Abstract
Water scarcity remains a critical global challenge, necessitating the advancement of sustainable water treatment technologies. Polymeric membranes have emerged as an indispensable solution for desalination and wastewater treatment due to their high efficiency and low energy consumption. However, conventional membrane fabrication relies on petroleum-derived polymers and toxic solvents, generating significant environmental concerns. This review sheds light on the state-of-the-art approaches to sustainable membrane development, focusing on green chemistry principles and circular economy strategies. Mechanosynthesis offers a solvent-free alternative for synthesizing advanced membrane materials, including metal–organic frameworks, covalent organic frameworks, and polymers of intrinsic microporosity. Additionally, the adoption of biobased green solvents, such as Cyrene and γ-valerolactone, provides viable substitutes for hazardous dipolar aprotic solvents traditionally used in membrane fabrication. The incorporation of biopolymers, including cellulose derivatives and polyhydroxyalkanoates, further enhances the sustainability of polymeric membranes. To mitigate membrane waste, circular economy strategies, including downcycling, upcycling, and repreparation via covalent adaptable networks, offer promising pathways for extending membrane lifecycles and minimizing environmental impact.
Keywords: mechanosynthesis, biopolymer, green solvents, upcycling and downcycling, covalent adaptable network membranes
1. Introduction
Water scarcity is a pressing global challenge, with approximately 10% of the global population (around 720 million people) living in regions experiencing high or critical water stress levels as of 2021. The agricultural sector accounts for the majority of global water withdrawals (72%), followed by municipal use for households and services (16%) and industrial activities (12%). In this context, membrane technology has emerged as a critical solution for mitigating water scarcity by enabling the conversion of saline and contaminated water sources into potable water, aligning with the United Nations’ (UN) Sustainable Development Goal 6 (SDG 6, Clean Water and Sanitation) to ensure availability and sustainable management of water and sanitation for all by 2030. Technologies such as reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), and microfiltration (MF) are now integral to desalination and water treatment processes. With nearly 16,000 operational desalination plants worldwide producing approximately 95 million m3/day of desalinated water for human use, membrane technology has become indispensable in addressing global water shortages, and advancing the ambitious targets of SDG 6.
Polymeric membranes, in particular, are widely regarded as a green technology due to their low energy consumption and operational simplicity. However, the sustainability of membrane fabrication and disposal processes remains a significant concern. Conventional polymeric membranes are predominantly manufactured from petroleum-based polymers such as poly(vinylidene fluoride) (PVDF), polysulfone (PSf), and poly(ether sulfone) (PES), which pose considerable ecological challenges. Furthermore, the fabrication process relies heavily on toxic, dipolar aprotic and petroleum-based solvents like N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP) for phase inversion. This results in the annual generation of over 50 billion liters of wastewater contaminated with these solvents. The typical lifespan of RO and NF membranes ranges from 3 to 7 years, after which they are often disposed of via landfills or incineration. This practice contributes to significant environmental degradation, with an estimated 14,000 tonnes of RO membranes discarded annually, releasing substantial amounts of CO2 into the atmosphere. These issues highlight the urgent need to re-evaluate conventional membrane fabrication and disposal practices to align with the principles of sustainability and environmental duties.
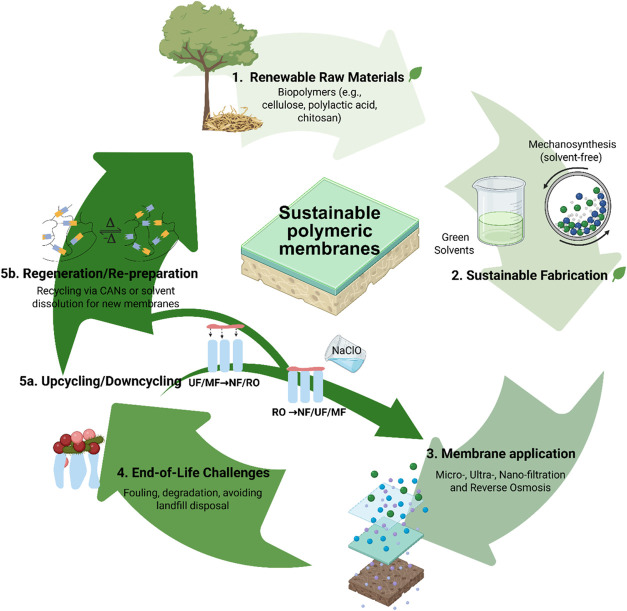
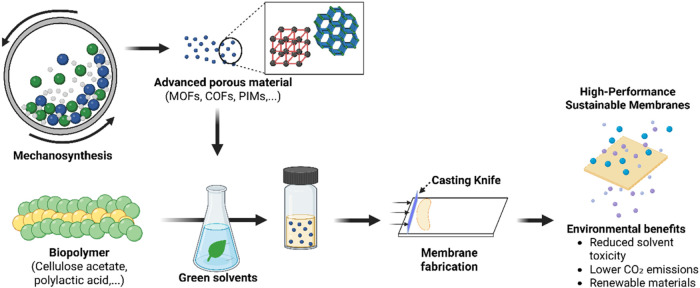
To address these challenges, an innovative framework integrating green chemistry and circular economy principles has been proposed in this study (Figure ). Advances in mechanosynthesis (a solvent-free synthesis using mechanical energy), the use of biobased green solvents (e.g., dihydrolevoglucosenone (Cyrene), γ-valerolactone), and renewable biopolymers (e.g., cellulose derivatives, chitosan, biopolyesters) offer a promising pathway for developing high-performance membranes with reduced environmental impact. Concurrently, circular economy strategies such as upcycling, downcycling, and repreparation of end-of-life (EoL) membranes, provide opportunities to extend membrane lifecycles and minimize waste. The growing body of research on sustainable membrane manufacturing for liquid separation underscores the importance of integrating sustainability into membrane fabrication processes (Figure ). These approaches align with SDG 6, supporting global efforts toward sustainable water management.
1.
Schematic diagram of the sustainable, closed-loop polymeric membrane fabrication coupling mechanosynthesis, green solvents, biopolymer, and circular economy strategies such as upcycling, downcycling and repreparation of polymeric membranes.
2.
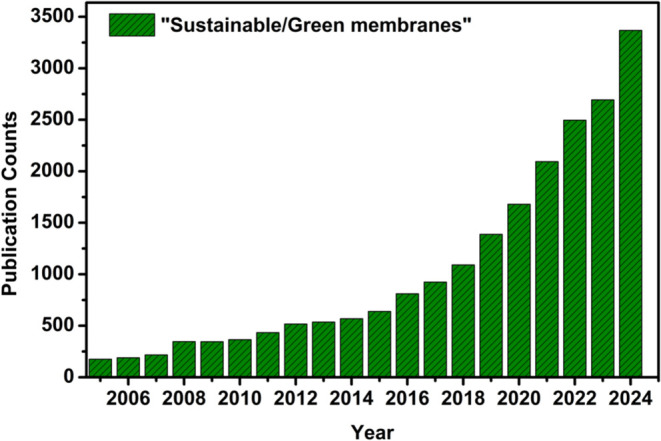
Publication trends on sustainable/green membranes for liquid separation from 2005–2024 (sourced from Web Of Science with the prompt: (“sustainable” OR “green”) AND “membrane” AND “water separation”).
In this review, the state-of-the-art in sustainable membrane fabrication for liquid separation will be critically examined, with a focus on green chemistry and circular economy principles. First, the integration of sustainability into polymeric membrane fabrication is discussed, including the application of mechanosynthesis, green solvents, and biopolymers. Next, the circular economy framework for EoL membranes is explored in detail, emphasizing upcycling, downcycling, and repreparation strategies. Finally, the challenges and future prospects of these technologies are analyzed to provide insights into their scalability and potential impact. By bringing together recent advancements and identifying research gaps, this work seeks to guide the transition toward environmentally responsible membrane technologies that address both water scarcity and ecological sustainability.
2. Sustainable Polymeric Membrane Fabrication: Mechanosynthesis, Green Solvent, and Biopolymer
Organic materials have emerged as a dominant class of membrane building blocks for addressing water treatment challenges. Among these, petroleum-based polymers have historically dominated the membrane market due to their cost-effectiveness, flexibility, and robust thermal and chemical stability. However, the processes associated with crude oil extraction, transportation, and refining pose significant risks of aquatic and atmospheric pollution, contributing to substantial environmental impacts. To address these challenges, it is crucial to advance membrane manufacturing processes by adopting sustainable practices and minimizing environmental footprints. In the current section, we will include the motivation of employing sustainable membrane technologies, the recent trend of mechanosynthesis of membrane materials, the involvement of green solvents in membrane fabrication, biopolymer-based membranes with their economic viability for scaling up, and the current policy and regulatory framework that affects the sustainable membrane technologies.
2.1. Motivations for Sustainable Polymeric Membranes
The shift toward sustainable polymeric membrane technologies is driven by pressing global challenges and the need for environmentally responsible solutions in water treatment. First, water scarcity affects over 2 billion people worldwide, necessitating efficient and sustainable membrane-based processes to provide clean water and meet SDG 6. Additionally, conventional membrane fabrication, reliant on toxic and petroleum-based solvents like NMP and DMF, contributes to environmental pollution, with solvent emissions accounting for significant volatile organic compound (VOC) releases and endangering terrestrial and aquatic life. These traditional membrane fabrication processes also depend on nonrenewable petroleum-based polymers, amplifying resource depletion and greenhouse gas emissions.
In terms of policies, regulatory frameworks, such as the European Union’s Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and the U.S. Toxic Substances Control Act (TSCA), are phasing out hazardous chemicals, creating an urgent need for green alternatives like biobased solvents and renewable biopolymers. Economically, sustainable approaches offer long-term benefits, including reduced waste disposal costs and energy savings. Furthermore, the growing demand for circular economy practices, driven by consumer and industry preferences for sustainable products, underscores the importance of recyclable membranes using strategies like upcycling, downcycling and membrane repreparation. By addressing these challenges, sustainable membrane technologies not only mitigate environmental harm but also enhance water security and support economic resilience.
2.2. Mechanosynthesis of Organic Membrane Building Blocks
Mechanosynthesis is a synthesis technique that utilizes physiochemical transformations induced by mechanical energy, such as shear, impact, extension, and compression. Unlike traditional solvothermal methods, mechanosynthesis operates under solvent-free or minimal-solvent conditions, significantly reducing the environmental footprint of chemical processes and aligning with the Twelve Principles of Green Chemistry. The technique promotes molecular collisions and bond formation through mechanical forces, enabling efficient reactions with reduced energy input and shorter reaction times compared to conventional methods. Key considerations in mechanosynthesis include optimizing reaction efficiency, ensuring reproducibility of material properties (e.g., porosity, surface area), and addressing scalability challenges for industrial applications.
Over the past decade, mechanosynthesis has garnered significant attention from organic chemists and is widely regarded as a green chemistry approach, primarily due to its solvent-free reaction conditions or minimal use of liquid reaction media. Although mechanochemistry aligns well with the principles of green chemistry, its application in the synthesis of conventional membrane polymers remains limited. This is primarily due to the mechanical forces involved, such as grinding and shearing, which induce external stress on polymer chains, often resulting in chain scission and stretching. − These effects reduce molecular weight, viscosity, and mechanical strength, properties critical for effective membrane fabrication. As high molecular weight is vital for achieving robust membrane-forming capabilities and mechanical performance, these limitations hinder the broader application of mechanochemistry in conventional polymer synthesis.
In contrast, novel organic porous materials such as metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and polymer of intrinsic microporosity (PIMs) offer promising alternatives for membrane fabrication due to their high specific surface areas, chemical and thermal stability, and tunable functional groups. However, the conventional synthesis of these materials often involves significant quantities of toxic and hazardous solvents and extended reaction times. For instance, the Innovative Medicines Initiative (IMI)–CHEM 21 has classified toluene and dimethylacetamide (DMAc) as hazardous and problematic, respectively, both of which are commonly used as reaction media in traditional PIM-1 synthesis. − Numerous studies have reported successful mechanosynthesis of novel organic porous materials in the past years (Table ), making mechanochemistry a viable option to substitute traditional synthesis. Therefore, this subsection focuses on recent advancements in the mechanosynthesis of membrane building blocks as a more sustainable approach (Figure ).
1. Recent Studies on Mechanosynthesis of Organic Membrane Building Blocks with Their Environmental Implications and Performance.
| name | mechanosynthesis method | reaction time (min) | yield | additive/solvent used | SBET (m2/g) | refs | |
|---|---|---|---|---|---|---|---|
| metal–organic framework | CuBTC | planetary mill | 60 | 50–60% | 1638 | ||
| MOF-303 | planetary mill | 180 | 50–60% | H2O | 1180 | ||
| MOF-801 | mixer mill | 90 | 87 mg | H2O | 540 | ||
| MOF-804 | mixer mill | 90 | 108 mg | H2O | 755 | ||
| UiO-66 | mixer mill | 120 | 95 mg | H2O | 1145 | ||
| UiO-66-NH2 | mixer mill | 90 | 111 mg | H2O | 815 | ||
| UiO-66-NH2 | planetary mill | 90 | 11.3 g | H2O | 885 | ||
| UiO-66-NH2 | twin screw extrusion | 1.4 kg/h | methanol | 610 | |||
| ZIF-8 | planetary mill | 70 | 50–60% | 1609 | |||
| ZIF-9 | hand-grinding | ∼10 mg | ethanol | ||||
| ZIF-UC-6 | mixer mill | 30 | DMF | 504 | |||
| covalent organic framework | COF-1 | mixer mill | 45 | 76 mg | mesitylene + dioxane/THF | 680 | |
| COF-102 | mixer mill | 45 | 50 mg | mesitylene + dioxane/THF | 2600 | ||
| DMTP-TPB | mixer mill | 60 | 0.9 g, 83% | acetic acid + acetonitrile | 1554 | ||
| Tp+Azo | hand-grinding | 15 | p-toluenesulfonic acid | 1707 | |||
| Tp+BD-(SO3H)2 | hand-grinding | 15 | 72% | 37 | |||
| Tp+Pa-1+MCNT | hand-grinding | 25 | p-toluenesulfonic acid + H2O | 218 | |||
| Tp+Pa-1+nanocellulose | ball mill | 20 | p-toluenesulfonic acid + H2O | 247 | |||
| polymer of intrinsic microporosity | PIM-1 | mixer mill | 15 | 0.9 g, 98% | 520 | ||
| PIM-1 | planetary mill | 20 | 545 | ||||
| PIM-1 | planetary mill | 60 | 856 | ||||
| PIM-4 | mixer mill | 15 | 179 | ||||
S BET: Brunauer–Emmett–Teller (BET) surface area.
3.
Schematic diagram for the comparison of traditional solvothermal synthesis and mechanosynthesis for producing organic membrane materials such as MOFs, COFs, and PIMs.
2.2.1. Metal–organic Frameworks (MOFs)
MOFs are a class of porous materials composed of individual metal centers or clusters linked by bridging organic ligands, offering unique advantages such as a uniform porous structure, large specific surface area, and abundant functional groups, making MOFs particularly attractive for membrane fabrication. The feasibility of MOF mechanosynthesis has been successfully demonstrated by various researchers. −
Mechanochemistry is a great synthesis tool for MOFs, but it can also alter the dimensions of the material. Saini and colleagues synthesized a two-dimensional (2D) cobalt-based zeolitic imidazolate framework, ZIF-9-III, using a mechanochemical process and subsequently incorporated it into a composite membrane (ZIF-9@PVDF) via a nonsolvent-induced phase inversion (NIPS) process. The mechanochemical synthesis involved manual grinding with a minimal amount of ethanol, whereas the conventional solvothermal method required toxic organic solvents, such as DMF, and high energy input (130 °C for 48 h). Interestingly, the two synthesis methods yielded distinct structural outcomes. The conventional solvothermal method produced ZIF-9-I with a three-dimensional (3D) sodalite structure characterized by large open pores, while the mechanochemical approach exfoliated ZIF-9-III into 2D nanosheets. This structural difference significantly influenced the materials’ performance in oil/water separation. Mechanochemically synthesized ZIF-9-III exhibited superhydrophobic properties (water contact angle: 144°) and achieved superior separation efficiency (removal efficiency: 99.8%) compared to conventionally synthesized ZIF-9-I (water contact angle: ∼92°).
The scalability of a technology is equally important to its efficiency and performance. Karadeniz’s group successfully developed a scalable water-assisted mechanosynthesis process for zirconium-based MOFs (UiO-66, UiO-66-NH2, MOF-801, and MOF-804), achieving good yields after reaction times of 30–90 min. The synthesized MOFs demonstrated comparable BET surface areas to those produced via solvothermal methods, maintaining the crucial characteristics of the material while eliminating the use of toxic organic solvents and reducing energy use for long reaction time. Importantly, the water-assisted mechanosynthesis achieved scalability, with 10 g-scale production using a laboratory planetary mill and over 100 g-scale production of UiO-66-NH2 via twin-screw extrusion technology, achieving a throughput of 1.4 kg/h. Therefore, this study revealed a potential scalable approach for mechanosynthesizing MOFs using twin-screw extrusion.
2.2.2. Covalent Organic Frameworks (COFs)
COFs are an emerging class of crystalline, porous materials composed entirely of light elements (H, C, O, N, and B) bonded covalently. Their low density, inherent porosity, large surface area, and tunable pore sizes make them appealing for membrane applications. − Recent advancements in mechanosynthesis provide a promising pathway to greener COF fabrication, circumventing the need for hazardous solvents and potentially reducing energy input.
Lin et al. reported a dual-functional COF with high adsorption capacity and fluorescence sensing ability, synthesized using a mortar and pestle with 2,4,6-triformylphloroglucinol (Tp) and 2,2′-benzidinedisulfonic acid (BD-(SO3H)2) as precursors. The resulting TpBD-(SO3H)2 COF provided abundant negatively charged adsorption sites, achieving a maximum norfloxacin adsorption capacity of 1709 mg/g. This facile and green mechanochemical synthesis highlighted its potential for environmental applications.
Aside from synthesizing pure COFs, Liu et al. fabricated a magnetic COF composite using mechanochemical reaction to remove microcystins in lake water. The facile mechanosynthesis involves a one-step hand-grinding of Tp, p-phenylenediamine (Pa-1) and carbon nanotubes encapsulated magnetic nanoparticles (MCNTs), where the magnetic COF composite exerts a high recovery of microcystins (>85%). Interestingly, this research opens up opportunities to prepare magnetic COFs with excellent properties via green mechanochemistry.
The mechanosynthesis-enabled COFs are not limited to water treatment membrane processes. Wu and colleagues fabricated a freestanding COF membrane by reacting Tp and 4,4′-azodianiline (Azo) via Schiff base reactions using a hand-grinding method. The membrane was subsequently modified with sulfonated poly(ether ether ketone) (s-PEEK) for application in flow batteries as a proton exchange membrane, achieving high proton conductivity (10.88 mS/cm). This innovative approach demonstrated the potential of mechanochemistry in fabricating greener membranes with excellent performance.
2.2.3. Polymers of Intrinsic Microporosity (PIMs)
PIMs are a class of highly porous organic materials characterized by rigid, contorted polymer backbones that create interconnected micropores. These unique structural attributes, including high surface area, excellent permeability, and solution processability, make PIMs highly promising for applications in gas separation, water treatment, and organic solvent nanofiltration (OSN). − Among the various PIMs, PIM-1 stands out as one of the most widely studied and utilized archetypes. Its synthesis typically involves the step-growth polymerization of 5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane (TTSBI) with tetrafluoroterephthalonitrile (TFTPN) in a solvent system comprising DMAc and toluene at 160 °C for approximately 60 min. However, this approach heavily relies on toxic solvents, such as DMAc, which pose significant environmental and health hazards. Therefore, developing greener synthesis routes for PIM-1 that align with the principles of green chemistry and sustainability is imperative.
Dai’s group demonstrated an innovative synthesis of PIM-1 using mechanochemistry. By employing ball-grinding, the reaction time was significantly reduced to 15 min compared to the 24–72 h required for conventional PIM-1 synthesis. The external mechanical force generated during ball milling enhances molecular collisions, facilitates rapid chain growth, and minimizes premature chain termination. Consequently, the mechanochemically synthesized PIM-1 exhibits a higher weight-average molecular weight (M w: 485,000 g/mol) and a low polydispersity index (M w/Mn : 1.4). Moreover, the resulting PIM-1 is readily soluble in organic solvents such as tetrahydrofuran (THF) and chloroform, improving its processability and broadening its potential applications.
In addition to synthesis efficiency and material properties, assessing the environmental impact of mechanochemical approaches is crucial to ensure sustainability. Loh et al. synthesized PIM-1 using a planetary ball mill, achieving a reaction time of under 60 min. The BET surface area (S BET) of the mechanochemically synthesized PIM-1 exceeded 800 m2/g, falling within the typical range for PIM-1 (S BET: 500–1000 m2/g). Furthermore, Xie’s group conducted a Life Cycle Assessment (LCA) comparing mechanochemical synthesis with the conventional solvent-based method. The LCA revealed that the environmental impact of conventional synthesis is 1.5 times greater than that of mechanochemical synthesis for PIM-1 production.
2.2.4. Economic Viability of Mechanochemistry
The economic viability of a technology is a critical factor influencing its potential for commercialization. Techno-economic analysis (TEA) is frequently employed as a robust tool to assess the commercial feasibility of emerging technologies. In a recent study, Wenger et al. conducted a TEA on the green mechanochemical synthesis of UiO-66-NH2. Using a batch mechanosynthesis approach via ball milling, they achieved a production rate of 3.01 g/h with a 43% yield. To improve the accuracy of their cost estimation, the authors incorporated labor costs into the analysis, resulting in a levelised production cost of approximately USD 6,498 per kilogram of UiO-66-NH2. This figure is notably lower than the current market price offered by commercial MOF producers, which exceeds USD 10,000 per kilogram. These findings highlight mechanosynthesis not only as an environmentally sustainable method but also as an economically competitive alternative for MOF production.
Energy consumption is also a fundamental factor in evaluating the economic viability of a technology, as it directly influences operational costs. Loh et al. compared the energy requirements for producing PIM-1 using mechanosynthesis and conventional wet chemical methods, reporting energy consumptions of 24.6 and 36.9 kWh/g, respectively. While this comparison is based on laboratory-scale synthesis, it clearly shows the significant difference in energy usage between the two methods. Specifically, the mechanochemical route consumes approximately 1.5 times less energy than the conventional method, indicating great potential for energy savings during scale-up of PIM-1 production and hence better economic value.
Despite its potential as a green and solvent-free synthesis route, mechanochemistry faces a significant challenge in terms of capital investment. A notable cost disparity exists between conventional hydrothermal synthesis and mechanosynthesis, particularly at the laboratory scale. For example, a planetary ball mill (e.g., RETSCH PM 100) typically costs around £10,000, whereas a hydrothermal setup, comprising a 100–500 mL Teflon-lined autoclave (£100–£1000) and a laboratory oven (approximately £2,000), requires substantially less investment. This 3-fold difference in cost makes mechanosynthesis less accessible, especially for small- and medium-sized enterprises. Therefore, to enhance its practical viability, further attention must be given to optimizing process parameters to improve both energy efficiency and product yield.
2.3. Green Solvents in Membrane Fabrication Processes
Having explored mechanosynthesis, we now turn to green solvents as a complementary strategy. As mentioned, one of the governing class of material used in membrane fabrication is represented by polymers, for example, polyacrylonitrile (PAN), PSf, PES, polyimide (PI), polyamides (PAs), cellulose and fluoropolymers. Commercial polymeric membranes are predominantly produced using the phase inversion methoda controlled process that transitions a polymer from a viscous liquid to a solid state. − This transformation is driven by polymer precipitation, induced by various mechanisms such as nonsolvent immersion, vapor phase exposure, solvent evaporation, or thermal changes. Among the key factors influencing membrane characteristics, the choice of solvent for polymer dissolution plays a critical role, directly affecting membrane properties, including morphology, porosity, mechanical strength, and pore size. These structural attributes, in turn, dictate performance metrics such as permeability and selectivity. ,
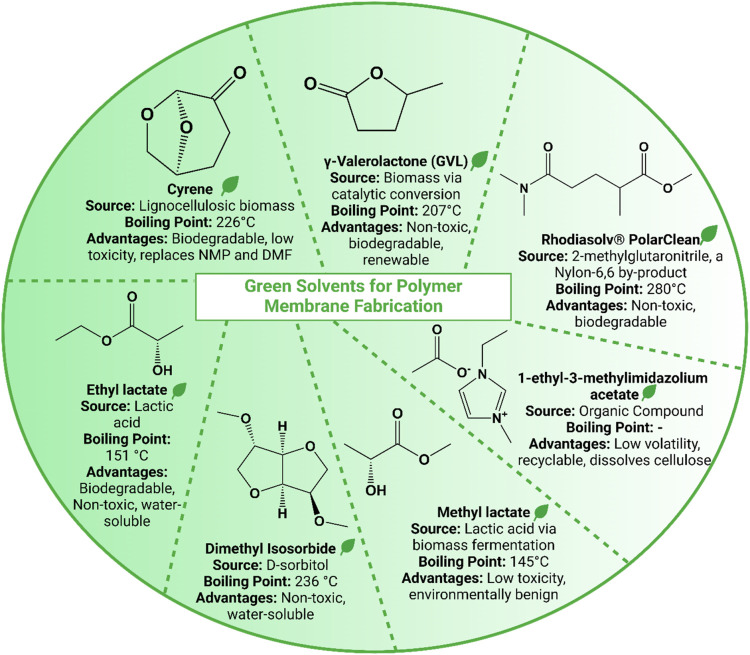
Traditionally, membrane fabrication has relied on toxic organic solvents and petroleum-based compounds, such as NMP, DMAc, DMF, and THF. , Notably, this manufacturing process generates over 50 billion liters of wastewater annually, contaminated with hazardous solvents. To address these environmental and health concerns, recent years have seen the emergence of alternative solvents with lower toxicity and reduced environmental impact (Table ). A summary of the solvent characteristics is illustrated in Figure . This section focuses on these green solvents, which hold promise for sustainable membrane fabrication processes.
2. Comparison between the Recent Studies on Sustainable Membrane Fabrication via Green Solvents and Petroleum-based/Toxic Solvents.
| solvents | polymer | cosolvent | membrane fabrication method | application | flux/permeance | rejection | refs | |
|---|---|---|---|---|---|---|---|---|
| green solvents | cyrene (biobased) | matrimid | THF | NIPS | gas separation | |||
| PES | spray coating + NIPS | UF, support layer | 68.9 | |||||
| PLA | NIPS | UF | 24.0 | |||||
| PSf | NIPS | UF | 29.1 | BSA: 96.7% | ||||
| PVDF | NIPS | UF | ||||||
| dimethyl isosorbide (biobased) | PES | VIPS + NIPS | UF | 2500 | ||||
| PLA | NIPS | UF | 389 | |||||
| PVDF | VIPS + NIPS | UF | 2100 | |||||
| ethyl lactate (biobased) | CA | ethanol | electrospinning | photosensitizer | ||||
| CA + GO | NIPS | UF | 6.0 | methyl orange: 38.9% | ||||
| PLA | NIPS | UF | 93.0 | |||||
| [EMIM]OAc (synthetic) | cellulose | acetone | NIPS | oil/water separation | 39.0 | emulsion retention: 99.0% | ||
| cellulose | DMSO + H2O | NIPS | gas separation | |||||
| CA | acetone | NIPS | UF | 110.0–330.0 | BSA: 91.0% | |||
| γ-globulin: 96.0–98.0% | ||||||||
| methyl lactate (biobased) | CA | 2-MeTHF | NIPS | NF | 2.4–12.8 | rose Bengal: 93.0–99.5% | ||
| CA | NIPS | NF | 0.2 | 100 kDa PEG: 100% | ||||
| PLA | NIPS | UF, MF | 21.9 | 10 kDa dextran: 99.9% | ||||
| rhodiasolv PolarClean (biobased) | PSf | NIPS | UF, support layer | 4000 | ||||
| PVDF-HFP | VIPS/NIPS | UF | 4000–10,000 | methylene blue: 72–89% | ||||
| γ-valerolactone (biobased) | CA | NIPS | NF | 1.7 | rose Bengal: 96.2 | |||
| CTA | NIPS | NF | 15.9 | rose Bengal: 94% | ||||
| polyimide | NIPS | NF | 2.6 | rose Bengal: 97.2% | ||||
| PES | NIPS | NF | 1.1 | rose Bengal: 96.2% | ||||
| PSf | NIPS | UF, support layer | 5,500 | |||||
| PSf | NIPS | UF | 15.6 | BSA: 92.7% | ||||
| PSf | NIPS | NF | 1.3 | rose Bengal: 98.3 | ||||
| petroleum-based/toxic solvents | DMAc | PES | NIPS | UF | 44.4 | powder milk proteins: 98% | ||
| PSf | NIPS | UF | 10.7 | BSA: 90.1% | ||||
| PSf | NIPS | UF | 15.8 | BSA: 96.0% | ||||
| PVDF | NIPS | UF | 32.9 | protein: 98.0% | ||||
| DMF | PSf | NIPS | UF | 3.2 | BSA: 95.1% | |||
| PSf | NIPS | UF | 55.8 | BSA: 95.2% | ||||
| PVDF | NIPS | UF | 17.0 | BSA: >80% | ||||
| NMP | CA | NIPS | UF | 49.3 | ||||
| CA | γ-butyrolactone | NIPS | UF | 1,78l | 100k dextran: 90% | |||
| PES | NIPS | UF | 39.4 | |||||
| PSf | NIPS | UF | 46.3 | BSA: >96.0% | ||||
| PVDF | NIPS | UF | 9.2 | river water TOC removal: 73% |
Data is only referenced for water separation membranes.
4.
Summary of green solvents for polymer membrane fabrication, highlighting sustainable alternatives to conventional solvents. The diagram includes solvents such as Cyrene, GVL, PolarClean, [EMIM]OAc, methyl lactate, DMI, and ethyl lactate, detailing their natural or renewable sources, boiling points, and eco-friendly advantages, for example, biodegradability, low toxicity, and water solubility.
2.3.1. Green Solvent Alternatives
2.3.1.1. Cyrene
Cyrene is a chiral, bicyclic cycloalkane (Figure ), which is a waste-derived and fully biodegradable aprotic dipolar solvent. It is an alternative solvent to the petroleum-based NMP and DMF, developed by Sherwood et al. The green solvent has a boiling point of 226 °C and high water solubility. Polymer materials such as PES, PVDF and cellulose acetate (CA) are highly dissolvable in Cyrene. Membranes fabricated using Cyrene typically exhibit sponge-like , or finger-like pore structures in the support layer, depending on the polymer and coagulation bath conditions. These morphologies closely resemble those of membranes produced using conventional solvents, indicating that Cyrene is a viable substitute in terms of membrane structure and formation characteristics.
To further assess the viability of Cyrene, a comparison test between conventional solvent and green alternative is necessary. Lau’s group employed Cyrene in the fabrication of PES ultrafiltration membranes. They demonstrated that PES ultrafiltration membranes fabricated via spray coating using Cyrene possess better performance than those prepared using the traditional knife cast method using NMP as the solvent, reaching a membrane permeance of 68 L/m2·h·bar, 7 times higher than the knife cast membrane. In addition to increasing membrane performance, the environmental impact of the membrane fabrication process was also reduced due to the usage of greener solvents.
However, a comprehensive assessment of Cyrene must consider its environmental footprint alongside its performance benefits. Also conducted by Lau’s group, the LCA have indicated that membranes produced using biorenewable solvents such as Cyrene may initially exhibit a higher environmental impact than those made with traditional solvents. This is primarily due to lower membrane yields and the limited scope of early stage assessments. Nevertheless, the higher permeance of green solvent-based membranes may offset these drawbacks during later stages of the product life cycle, leading to lower operational energy requirements and, ultimately, a reduced overall environmental burden.
2.3.1.2. γ-Valerolactone (GVL)
γ-Valerolactone (GVL) is another polar aprotic solvent (Figure ). It is a popular nontoxic solvent with high boiling point (207 °C) and has been used in chemical processes and as a flavor additive in perfumes. , In a typical synthetic process, biomass, such as lignocellulose, can be directly converted into GVL using a one-pot catalytic system, integrating all hydrolysis, dehydration, and hydrogenation processes. The steps included hydrolysis of cellulose or hemicellulose to sugar, dehydration of sugar to form furfural or levulinic acid and later formation of GVL from the hydrogenation of furfural and levulinic acid.
In membrane fabrication, GVL has demonstrated promising results as an alternative to petroleum-based solvents such as NMP. Wang’s group reported that membranes fabricated using GVL exhibit a sponge-like porous structure, contrasting with the finger-like structures often associated with NMP-based membranes. This morphological difference is attributed to the disparity in solubility parameters between the solvent and water: GVL (17.5 MPa1/2) has a significantly greater mismatch with water (47.8 MPa1/2) compared to NMP (23.3 MPa1/2). The reduced compatibility between GVL and water lowers the thermodynamic driving force for water in-diffusion during phase inversion, promoting sponge-like pore formation.
By using GVL as the green alternative solvent for NIPS method, Rasool and Vankelecom have managed to fabricate NF membranes with different polymeric materials, including CA, PES, PSf, PI and cellulose triacetate (CTA). All polymers yield reasonable permeance and high rejection toward a cationic dye, Rhodamine B (RB). The best-performing membrane material when using GVL as solvent is CTA, with a high permeance of 16 L/m2·h·bar and excellent RB-rejection of 94%. This study highlights the potential of green solvents for tight NF membranes and demonstrates their applicability in fabricating ultrafiltration membranes or support layers for thin-film composites.
While green solvents such as GVL are generally assumed to provide environmental benefits, LCA studies have challenged this assumption. Lu et al. conducted an LCA comparing the environmental impacts of membranes fabricated using GVL and NMP. Similar to the case in Cyrene-based membrane, their findings revealed that PSf membranes fabricated with GVL incurred higher environmental burdens in most impact categories compared to those made with NMP. These results highlight that bioderived solvents do not necessarily yield lower environmental footprints, as secondary materials and emissions associated with their production can offset their ecological advantages.
2.3.1.3. Rhodiasolv PolarClean
Methyl-5-(Dimethylamino)-2-Methyl-5-Oxopentanoate (commonly known as PolarClean, Figure ) is a nonionic, water-soluble, biodegradable, and eco-friendly synthetic organic solvent. PolarClean has been reported to pose no health hazards during PVDF membrane casting, making it a promising alternative to traditional solvents. , Commercially available from Solvay Novecare, PolarClean is derived from the valorisation of 2-methylglutaronitrile, a byproduct generated during the synthesis of Nylon-6,6. , Fionah et al. successfully demonstrated the use of PolarClean in the fabrication of biochar-incorporated PSf support layer membranes. Compared to membranes synthesized using NMP, which exhibits a finger-like pore structure, PolarClean-fabricated membranes displayed a sponge-like pore structure, enhancing surface wettability. Additionally, membranes synthesized with PolarClean showed higher leaching compared to their NMP counterparts.
Russo’s group further explored the application of PolarClean in the fabrication of flat-sheet poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) membranes via vapor-induced phase inversion (VIPS) and NIPS. The resulting PVDF-HFP membranes demonstrated a high water permeance of up to 3200 L/m2·h·bar and an excellent rejection efficiency for methylene blue (MB) dye at 89%. Their study also highlighted the ability to control critical membrane properties (surface roughness, morphology, and pore size) by modifying parameters including the use of pore-forming additives and variations in the coagulation bath composition.
2.3.1.4. Lactate Esters
Methyl lactate (Figure ), a biodegradable derivative of lactic acid, is a versatile solvent with the ability to dissolve PVDF and CA polymers. , By tuning the types of polymer and polymer concentration, sponge-like and finger-like , pore structure of the membrane can be achieved using methyl lactate as the solvent, showing the versatility of the green solvent. Rasool et al. successfully utilized methyl lactate as the primary solvent to fabricate an NF-based CA membrane. The resulting membrane demonstrated a high rejection efficiency of 92% for rose Bengal dye, with a permeance of 3.5 L/m2·h·bar. Additionally, the membrane achieved efficient removal of magnesium sulfate (MgSO4), with a rejection rate of 97% and permeance of 1.3 L/m2·h·bar. These results underscore the potential of green solvents like methyl lactate to facilitate high-performance membrane fabrication while reducing environmental impact. However, it is important to note that the CA membrane fabricated in this study did not surpass the performance of commercially available membranes, highlighting an area for further optimization.
Ethyl lactate (Figure ), were also explored for polylactic acid (PLA) membrane fabrication. Ethyl lactate, synthesized through the esterification of ethanol and lactic acid derived from biomass via fermentation, is recognized as a sustainable solvent. It exhibits desirable properties such as low volatility, low viscosity, and a high boiling point of 154 °C, alongside its environmentally benign nature. These characteristics underscore its potential as a safe and effective alternative for membrane fabrication processes. Interestingly, the membrane morphology of the ethyl lactate-based PLA membrane shows a finger-like macro-void structure, which is similar to employing Cyrene and dimethyl isosorbide (DMI) green solvents.
2.3.1.5. Ionic Liquids
Ionic liquids (ILs) are liquid salts with melting points below 100 °C, typically composed of a bulky, asymmetric organic cation paired with a weakly coordinating organic anion (Figure ). Although they are synthetic solvents, they possess green properties, such as recyclable and low volatility. ILs have been extensively used as solvents for cellulose, one of the most abundant natural polymers, which presents significant challenges in dissolution. Cellulose membranes are often fabricated via the NIPS method, a process facilitated by the high miscibility of ILs with water. Chen et al. demonstrated the successful use of 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc) as a solvent for cellulose-based membranes in oil–water separation. The incorporation of acetone as a cosolvent significantly enhanced the water permeability of the membrane by 178%, while maintaining a high emulsion retention efficiency of 99.0%.
In addition to dissolving pure cellulose, the ionic liquid [EMIM]OAc can also dissolve cellulose derivatives such as CA. Kim et al. employed [EMIM][OAc] as the primary solvent in the fabrication of CA ultrafiltration (UF) membranes. The resulting membrane morphology varied significantly, ranging from sponge-like pore structures to macrovoids, depending on the presence of a cosolvent and the concentration of the polymer. The CA membranes fabricated using [EMIM][OAc] exhibited promising separation performance, achieving a permeance of 110 L m–2 h–1 bar–1 and a protein rejection rate of up to 98%. This performance is comparable to that of CA membranes prepared using conventional NMP, which typically demonstrate a permeance of 100 L m–2 h–1 bar–1 and similarly high protein rejection. These findings suggest that [EMIM][OAc] is a viable green solvent alternative for CA membrane fabrication, offering competitive separation efficiency with potentially reduced environmental impact.
Despite their advantages, the use of ILs is not without drawbacks. Their high production costs, energy-intensive synthesis processes, and potential environmental impact undermine their reputation as green chemicals. Studies have reported the toxicity of certain ILs and their slow biodegradability, raising concerns about their suitability as sustainable alternatives to conventional solvents. − Therefore, the adoption of ILs as green solvents requires careful evaluation of their environmental and economic implications.
2.3.1.6. Other
Dimethyl isosorbide (DMI, Figure ) is a nonhazardous, water-soluble solvent derived from D-sorbitol, a renewable sugar-based starting material. It has emerged as a promising green alternative to many dipolar aprotic solvents. DMI-based membranes possess similar morphological structure as of Cyrene-based membranes when using PLA as the polymeric material. The DMI-based PLA membrane presents a macrovoid with finger-like pore structure in the support layer, similar to the Cyrene- and ethyl lactate-based PLA membrane.
Employing DMI on membrane fabrication also provide good filtration performances. Gomez d’Ayala et al. demonstrated the sustainable fabrication of PLA membranes using DMI, which exhibited excellent properties. The membranes had pore sizes ranging from 0.02 to 0.09 μm and achieved a pure water permeance of 314 L/m2·h·bar, highlighting their high performance.
2.3.2. Compatibility of Green Solvents
While these green solvents show promise, their practical adoption hinges on compatibility with membrane polymers. Hansen solubility parameters (HSP) theory is a powerful tool for predicting polymer solubility in solvents to achieve uniform solutions. ,, HSP theory is based on three key physiochemical components of molecular interactions: dispersive interactions (van der Waals’ force, δd), polar interactions (δp) and hydrogen bonding (δh). Table shows the HSP values for various solvents. Using these parameters, the Hansen solubility sphere can be modeled to determine the compatibility of a solvent for dissolving a specific polymer. This compatibility is assessed by calculating the interaction distance (R a) between the polymer and the solvent, as described by eq
| 1 |
where P and S are the polymer and solvent, respectively.
3. HSP Values for Various Solvents.
| Hansen
solubility parameters |
||||
|---|---|---|---|---|
| solvents | δd (MPa) | δp (MPa) | δh (MPa) | refs |
| water | 15.6 | 16.0 | 42.3 | |
| DMF | 17.4 | 13.7 | 11.3 | |
| NMP | 18.0 | 12.3 | 7.2 | |
| rhodiasolv PolarClean | 15.8 | 10.7 | 9.2 | |
| cyrene | 18.8 | 10.6 | 6.9 | |
| dimethyl isosorbide | 17.6 | 7.1 | 7.5 | |
| methyl lactate | 15.5 | 7.2 | 7.6 | |
| ethyl lactate | 16.0 | 7.6 | 12.5 | |
| γ-valerolactone | 17.1 | 11.9 | 6.2 | |
The Hansen solubility sphere is constructed by defining a radius (R 0) that corresponds to the maximum solubility distance from the polymer center in a three-dimensional (3D) space (Figure ). A solvent with a lower R a value indicates better compatibility with the polymer and a higher likelihood of forming a homogeneous and stable doped solution. Conversely, if R a exceeds R 0, the solvent is considered a bad or undesirable solvent to be used for dissolving the polymer.
5.
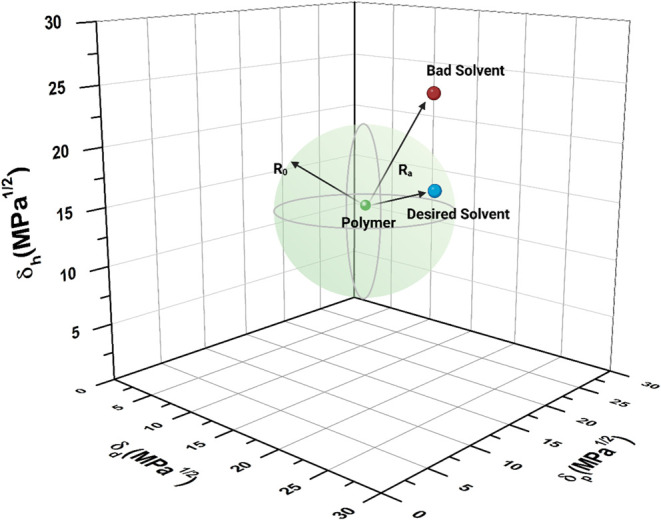
Hansen solubility sphere (green sphere) and R a using a three-dimensional graph. The green dot at the center denotes the polymer’s solubility parameters, with the sphere’s radius (R 0) defining the solubility boundary. The blue dot (Desired Solvent) lies within the sphere (R a < R 0), indicating good compatibility, while the red dot (Bad Solvent) lies outside (R a > R 0), indicating poor compatibility. R a represents the distance between the polymer and solvent in solubility parameter space.
Furthermore, the miscibility of the polymer in a solvent can be determined by calculating the relative energy difference (RED), defined as
| 2 |
The RED value provides insights into solvent performance. If RED < 1, the solvent is considered good, exhibiting high miscibility with the polymer. When RED > 1, the solvent is unsuitable, leading to a nonhomogeneous polymer mixture. In rare cases, when RED = 1, the solvent is at a boundary condition and may only marginally dissolve the polymer.
2.3.3. Economic Challenges in Adopting Green Solvents for Membrane Fabrication
The shift toward green solvents in polymeric membrane fabrication is driven by environmental and health considerations. However, economic viability remains a major barrier to widespread adoption. While green solvents are often biodegradable, nontoxic, and derived from renewable sources, their high-cost relative to conventional petroleum-based solvents limits their industrial scalability.
Ethyl lactate, derived from renewable biomass, is among the least expensive green solvents, costing approximately £38/kg (data sourced from Merck). However, its applicability is restricted, as it is primarily effective for dissolving CA and PLA. More versatile green solvents, such as Cyrene and GVL, have gained attention in membrane research due to their compatibility with a wider range of polymers. Nevertheless, Cyrene is almost twice as costly as traditional solvents like NMP and DMF, while GVL is nearly five times more expensive, posing a considerable economic challenge.
Additionally, emerging green solvents such as [EMIM][OAc] and methyl lactate are significantly more costly, exceeding £18,000/kg and £2000/kg, respectively (data sourced from Merck). Leading from that, the high cost made them impractical for routine use in large-scale membrane fabrication.
2.4. Biopolymers as Green Alternatives to Fossil-derived Polymers
Biopolymers are polymers derived from living organisms such as microorganisms, plants, or animals and are composed of biodegradable, renewable monomers. Unlike synthetic polymers, biopolymers are inherently sustainable and environmentally friendly, making them an attractive alternative to petroleum-based polymers. In recent years, their potential as a sustainable material has garnered significant attention in membrane fabrication, particularly as the membrane market and research community shift toward addressing global challenges such as plastic waste accumulation and resource depletion. , Biopolymers exhibit unique characteristics, including biodegradability and versatility in material design, which positions them as a promising class of materials for sustainable applications. Broadly, biopolymers can be categorized into three main types: polysaccharides, polyesters, and proteins. This section highlights recent advancements in membrane fabrication using biopolymers, with a focus on membrane structure, material characteristics, and filtration performance.
2.4.1. Polysaccharides
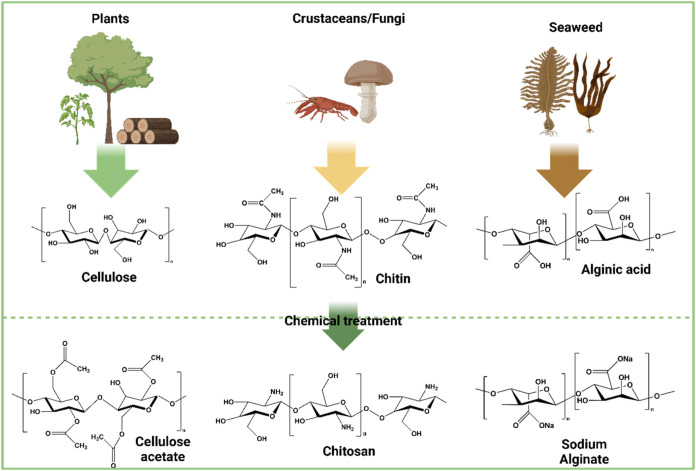
Polysaccharides are long-chain polymeric carbohydrates composed of monosaccharide units linked by glycosidic bonds. Figure illustrates the various types of polysaccharides with their sources. These polymers often occur in heterogeneous forms, with slight modifications in their repeating units. Depending on their structure and composition, polysaccharides exhibit diverse properties that differ significantly from their monosaccharide building blocks, including variations in crystallinity, solubility, and mechanical behavior. Certain polysaccharides are amorphous, while others are water-insoluble, offering a wide range of functionalities for membrane applications.
6.
Schematic diagram of polysaccharide-based membrane materials derived from various natural sources, including plants, crustaceans, fungi, and algae. The diagram depicts the extraction and processing of biopolymers such as cellulose, chitin, and alginate from these sources, which are then combined into a unified polysaccharide structure for membrane fabrication, emphasizing a sustainable and biobased approach to material design for potential applications in wastewater treatment.
2.4.1.1. Cellulose-based Derivatives
Cellulose is a polysaccharide predominantly found in plants or produced via the microbial fermentation of sugars, making it one of the most abundant biopolymers in nature. Its structure consists of long macromolecular chains of β-d-glucose units. Due to its abundance of hydroxyl groups, cellulose exhibits exceptional hydrophilicityan essential property for water treatment membranes. However, pristine cellulose is rarely utilized in membrane fabrication because of its low solubility in most solvents and its limited mechanical and thermal stability. To overcome these limitations, various cellulose derivatives can be synthesized through chemical modifications. For example, CA is produced by treating cellulose with acetic acid, acetic anhydride, and sulfuric acid as a catalyst, while hydroxyethyl cellulose (HEC) is obtained by reacting ethylene oxide with alkali-treated cellulose.
Although pristine cellulose is not widely applied in membrane fabrication, some progress has been made in dissolving cellulose. Li’s group developed a cellulose-based NF membrane using the layer-by-layer (LbL) technique for sodium chloride (NaCl) removal. Bamboo cellulose and chitosan were dissolved in N-methylmorpholine-N-oxide (NMMO) solvent and processed into cellulose support membranes using the immersion gel method. Chitosan introduced positive charges into the support matrix, while sodium carboxymethyl cellulose, an anionic cellulose ether, was applied as a top-coating layer. The resulting composite membrane demonstrated a rejection rate of 36% and a flux of 121 L/m2·h for 500 ppm of NaCl at 0.3 MPa, showcasing a scalable and convenient method for NF membrane production.
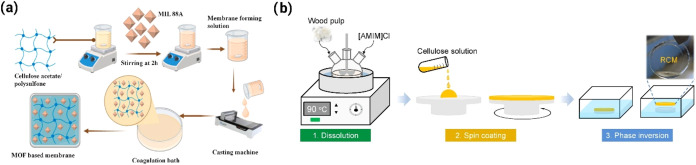
CA, an ester derivative of cellulose, is one of the most frequently used cellulose-based materials in membrane fabrication. Recently, Ananthi et al. fabricated a CA-based UF membrane incorporating MOFs for the removal of micropollutants (Figure a). Using NIPS method, CA, polystyrene, and MIL-88A (a MOF composed of Fe clusters and fumarate linkers) were dissolved in an acetone/chloroform mixture and cast into a UF membrane. The fabricated membrane exhibited excellent separation performance, achieving flux values of 75.2 L/m2·h with an 80% removal rate for diclofenac and 86.9 L/m2·h with a 78% removal rate for ciprofloxacin.
7.
Schematic diagram of (a) CA/MOF/PSf membrane fabrication via NIPS and (b) regenerated cellulose membrane fabrication via spin-coating coupled with NIPS.
Modified CA membranes have also been developed for specific applications. Ning et al. prepared deacetylated cellulose acetate (d-CA) nanofibers via electrospinning for oily water purification. CA membranes were treated with a NaOH mixture to produce d-CA membranes, which were then reinforced with bacterial cellulose for improved performance. The resultant membranes demonstrated remarkable hydrophilicity (water contact angle >40°) and achieved exceptional separation performance in n-hexane/water emulsions, with permeance values of 5479 L/m2·h·bar and 99.4% removal efficiency (with emulsifier) and 9364 L/m2·h·bar with 98.3% removal efficiency (without emulsifier).
To achieve sustainability, recycling cellulose-based materials is another promising approach. Torkashvand et al. fabricated CA membranes for forward osmosis (FO) applications using recycled cigarette butts as the source material. Cigarette filters, which consist of CA, were manually separated, purified, dissolved in NMP, and cast using the NIPS method. The recycled membranes demonstrated effective heavy metal removal, achieving a flux of 13.2 L/m2·h and removal efficiencies of 85–90% for chromium(IV), cadmium, and lead ions.
Regenerated cellulose (RC), another cellulose subclass, involves converting natural cellulose into soluble derivatives through chemical treatment. Song et al. employed RC to fabricate membranes for OSN (Figure b). The RC was sourced from a processed wood pulp board from a paper mill. Using a green ionic liquid, 1-allyl-3-methylimidazolium chloride ([AMIM]Cl), RC was dissolved and fabricated into membranes via spin coating and NIPS. The membranes, with thicknesses ranging from 150 to 350 μm, exhibited ethanol permeance of 30 L/m2·h·bar and high molecular selectivity for Alcian blue/Rifampicin (294) and Alcian blue/Tetracycline (68) mixtures, showcasing their potential for OSN applications.
2.4.1.2. Chitosan
Chitosan is a biopolymer composed of linear amino polysaccharides consisting of β-d-glucosamine and N-acetyl glucosamine units. Typically, it appears as a white, hard, and inelastic material. Chitosan is derived from the alkaline deacetylation of chitin, which is abundant in the exoskeletons of crustaceans and the cell walls of fungi. Its biodegradability, biocompatibility, and nontoxic nature make it a promising candidate for membrane fabrication. ,
Chitosan’s inherent hydrophilicity, attributed to its abundance of amine and hydroxyl functional groups, enhances its adsorption capabilities for charged contaminants. For instance, Wu et al. developed a chitosan-based cellulosic nanofibril membrane composite for dye adsorption. Anionic cellulose nanofibrils were incorporated to improve the acid resistance of the cationic chitosan via ionic and hydrogen bonding. The composite membrane was fabricated using a gel-casting method. The resulting chitosan/cellulose composite exhibited a high adsorption capacity for methylene blue, a positively charged dye, achieving an adsorption rate of 14.7 mg/g under strongly acidic conditions. Furthermore, the membrane demonstrated excellent reusability, maintaining an 85% desorption efficiency after multiple adsorption–desorption cycles.
In addition to dye removal, chitosan has been applied in nuclear wastewater decontamination. A chitosan-based thin-film composite NF membrane was synthesized via interfacial polymerization (IP) followed by polyethylenimine (PEI) postfunctionalization. In this study, chitosan was cross-linked with trimesoyl chloride to form a PA selective layer, and surface functionalization with PEI introduced cationic functional groups. The fabricated membrane exhibited high water permeance (17.4 L/m2·h·bar) and excellent rejection rates for CoCl2 (94.8%), SrCl2 (93.1%), and CsCl2(32.3%).
2.4.1.3. Alginate
Alginate is a seaweed-derived, hydrophilic polysaccharide consisting of alternating blocks of β-d-mannuronic acid (M) and α-l-guluronic acid (G) residues arranged in homopolymeric (MM or GG) or heteropolymeric (MG) sequences. Alginates are widely used due to their biocompatibility and gel-forming properties. Xia et al. utilized sodium alginate (SA) as a hydrogel membrane coupled with Cu2+ metal ion for surface modification to the RO thin film composite (TFC) via LbL method. SA is a natural polyelectrolyte with high density of hydroxyl group and carboxyl groups, making it easy to form ionic bonds with metal ions to form polyelectrolyte hydrogel. The surface-modified RO membrane possesses water flux up to 71.4 L/m2·h, which is 93% better than the unmodified membranes without any significant decrease in salt rejection. Furthermore, the flux recovery rate of the surface-modified membrane is 26% better than that of the unmodified membrane using bovine serum albumin (BSA) as the modeled fouling tests. The excellent antifouling capabilities of the membrane can be attributed to the synergy of the polyelectrolyte hydrogel cross-linked with metal ions.
Due to the excellent hydrophilicity of alginate compounds, they were extensively used in oil/water separation. Wang’s group prepared a SA electrospun membrane incorporated with tannic acid (TA) to form TA-Fe(III) complexes via a coordination reaction. Then a layer of β-FeOOH nanoparticles was constructed on the surface of the membrane. The resulting nanofibrous membrane exerts excellent underwater superoleophobicity and anticrude oil fouling properties. The nanofibrous membrane has high flux (∼1900 L/m2·h) and separation efficiency (99.6%) for crude oil-in-water emulsions. Interestingly, the membrane also possesses photo-Fenton self-cleaning that can degrade the absorbed pollutants on the membrane surface.
2.4.2. Biopolyesters
Shifting from polysaccharide-based solutions, biopolyesters offer distinct advantages and challenges in membrane design. Polyesters are a class of polymers characterized by one or more repeated ester linkages in their main molecular backbone, and they are most commonly associated with synthetic fibers. In contrast, biopolyesters are a subclass of polyesters that are biodegradable and biocompatible, derived from renewable biological sources. Biopolyesters have garnered significant attention as sustainable substitutes for petroleum-based polymers. They can be synthesized through various methods, including microbial fermentation, , chemical synthesis using biobased monomers or direct extraction from natural sources.
2.4.2.1. Polylactic Acid
Polylactic acid (PLA) is a biopolymer composed of lactic acid monomers, where the lactic acid is typically derived from the fermentation of sugars or starches. Among many biopolymers, PLA stands out as a promising material for membrane fabrication due to its processability, low environmental impact, high mechanical and thermal strength, and biocompatibility. −
Despite its potential as a sustainable alternative to conventional polymers, PLA is intrinsically hydrophobic, which limits its applicability in water treatment. To address this limitation, a PLA-based hollow fiber membrane was synthesized using the NIPS method, with a surfactant (Tween-80) and poly(vinylpyrrolidone) (PVP) as coadditives to enhance the hydrophilicity of the membrane surface. The modified PLA membrane exhibited a significant improvement in water permeance, increasing by 816.4% (151.2 L/m2·h·bar) compared to pristine PLA membranes. Tween-80 improved the dispersity of PVP, facilitating its migration toward the membrane surface via hydrogen bonding, which altered the surface properties and pore structure.
Given the challenges posed by membrane fouling, recent studies have focused on incorporating antifouling mechanisms into membrane fabrication. For instance, Khalil et al. developed an asymmetrical UF PLA membrane for the removal of organic substances from wastewater. Using the NIPS method, the fabricated membrane achieved a high separation efficiency of 92% for organic contaminants and demonstrated excellent antifouling properties, with a flux recovery ratio (FRR) of 89%.
In recent years, adsorptive membranes have gained prominence due to the integration of adsorption processes with membrane technology. Nassar et al. fabricated an adsorptive PLA-based membrane incorporating positively charged carbon nanotubes/graphene oxide nanohybrids via the NIPS method. This membrane was applied for the adsorptive removal of nitrogen and phosphorus nutrients from wastewater, achieving separation efficiencies of 90.1% for ammonium-nitrogen and 71.3% for phosphate ions in raw municipal wastewater. Moreover, the membrane demonstrated excellent regenerative ability, maintaining stability and reusability after simple washing with water, underscoring its potential for real-world wastewater applications.
2.4.2.2. Polyhydroxyalkanoate
Similar to PLA, polyhydroxyalkanoates (PHAs) are a family of biodegradable polyesters naturally produced by microorganisms as intracellular energy and carbon storage materials. PHAs can be categorized into three groups based on the carbon chain length of the hydroxylalkanoate (HA) unit: short-chain length (scl-PHAs, fewer than six carbons), medium-chain length (mcl-PHAs, six to 14 carbons), and long-chain length (lcl-PHAs, 14 or more carbons). The molecular structure and properties of PHAs vary depending on factors such as synthesis conditions, the type of carbon source fed to microorganisms, and the specific microorganisms employed. − The most common and widely used PHAs are polyhydroxybutyrate (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) in membrane fabrication, though over 150 types of PHAs have been synthesized to date.
MF membranes made of PHAs can also perform well in filtration applications compared to conventional polymers. Tomietto et al. fabricated an MF membrane using PHBV via the NIPS method. They also added hydrophilic additives such as ethylene glycol and poly(ethylene glycol) into the fabrication process, altering the morphology of the membrane structure. Due to the increased pore size, the permeance was significantly increased by adding the additives at 480 L/m2·h·bar with decent clay dispersion rejection.
An environmentally friendly PHB-based biodegradable nanofibre coupled with SiO2 nanoparticles for gravity-driven oil–water separation was fabricated by Sariipek’s group. The nanofibers were prepared via electrospinning and they possess superhydrophobic properties, with water contact angle of 160.2°. Oil–water dispersion can be separated via gravity using the SiO2/PHB membrane, and the separation performance is excellent as well, with 99.4% removal efficiency and a permeance of 5000 L/m2·h.
2.4.2.3. Poly(butylene succinate)
Poly(butylene succinate) (PBS) is an aliphatic biopolyester that can be synthesized via polycondensation of biobased succinic acid and 1,4-butanediol. The feedstock can be derived from renewable sources, such as corn or sugar cane. Although biobased PBS currently remains more expensive than fossil fuel-derived PBS, there is still some research revolving around the use of biobased PBS. Bang et al. prepared a nanofibrous membrane with biobased PBS using a solution blow spinning method, yielding 130 nm fiber diameters and high porosity (97.4%). The PBS nanofibrous membrane exerts high oil adsorption capacity (18.7–38.5 mg/g) and excellent separation efficiency for water/oil mixtures (99.4–99.98%) and emulsions (98.1–99.5%). In the degradation tests, the PBS membrane can be degraded either through hydrolysis or biodegradation processes.
2.4.3. Proteins
2.4.3.1. Silk Fibroin
Silk Fibroin (SF) is a natural protein polymer produced from the silk of silkworms () or certain spider species. It is composed of long polypeptide chains primarily made up of amino acids. In addition to its biocompatibility, SF is renowned for its excellent mechanical strength, high thermal stability, and strong performance in membrane-based water filtration applications. − For dye removal, a biodegradable multilayered nanofibrous membrane incorporating lignin and SF was developed. The resultant lignin/SF membrane achieved a remarkable dye removal efficiency of 99.5% for contaminants such as crystal violet, methylene blue, and brilliant green via adsorption. Furthermore, the membrane demonstrated versatility by efficiently retaining metal ions such as copper and cadmium, highlighting its potential as a biobased membrane with minimal environmental impact.
SF is also an effective surface modifier in TFC membrane fabrication. Lee’s group utilized the abundance of hydroxyl groups in SF to coat PA TFC membranes. The SF-coated PA membranes exhibited a smaller surface ζ-potential compared to pristine PA membranes, which reduced carboxyl group scaling on the surface. Additionally, the SF-coated membranes demonstrated exceptional antifouling properties, including five times lower irreversible scaling resistance, 50% higher FRR, and a filtration duration four times longer than unmodified membranes.
2.4.3.2. Collagen
Collagen is a structural protein abundantly found in the human body, accounting for approximately 25–35% of total protein content. It is widely employed as a biomedical material due to its biodegradability, biocompatibility, and low immunogenicity. , Collagen is commonly extracted from animal byproducts, with its molecular structure comprising three twisted polypeptide α-chains that form a triple helix.
The molecular structure of collagen contains numerous hydrophilic groups (−OH, −COOH, –NH2), making it a promising material for membrane fabrication. Desiriani et al. incorporated collagen and polyphenols from green tea into a polymeric membrane matrix to enhance the antifouling and antibacterial properties of PES membranes. The addition of 1 wt % collagen and 3 wt % polyphenols significantly improved membrane performance compared to the pristine PES membrane. The modified membrane achieved a pure water flux of 359.9 L/m2·h, an FRR of 85.1% for BSA solutions, and a 93.5% removal efficiency for . The incorporation of polyphenols improved the membrane’s antibiofouling properties by providing antibacterial effects, while collagen enhanced antifouling performance through its intrinsic hydrophilicity. The combined effects of these additives resulted in improved water uptake, porosity, hydrophilicity, solute rejection, and flux recovery, demonstrating the potential of collagen-polyphenol-modified membranes for practical water treatment applications.
2.4.4. Economic Viability of Biopolymers
Biopolymers present a sustainable alternative to conventional polymers for membrane manufacturing, offering environmental benefits due to their biodegradability. However, their economic viability is constrained by high production costs, limited feedstock options, and challenges in waste management.
As compared to conventional polymers, biopolymers have higher production costs and poses as a major challenge toward the widespread use of it. For instance, conventional polymers costs around $1000–1500/ton, while the most commonly used polymer, PLA, cost around $4,000/ton and can reach as high as $15,000/ton for PHA. It also has been reported that biopolymers are 7.5 times more expensive than conventional petroleum-derived products. Even the biobased ($5/kg-$6/kg) and petroleum-based ($4/kg-$4.5/kg) PBS have a slight discrepancy in their cost, leading to a more favored economic position for the latter product.
Feedstock availability and type play a critical role in determining the cost and sustainability of biopolymer production. There are currently three generations of carbon sources for biopolymer production and synthesis. first generation feedstocks, derived from carbohydrate-rich food crops like maize and sugar cane, are widely used but compete with human food supplies and require significant land, raising concerns about food security and environmental impact. second generation feedstocks, sourced from nonfood biomass such as bagasse, husks, and bone waste, repurpose agricultural residues, reducing competition with food resources and lowering production costs by minimizing cultivation needs. third generation feedstocks, based on algae, offer high biomass productivity due to efficient CO2 uptake and can be cultivated in nonarable environments, enhancing yield and sustainability. Current research prioritises second- and third-generation feedstocks to improve economic and environmental outcomes. By repurposing waste, second-generation feedstocks enhance the economic value of biopolymers, while algae-based third-generation sources promise higher yields with minimal resource inputs. Transitioning to these feedstocks could lift up the cost barrier and improve the scalability of biopolymer production.
Biopolymers are known for their biodegradability, which simplifies EoL waste management compared to conventional polymers. However, the degradation process varies significantly depending on environmental conditions and biopolymer type. For instance, PLA can decompose into its constituent parts within three months in a controlled composting environment with adequate heat, moisture, and microbial activity. In contrast, under natural conditions lacking excessive light and oxygen, a PLA bottle may persist for 100 to 1000 years. This variability complicates waste management and increases disposal costs, particularly for membrane applications where biopolymer dismantling from modules would need additional expenses.
2.5. Integration of Sustainable Approaches in Membrane Fabrication
To maximize the sustainability of polymeric membrane fabrication, the integration of mechanosynthesis, green solvents and biopolymers offers a synergistic pathway to reduce environmental impact while maintaining high performance. By combining this approach, it is possible to create membranes that leverages the solvent-free synthesis of advanced materials, the low toxicity profile of green solvents and the renewability of biopolymers. This integrated framework aligns with the principles of green chemistry and supports the closed-loop membrane production system, as illustrated in Figure .
One promising and straightforward approach is the use of mechanosynthesized materials, such as MOFs, as fillers or building blocks in biopolymer-based membranes fabricated with green solvents (Figure ). For instance, mechanochemically synthesized water stable UiO-66 can be incorporated into CA membranes using ethyl lactate as a solvent via NIPS. The high surface area, tunable porosity and diversified derivatives of UiO-66 enhance the membrane’s permeance and selectivity for contaminants like dyes or micropollutants, while ethyl lactate ensures a low-toxicity fabrication process, and CA provides a renewable polymer matrix. This combination could yield membranes with enhanced separation efficiency and reduced environmental footprint. Recently, a mixed matrix membrane consisting CA and various MOFs was fabricated using dimethyl carbonate as the green solvent for gas separation application. This study sheds light to the potential of incorporating mechanosynthesized MOFs or COFs into the polymer matrix as a water separation membrane, which was discussed earlier.
8.
Schematic diagram illustrating the integration of mechanosynthesis (e.g., MOFs, COFs, PIMs), green solvents (e.g., Cyrene, GVL), and biopolymers (e.g., CA, PLA) in the fabrication of high-performance membranes via processes like NIPS.
Although the integration of the sustainable practices may seem ideal, some challenges persist in the adoption of this integration. Challenges in integration include ensuring compatibility between mechanosynthesized materials and green solvents, as well as optimizing biopolymer stability for high-pressure applications, such as NF/RO. Additionally, green solvents could be explored for dissolving mechanosynthesized COFs to form composite membranes with enhanced selectivity.
2.6. Policy and Regulatory Framework for Sustainable Membranes Manufacturing
A successful transition to sustainable membrane manufacturing requires a robust policy and regulatory framework that supports the adoption of green chemistry and circular economy principles. These frameworks, comprising chemical regulations, environmental legislation, voluntary standards, and funding incentives, create an enabling environment for the integration of sustainable practices, as discussed in previous sections, while also facilitating alignment with circular economy objectives explored later in the next section.
Chemical regulations are instrumental in driving the shift away from hazardous substances toward more environmentally benign alternatives. Within the European Union, the REACH regulation restricts the use of toxic solvents such as NMP and DMF, thereby encouraging the adoption of greener alternatives including biobased solvents such as Cyrene and GVL, as previously highlighted. In the United States, the Environmental Protection Agency (EPA) enforces the TSCA, which regulates the production and use of chemicals across various industries, including membrane manufacturing. Additionally, the US Clean Water Act mandates the treatment of solvent-contaminated effluents, thereby promoting the uptake of solvent-free and wastewater recycling technologies. Consequently, these regulatory measures foster the use of biobased solvents and innovative synthesis approaches such as mechanosynthesis, helping to reduce the environmental footprint of membrane production.
In addition to legislative instruments, voluntary standards and strategic funding mechanisms play a vital role in advancing sustainable manufacturing. International standards such as ISO 14001 provide a framework for environmental management systems, guiding organisations toward more resource-efficient and environmentally responsible operations. Furthermore, funding initiatives such as Horizon Europe and the UK’s Engineering and Physical Sciences Research Council (EPSRC) have significantly supported research into green chemistry, including the development of biopolymers and sustainable solvents for membrane fabrication.
The incorporation of circular economy principles, which will be discussed in the next major section, is also a major driving force for sustainable membrane production. Policies such as the European Union’s Circular Economy Action Plan promote material recovery and recycling strategies, which are directly relevant to the advancement of EoL membrane recycling. These measures contribute to waste reduction and improve material reusability, aligning with the SDG 12 on responsible consumption and production.
In summary, a comprehensive and forward-looking policy and regulatory landscape, characterized by stringent legislation, voluntary compliance standards, and targeted financial support, is essential to fostering innovation and accelerating the transition to sustainable membrane manufacturing in line with global environmental and socio-economic priorities.
3. Polymeric Membrane for Circular Economy: Downcycling, Upcycling and Repreparation
To ensure the long-term viability of membrane technology, it is crucial to evaluate the sustainability of both the fabrication processes and the management of EoL membranes. Synthetic polymeric membranes are commonly classified into four categories based on pore size: MF, UF, NF, and RO, with pore size decreasing progressively across these types. Despite their efficacy, all synthetic membranes inevitably reach the end of their functional lifespan, compelling the development of sustainable solutions for their disposal or recycling. Landaburu-Aguirre et al. estimated that by 2025, nearly 30,000 tonnes of discarded RO membranes would accumulate in landfills worldwide. Additionally, regional differences in membrane management practices can result in significantly shorter operational lifespans for membranes compared to reported averages, worsening the problem of membrane waste generation.
A circular economy framework, which emphasizes closing material loops to prevent waste and minimize environmental impact, offering a promising pathway for addressing the sustainability challenges associated with membrane technology. Recycling EoL membranes plays a critical role in advancing a circular economy by extending the functional lifespan of membranes and reducing waste. Membrane recycling can generally be categorized into three types: upcycling, downcycling, and repreparation. Upcycling involves converting EoL membranes into materials of higher quality than the original, while downcycling produces materials of lower quality. On the other hand, repreparation restores EoL membranes to a state comparable to that of pristine membranes. In this section, we highlight the trend of downcycling, upcycling, and the repreparation of EoL membranes. These circular strategies collectively pave the way for sustainable membrane lifecycles, setting the stage for future challenges and opportunities.
3.1. Downcycling
Membrane downcycling represents a promising recycling strategy for EoL RO membranes, enabling their transformation into lower-pressure filtration systems, such as NF, UF, or MF membranes. This approach typically employs sodium hypochlorite (NaClO) treatment to selectively degrade or remove the PA selective layer, which is the primary functional component of RO membranes (Figure a). The oxidative action of NaClO disrupts the molecular structure of the PA layer, facilitating its controlled removal and allowing the underlying support structure to be repurposed for less selective filtration applications. Alternative oxidative agents, such as hydrogen peroxide (H2O2) and potassium permanganate (KMnO4), have also been explored for PA layer removal. However, these compounds often generate oxidative residues that complicate downstream applications and increase the complexity of water treatment processes, making NaClO the preferred reagent for downcycling.
9.
(a) Schematic diagram for a typical membrane downcycling process. (b) Downcycling of RO membrane to UF- or NF-like membranes using NaClO treatment for different treatment times. Reprinted from Wang et al., Copyright 2024, with permission from Elsevier.
Recent advances in membrane downcycling have demonstrated its versatility and efficacy. For instance, RO membranes fouled with Si–Al complexes have been successfully converted into loose NF- or UF-like membranes through NaClO treatment (Figure b). Alkaline NaClO exposure gradually deconstructs the Si–Al fouling layer while simultaneously degrading the PA selective layer. Short chemical treatment results in a loose NF-like structure, whereas sufficient chlorine oxidation can fully eliminate the PA layer, yielding a UF-like membrane. Both resultant membranes exhibit satisfactory separation performance and enhanced antifouling properties, highlighting the adaptability of this method to fouled EoL membranes.
Downcycled membranes have also shown potential for specialized applications, such as the removal of contaminants like uranium from groundwater to produce potable water. Two RO membranes were treated with varying NaClO concentrations to produce recycled NF-based membranes. A membrane exposed to 220,000 ppm·h of NaClO achieved a uranium removal efficiency of 71.6%. However, this membrane exhibited susceptibility to fouling, with a 53% reduction in flux from an initial value of 10.3 L/m2·h, underscoring a trade-off between selectivity and long-term performance that warrants further optimization.
An alternative downcycling strategy integrates cleaning, healing, and IP with NaClO treatment to enhance the functionality of recycled membranes. In this method, EoL RO membranes are first cleaned with NaClO to remove fouling, followed by complete PA layer removal to produce a UF-like support membrane. A healing step employing polyelectrolyte deposition via the LbL technique reduces surface hydrophobicity, facilitating subsequent IP to reintroduce a PA layer. The resulting tight-NF membrane demonstrates exceptional performance, including a pure water permeance of 7.3 L/m2·h·bar and a Na2SO4 rejection rate of 99.5%, alongside improved long-term stability and fouling resistance. This multistep approach exemplifies how downcycling can not only repurpose EoL membranes but also restore or enhance their utility for high-efficiency separations.
3.2. Upcycling
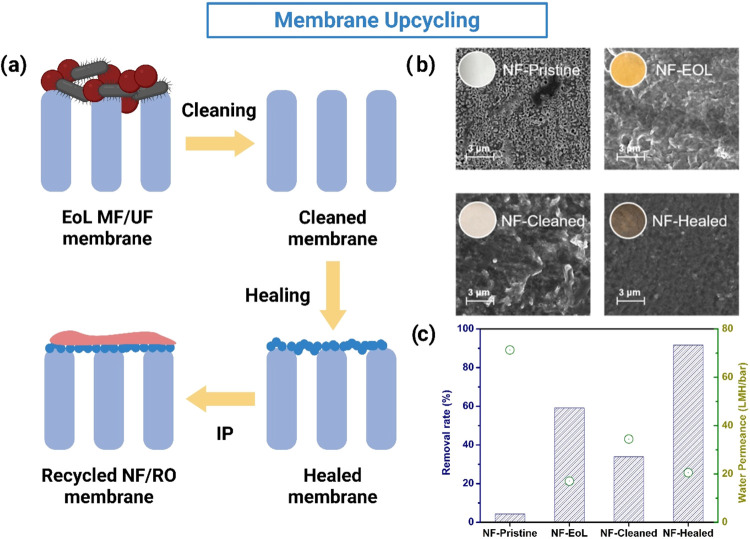
Similar to membrane downcycling, the upcycling of EoL membranes represents a transformative approach to membrane recycling. This process involves reprocessing retired MF/UF membranes into higher-performance NF/RO membranes through the introduction of a PA selective layer via IP, thereby forming TFC membranes (Figure a).
10.
(a) Schematic diagram for cleaning-healing-IP method for membrane upcycling. (b) SEM diagram for the surface and (c) filtration performance of NF membrane made of pristine MF substrate, EoL MF substrate, cleaned EoL MF substrate and cleaned-healed EoL MF membrane. Reprinted with permission from Dai et al. Copyright 2021 American Chemical Society.
A widely adopted upcycling methodology follows a sequential cleaning-healing-IP protocol. The initial cleaning phase is critical for removing organic and inorganic foulants, while the subsequent healing step ensures uniform PA layer deposition by mitigating defects and preventing undesirable selective layer growth (Figure b). For instance, the efficacy of this approach was demonstrated through enhanced salt rejection performance: cleaned membranes exhibited ∼60% Na2SO4 rejection, whereas healed membranes modified with a hydrophilic polydopamine interlayer achieved 92.4% rejection (Figure c). Moreover, recent advancements have streamlined the healing process by substituting polydopamine with a tannic acid-Fe complex, reducing total processing time to <20 min while maintaining comparable separation performance. Economic analyses further underscore the viability of this approach, as the chemical costs for healing are offset by savings from avoided EoL membrane disposal and new membrane procurement.
Innovative strategies have also emerged that bypass traditional cleaning and healing steps. , Biofouling residues on EoL MF membranes have been shown to regulate IP dynamics, facilitating controlled PA layer formation while simultaneously creating interfacial water channels between the selective layer and substrate. Additionally, surfactant incorporation during recycling has proven effective in regulating IP by enhancing surface wettability, thereby optimizing PA layer morphology and separation efficiency. Such simplified protocols not only reduce operational complexity but also improve cost-effectiveness, further advocating for membrane upcycling as a sustainable alternative.
These advancements highlight the technical and economic potential of membrane upcycling. By transforming EoL membranes into high-value TFC membranes through tailored chemical and structural modifications, this approach aligns with circular economy principles, offering a scalable and environmentally responsible solution for membrane lifecycle management.
3.3. Repreparation
Beyond conventional upcycling and downcycling, membrane repreparation offers a forward-looking pathway to align membrane EoL management with circular economy principles. This approach integrates sustainability into the membrane lifecycle by prioritising material reprocessability at the design stage. Repreparation involves dissolving retired polymeric membranes in organic solvents to form regenerated dopant solutions, which are subsequently reprocessed into new membranes with comparable or enhanced performance.
3.3.1. Covalent Adaptable Network (CAN)
Covalent adaptable networks (CANs) have emerged as a groundbreaking class of materials for advancing recyclable membrane technologies. − CANs are thermoset polymers engineered with dynamic covalent bonds, which undergo reversible cleavage or exchange reactions in response to external stimuli (e.g., heat, light, pH). This inherent dynamicity enables structural reconfiguration, self-healing, and closed-loop recyclingproperties that address key challenges in membrane sustainability (Figure a).
11.
(a) Schematic diagram of the mechanism of bond-forming/breaking in CANs for Diels–Alder chemistry. (b) (i) The SEM diagram, (ii) mechanical properties, and (iii) filtration performance of the recyclable NF membrane. Copyright 2023 National Academy of Sciences. (c) (i) The captured image and SEM image, (ii) the water contact angle, and (iii) the oil flux of the recyclable electrospun membrane for oil/water filtration. Reprinted from Li et al., Copyright 2020, with permission from Elsevier. (d) (i) The SEM image, (ii) the captured image for mechanical properties, and (iii) the filtration performance for the disulfide bonds enabled recycled membrane for OSN applications. Reprinted with permission from Ramírez-Martínez et al. Copyright 2024 American Chemical Society.
An innovative example is the Diels–Alder (DA) reaction, a thermally reversible dynamic covalent chemistry. In DA-based CANs, a conjugated diene reacts with a dienophile to form a six-membered cycloadduct, which can dissociate via retro-DA reactions under elevated temperatures. Leveraging this mechanism, Li et al. demonstrated the fabrication of a fully recyclable CAN membrane utilizing furan-maleimide DA adduct for water purification (Figure b). The pristine membrane exhibited robust separation performance, with a water permeance of 28.5 L/m2·h·bar and a molecular weight cutoff (MWCO) of 4581 g/mol, enabling efficient dye rejection. The pristine membrane exhibited robust separation performance, with a water permeance of 28.5 L/m2·h·bar and a molecular weight cutoff (MWCO) of 4581 g/mol, enabling efficient dye rejection. After using, the membrane was depolymerised at high temperature (90 °C) and reprocessed into new membranes via NIPS solvent-casting (Figure b(i)). Remarkably, the recycled membranes retained their chemical integrity, mechanical strength (Figure b(ii)), and filtration performance (Figure b(iii)) over three reprocessing cycles, with no significant degradation in filtration efficiency. Moreover, the absorbed dye on the membrane before recycling has been removed using liquid extraction method, confirming that the fouled substances can be removed easily.
Electrospinning has emerged as a promising technique to integrate CANs into nanofibrous architectures, focusing on their dynamic covalent chemistry to enhance recyclability. For instance, CAN-based electrospun membranes exhibit exceptional hydrophobicity, high porosity, and robust chemical stability, enabling efficient oil/water separation with >99% oil removal efficiency (Figure c(i)). The inherent recyclability of these membranes was demonstrated through thermal depolymerization via retro-Diels–Alder (retro-DA) reactions, which effectively disintegrated oil-fouling layers while preserving nanofiber integrity. Remarkably, reprocessed membranes retained their initial separation efficiency over two recycling cycles (Figure c(ii,iii)), showing their durability in aggressive hydrocarbon environments. This approach not only addresses fouling challenges but also aligns with circular economy principles by minimizing material waste.
CANs have also been engineered for OSN, where solvent-resistant, recyclable membranes are critical for sustainable chemical processing. A notable example involves a furan-modified poly(amide-imide)/bismaleimide DA system, which achieved an acetone permeance of 3.68 L·m–2·h–1·bar–1 and 95% rejection of Rose Bengal. While the original asymmetric membrane structure transitioned to a dense morphology postrecycling via solvent evaporation, the reprocessed membranes maintained mechanical integrity over three cycles, with negligible changes in tensile strength. However, the absence of postrecycling filtration data highlights a critical gap in current research.
Beyond thermally triggered systems, redox-responsive CANs leveraging disulfide dynamics offer a unique pathway for membrane recycling under mild conditions. Ramírez-Martínez et al. demonstrated this with a cysteamine-cross-linked poly(ether imide) OSN membrane, which exhibited 99% rejection of Direct Red 80 in methanol (permeance: 1.4 L·m–2·h–1·bar–1) (Figure d). Chemical recycling was achieved via disulfide bond reduction using 1,4-dithiothreitol, which cleaved the polymer network while retaining the original mechanical strength postreprocessing (Figure d(i)). Although dye rejection was decreased in recycled membranes, the retained solvent resistance and mechanical robustness emphasize the viability of redox-driven CANs for industrial OSN applications (Figure d(ii,iii)). This approach bridges the gap between covalent adaptability and practical solvent stability, offering a blueprint for designing chemically resilient yet recyclable membranes. The absence of comprehensive filtration performance data postrecycling in CAN studies underscores the need for standardized testing protocols to validate their practical utility in desalination.
3.4. Economic Viability of Membrane Recycling
Membrane recycling offers significant environmental benefits, but its industrial adoption hinges on economic viability. Recent studies demonstrate that membrane recycling is not only sustainable but also highly cost-effective, making it an attractive option for water treatment industries. de Paula et al. highlight the eco-efficiency of membrane recycling, showing substantial cost savings alongside environmental benefits. Their analysis indicates that replacing 9000 UF membranes with recycled ones can yield annual savings of $10 million, representing a 98.9% cost reduction compared to purchasing new UF membranes. This dramatic cost advantage underscores the economic potential of membrane recycling. Further supporting these findings, another study reports a 27.1% cost reduction through closed-loop recycling of UF and NF membranes. The recycling process itself is cost-efficient, with associated costs estimated at only 0.7–2.1% of the total expenditure. These low recycling costs, combined with reduced expenses for membrane replacement and waste disposal, make recycling EoL membranes a financially superior alternative to procuring new ones. In summary, the economic advantages of membrane recycling, driven by significant cost reductions and minimal recycling expenses, position it as a compelling strategy for sustainable and cost-effective water treatment solutions.
4. Outlook and Perspective
This review highlights advancements in mechanosynthesis, green solvents, and circular economy integration for sustainable membrane fabrication. By comparing the data and information from the literatures, a comparative evaluation was illustrated to analyze the industrial adoption of green membrane fabrication technologies and the current conventional membrane fabrication methods (Table ). While these innovations offer promising pathways to reduce environmental impact, several challenges must be addressed to enable their widespread adoption in industrial membrane manufacturing and desalination applications.
4. Comparative Evaluation of Sustainable Membrane Technologies and Conventional Membrane Fabrication Methods for Industrial Adoption.
Ratings are based
on subjective
evaluation of scalability, cost, and compatibility with existing industrial
processes; ( :low,
:low,  : high).
: high).
4.1. Challenges
Mechanochemical reactions provide energy-efficient, solvent-free routes for synthesizing advanced materials such as MOFs, COFs and PIMs. However, transitioning from lab-scale synthesis to industrial production remains a critical barrier. For instance, while twin-screw extrusion has enabled UiO-66-NH2 production at 1.4 kg/h, the high capital costs of specialized equipment (e.g., planetary mills, extruders) and the need for precise control over mechanical energy inputs hinder scalability. Furthermore, batch-to-batch variability in material properties (e.g., BET surface area, pore size distribution) may compromise membrane performance consistency in separation processes. Concurrently, green solvents, valued for their biodegradability and low toxicity, face compatibility challenges with conventional membrane polymers. For example, their application to PA-based TFC membranes remains underexplored. Moreover, membranes fabricated with green solvents frequently exhibit trade-offs between selectivity and permeance compared to those derived from traditional solvents (e.g., NMP, DMF), underscoring the need to balance sustainability with separation efficiency. Aside from that, biopolymers such as cellulose derivatives, PLA and PHAs present eco-friendly alternatives to petroleum-based polymers. However, their limited mechanical strength and thermal stability restrict their utility in high-pressure environments (e.g., RO- and NF-systems). For example, pristine cellulose suffers from poor solubility and durability, while PLA’s inherent hydrophobicity necessitates surface modifications to enhance water permeance, a process that increases manufacturing complexity and cost.
Sustainable materials and processes often incur higher costs than conventional counterparts. For instance, GVL (£368/kg, Sigma-Aldrich) and biobased PBS ($5/kg-$6/kg) are significantly more expensive than NMP (£82/kg, Sigma-Aldrich) and petroleum-based PBS ($4-$4.5/kg). Additionally, the capital investment required for mechanosynthesis infrastructure (e.g., twin-screw extruders) poses economic barriers to scaling. Without cost-competitive incentives, industrial adoption may lag, particularly in sectors prioritising operational efficiency. Further, circular economy strategies such as CANs depolymerization face practical challenges: industrial RO/NF modules, often encased in sealed shells, require costly dismantling and reprocessing steps for reuse.
While membrane recycling aligns with circular economy principles, performance degradation postrecycling remains a concern. Downcycled NF membranes exhibit reduced flux and increased fouling susceptibility, while upcycling processes demand multistep healing to restore filtration efficiency. Residual contaminants in upcycled membranes further limit their applicability in sensitive processes like desalination. Additionally, the lack of comprehensive data on the long-term stability and scalability of recycled CAN-based membranes raises uncertainties about their industrial viability. Furthermore, the increased logistical cost for membrane recycling could also be a potential economic issue to scale up the technology.
Aside from water treatment application, a critical challenge in advancing sustainable high-performance polymeric membranes for electrochemical and separation processes lies in addressing compatibility issues between polymers, green solvents, and operational environments. High-performance polymers, such as Nafion and s-PEEK, are prized for their thermal and chemical stability in applications like ion exchange membranes for flow batteries. However, transitioning to green solvents introduces compatibility challenges, as they can lead to undesired or suboptimal membrane morphologies. In electrochemical applications, membranes must withstand harsh electrolytes, where chemical degradation or fouling can compromise ionic conductivity and selectivity. These compatibility issues necessitate tailored solvent–polymer matching and surface modifications to ensure performance aligns with sustainability goals.
4.2. Future Potential
Convergence of advanced manufacturing, computational tools, and material science offers transformative opportunities for sustainable membrane technologies. Machine learning (ML) and artificial intelligence (AI) offer revolutionary potential for sustainable membrane manufacturing, addressing challenges in material selection, design and process optimization. For instance, graph neural networks (GNNs) can accelerate the discovery of biobased polymers and green solvents by predicting molecular properties, reducing reliance on toxic solvents. In addition, GNNs can also potentially model the degradation pathways of recyclable membranes, optimizing chemical processes for recycling strategies especially for vitrimer-based membranes. Besides GNNs, reinforcement learning (RL) and deep neural networks (DNNs) can streamline manufacturing processes, like mechanosynthesis and phase inversion processes, minimizing energy and solvent use. By integrating ML/AI with experimental and industrial data, researchers can overcome scalability barriers. Therefore, future efforts can be focused on curating comprehensive data sets and validating ML predictions to ensure practical implementation.
Biopolymers are promising sustainable alternatives for water treatment membranes but often exhibit insufficient mechanical strength for high-pressure applications. A facile strategy to address this limitation involves blending biopolymers with mechanically robust polymers (e.g., PVDF, PES, chitosan). Incorporating a small percentage of PVDF or PES into the biopolymer matrix can significantly enhance mechanical strength while preserving biodegradability. Additionally, the hydrogen bonding capacity of chitosan facilitates the formation of robust intermolecular interactions, yielding a stable membrane complex. Furthermore, reinforcing biopolymer matrices with nanomaterials (e.g., graphene oxide, carbon nanotubes) or developing hybrid organic–inorganic composites with inorganic materials (e.g., zeolites, MOFs) can substantially improve mechanical resilience, making them suitable for demanding applications like RO. Moreover, physical reinforcement through advanced fabrication techniques, such as electrospinning or LbL assembly, can further enhance the structural integrity of biopolymer membranes, optimizing their performance in water treatment processes.
Although membrane recycling presents a sustainable alternative to conventional EoL membrane disposal (landfill/incineration), it is often overlooked due to the logistical and operational challenges associated with transporting membranes to dedicated recycling facilities. These additional logistics not only increase costs but also contribute to the overall environmental footprint. To address these limitations, cost-effective circular economy strategies, such as on-site membrane upcycling or downcycling, or in situ CAN polymers repreparation, could revolutionize EoL membrane management. By localizing the recycling process, such approaches would streamline operations, reduce costs, and minimize environmental impacts, making sustainable membrane disposal more viable.
In addition to polymer-based membranes, inorganic materials such as ceramics, zeolites, and metal oxides have emerged as promising green alternatives for membrane applications. In contrast to their polymeric counterparts, inorganic membranes exhibit superior thermal and chemical resistance, along with enhanced mechanical strength and long-term durability. These properties significantly reduce the frequency of membrane replacement, particularly under harsh operating conditions involving aggressive cleaning agents such as NaClO. Despite these advantages, the fabrication of inorganic membranes often requires energy-intensive processes, such as sintering or pyrolysis, and may involve the use of volatile and hazardous chemicals through methods like chemical vapor deposition or sol–gel synthesis. These factors present environmental and safety challenges that must be addressed when evaluating the sustainability of inorganic membranes. Nevertheless, the successful commercialization of inorganic NF membranes, such as those developed by Inopor, demonstrates the growing viability of inorganic materials in the membrane technology market and their potential role in advancing sustainable separation processes.
To accelerate adoption, interdisciplinary collaboration among academia, industry, and policymakers will be essential to address technical bottlenecks, reduce costs, and establish standardized frameworks for sustainable membrane design and recycling.
By prioritising scalability, performance optimization, and lifecycle sustainability, next-generation membranes could achieve the dual objectives of environmental stewardship and industrial feasibility, which is critical for advancing green membrane technologies in a resource-constrained world.
5. Conclusions
This review underscores the progress in sustainable polymeric membranes through green chemistry and circular economy principles. Mechanosynthesis enables solvent-free synthesis of advanced materials, such as MOFs, COFs, and PIMs, reducing environmental impact. Green solvents, in particular, Cyrene and GVL offer low-toxicity alternatives for membrane fabrication, while biopolymers like cellulose derivatives and PLA provide renewable options, despite limitations in mechanical and thermal stability. These innovations collectively shift membrane technology toward sustainability.
Circular strategies such as downcycling, upcycling, and repreparation with CANs, extend membrane lifecycles, minimizing waste and conserving resources. However, challenges in scalability, economic viability, and performance persist, requiring further optimization. By advancing these eco-friendly approaches, sustainable membranes hold promise for water treatment and desalination, meeting industrial demands while prioritising environmental stewardship. Ongoing research and collaboration will be key to unlocking their full potential in a resource-limited future.
Acknowledgments
C.Y.L. would like to thank the EPSRC for PhD studentship and the Royal Society of Chemistry’s Research Collaboration Grant (C24-9732941606). The authors also acknowledge financial support from the Royal Society International Exchange (IEC\NSFC\242089) and the Royal Society Research Grant (RG\R1\251471).
The authors declare no competing financial interest.
References
- Biancalani, R. ; Hamouda, G. B. ; Marinelli, M. ; Chocholata, L. . Progress on the Level of Water Stress – Mid-term Status of SDG Indicator 6.4.2 and Acceleration Needs, with Special Focus on Food Security, FAO; 2024. [Google Scholar]
- UN-Water . Summary Progress Update 2021: SDG 6 Water and Sanitation for All, UN-water; 2021. [Google Scholar]
- Jones E., Qadir M., van Vliet M. T. H., Smakhtin V., Kang S.-m.. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019;657:1343–1356. doi: 10.1016/j.scitotenv.2018.12.076. [DOI] [PubMed] [Google Scholar]
- Sholl D. S., Lively R. P.. Seven chemical separations to change the world. Nature. 2016;532(7600):435–437. doi: 10.1038/532435a. [DOI] [PubMed] [Google Scholar]
- Yadav P., Ismail N., Essalhi M., Tysklind M., Athanassiadis D., Tavajohi N.. Assessment of the environmental impact of polymeric membrane production. J. Membr. Sci. 2021;622:118987. doi: 10.1016/j.memsci.2020.118987. [DOI] [Google Scholar]
- Razali M., Kim J. F., Attfield M., Budd P. M., Drioli E., Lee Y. M., Szekely G.. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015;17(12):5196–5205. doi: 10.1039/C5GC01937K. [DOI] [Google Scholar]
- Nunes S. P., Culfaz-Emecen P. Z., Ramon G. Z., Visser T., Koops G. H., Jin W., Ulbricht M.. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020;598:117761. doi: 10.1016/j.memsci.2019.117761. [DOI] [Google Scholar]
- Galiano F., Briceño K., Marino T., Molino A., Christensen K. V., Figoli A.. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018;564:562–586. doi: 10.1016/j.memsci.2018.07.059. [DOI] [Google Scholar]
- Shanmugaratnam, T. ; Mazzucato, M. ; Okonjo-Iweala, N. ; Rockström, J. ; Ovink, H. . The Economics of Water: Valuing the Hydrological Cycle as a Global Common Good; Global Commission on the Economics of Water, 2024. [Google Scholar]
- Baláž P., Achimovičová M., Baláž M., Billik P., Cherkezova-Zheleva Z., Criado J. M., Delogu F., Dutková E., Gaffet E., Gotor F. J.. et al. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013;42(18):7571–7637. doi: 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]
- Ardila-Fierro K. J., Hernández J. G.. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem. 2021;14(10):2145–2162. doi: 10.1002/cssc.202100478. [DOI] [PubMed] [Google Scholar]
- Friščić T., Childs S. L., Rizvi S. A. A., Jones W.. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm. 2009;11(3):418–426. doi: 10.1039/B815174A. [DOI] [Google Scholar]
- Staudinger H., Leupold E. O.. Über Isopren und Kautschuk, 18. Mitteil.: Viscositäts-Untersuchungen an Balata. Ber. Dtsch. Chem. Ges. A/B Ser. 1930;63(3):730–733. doi: 10.1002/cber.19300630329. [DOI] [Google Scholar]
- Staudinger H., Heuer W.. Über hochpolymere Verbindungen, 93. Mitteil.: Über das Zerreißen der Faden-Moleküle des Poly-styrols. Ber. Dtsch. Chem. Ges. A/B Ser. 1934;67(7):1159–1164. doi: 10.1002/cber.19340670708. [DOI] [Google Scholar]
- Staudinger H., Bondy H. F.. Über Isopren und Kautschuk, 19. Mitteil.: Über die Molekülgröße des Kautschuks und der Balata. Ber. Dtsch. Chem. Ges. A/B Ser. 1930;63(3):734–736. doi: 10.1002/cber.19300630330. [DOI] [Google Scholar]
- Krusenbaum A., Grätz S., Tigineh G. T., Borchardt L., Kim J. G.. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022;51(7):2873–2905. doi: 10.1039/D1CS01093J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat D., Wells A., Hayler J., Sneddon H., McElroy C. R., Abou-Shehada S., Dunn P. J.. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016;18(1):288–296. doi: 10.1039/C5GC01008J. [DOI] [Google Scholar]
- Wongwilawan S., Nguyen T. S., Nguyen T. P. N., Alhaji A., Lim W., Hong Y., Park J. S., Atilhan M., Kim B. J., Eddaoudi M., Yavuz C. T.. Non-solvent post-modifications with volatile reagents for remarkably porous ketone functionalized polymers of intrinsic microporosity. Nat. Commun. 2023;14(1):2096. doi: 10.1038/s41467-023-37743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J., Wang X., Du J., Liang Q., He W., Liu Y., Ma J., Zhang C., Liu J.. Surface-engineered PIM-1 membranes for facile CO2 capture. Chem. Eng. J. 2023;477:147017. doi: 10.1016/j.cej.2023.147017. [DOI] [Google Scholar]
- Ameen A. W., Ji J., Tamaddondar M., Moshenpour S., Foster A. B., Fan X., Budd P. M., Mattia D., Gorgojo P.. 2D boron nitride nanosheets in PIM-1 membranes for CO2/CH4 separation. J. Membr. Sci. 2021;636:119527. doi: 10.1016/j.memsci.2021.119527. [DOI] [Google Scholar]
- Wang J., Zhou Y., Liu X., Liu Q., Hao M., Wang S., Chen Z., Yang H., Wang X.. Design and application of metal–organic framework membranes for gas and liquid separations. Sep. Purif. Technol. 2024;329:125178. doi: 10.1016/j.seppur.2023.125178. [DOI] [Google Scholar]
- Saini H., Kallem P., Otyepková E., Geyer F., Schneemann A., Ranc V., Banat F., Zbořil R., Otyepka M., Fischer R. A., Jayaramulu K.. Two-dimensional MOF-based liquid marbles: surface energy calculations and efficient oil–water separation using a ZIF-9-III@PVDF membrane. J. Mater. Chem. A. 2021;9(41):23651–23659. doi: 10.1039/D1TA05835E. [DOI] [Google Scholar]
- Li N., Ma C., Ye M., Guo X., Qiao Z., Zhong C.. Mechanochemical synthesized amino-functionalized ultramicroporous ZIF based mixed-matrix membranes for CO2 separation. J. Membr. Sci. 2023;680:121733. doi: 10.1016/j.memsci.2023.121733. [DOI] [Google Scholar]
- Karadeniz B., Howarth A. J., Stolar T., Islamoglu T., Dejanović I., Tireli M., Wasson M. C., Moon S.-Y., Farha O. K., Friščić T., Užarević K.. Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal–Organic Frameworks by Water-Assisted Mechanochemistry. ACS Sustainable Chem. Eng. 2018;6(11):15841–15849. doi: 10.1021/acssuschemeng.8b04458. [DOI] [Google Scholar]
- Głowniak S., Szczęśniak B., Choma J., Jaroniec M.. Mechanochemical Synthesis of MOF-303 and Its CO2 Adsorption at Ambient Conditions. Molecules. 2024;29(11):2698. doi: 10.3390/molecules29112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu B., Wang M., Jiang J., Jiang Z.. Molecular design of covalent–organic framework membranes for Li+/Mg2+ separation: Significant charge effect. J. Membr. Sci. 2022;662:120976. doi: 10.1016/j.memsci.2022.120976. [DOI] [Google Scholar]
- Dawson R., Cooper A. I., Adams D. J.. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012;37(4):530–563. doi: 10.1016/j.progpolymsci.2011.09.002. [DOI] [Google Scholar]
- Wan H., Yan X., Yang J., Yan G., Zhang G.. How to transform microporous organic polymers for membrane-based separation: A review. Sep. Purif. Technol. 2024;348:127755. doi: 10.1016/j.seppur.2024.127755. [DOI] [Google Scholar]
- Lin S., Su M., Li X., Liang S.-x.. Solvent-free mechanochemical synthesis of a sodium disulfonate covalent organic framework for simultaneous highly efficient selective capture and sensitive fluorescence detection of fluoroquinolones. Sep. Purif. Technol. 2024;336:126167. doi: 10.1016/j.seppur.2023.126167. [DOI] [Google Scholar]
- Liu G., Chen H., Zhang W., Ding Q., Wang J., Zhang L.. Facile mechanochemistry synthesis of magnetic covalent organic framework composites for efficient extraction of microcystins in lake water samples. Anal. Chim. Acta. 2021;1166:338539. doi: 10.1016/j.aca.2021.338539. [DOI] [PubMed] [Google Scholar]
- Wu J., Wang Y., Wu Y., Xu W., Wang J., Li S., Xu Z.. Freestanding covalent organic framework membranes with enhanced proton perm-selectivity for flow batteries. J. Membr. Sci. 2023;687:122091. doi: 10.1016/j.memsci.2023.122091. [DOI] [Google Scholar]
- Yang Y., Wang Z., Song Z., Liu D., Zhang J., Guo L., Fang W., Jin J.. Thermal treated amidoxime modified polymer of intrinsic microporosity (AOPIM-1) membranes for high permselectivity reverse osmosis desalination. Desalination. 2023;551:116413. doi: 10.1016/j.desal.2023.116413. [DOI] [Google Scholar]
- Liu M.-L., Chen Y., Hu C., Zhang C.-X., Fu Z.-J., Xu Z., Lee Y. M., Sun S.-P.. Microporous membrane with ionized sub-nanochannels enabling highly selective monovalent and divalent anion separation. Nat. Commun. 2024;15(1):7271. doi: 10.1038/s41467-024-51540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Zhu J., Jin J., Wang Y., Zhang Y., Van der Bruggen B.. Polymers of intrinsic microporosity for membrane-based precise separations. Prog. Mater. Sci. 2024;144:101285. doi: 10.1016/j.pmatsci.2024.101285. [DOI] [Google Scholar]
- Loh C. Y., Burrows A. D., Zhang X., Xie M.. Polymer of Intrinsic Microporosity Enabled pH-Responsive Adsorptive Membrane: Selectivity and Mechanism. ACS Appl. Eng. Mater. 2024;2(2):404–414. doi: 10.1021/acsaenm.3c00715. [DOI] [Google Scholar]
- Loh C. Y., Huang R., Bell R., Xie M.. Solvent-free mechanosynthesis of polyethyleneimine-grafted polymers of intrinsic microporosity with enhanced adsorption capacity. Environ. Technol. Innovation. 2024;34:103551. doi: 10.1016/j.eti.2024.103551. [DOI] [Google Scholar]
- Budd, P. M. Polymers of Intrinsic Microporosity and Their Potential in Process Intensification. In Sustainable Nanoscale Engineering; Szekely, G. ; Livingston, A. , Eds.; Elsevier, 2020; Chapter 9, pp 231–264. [Google Scholar]
- Zhang P., Jiang X., Wan S., Dai S.. Advancing polymers of intrinsic microporosity by mechanochemistry. J. Mater. Chem. A. 2015;3(13):6739–6741. doi: 10.1039/C4TA07196D. [DOI] [Google Scholar]
- Loh C. Y., Huang R., Bell R., Xie M.. Towards sustainable synthesis: a life cycle assessment of polymer of intrinsic microporosity (PIM-1) by green mechanosynthesis. RSC Sustainability. 2023;1(9):2287–2295. doi: 10.1039/D3SU00340J. [DOI] [Google Scholar]
- Budd P. M., Ghanem B. S., Makhseed S., McKeown N. B., Msayib K. J., Tattershall C. E.. Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004;(2):230–231. doi: 10.1039/b311764b. [DOI] [PubMed] [Google Scholar]
- Hamzehpoor E., Effaty F., Borchers T. H., Stein R. S., Wahrhaftig-Lewis A., Ottenwaelder X., Friščić T., Perepichka D. F.. Mechanochemical Synthesis of Boroxine-linked Covalent Organic Frameworks. Angew. Chem., Int. Ed. 2024;63(51):e202404539. doi: 10.1002/anie.202404539. [DOI] [PubMed] [Google Scholar]
- Brown N., Alsudairy Z., Behera R., Akram F., Chen K., Smith-Petty K., Motley B., Williams S., Huang W., Ingram C., Li X.. Green mechanochemical synthesis of imine-linked covalent organic frameworks for high iodine capture. Green Chem. 2023;25(16):6287–6296. doi: 10.1039/D3GC01927F. [DOI] [Google Scholar]
- Zhu C., Pang S., Chen Z., Bi L., Wang S., Liang C., Qin C.. Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR. Polymers. 2022;14(15):3158. doi: 10.3390/polym14153158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger S. R., Kearns E. R., Miller K. L., D’Alessandro D. M.. Green, One-Step Mechanochemical Synthesis and Techno-economic Analysis of UiO-66-NH2. ACS Appl. Energy Mater. 2023;6(18):9074–9083. doi: 10.1021/acsaem.2c02460. [DOI] [Google Scholar]
- Ulbricht M.. Advanced functional polymer membranes. Polymer. 2006;47(7):2217–2262. doi: 10.1016/j.polymer.2006.01.084. [DOI] [Google Scholar]
- Guillen G. R., Pan Y., Li M., Hoek E. M. V.. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011;50(7):3798–3817. doi: 10.1021/ie101928r. [DOI] [Google Scholar]
- Ren, J. ; Wang, R. . Preparation of Polymeric Membranes. In Membrane and Desalination Technologies; Wang, L. K. ; Chen, J. P. ; Hung, Y.-T. ; Shammas, N. K. , Eds.; Humana Press, 2011; pp 47–100. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Springer science & business media, 2012. [Google Scholar]
- Kahrs C., Schwellenbach J.. Membrane formation via non-solvent induced phase separation using sustainable solvents: A comparative study. Polymer. 2020;186:122071. doi: 10.1016/j.polymer.2019.122071. [DOI] [Google Scholar]
- Russo F., Galiano F., Pedace F., Aricò F., Figoli A.. Dimethyl Isosorbide As a Green Solvent for Sustainable Ultrafiltration and Microfiltration Membrane Preparation. ACS Sustainable Chem. Eng. 2020;8(1):659–668. doi: 10.1021/acssuschemeng.9b06496. [DOI] [Google Scholar]
- Russo, F. ; Vigile, M. F. ; Galiano, F. ; Figoli, A. . Green Solvents for Membrane Fabrication. In Green Membrane Technologies towards Environmental Sustainability; Dumée, L. F. ; Sadrzadeh, M. ; Shirazi, M. M. A. , Eds.; Elsevier, 2023; Chapter 2, pp 9–44. [Google Scholar]
- Sherwood J., De bruyn M., Constantinou A., Moity L., McElroy C. R., Farmer T. J., Duncan T., Raverty W., Hunt A. J., Clark J. H.. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014;50(68):9650–9652. doi: 10.1039/C4CC04133J. [DOI] [PubMed] [Google Scholar]
- Cardoso A. P., Giacobbo A., Bernardes A. M., Ferreira C. A.. Performance evaluation of polysulfone-based membranes produced with a green solvent. J. Mater. Res. 2024;39(10):1525–1536. doi: 10.1557/s43578-024-01327-3. [DOI] [Google Scholar]
- Hackett C., Hale D., Bair B., Manson-Endeboh G. s.-D., Hao X., Qian X., Wickramasinghe S. R., Thompson A.. Polysulfone ultrafiltration membranes fabricated from green solvents: Significance of coagulation bath composition. Sep. Purif. Technol. 2024;332:125752. doi: 10.1016/j.seppur.2023.125752. [DOI] [Google Scholar]
- Lin S., He S., Sarwar S., Milescu R. A., McElroy C. R., Dimartino S., Shao L., Lau C. H.. Spray coating polymer substrates from a green solvent to enhance desalination performances of thin film composites. J. Mater. Chem. A. 2023;11(2):891–900. doi: 10.1039/D2TA07200A. [DOI] [Google Scholar]
- Arif A., Chanchaona N., Lau C. H.. Comparing the environmental impacts of using bio-renewable and fossil-derived solvent in polymer membrane fabrications. Adv. Membr. 2023;3:100079. doi: 10.1016/j.advmem.2023.100079. [DOI] [Google Scholar]
- Rasool M. A., Vankelecom I. F. J.. Use of γ-valerolactone and glycerol derivatives as bio-based renewable solvents for membrane preparation. Green Chem. 2019;21(5):1054–1064. doi: 10.1039/C8GC03652G. [DOI] [Google Scholar]
- Andresen-Streichert H., Jungen H., Gehl A., Müller A., Iwersen-Bergmann S.. Uptake of Gamma-ValerolactoneDetection of Gamma-Hydroxyvaleric Acid in Human Urine Samples. J. Anal. Toxicol. 2013;37(4):250–254. doi: 10.1093/jat/bkt013. [DOI] [PubMed] [Google Scholar]
- Huber G. W., Iborra S., Corma A.. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006;106(9):4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- Jia Y.-X., Xie L., Xu X.-G., Wang M.. Using γ-Valerolactone as a Nontoxic Solvent for Fabricating Anion-Exchange Membrane via Nonsolvent-Induced Phase Separation. J. Appl. Polym. Sci. 2025;142(22):e56952. doi: 10.1002/app.56952. [DOI] [Google Scholar]
- Rasool M. A., Vankelecom I. F. J.. γ-Valerolactone as Bio-Based Solvent for Nanofiltration Membrane Preparation. Membranes. 2021;11(6):418. doi: 10.3390/membranes11060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Jung M., Escobar I. C., Harris T. A. L.. Advances in applying sustainable materials and manufacturing scale-up in polymeric membrane fabrication. Curr. Opin. Chem. Eng. 2025;47:101085. doi: 10.1016/j.coche.2024.101085. [DOI] [Google Scholar]
- Hassankiadeh N. T., Cui Z., Kim J. H., Shin D. W., Lee S. Y., Sanguineti A., Arcella V., Lee Y. M., Drioli E.. Microporous poly(vinylidene fluoride) hollow fiber membranes fabricated with PolarClean as water-soluble green diluent and additives. J. Membr. Sci. 2015;479:204–212. doi: 10.1016/j.memsci.2015.01.031. [DOI] [Google Scholar]
- Jung J. T., Kim J. F., Wang H. H., di Nicolo E., Drioli E., Lee Y. M.. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS) J. Membr. Sci. 2016;514:250–263. doi: 10.1016/j.memsci.2016.04.069. [DOI] [Google Scholar]
- Marino T., Blasi E., Tornaghi S., Di Nicolò E., Figoli A.. Polyethersulfone membranes prepared with Rhodiasolv Polarclean as water soluble green solvent. J. Membr. Sci. 2018;549:192–204. doi: 10.1016/j.memsci.2017.12.007. [DOI] [Google Scholar]
- Randová A., Bartovská L., Morávek P., Matějka P., Novotná M., Matějková S., Drioli E., Figoli A., Lanč M., Friess K.. A fundamental study of the physicochemical properties of Rhodiasolv Polarclean: A promising alternative to common and hazardous solvents. J. Mol. Liq. 2016;224:1163–1171. doi: 10.1016/j.molliq.2016.10.085. [DOI] [Google Scholar]
- Fionah A., Oluk I., Brady L., Byrne D. M., Escobar I. C.. Performance and Environmental Assessment of Biochar-Based Membranes Synthesized from Traditional and Eco-Friendly Solvents. Membranes. 2024;14(7):153. doi: 10.3390/membranes14070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F., Ursino C., Sayinli B., Koyuncu I., Galiano F., Figoli A.. Advancements in Sustainable PVDF Copolymer Membrane Preparation Using Rhodiasolv PolarClean As an Alternative Eco-Friendly Solvent. Clean Technol. 2021;3(4):761–786. doi: 10.3390/cleantechnol3040045. [DOI] [Google Scholar]
- Medina-Gonzalez Y., Aimar P., Lahitte J. F., Remigy J. C.. Towards green membranes: preparation of cellulose acetate ultrafiltration membranes using methyl lactate as a biosolvent. Int. J. Sustainable Eng. 2011;4(1):75–83. doi: 10.1080/19397038.2010.497230. [DOI] [Google Scholar]
- Rasool M. A., Van Goethem C., Vankelecom I. F. J.. Green preparation process using methyl lactate for cellulose-acetate-based nanofiltration membranes. Sep. Purif. Technol. 2020;232:115903. doi: 10.1016/j.seppur.2019.115903. [DOI] [Google Scholar]
- Zhu M., Han D., Yang S., Zhang Y., Zhang H.. Fabrication of PVDF ultrafiltration membranes with methyl lactate: enhancing performance through green solvent practices. Green Chem. 2025;27(18):5149–5162. doi: 10.1039/D4GC05922K. [DOI] [Google Scholar]
- d’Ayala G. G., Marino T., de Almeida Y. M. B., de Matos Costa A. R., da Silva L. B., Argurio P., Laurienzo P.. Enhancing Sustainability in PLA Membrane Preparation through the Use of Biobased Solvents. Polymers. 2024;16(14):2024. doi: 10.3390/polym16142024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Hua D., Hong Y., Ibrahim A.-R., Yao A., Pan J., Zhan G.. Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes. 2020;10(12):395. doi: 10.3390/membranes10120395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Kim D., de Vos W. M.. Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent. Membranes. 2024;14(9):202. doi: 10.3390/membranes14090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Le N. L., Nunes S. P.. The effects of a co-solvent on fabrication of cellulose acetate membranes from solutions in 1-ethyl-3-methylimidazolium acetate. J. Membr. Sci. 2016;520:540–549. doi: 10.1016/j.memsci.2016.08.015. [DOI] [Google Scholar]
- Pham T. P. T., Cho C.-W., Yun Y.-S.. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010;44(2):352–372. doi: 10.1016/j.watres.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Ratti R.. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014;2014(1):729842. doi: 10.1155/2014/729842. [DOI] [Google Scholar]
- Ventura S. P. M., Gonçalves A. M. M., Sintra T., Pereira J. L., Gonçalves F., Coutinho J. A. P.. Designing ionic liquids: the chemical structure role in the toxicity. Ecotoxicology. 2013;22(1):1–12. doi: 10.1007/s10646-012-0997-x. [DOI] [PubMed] [Google Scholar]
- Dong X., Lu D., Harris T. A. L., Escobar I. C.. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes. 2021;11(5):309. doi: 10.3390/membranes11050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A., Santos A., Tojo J., Rodríguez A.. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008;151(1):268–273. doi: 10.1016/j.jhazmat.2007.10.079. [DOI] [PubMed] [Google Scholar]
- Bridge A. T., Wamble N. P., Santoso M. S., Brennecke J. F., Freeman B. D.. Defect-free asymmetric Matrimid gas separation membranes using dihydrolevoglucosenone (Cyrene) as a greener polar aprotic solvent than traditional solvents. J. Membr. Sci. 2024;691:122221. doi: 10.1016/j.memsci.2023.122221. [DOI] [Google Scholar]
- Rizqi R. A., Hartono Y. V., Shalahuddin I., Nugroho W. A., Bilad M. R., Arif C., Wibisono Y.. Green synthesis of polyvinylidene fluoride ultrafiltration membrane with upgraded hydrophilicity. Results Mater. 2023;19:100417. doi: 10.1016/j.rinma.2023.100417. [DOI] [Google Scholar]
- Yoon J., Lee J., Hong S. P., Park H.-J., Kim J., Lee J., Lee C., Oh S.-G.. Fabrication of biodegradable cellulose acetate nanofibers containing Rose Bengal dye by electrospinning technique and their antiviral efficacy under visible light irradiation. Chemosphere. 2024;349:140897. doi: 10.1016/j.chemosphere.2023.140897. [DOI] [PubMed] [Google Scholar]
- Chai, P. ; Ku, Y. ; Chan, W. In Preparation of Enhanced Antifouling Graphene Oxide Cellulose Acetate Mixed-matrix Membrane Using Ethyl Lactate Green Solvent, AIP Conference Proceedings; AIP Publishing, 2023. [Google Scholar]
- Lei L., Lindbråthen A., Sandru M., Gutierrez M. T. G., Zhang X., Hillestad M., He X.. Spinning Cellulose Hollow Fibers Using 1-Ethyl-3-methylimidazolium Acetate–Dimethylsulfoxide Co-Solvent. Polymers. 2018;10(9):972. doi: 10.3390/polym10090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Thi H. N., Kang J., Hwang J., Kim S., Park S., Lee J.-H., Abdellah M. H., Szekely G., Lee J. S., Kim J. F.. Sustainable fabrication of solvent resistant biodegradable cellulose membranes using green solvents. Chem. Eng. J. 2024;494:153201. doi: 10.1016/j.cej.2024.153201. [DOI] [Google Scholar]
- Otitoju T. A., Kim C.-H., Ryu M., Park J., Kim T.-K., Yoo Y., Park H., Lee J.-H., Cho Y. H.. Exploring green solvents for the sustainable fabrication of bio-based polylactic acid membranes using nonsolvent-induced phase separation. J. Cleaner Prod. 2024;467:142905. doi: 10.1016/j.jclepro.2024.142905. [DOI] [Google Scholar]
- Gholami F., Zinadini S., Zinatizadeh A. A., Abbasi A. R.. TMU-5 metal-organic frameworks (MOFs) as a novel nanofiller for flux increment and fouling mitigation in PES ultrafiltration membrane. Sep. Purif. Technol. 2018;194:272–280. doi: 10.1016/j.seppur.2017.11.054. [DOI] [Google Scholar]
- Arthanareeswaran G., Velu S., Muruganandam L.. Performance enhancement of polysulfone ultrafiltration membrane by blending with polyurethane hydrophilic polymer. J. Polym. Eng. 2011;31(2–3):125–131. doi: 10.1515/polyeng.2011.029. [DOI] [Google Scholar]
- Dong X., Al-Jumaily A., Escobar I. C.. Investigation of the Use of a Bio-Derived Solvent for Non-Solvent-Induced Phase Separation (NIPS) Fabrication of Polysulfone Membranes. Membranes. 2018;8(2):23. doi: 10.3390/membranes8020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimpour A., Madaeni S. S., Amirinejad M., Mansourpanah Y., Zereshki S.. The effect of heat treatment of PES and PVDF ultrafiltration membranes on morphology and performance for milk filtration. J. Membr. Sci. 2009;330(1):189–204. doi: 10.1016/j.memsci.2008.12.059. [DOI] [Google Scholar]
- Habibi S., Nematollahzadeh A.. Enhanced water flux through ultrafiltration polysulfone membrane via addition-removal of silica nano-particles: Synthesis and characterization. J. Appl. Polym. Sci. 2016;133(25):43556. doi: 10.1002/app.43556. [DOI] [Google Scholar]
- Cheng L., Zhou Z., Li L., Xiao P., Ma Y., Liu F., Li J.. PVDF/MOFs mixed matrix ultrafiltration membrane for efficient water treatment. Front. Chem. 2022;10:985750. doi: 10.3389/fchem.2022.985750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljoughi E., Sadrzadeh M., Mohammadi T.. Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes. J. Membr. Sci. 2009;326(2):627–634. doi: 10.1016/j.memsci.2008.10.044. [DOI] [Google Scholar]
- Li Z., Ren J., Fane A. G., Li D. F., Wong F.-S.. Influence of solvent on the structure and performance of cellulose acetate membranes. J. Membr. Sci. 2006;279(1):601–607. doi: 10.1016/j.memsci.2005.12.054. [DOI] [Google Scholar]
- Gohari R. J., Lau W. J., Matsuura T., Ismail A. F.. Fabrication and characterization of novel PES/Fe–Mn binary oxide UF mixed matrix membrane for adsorptive removal of As(III) from contaminated water solution. Sep. Purif. Technol. 2013;118:64–72. doi: 10.1016/j.seppur.2013.06.043. [DOI] [Google Scholar]
- Zhao S., Wang Z., Wang J., Yang S., Wang S.. PSf/PANI nanocomposite membrane prepared by in situ blending of PSf and PANI/NMP. J. Membr. Sci. 2011;376(1):83–95. doi: 10.1016/j.memsci.2011.04.008. [DOI] [Google Scholar]
- Van Krevelen D. W., Hoftyzer P. J.. Practical evaluation of the [η]–M relationship. J. Appl. Polym. Sci. 1967;11(3):473. doi: 10.1002/app.1967.070110314. [DOI] [Google Scholar]
- Scott G.. Properties of polymers. Their correlation with chemical structure; their numerical estimation and prediction from additive group contributions. Endeavour. 1992;16(2):97–98. doi: 10.1016/0160-9327(92)90023-I. [DOI] [Google Scholar]
- Nardella F., Prothmann J., Sandahl M., Spégel P., Ribechini E., Turner C.. Native lignin extraction from soft- and hardwood by green and benign sub/supercritical fluid extraction methodologies. RSC Adv. 2023;13(32):21945–21953. doi: 10.1039/D3RA01873C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Jiménez M., Palacio D. A., Palencia M., Meléndrez M. F., Rivas B. L.. Bio-Based Polymeric Membranes: Development and Environmental Applications. Membranes. 2023;13(7):625. doi: 10.3390/membranes13070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. N., Dowd M. K., Selling G. W., Biswas A.. Synthesis of cellulose acetate from cotton byproducts. Carbohydr. Polym. 2010;80(2):449–452. doi: 10.1016/j.carbpol.2009.11.048. [DOI] [Google Scholar]
- El-Sheikh M. A., El-Rafie S. M., Abdel-Halim E. S., El-Rafie M. H.. Green Synthesis of Hydroxyethyl Cellulose-Stabilized Silver Nanoparticles. J. Polym. 2013;2013(1):650837. doi: 10.1155/2013/650837. [DOI] [Google Scholar]
- Li S., Wang D., Xiao H., Zhang H., Cao S., Chen L., Ni Y., Huang L.. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2021;255:117352. doi: 10.1016/j.carbpol.2020.117352. [DOI] [PubMed] [Google Scholar]
- P A., K H., S M., Pius A.. Cellulose acetate based-membrane supported by metal-organic frameworks for the removal of diclofenac and ciprofloxacin from polluted water. Groundwater Sustainable Dev. 2024;26:101308. doi: 10.1016/j.gsd.2024.101308. [DOI] [Google Scholar]
- Ning D., Lu Z., Tian C., Yan N., Xie F., Li N., Hua L.. Superwettable cellulose acetate-based nanofiber membrane with spider-web structure for highly efficient oily water purification. Int. J. Biol. Macromol. 2023;253:126865. doi: 10.1016/j.ijbiomac.2023.126865. [DOI] [PubMed] [Google Scholar]
- Torkashvand J., Saeedi-Jurkuyeh A., Kalantary R. R., Gholami M., Esrafili A., Yousefi M., Farzadkia M.. Preparation of a cellulose acetate membrane using cigarette butt recycling and investigation of its efficiency in removing heavy metals from aqueous solution. Sci. Rep. 2022;12(1):20336. doi: 10.1038/s41598-022-24432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Chen R., Luo S., Yu W., Yuan J., Lin F., Wang M., Cao X., Liao Y., Huang B., You X.. Regenerated cellulose membranes for efficient separation of organic mixtures. Sep. Purif. Technol. 2024;328:125118. doi: 10.1016/j.seppur.2023.125118. [DOI] [Google Scholar]
- Salehi E., Khajavian M., Sahebjamee N., Mahmoudi M., Drioli E., Matsuura T.. Advances in nanocomposite and nanostructured chitosan membrane adsorbents for environmental remediation: A review. Desalination. 2022;527:115565. doi: 10.1016/j.desal.2022.115565. [DOI] [Google Scholar]
- Vedula S. S., Yadav G. D.. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021;98(2):100017. doi: 10.1016/j.jics.2021.100017. [DOI] [Google Scholar]
- Wu J., Dong Z., Li X., Li P., Wei J., Hu M., Geng L., Peng X.. Constructing acid-resistant chitosan/cellulose nanofibrils composite membrane for the adsorption of methylene blue. J. Environ. Chem. Eng. 2022;10(3):107754. doi: 10.1016/j.jece.2022.107754. [DOI] [Google Scholar]
- Wang Y., Li H., Zhao J., Li Z., Li J., Li J.. Polyethyleneimine functionalized chitosan nanofiltration membrane for nuclear wastewater decontamination. J. Ind. Eng. Chem. 2024;132:318–325. doi: 10.1016/j.jiec.2023.11.024. [DOI] [Google Scholar]
- Xia Y., Wang Z., Chen L.-Y., Xiong S.-W., Zhang P., Fu P.-G., Gai J.-G.. Nanoscale polyelectrolyte/metal ion hydrogel modified RO membrane with dual anti-fouling mechanism and superhigh transport property. Desalination. 2020;488:114510. doi: 10.1016/j.desal.2020.114510. [DOI] [Google Scholar]
- Wang Y., He Y., Yu J., Zhang L., Li S., Li H.. Alginate-based nanofibrous membrane with robust photo-Fenton self-cleaning property for efficient crude oil/water emulsion separation. Sep. Purif. Technol. 2022;287:120569. doi: 10.1016/j.seppur.2022.120569. [DOI] [Google Scholar]
- Thu N. T. T., Hoang L. H., Cuong P. K., Viet-Linh N., Nga T. T. H., Kim D. D., Leong Y. K., Nhi-Cong L. T.. Evaluation of polyhydroxyalkanoate (PHA) synthesis by Pichia sp. TSLS24 yeast isolated in Vietnam. Sci. Rep. 2023;13(1):3137. doi: 10.1038/s41598-023-28220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H. R., Ebrahimi F.. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2023;63(1):22–43. doi: 10.1002/pen.26193. [DOI] [Google Scholar]
- Vatanpour V., Dehqan A., Paziresh S., Zinadini S., Zinatizadeh A. A., Koyuncu I.. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022;296:121433. doi: 10.1016/j.seppur.2022.121433. [DOI] [Google Scholar]
- Kaseem M., Ur Rehman Z., Hossain S., Singh A. K., Dikici B.. A Review on Synthesis, Properties, and Applications of Polylactic Acid/Silica Composites. Polymers. 2021;13(18):3036. doi: 10.3390/polym13183036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaba N. F., Jaafar M.. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020;60(9):2061–2075. doi: 10.1002/pen.25511. [DOI] [Google Scholar]
- Zhang J., Jiao Y., Zhang Y., Wang K., Sui X., Song D., Drioli E., Cheng X.. Development of Hydrophilic Polylactic Acid Hollow-Fiber Membranes for Water Remediation. Ind. Eng. Chem. Res. 2022;61(48):17479–17487. doi: 10.1021/acs.iecr.2c03100. [DOI] [Google Scholar]
- Khalil H., Hegab H. M., Nassar L., Wadi V. S., Naddeo V., Yousef A. F., Banat F., Hasan S. W.. Asymmetrical ultrafiltration membranes based on polylactic acid for the removal of organic substances from wastewater. J. Water Process Eng. 2022;45:102510. doi: 10.1016/j.jwpe.2021.102510. [DOI] [Google Scholar]
- Nassar L., Wadi V. S., Hegab H. M., Khalil H., Banat F., Naddeo V., Hasan S. W.. Sustainable and green polylactic acid-based membrane embedded with self-assembled positively charged f-MWCNTs/GO nanohybrids for the removal of nutrients from wastewater. npj Clean Water. 2022;5(1):57. doi: 10.1038/s41545-022-00206-w. [DOI] [Google Scholar]
- Tomietto, P. ; Loulergue, P. ; Paugam, L. ; Audic, J.-L. . Polyhydroxyalkanoates (PHAs) for the Fabrication of Filtration Membranes. In Advances in Science, Technology Innovation; Zhang, Z. ; Zhang, W. ; Chehimi, M. M. , Eds.; Springer International Publishing, 2021; pp 177–195. [Google Scholar]
- Mekonnen T., Mussone P., Khalil H., Bressler D.. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A. 2013;1(43):13379–13398. doi: 10.1039/c3ta12555f. [DOI] [Google Scholar]
- Anjum A., Zuber M., Zia K. M., Noreen A., Anjum M. N., Tabasum S.. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016;89:161–174. doi: 10.1016/j.ijbiomac.2016.04.069. [DOI] [PubMed] [Google Scholar]
- Masood F., Yasin T., Hameed A.. Polyhydroxyalkanoates – what are the uses? Current challenges and perspectives. Crit. Rev. Biotechnol. 2015;35(4):514–521. doi: 10.3109/07388551.2014.913548. [DOI] [PubMed] [Google Scholar]
- Tomietto P., Carré M., Loulergue P., Paugam L., Audic J.-L.. Polyhydroxyalkanoate (PHA) based microfiltration membranes: Tailoring the structure by the non-solvent induced phase separation (NIPS) process. Polymer. 2020;204:122813. doi: 10.1016/j.polymer.2020.122813. [DOI] [Google Scholar]
- Sariipek F. B., Gündoğdu Y., Kiliç H. Ş.. Fabrication of eco-friendly superhydrophobic and superoleophilic PHB-SiO bionanofiber membrane for gravity-driven oil/water separation. J. Appl. Polym. Sci. 2023;140(9):e53542. doi: 10.1002/app.53542. [DOI] [Google Scholar]
- Bang J., Park S., Hwang S.-W., Oh J.-K., Yeo H., Jin H.-J., Kwak H. W.. Biodegradable and hydrophobic nanofibrous membranes produced by solution blow spinning for efficient oil/water separation. Chemosphere. 2023;312:137240. doi: 10.1016/j.chemosphere.2022.137240. [DOI] [PubMed] [Google Scholar]
- Ling S., Qin Z., Huang W., Cao S., Kaplan D. L., Buehler M. J.. Design and function of biomimetic multilayer water purification membranes. Sci. Adv. 2017;3(4):e1601939. doi: 10.1126/sciadv.1601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X., Han Y., Cheng H.. Biomedical applications of chitosan/silk fibroin composites: A review. Int. J. Biol. Macromol. 2023;240:124407. doi: 10.1016/j.ijbiomac.2023.124407. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Peng S., Li X., Wang X., Jiang J., Liu X., Wang L.. Design and function of lignin/silk fibroin-based multilayer water purification membranes for dye adsorption. Int. J. Biol. Macromol. 2023;253:126863. doi: 10.1016/j.ijbiomac.2023.126863. [DOI] [PubMed] [Google Scholar]
- Lee S., Kim H.-J., Tian M., Khang G., Kim H.-W., Bae T.-H., Lee J.. Silk fibroin-coated polyamide thin-film composite membranes with anti-scaling properties. Desalination. 2023;546:116195. doi: 10.1016/j.desal.2022.116195. [DOI] [Google Scholar]
- Chowdhury, S. R. ; Mh Busra, M. F. ; Lokanathan, Y. ; Ng, M. H. ; Law, J. X. ; Cletus, U. C. ; Binti Haji Idrus, R. . Collagen Type I: A Versatile Biomaterial. In Advances in Experimental Medicine and Biology; Chun, H. J. ; Park, K. ; Kim, C.-H. ; Khang, G. , Eds.; Springer Singapore, 2018; pp 389–414. [DOI] [PubMed] [Google Scholar]
- Desiriani R., Susanto H., Aryanti N., Abriyanto H.. Improvement of the antifouling and antibacterial properties of polyethersulfone membrane by incorporating the natural additives collagen and green tea. Results Eng. 2023;18:101176. doi: 10.1016/j.rineng.2023.101176. [DOI] [Google Scholar]
- Mohammadi R., Mohammadifar M. A., Mortazavian A. M., Rouhi M., Ghasemi J. B., Delshadian Z.. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM) Food Chem. 2016;190:186–193. doi: 10.1016/j.foodchem.2015.05.073. [DOI] [PubMed] [Google Scholar]
- Alias, N. H. ; Abdullah, N. ; Othman, N. H. ; Marpani, F. ; Zainol, M. M. ; Shayuti, M. S. M. . Sustainability Challenges and Future Perspectives of Biopolymer. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A. K. ; Sharma, S. ; Bhat, R. , Eds.; Springer International Publishing, 2022; pp 373–389. [Google Scholar]
- Barletta M., Aversa C., Ayyoob M., Gisario A., Hamad K., Mehrpouya M., Vahabi H.. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022;132:101579. doi: 10.1016/j.progpolymsci.2022.101579. [DOI] [Google Scholar]
- Kartik A., Akhil D., Lakshmi D., Gopinath K. P., Arun J., Sivaramakrishnan R., Pugazhendhi A.. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021;329:124868. doi: 10.1016/j.biortech.2021.124868. [DOI] [PubMed] [Google Scholar]
- Torre-Celeizabal A., Russo F., Galiano F., Figoli A., Casado-Coterillo C., Garea A.. Green Synthesis of Cellulose Acetate Mixed Matrix Membranes: Structure–Function Characterization. ACS Sustainable Chem. Eng. 2025;13(3):1253–1270. doi: 10.1021/acssuschemeng.4c07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission, E. . REACH Regulation. 2023.
- Agency, E. P. . Toxic Substances Control Act of 1976. 2016.
- Agency, E. P. . Clean Water Act. 2018.
- Organisation, I. S. . ISO 14001:2015. 2015.
- Commission, E. . Circular Economy Action Plan. 2020.
- Landaburu-Aguirre J., García-Pacheco R., Molina S., Rodríguez-Sáez L., Rabadán J., García-Calvo E.. Fouling prevention, preparing for re-use and membrane recycling. Towards circular economy in RO desalination. Desalination. 2016;393:16–30. doi: 10.1016/j.desal.2016.04.002. [DOI] [Google Scholar]
- Grossi L. B., da Silva B. R. S., Neves E. F. O., Lange L. C., Amaral M. C. S.. Reverse osmosis elements waste assessment: Screening and forecasting of emerging waste in Brazil. Desalination. 2021;517:115245. doi: 10.1016/j.desal.2021.115245. [DOI] [Google Scholar]
- García-Pacheco R., Landaburu-Aguirre J., Molina S., Rodríguez-Sáez L., Teli S. B., García-Calvo E.. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Membr. Sci. 2015;495:305–315. doi: 10.1016/j.memsci.2015.08.025. [DOI] [Google Scholar]
- Liu C., Wang W., Yang B., Xiao K., Zhao H.. Separation, anti-fouling, and chlorine resistance of the polyamide reverse osmosis membrane: From mechanisms to mitigation strategies. Water Res. 2021;195:116976. doi: 10.1016/j.watres.2021.116976. [DOI] [PubMed] [Google Scholar]
- de Paula E. C., Gomes J. C. L., Amaral M. C. S.. Recycling of end-of-life reverse osmosis membranes by oxidative treatment: a technical evaluation. Water Sci. Technol. 2017;76(3):605–622. doi: 10.2166/wst.2017.238. [DOI] [PubMed] [Google Scholar]
- Fujioka T., Ngo M. T. T., Boivin S., Kawahara K., Takada A., Nakamura Y., Yoshikawa H.. Controlling biofouling and disinfection by-product formation during reverse osmosis treatment for seawater desalination. Desalination. 2020;488:114507. doi: 10.1016/j.desal.2020.114507. [DOI] [Google Scholar]
- Wang H., Xu Y., Ma B., Zou W., Zeng J., Dai R., Wang Z.. Alkaline pre-treatment enables controllable downcycling of Si-Al fouled end-of-life RO membrane to NF and UF membranes. J. Membr. Sci. 2024;690:122209. doi: 10.1016/j.memsci.2023.122209. [DOI] [Google Scholar]
- Moreira V. R., Grossi L. B., Guimaraes R. N., Amaral M. C. S.. Recycled nanofiltration membrane as a low-cost alternative to remove uranium from drinking water in remote communities. Desalination. 2024;586:117820. doi: 10.1016/j.desal.2024.117820. [DOI] [Google Scholar]
- Cui J., Chen Y., Guo P., Su W., Xu L., Zhang Y.. Recycling End-of-Life RO Membranes for NF Membranes via Layer-by-Layer Assembly and Interfacial Polymerization. Ind. Eng. Chem. Res. 2023;62(25):9837–9848. doi: 10.1021/acs.iecr.3c01042. [DOI] [Google Scholar]
- Dai R., Han H., Wang T., Li J., Wu Z., Tang C. Y., Wang Z.. Cleaning–Healing–Interfacial Polymerization Strategy for Upcycling Real End-of-Life Polyvinylidene Fluoride Microfiltration Membranes. ACS Sustainable Chem. Eng. 2021;9(30):10352–10360. doi: 10.1021/acssuschemeng.1c03481. [DOI] [Google Scholar]
- Wang X., Han H., Zhou H., Wang T., Dai R., Wang Z.. Rapid Upcycling of End-of-Life Microfiltration Membrane Mediated by the Healing of Metal–Organic Complex. ACS Sustainable Chem. Eng. 2022;10(30):9841–9849. doi: 10.1021/acssuschemeng.2c01697. [DOI] [Google Scholar]
- Dai R., Han H., Wang T., Li J., Tang C. Y., Wang Z.. Fouling is the beginning: upcycling biopolymer-fouled substrates for fabricating high-permeance thin-film composite polyamide membranes. Green Chem. 2021;23(2):1013–1025. doi: 10.1039/D0GC03340E. [DOI] [Google Scholar]
- Dai R., Chen J., Han H., Zhou H., Wang Z.. Interfacial Wettability Regulation Enables One-Step Upcycling of the End-of-Life Polymeric Microfiltration Membrane. ACS ES&T Eng. 2023;3(4):479–486. doi: 10.1021/acsestengg.2c00329. [DOI] [Google Scholar]
- Li B., Wang S., Loh X. J., Li Z., Chung T.-S.. Closed-loop recyclable membranes enabled by covalent adaptable networks for water purification. Proc. Natl. Acad. Sci. U.S.A. 2023;120(15):e2301009120. doi: 10.1073/pnas.2301009120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Qu C., Wang S., Yeo J. C. C., Surat’man N. E. B., Loh X. J., Li Z., Chung T.-S.. Closed-loop recyclable dynamic covalent crosslinked nanofibrous membranes for efficient oil/water separation. J. Membr. Sci. 2024;693:122378. doi: 10.1016/j.memsci.2023.122378. [DOI] [Google Scholar]
- Poniatowska J., Houben M., Borneman Z., Nijmeijer K.. Towards organic solvent nanofiltration (OSN) membrane recyclability through Diels-Alder (DA) dynamic covalent bonds. Sep. Purif. Technol. 2025;362:131812. doi: 10.1016/j.seppur.2025.131812. [DOI] [Google Scholar]
- Ramírez-Martínez M., Aristizabal S., Upadhyaya L., Emwas A.-H., Hadjichristidis N., Nunes S. P.. Recyclable Membranes through Reversible and Dynamic Crosslinking. ACS Appl. Polym. Mater. 2024;6(21):13120–13131. doi: 10.1021/acsapm.4c02286. [DOI] [Google Scholar]
- Gupta R. S., Mandal S., Malakar A., Rege S., Islam S. S., Samanta K., Misra A., Bose S.. Graphene oxide offers precise molecular sieving, structural integrity, microplastic removal, and closed-loop circularity in water-remediating membranes through a covalent adaptable network. J. Mater. Chem. A. 2023;12(1):321–334. doi: 10.1039/D3TA04539K. [DOI] [Google Scholar]
- Wang S., Wang N., Kai D., Li B., Wu J., Yeo J. C. C., Xu X., Zhu J., Loh X. J., Hadjichristidis N., Li Z.. In-situ forming dynamic covalently crosslinked nanofibers with one-pot closed-loop recyclability. Nat. Commun. 2023;14(1):1182. doi: 10.1038/s41467-023-36709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula E. C., Amaral M. C. S.. Environmental and economic evaluation of end-of-life reverse osmosis membranes recycling by means of chemical conversion. J. Cleaner Prod. 2018;194:85–93. doi: 10.1016/j.jclepro.2018.05.099. [DOI] [Google Scholar]
- Chen J., Dai R., Wang Z.. Closing the loop of membranes by recycling end-of-life membranes: Comparative life cycle assessment and economic analysis. Resour., Conserv. Recycl. 2023;198:107153. doi: 10.1016/j.resconrec.2023.107153. [DOI] [Google Scholar]
- Reiser P., Neubert M., Eberhard A., Torresi L., Zhou C., Shao C., Metni H., van Hoesel C., Schopmans H., Sommer T., Friederich P.. Graph neural networks for materials science and chemistry. Commun. Mater. 2022;3(1):93. doi: 10.1038/s43246-022-00315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]