaluated. This rate is within the range (14 to 36%) of data reported in previous nasal colonization studies (1, 12, 30; J. Bustos-Martinez and M. Gutierrez, Abstr. Int. Soc. Microb. Ecol., abstr. 1523, 2004). A higher percentage of nasal isolates than of vaginal isolates was found to be toxigenic vagina (6% versus 1%), consistent with previous findings (1, 12).
The antibody prevalence results reported here are generally in agreement with previous studies among females. Antibody prevalences of 98% among women aged 18 to 45 (22) and 87% among female adolescents aged 10 to 19, using lower antibody titer levels as the standard (≥1:8 versus ≥ 1:32 in this study) (Parsonnet et al., 33rd ICAAC), have been reported previously. The same study of adolescent females reported the antibody prevalence among a subset of TSST-1 carriers to be 100%. In the present study of women aged 13 to 40, we found 85% to have antibody titers (≥1:32), including 81% among those aged 13 to 18 and 100% among TSST-1 carriers between the ages of 13 and 18 years. Only 8% of the study population could be classified as antibody “negative,” or having a titer of ≤1:4, which is somewhat lower than the value (11.8%) reported for male and female Air Force recruits in 1982 (Vergeront et al., 24th ICAAC).
Only 7% of the study population was found to carry toxigenic S. aureus at any body site at the time of the study, but a much higher percentage (85%) was found to have positive antibody titers to TSST-1. This is consistent with findings from previous studies that evaluated both colonization and antibody prevalence in the same study populations (12, 22; Parsonnet et al., 33rd ICAAC). These trends could indicate that a higher percentage of the subjects had been exposed to toxigenic S. aureus at one time than were actually found to be colonized at the time of the study. These findings could also be attributed to different methods used to detect S. aureus and serum antibody titers. It has been shown that culture-based methods for the detection of S. aureus can be less sensitive than other molecular and DNA-based techniques (31). In addition, cultures found to contain S. aureus in this study were then used to determine colonization of toxigenic strains. As a result, the low level of toxigenic strains found in the study population could also be attributed to lower sensitivity of the culture-based methods used here. Other findings suggest that both factors probably contribute to these results. It has been demonstrated that S. aureus culture-negative women can become culture positive within a 1- to 2-year period (J. Parsonnet, M. Delaney, A. Dubois, P. Modern, W. Wieland-Alter, M. Hansmann, J. Seymour, J. Wild, and A. Onderdonk, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1437, 2002). In the present study, we found that a disproportionately higher percentage of subjects in the first 10 days of their menstrual cycle were colonized vaginally with S. aureus. Previous studies noted that menstruation at the time of sample collection was one predictor of vaginal S. aureus colonization (22; Parsonnet et al., 33rd ICAAC). A recent in vivo study has shown that the presence of menses in the vagina provides oxygen to the normally anaerobic vaginal environment (D. R. Hill, D. C. Schmitz, M. Brunner, C. C. Davis, J. A. Flood, M. B. Jones, and T. W. Osborn, Abstr. Biofilms 2003, abstr. 327C, 2003). It is possible that the presence of oxygen in the menses could allow better recovery of S. aureus via culture techniques because the samples would be incubated under aerobic conditions. In total, these findings would be consistent with the notion that vaginal colonization by S. aureus can fluctuate over time in response to environmental factors, such as the presence of menses, and that culture-based methods may not have the sensitivity to detect the presence of S. aureus during times of low colonization.
It is interesting that of the subjects who had positive nasal cultures for S. aureus, only 24% also tested positive as vaginal and/or anal carriers. Toxigenic isolates constituted 32% of the nasal isolates but only 12% of the vaginal and 15% of the anal isolates. These findings suggest that localized colonization areas may occur and that nasal cultures should not be considered predictive of positive screening for vaginal S. aureus colonization. Nineteen subjects colonized with toxigenic strains in one body site were also colonized with nontoxigenic strains in another body site, indicating that colonization with different strains of S. aureus may occur within the same individual.
mTSS incidence rates have been reported to vary according to geographical location, with the highest incidence rates in the Mountain and North Central states and the lowest in the Southeast in the United States (5, 16). Significantly higher rates of seronegativity (antibody titers of ≤1:4) reported for male and female Air Force recruits from the Mountain and Pacific regions compared to those from the South Atlantic and East-Southern Central regions appear to be consistent with reported TSS incidence rates (Vergeront et al., 24th ICAAC). In other work, females aged 10 to 19 in Phoenix, Arizona, have been reported to be less likely to have protective antibodies than residents of Indianapolis, Indiana, or Tampa, Florida (Parsonnet et al., 33rd ICAAC). Based on these previous findings, we expected to find lower colonization rates and/or higher antibody titers among subjects from Florida than among those from the other geographical regions. The results from this study did not support these expectations. We found rates of colonization of toxigenic S. aureus to be lowest in Florida, but the percentage of subjects with protective antibody titers also tended to be lowest in Florida. Subjects from Manitoba, Canada, were found have significantly higher levels of antibody than subjects from any of the other locations studied, with the exception of Arizona.
Epidemiology studies conducted in the late 1980s showed that women who develop mTSS tended to be predominately white (10, 5, 16). Since then, few clinical studies that evaluated colonization and/or antibody prevalence among different racial and ethnic groups have been published (14; Vergeront et al., 24th ICAAC). As a result, it has generally been accepted that women of other races are less susceptible to developing mTSS than white women.
In this study, we found equal rates of S. aureus colonization across all body sites for all groups studied; however, white subjects were more likely to be carriers of toxigenic S. aureus than black, Hispanic, or Asian subjects. Vaginal colonization of S. aureus was found to be significantly higher for black subjects than for white subjects, consistent with findings from a previous study (14). Of those colonized vaginally, more than twice the percentage of white subjects were found to be carriers of toxigenic strains. These results suggest that blacks may have higher vaginal colonization rates than whites, but they tend to be colonized more frequently with nontoxigenic strains of S. aureus.
Overall, we found the percentage of black subjects with positive antibody titer to be significantly lower than that of white subjects. This finding is inconsistent with data reported by Vergeront et al. (24th ICAAC) and could be related to the small number of black women (n = 26) in this study population. Importantly, of those found to be carriers of toxigenic S. aureus, significantly fewer black subjects than white or Hispanic subjects were found to have positive antibody titers (≥1:32). These findings indicate that black women may be more susceptible to mTSS than white women. None of the Hispanic or Asian subjects in the study were found to be colonized vaginally with toxigenic S. aureus, but as a group they were found to have high positive antibody titers. These results suggest that subjects could have been exposed previously to either toxigenic S. aureus or cross-reactive antigens. It is also possible that vaginal carriage of toxigenic S. aureus was not detected due to an expected carriage rate of 1% and the smaller sizes of these groups (Asians, n = 109; Hispanics, n = 159).
There appear to be conflicting reports in the literature regarding the susceptibility of teenagers to mTSS. It has been reported that younger women have an increased risk of developing mTSS (10). It has also been reported that that most individuals develop antibodies to toxigenic S. aureus in the first decade of life (32). Vergeront et al. reported that approximately 70% of young females between the ages of 14 and 15 (n = 41) had developed antibody titer (≥1:100) to staphylococcal enterotoxin F. We found that the vast majority (81%) of teenagers (13 to 18 years of age) had already developed positive antibody titers (≥1:32) to TSST-1, which is in agreement with a previous study (Parsonnet et al., 33rd ICAAC). While this value was found to be significantly lower than that for older women, this small difference may not be clinically relevant. It is important to note that 100% of teenaged carriers of toxigenic S. aureus were found to have positive antibody. This represents a rate of protection equal to, if not higher than, that found for the older women in this study. Based on these findings, teenagers may not have a meaningfully higher risk of developing mTSS than older women.
In conclusion, results from this study support the proposed hypothesis that the low incidence of mTSS can be attributed to the fact that the majority of women colonized with vaginal S. aureus are colonized with nontoxigenic strains and that the vast majority of women are protected by serum antibodies to TSST-1. These findings also suggest that black women may be more susceptible and teenagers less susceptible to mTSS than previously thought.
Acknowledgments
This work was sponsored by The Procter & Gamble Company, Feminine Care Global Business Unit.
We thank Wendy Osterling and Matthew Lawlor (Channing Laboratory, Brigham & Women's Hospital) for providing microbial analysis used in this study, Anita L. Guy of ALG Technical Communications for assistance in preparing the manuscript, and Catherine Davis (Procter & Gamble) for technical review of the manuscript.
REFERENCES
- 1.Adesium, A. A., D. Singh, and R. I. Guness. 1994. Toxic shock syndrome toxin-1 (TSST-1) production and phage susceptibility of Staphylococcus aureus strains from human vaginas and anterior nares in Trinidad. Zentralbl. Bakteriol. 280:371-381. [DOI] [PubMed] [Google Scholar]
- 2.Bergdoll, M. S., B. A. Crass, R. F. Reiser, R. N. Robbins, and J. P. Davis. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock syndrome Staphylococcus aureus isolates. Lancet i:1017-1021. [DOI] [PubMed] [Google Scholar]
- 3.Bergdoll, M. S., B. A. Crass, R. F. Reiser, R. N. Robbins, A. C. Lee, P. J. Chesney, J. P. Davis, J. M. Vergeront, and P. J. Wand. 1982. An enterotoxin-like protein in Staphylococcus aureus strains from patients with toxic shock syndrome. Ann. Intern. Med. 96:969-971. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre, P. F., M. R. Thompson, L. E. Adinolfi, Z. A. Gillis, and J. Parsonnet. 1988. Neutralization of toxic shock syndrome toxin-1 by monoclonal antibodies in vitro and in vivo. Infect. Immun. 56:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broome, C. V. 1989. Epidemiology of toxic shock syndrome in the United States: overview. Rev. Infect. Dis. 11(Suppl. 1):S14-S21. [DOI] [PubMed] [Google Scholar]
- 6.Chesney, P. J. 1989. Clinical aspects and spectrum of illness of toxic shock syndrome: overview. Rev. Infect. Dis. 11(Suppl. 1):S1-S7. [PubMed] [Google Scholar]
- 7.Chow, A. W., K. H. Bartlett, R. Percival-Smith, and B. J. Morrison. 1984. Vaginal colonization with Staphylococcus aureus, positive for toxic-shock marker protein, and Escherichia coli in healthy women. J. Infect. Dis. 150:80-84. [DOI] [PubMed] [Google Scholar]
- 8.Czerwinski, B. S. 2000. Variation in feminine hygiene practices as a function of age. J. Obstet. Gynecol. Neonatal Nurs. 29:625-633. [DOI] [PubMed] [Google Scholar]
- 9.Davis, C. C., M. J. Kremer, P. M. Schlievert, and C. A. Squier. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution and tissue damage. Am. J. Obstet. Gynecol. 189:1785-1791. [DOI] [PubMed] [Google Scholar]
- 10.Gaventa, S., A. L. Reingold, A. W. Hightower, C. V. Broome, B. Schwartz, C. Hoppe, J. Harwell, L. K. Lefkowitz, S. Makintubee, D. R. Cundiff, S. Sitze, and the Toxic Shock Syndrome Study Group. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev. Infect. Dis. 11(Suppl. 1):S28-S34. [DOI] [PubMed] [Google Scholar]
- 11.Haijeh, R. A., A. Reingold, A. Weil, K. Shutt, A. Schuchat, and B. A. Perkins. 1999. Toxic shock syndrome in the United States: surveillance update, 1979-1996. Emerg. Infect. Dis. 5:807-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson, J. A., E. M. Kasworm, B. A. Crass, and M. S. Bergdoll. 1986. Nasal carriage of toxigenic Staphylococcus aureus and prevalence of serum antibody to toxic-shock-syndrome toxin 1 in Utah. J. Infect. Dis. 153:356-359. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, W. M., and S. D. Tyler. 1993. PCR Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin-1 in Staphylococcus aureus, p. 294-299. In D. M. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology principles and applications. American Society for Microbiology, Washington, D.C.
- 14.Linnemann, C. C., Jr., J. L. Staneck, S. Hornstein, T. P. Barden, J. L. Rauh, P. F. Boneventre, C. R. Buncher, and A. Benting. 1982. The epidemiology of genital colonization with Staphylococcus aureus. Ann. Intern. Med. 96:940-944. [DOI] [PubMed] [Google Scholar]
- 15.Martin, R. R., V. Buttram, P. Besch, J. J. Kirkland, and G. P. Petty. 1982. Nasal and vaginal Staphylococcus aureus in young women: quantitative studies. Ann. Int. Med. 96:951-953. [DOI] [PubMed] [Google Scholar]
- 16.Miday, R. K., and E. R. Wilson. 1988. Toxic shock syndrome: incidence and geographic distribution from a hospital medical records reporting system. Am. J. Public Health 78:578-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson, E., and M. Jordan. 2000. Sensitive export: seeking new markets for tampons, P&G faces cultural barriers. Wall Street J., December 8, p. A1.
- 18.Onderdonk, A. B., G. R. Zamarchi, J. A. Walsh, R. D. Mellor, A. Muñoz, and E. H. Kass. 1986. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl. Environ. Microbiol. 51:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onderdonk, A. B., G. R. Zamarchi, M. L. Rodriguez, M. L. Hirsch, A. Muñoz, and, E. H., Kass. 1987. Quantitative assessment of vaginal microflora during use of tampons of various compositions. Appl. Environ. Microbiol. 53:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterholm, M. T., and J. C. Forfang. 1982. Surveillance of toxic shock syndrome in Minnesota. Ann. Int. Med. 96:887-890. [DOI] [PubMed] [Google Scholar]
- 21.Osterholm, M. T., J. P. Davis, R. W. Gibson, J. S. Mandel, L. A. Wintermeyer, C. M. Helms, J. C. Forfang, J. Rondeau, and J. M. Vergeront. 1982. Tri-state toxic-shock syndrome study. I. Epidemiologic findings. J. Infect. Dis. 145:431-439. [DOI] [PubMed] [Google Scholar]
- 22.Parsonnet, J., C. Sullivan, P. Modern, and K. Wissemann. 1997. Risk factors for TSS among European women: vaginal colonization with S. aureus and antibody to TSST-1, p. 72. In J. Arbuthnott and B. Furman (ed.), Proceedings of the European Conference on Toxic Shock Syndrome. The Royal Society of Medicine Press, Ltd., London, United Kingdom.
- 23.Parsonnet, J., J. T. Mills, Z. A. Gillis, and G. B. Pier. 1985. Competitive, enzyme-linked immunosorbent assay for toxic shock syndrome toxin-1. J. Clin. Microbiol. 22:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reingold, A. L., C. V. Bromme, S. Gaventa, and A. W. Hightower. 1989. Risk factors for menstrual toxic shock syndrome: results of a multistate case-control study. Rev. Infect. Dis. 11(Suppl. 1):S35-S42. [DOI] [PubMed] [Google Scholar]
- 25.Sarafian, S. K., and S. A. Morse. 1987. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J. Med. Microbiol. 24:75-81. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert, P. M., K. N. Shands, B. B. Dan, G. P. Schmid, and R. D. Nishimura. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143:509-516. [DOI] [PubMed] [Google Scholar]
- 27.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser,. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical feature in 52 cases. N. Engl. J. Med. 303:1436-1442. [DOI] [PubMed] [Google Scholar]
- 28.Taylor, D., and K. T. Holland. 1988. Effect of dilution rate and Mg+2 limitation on toxic shock syndrome toxin-1 production by Staphylococcus aureus grown in defined continuous culture. J. Gen. Microbiol. 134:719-723. [DOI] [PubMed] [Google Scholar]
- 29.Todd, J. K., A. M. Wisenthal, M. Ressman, S. A. Caston, and R. S. Hopkins. 1985. Toxic shock syndrome. II. Estimated occurrence in Colorado as influenced by case ascertainment methods. Am. J. Epidemiol. 122:857-867. [DOI] [PubMed] [Google Scholar]
- 30.Uemura, E., S. Kakinohana, N. Higa, C. Toma, and N. Nakasone. 2004. Comparative characterization of Staphylococcus aureus isolates from throats and noses of healthy volunteers. Jpn. J. Infect. Dis. 57:21-24. [PubMed] [Google Scholar]
- 31.Veeh, R. H., M. E. Shirtliff, J. R. Petik, J. A. Flood, C. C. Davis, J. L. Seymour, M. A. Hansmann, K. M. Kerr, M. E. Pasmore, and J. W. Costeron. 2003. Detection of Staphylococcus aureus biofilm on tampons and menses components. J. Infect. Dis. 188:519-530. [DOI] [PubMed] [Google Scholar]
- 32.Vergeront, J. M., S. J. Stolz, B. A. Crass, D. B. Nelson, J. P. Davis, and M. S. Bergdoll. 1983. Prevalence of serum antibody to staphyloccocal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J. Infect. Dis. 148:692-698. [DOI] [PubMed] [Google Scholar]
- 33.Wong, A. C., and M. S. Bergdoll. 1990. Effect of environmental conditions on production of toxic shock syndrome toxin 1 by Staphylococcus aureus. Infect. Immun. 58:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide: regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]