Abstract
Two oligonucleotide primer sets for the discrimination of Streptococcus pneumoniae from “pneumococcus-like” oral streptococcal isolates by PCR were developed. Genomic subtractive hybridization was performed to search for differences between Streptococcus pneumoniae strain WU2 and the most closely related oral streptococcus, Streptococcus mitis strain 903. We identified 19 clones that contained S. pneumoniae-specific nucleotide fragments that were absent from the chromosomal DNA of typical laboratory strains of S. mitis and other oral bacteria. Subsequently, oligonucleotide PCR primers for the detection of S. pneumoniae were designed from the sequences of the subtracted DNA fragments, and the specificities of the 19 primer sets were evaluated by PCR using chromosomal DNAs extracted from four S. pneumoniae clinical isolates and from 20 atypical organisms classified as S. mitis or S. oralis, which harbored genes encoding the pneumococcal virulence factors autolysin (lytA) or pneumolysin (ply), as templates. Of the 19 primer sets, two (Spn9802 and Spn9828) did not amplify PCR products from any of the pneumococcus-like streptococcal strains that we examined. The genes containing the Spn9802 and Spn9828 sequences encoded proteins of unknown function that did not correspond to any previously described proteins in other bacteria. These new oligonucleotide primers may be very useful for early and correct diagnosis of S. pneumoniae infections.
Streptococcus pneumoniae is a major cause of bacterial disease in humans, including pneumonia, otitis media, septicemia, and meningitis. Two naturally transformable viridans group streptococci, Streptococcus mitis and Streptococcus oralis, are closely related to S. pneumoniae, and classification of these organisms has long been considered difficult (32). Indeed, the nucleotide sequences of the 16S rRNA genes from S. mitis and S. oralis are over 99% identical to that of S. pneumoniae (17). As the potentially pathogenic S. pneumoniae is frequently detected in the oral cavity (31), and the commensals S. mitis and S. oralis are almost always detected (18), it is important to accurately discriminate among them in order to provide accurate diagnoses and treatments.
Various molecular assays have been employed to identify pneumococcal strains and to detect pneumococci directly from clinical samples. PCR-based assays for identifying S. pneumoniae have frequently targeted genes encoding pneumococcal virulence factors, including pneumolysin (ply) (7), autolysin (lytA) (20), pneumococcal surface antigen A (21), manganese-dependent superoxide dismutase (sodA) (16), and penicillin binding protein (24). Ideally, amplification of these target genes should be specific for S. pneumoniae isolates only. However, the occasional occurrence of viridans group identified as S. mitis or S. oralis harbor genes encoding S. pneumoniae virulence factors has been reported (31).
In this study, genomic subtractive hybridization was performed to identify genomic differences between the two type strains, S. pneumoniae WU2 and S. mitis 903. In addition, we designed primers from the sequences of the DNA fragments obtained by subtractive hybridization and examined their diagnostic utility in PCR assays using chromosomal DNA from clinical S. mitis and S. oralis isolates harboring the lytA and/or the ply gene. The technique of subtractive hybridization was initially developed to isolate differences in cDNA pools, but it has also been successfully used to identify genomic differences between closely related strains of Helicobacter pylori (1), Mycobacterium tuberculosis (19), and Neisseria meningitidis (25). We describe here the application of this technique to S. pneumoniae and the identification of specific nucleotide sequences for discrimination of this pathogenic organism from pneumococcus-like isolates.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pneumoniae WU2 and S. mitis 903 were grown anaerobically (85% N2, 10% H2, 5% CO2) at 37°C in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) or on 2% Todd-Hewitt broth agar plates. Chromosomal DNA was purified using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.) according to the manufacturer's instructions. In addition to S. pneumoniae WU2 and S. mitis 903, chromosomal DNAs extracted from the following oral bacterial strains were used in PCR assays to evaluate the specificity of the DNA sequences amplified by two newly designed primers: S. oralis ATCC 10557, Streptococcus gordonii DL1, Streptococcus sanguinis ATCC 10556, Streptococcus mutans Xc, Streptococcus sobrinus 6715, Streptococcus salivarius HT9R, Streptococcus anginosus FW73, Fusobacterium nucleatum ATCC 10953, Haemophilus aphrophilus NCTC 5908, Eikenella corrodens 1085, Actinomyces naeslundii ATCC 51655, Actinomyces viscosus ATCC 43146, Porphyromonas gingivalis ATCC 33277, Tannerella forsythensis ATCC 43037, Prevotella intermedia ATCC 25611, Actinobacillus actinomycetemcomitans Y4, and Treponema denticola ATCC 35404. Escherichia coli DH5α [F- φ 80 lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rk- mk+) phoA supE44 λ- thi-1, gyrA96, relA1] (Takara Bio Co., Shiga, Japan) was used for the DNA manipulations.
Subtractive hybridization.
Genomic subtractive hybridization was performed as described by Tinsley and Nassif (30). Briefly, 200 ng of chromosomal DNA from S. pneumoniae WU2 was completely digested with Sau3AI, precipitated with ethanol-sodium acetate, and ligated with 0.2 nmol of an oligonucleotide R-adaptor pair (RBam12, 5′-GATCCTCGGTGA-3′, RBam24, 5′-AGCACTCTCCAGCCTCTCACCGAG-3′) for 16 h at 11°C. The ligated DNA fragments were purified using the QIAquick PCR purification kit (QIAGEN, Tokyo, Japan) and stored at −20°C. To prepare the subtractive driver DNA, chromosomal DNA from S. mitis 903 was digested with restriction enzymes, including HindIII, EcoRI, and MspI, to yield fragments ranging from approximately 3 to 10 kb. After digestion, the DNA was precipitated with ethanol-sodium acetate and dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0).
The first subtractive hybridization was performed in 10 μl of a reaction mixture containing 2 μg of the driver DNA from S. mitis, 20 ng of the R-adaptor-linked Sau3AI fragments from S. pneumoniae, 10 mM EPPS (3-[4-(2-hydroxyethyl)-1-piperazinyl] propanesulfonic acid; Wako, Tokyo, Japan), 1 mM EDTA, and 1 M NaCl. The DNA mixture was denatured at 100°C for 2 min and then hybridized at 55°C for 18 h. A 2-μl aliquot of the reaction mixture was placed on ice and diluted to 40 μl with the PCR mixture containing 100 pmol of RBam24, 0.25 mM of each deoxynucleoside triphosphate, 5 U of TaKaRa ExTaq and 1× ExTaq buffer (Takara Bio Co.) to fill in the ends corresponding to the RBam24 adaptor.
After denaturation at 94°C for 5 min, amplification by PCR was performed in a T3 thermocycler (Biometra, Göttingen, Germany) as follows: a total of 30 cycles, each of which comprised 94°C for 1 min, 70°C for 1 min, and 72°C for 3 min, followed by a final extension step at 72°C for 10 min. The RBam24 adaptor was removed from the PCR products by digestion with Sau3AI and the DNA fragments were purified using the QIAquick PCR purification kit. In a 40-μl volume, 1 nmol of the second adaptors (JBam12, 5′-GATCCGTTCATG-3′; JBam24, 5′-ACCGACGTCGACTATCCATGAACG-3′) were ligated for 16 h at 11°C. The second-round subtractive hybridization was performed with 2 ng of DNA from the first round PCR products and 2 μg of the driver DNA from S. mitis, ranging in size from 3 to 10 kb, prepared as described above. The amplification products of the second-round PCR, which appeared as a smear of products smaller than 800 bp upon electrophoresis with ethidium bromide, were digested with Sau3AI, cloned into BamHI-digested pBluescript II SK+ vector (Stratagene, La Jolla, Calif.), and used to transform E. coli DH5α by heat shock. The nucleotide sequences of the inserts were determined using an ABI PRISM 310 automatic sequencer (Applied Biosystems, Foster City, Calif.) and the manufacturer's services (Genenet, Saga, Japan).
Analysis of clones and design of PCR primers.
The homologies of the sequences of the genomic subtracted fragments with sequences in the GenBank database were determined using BLAST (2) by means of the server at the National Center for Biotechnology Information, Bethesda, Md. (http://www.ncbi.nlm.nih.gov/BLAST). The predicted complete open reading frames (ORFs) of S. pneumoniae R6 (GenBank accession number AE 007317) and S. pneumoniae TIGR4 (GenBank accession number AE 005672) corresponding to the subtracted genomic fragments from S. pneumoniae WU2 were used to search for protein homologies using BlastP (2). Oligonucleotide primer pairs were designed for 46 sequences of interest from the subtracted genomic S. pneumoniae WU2 DNA using Primer Express 1.5 software (Applied Biosystems).
The oligonucleotide primers for the lytA and ply genes were designed as previously described by Nagai et al. (22) and Salo et al. (26), respectively. The primers for detection lytA were 5′-CAACCGTACAGAATGAAGCGG-3′ (sense) and 5′-TTATTCGTGCAATACTCGTGCG-3′ (antisense). The primers for detection ply were 5′-ATTTCTGTAACAGCTACCAACGA-3′ (sense) and 5′-GAATTCCCTGTCTTTTCAAAGTC-3′ (antisense).
PCR assays with candidate primers.
The amplification reactions were conducted using a T3 thermocycler (Biometra) with the following temperature profile: initial denaturation at 94°C for 2 min, then 25 cycles consisting of 94°C for 10 seconds, 58°C for 15 seconds, and 72°C for 1 min, followed by a final extension step at 72°C for 5 min. The amplification products were loaded onto 1.5% (wt/vol) agarose gels, separated by electrophoresis, stained with ethidium bromide (0.5 μl/ml), and photographed under UV light.
Southern hybridization.
Southern hybridization was performed 6 h under stringent conditions (hybridization fluid with 50% formamide at 50°C). Posthybridization washes were performed twice with 2× SCC (1× SCC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% (wt/vol) sodium dodecyl sulfate (SDS) at room temperature for 15 min per wash and twice with 0.1× SCC-0.1% (wt/vol) SDS at room temperature for 15 min per wash. All other procedures that involved Southern hybridization were performed by the methods of Sambrook et al. (27).
Clinical specimens.
Oral mucosa swab samples from 432 preschool children (5 to 6 years old) were screened by PCR using lytA and ply as primers. Prior to this study the parents of preschool children were explained the procedure of taking oral mucosa swabs and informed consents were obtained from the parents of all children involved in this study. From 14 oral mucosa swab samples of systematically healthy preschool children, a total of 24 streptococcal isolates suspected of being pneumococci were identified on the basis of colony morphology of alpha-hemolytic streptococci on blood agar plates.
Although PCR amplification using the lytA- and/or the ply-specific primer gave positive results for all of these isolates, most could not be classified as S. pneumoniae based on optochin resistance and bile insolubility in addition to the results of the biotyping tests from the API 20 STREP system (BioMérieux, Tokyo, Japan). Optochin sensitivity and bile solubility tests were performed as previously described (31). API 20 STREP was used according to the manufacturer' s instructions. Of the 24 strains tested, four were classified as S. pneumoniae on the basis of the total consideration through these conventional identification methods and 20 were classified as S. mitis or S. oralis.
RESULTS
Subtractive hybridization.
Approximately 200 transformed E. coli colonies were obtained from cloning the second-round PCR products by direct genomic subtraction of S. mitis 903 from S. pneumoniae WU2. The nucleotide sequences of 92 randomly selected clones were determined. The inserted fragments of genomic subtracted DNA varied from 150 to 600 bp and contained diverse sequences; only three clones were identical and additional four clones contained two distinct Sau3AI fragments in a single clone. A nucleotide database search using BlastN (2) revealed that 68 of the Sau3AI fragments had high homology with the genome sequence of other S. pneumoniae strains, and 46 of these had no significant sequence homology with any DNA sequence in the GenBank database other than S. pneumoniae.
Initially, 46 pairs of S. pneumoniae-specific primers were designed from the sequences of the subtracted genomic fragments. The nucleotide sequences amplified by each primer pair shared no significant identity with any previously reported nucleotide sequences in a nucleotide database search (BlastN) (2). The specificities of the primers were examined by PCR using chromosomal DNA extracted from 18 oral bacterial species, including S. mitis 903 in addition to E. coli DH5α, as listed in Materials and Methods. The PCR analysis showed that 19 primer pairs amplified only the chromosomal DNA from S. pneumoniae WU2 (data not shown). The oligonucleotide sequences of these primer sets are listed in Table 1. The predicted sizes of the PCR amplicons ranged from 130 to 351 bp.
TABLE 1.
Sequences of oligonucleotide primers
| Primer | Sequence | Amplicon size (bp) |
|---|---|---|
| lytA sense | 5′-CAA CCG TAC AGA ATG AAG CGG-3′ | 308 |
| lytA antisense | 5′-TTA TTC GTG CAA TAC TCG TGC G-3′ | |
| ply sense | 5′-ATT TCT GTA ACA GCT ACC AAC GA-3′ | 329 |
| ply antisense | 5′-GAA TTC CCT GTC TTT TCA AAG TC-3′ | |
| Spn9802-143F | 5′-CAA GTC GTT CCA AGG TAA CAA GTC T-3′ | 162 |
| Spn9802-304R | 5′-CTA AAC CAA CTC GAC CAC CTC TTT-3′ | |
| Spn14.1-27F | 5′-CAC CAT CAT AAA TGC TAG CCT TGA-3′ | 301 |
| Spn14.1-327R | 5′-TTG GAA GAT AGA ACC ATA GTT GCT TG-3′ | |
| Spn9822-17F | 5′-CAG GGA TTC GCT TAA TAG CTG GT-3′ | 202 |
| Spn9822-218R | 5′-CCT GCC AAG GTC AAT CCA TAA A-3′ | |
| Spn24-4F | 5′-TGT CCC AAT CTC TAA AGG ATG GAG-3′ | 225 |
| Spn24-228R | 5′-CAA CTT GCC CGA TTC ACT TTT C-3′ | |
| Spn25.1-12F | 5′-TAA CCA AAA CCT TTA GTT TAG GTT GAT TTT-3′ | 135 |
| Spn25.1-146R | 5′-CTC ACT ATT TAG AGA CTC TTG AGG TTT GTT-3′ | |
| Spn25.2-6F | 5′-GAG ACT CCG TGG AAC CTC TTT G-3′ | 130 |
| Spn25.2-135R | 5′-GAC GAA CTG CCA TAT AAG AAC CAT T-3′ | |
| Spn27-11F | 5′-CAA GAT TGA ACG TAT CCG CGA-3′ | 285 |
| Spn27-295R | 5′-TGG CCA GTT GAT TCG AGT TTG-3′ | |
| Spn9827-70F | 5′-TGG CCC TAA TAC AAC GAT GGA-3′ | 208 |
| Spn9827-277R | 5′-AGC AAG TGA GGG AAA GAC CGT-3′ | |
| Spn9828-19F | 5′-GGC ATT GTG AAT GGA TTG ATT G-3′ | 227 |
| Spn9828-245R | 5′-TCA TGT GCA TCC CAA GCT ACA-3′ | |
| Spn34-5F | 5′-GGG CCT GTA TCG TTC TAC TAG GG-3′ | 231 |
| Spn34-235R | 5′-TTT CTC TCC AAT CTA CCT TAC CCG-3′ | |
| Spn38-5F | 5′-AAT GGT CGT ATT GTT GCC GG-3′ | 266 |
| Spn38-270R | 5′-AAG TAA TCA ATC GCA TCT TGT AGG G-3′ | |
| Spn53-415F | 5′-GGG ATT CAA AAT ACA GTA GAG TCC TTG-3′ | 346 |
| Spn53-760R | 5′-TGA GCT TTG CAG CAT AAC GG-3′ | |
| Spn63-191F | 5′-GGA CTT TCT TTT TCC CAT CGG-3′ | 339 |
| Spn63-529R | 5′-CCC AGA TTT GTT TGA GCC AAC-3′ | |
| Spn66-10F | 5′-AAC AAT CGA ATC CCC TGG G-3′ | 312 |
| Spn66-321R | 5′-AAC TCC ACC AGT CAG GGA TGA-3′ | |
| Spn70-49F | 5′-CCT GCG TAG CCC ATA TCT CG-3′ | 253 |
| Spn70-301R | 5′-GGC AGG ATG CCA TTC GAC T-3′ | |
| Spn76-33F | 5′-CAT AGA TTC CCC AGA TCT CGG T-3′ | 351 |
| Spn76-383R | 5′-CAG GTC AGA GTG AGC TTT TGG TG-3′ | |
| Spn79-68F | 5′-CGC TAG TGA TTG GTG GTG AGC-3′ | 261 |
| Spn79-328R | 5′-CAA GTT CCT GCA AGG TTT CAA GA-3′ | |
| Spn84-93F | 5′-CTT GTC AGC CTT TGC TTG GAA-3′ | 332 |
| Spn84-424R | 5′-GCG CTA GAC AGA TCA GCA GGA-3′ | |
| Spn86-24F | 5′-CGA AAC ACC AGC AGA AGA GCT-3′ | 184 |
| Spn86-207R | 5′-AAT TTC GCC ACT ATT TAG CCA GAT-3′ |
Sequence homologies.
The characteristics of the 19 S. pneumoniae-specific sequences that were shown to be absent in the other tested oral bacteria are summarized in Table 2. All 19 subtracted genomic fragments showed high sequence identity with the genomic sequences of S. pneumoniae R6 (15) in the GenBank database. A search of the protein database using BlastP (2) was performed using the predicted complete ORFs of S. pneumoniae R6 corresponding to each of the 19 subtracted genomic fragments from S. pneumoniae WU2. Of the five ORFs corresponding to the sequences of clones spn9802, spn9822, spn9828, spn14.1, and spn63 that encoded proteins of unknown function, two ORFs corresponding to spn9802 and spn9828 showed no significant homologies with any organisms other than S. pneumoniae. A DNA sequence identical to that of clone spn9802 was found in S. pneumoniae R6; however, its 107-amino-acid ORF constituted has not been deposited in the GenBank database. In S. pneumoniae TIGR4, a similar ORF that shared 93% identity with that derived from spn9802 was identified. The ORF was six amino acids longer than that of the strain R6 at the N terminus.
TABLE 2.
Characteristics of differential clones of S. pneumoniae genomic DNA
| Clone | Gene no.a | ORF in strain R6 containing differential clone
|
Other homologues
|
|||
|---|---|---|---|---|---|---|
| ORF size (aa) | % GC | Gene product and function in strain R6 (accession no.) | Gene product and function (homologues) | E valueb (% identity) | ||
| spn9802c | 107 | 29.6 | NDd | Unknown (S. pneumoniae TIGR4) | 3e-35 (93%) | |
| spn9822 | spr1802 | 680 | 43.4 | Unknown (NP_359394) | Unknown (Lactococcus lactis) | 4e-59 (25%) |
| spn9827 | spr1781 | 427 | 46.9 | UDP-N-acetylglucosamine l-carboxyvinyltransferase (NP_359373) | ||
| spn9828c | spr1523 | 114 | 39.1 | Unknown (NP_359116) | Unknown (S. pneumoniae TIGR4) | 8e-41 (90%) |
| spn14.1 | spr0087 | 320 | 40.6 | Unknown (NP_357681) | Unknown (Streptococcus mutans) | 5e-7 (23%) |
| spn24 | spr1092 | 292 | 44.9 | tRNA pseudouridine 5S synthase (NP_358685) | ||
| spn25.1 | spr1622 | 512 | 30.0 | Unknown (NP_359214) | Transcriptional activator, putative (S. pneumoniae TIGR4) | 0.0 (99%) |
| spn25.2 | spr1179 | 334 | 52.9 | Unknown (NP_358772) | Predicted iron-dependent peroxidase (Actinobacillus pleuropneumoniae) | 1e-101 (53%) |
| spn27 | spr0058 | 252 | 37.3 | Unknown (NP_357652) | Transcriptional regulator, GntR family (S. pneumoniae TIGR4) | 1e-135 (100%) |
| spn34 | spr1617 | 439 | 40.0 | Sucrose-6-phosphate hydrolase (NP_359209) | ||
| spn38 | spr1782 | 345 | 41.8 | Unknown (NP_3593740 | PDZ domain protein (Enterococcus faecalis) | 1e-85 (48%) |
| spn53 | spr0897 | 589 | 37.1 | Unknown (NP_358491) | Glycerophosphoryl diester phosphodiesterase family protein (E. faecalis) | 3e-75 (40%) |
| spn63 | spr0367 | 182 | 42.6 | Unknown (NP_357961) | Unknown (Streptococcus agalactiae) | 3e-37 (39%) |
| spn66 | spr2025 | 279 | 40.5 | ABC transporter ATP-binding protein—unknown substrate (NP_359616) | ABC transporter (S. mutans) | 1e-107 (70%) |
| spn70 | spr1496 | 924 | 43.8 | Unknown (NP_359089) | ABC transporter permease protein (L. lactis) | 0.0 (39%) |
| spn76 | spr1933 | 283 | 37.2 | Positive transcriptional regulator of glucosyltransferase and Spp phenotype (NP_359524) | RggD (Streptococcus gordonii) | 1e-62 (45%) |
| spn79 | spr0072 | 179 | 44.8 | Unknown (NP_357666) | Glycosyltransferase involved in cell wall biogenesis (Clostridium acetobutylicum) | 5e-42 (44%) |
| spn84 | spr1685 | 318 | 43.2 | ABC transporter membrane-spanning permease—ferric iron transport (NP_359277) | ABC-type enterochelin transport system, permease component (Enterococcus faecium) | 2e-56 (43%) |
| spn86 | spr1715 | 311 | 36.2 | Biotin-(acetyl-coenzyme A-carboxylase) ligase (NP_359307) | ||
The gene number named by Hoskins et al. (15).
The significance of the protein sequence homologies in GenBank with the BlastX algorithm.
Insertion sequences absent from DNA of any tested pneumococcus-like oral streptococci.
ND, none detected.
The spn25.1 and spn25.2 fragments were isolated in the same clone but represented independent Sau3AI fragments corresponding to 1596789 to 1598270 and 1177462 to 1178466, respectively, in the S. pneumoniae R6 genome sequence. The ORF containing spn25.1 showed high amino acid sequence identity with a putative transcriptional activator of S. pneumoniae TIGR4 and had no significant homology with known proteins from any other species. The ORF containing spn25.2 had 53% identity with a predicted iron-dependent peroxidase of Actinobacillus pleuropneumoniae, the etiologic agent of swine pleuropneumonia (10).
The two ORFs containing clones spn9827 and spn79 showed high identity with predicted proteins involved in cell wall biosynthesis. The gene containing clone spn9827 encoded UDP-N-acetylglucosamine 1-carboxyvinyltransferase, which catalyzes the first committed step of biosynthesis of cell wall peptidoglycan (6). The derived amino acid sequence from the ORF containing clone spn79 shared 44% identity with a glycosyltransferase involved in cell wall biogenesis in Clostridium acetobutylicum (23). Three clones (spn66, spn70, and spn84) encoding ABC-type transporters and two clones (spn27 and spn76) encoding transcriptional regulators were also found. The ORF containing spn53, which was a protein of unknown function from S. pneumoniae, shared 40% identity with the periplasmic enzyme GlpQ of Enterococcus faecalis. The glpQ gene encodes a glycerophosphoryl diester phosphodiesterase, which is involved in the hydrolysis of deacetylated phospholipids and is expressed strongly after phosphate starvation (3).
The GC contents of the 19 ORFs examined ranged from 29.6 to 46.5% and most were similar to the predicted GC percentage (40%) of the S. pneumoniae R6 genome (15). However, the finding that the GC contents of spn9802 and spn25.1 were below 30% suggests that interspecific transfer of these genes from other species with lower GC content than the S. pneumoniae strain may have occurred.
The DNA fragments involved in the lytA and ply genes have not been isolated in 92 random selected colonies.
Clinical specimens.
Nineteen primer sets designed from the subtracted DNA sequences were examined by PCR using as templates DNAs extracted from a total of 20 atypical S. mitis or S. oralis isolates harboring the lytA or ply genes classically associated with S. pneumoniae and four clinical isolates classified as S. pneumoniae (Table 3). Although chromosomal DNA extracted from S. mitis 903 was not amplified with these 19 primer sets, several primer sets resulted in amplification of DNA from the pneumococcus-like clinical isolates. The primers for clones spn63 and spn66 yielded PCR products of the predicted size with all of the clinical specimens. The primer set designed from clone spn25.2 yielded no amplification with any S. pneumoniae clinical isolates, but the chromosomal DNAs from two pneumococcus-like isolates were amplified.
TABLE 3.
Specificity of the primers designed from subtractive DNA
| Primer set | Result for type strain
|
Result for atypical oral streptococcal strains
|
Result for clincal pneumococcal isolate:
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WU2 | 903 | 1a | 2 | 3 | 4 | 5 | 6a | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14a | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| lytA | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | + | + | + |
| ply | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| Spn9802b | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + |
| Spn9822 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | + | − | + | + | + | + | + |
| Spn9827 | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + |
| Spn9828b | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + |
| Spn14.1 | + | − | − | + | − | − | − | − | − | − | − | − | − | − | + | + | − | + | − | − | + | − | + | + | + | + |
| Spn24 | + | − | + | − | + | + | + | − | + | + | + | + | + | + | − | + | + | + | − | − | − | + | + | + | + | + |
| Spn25.1 | + | − | − | − | + | + | + | − | + | + | − | + | − | − | − | − | − | − | − | − | − | + | + | + | + | + |
| Spn25.2 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − |
| Spn27 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | + | + | + | + |
| Spn34 | + | − | + | − | − | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | + | + | + |
| Spn38 | + | − | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Spn53 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | + | + |
| Spn63 | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Spn66 | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Spn70 | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Spn76 | + | − | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + |
| Spn79 | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| Spn84 | + | − | + | + | + | + | + | + | + | + | + | + | + | − | + | + | − | + | + | + | + | − | + | + | + | + |
| Spn86 | + | − | + | − | + | + | + | + | + | + | − | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
Strains 1, 6, and 14 are classified as S. oralis on the basis of phenotypic tests and API biotyping and additional biochemical tests. The other strains are classified as S. mitis.
No amplifying DNA from any atypical streptococci tested but amplifying DNA from all typical and clinically isolated pneumococci tested.
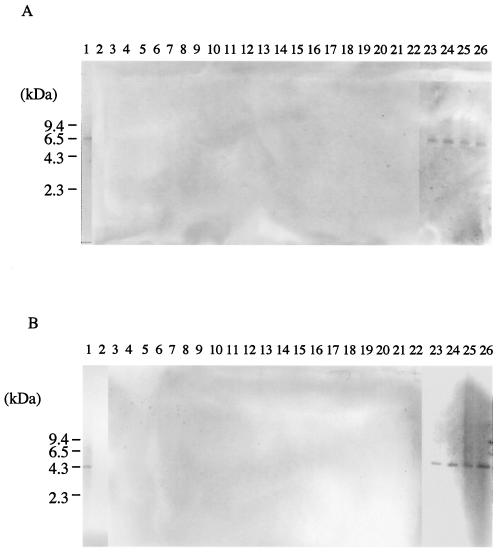
In contrast, none of the DNAs from pneumococcus-like isolates were amplified with the primers for clones spn9802 and spn9828, and the expected bands of 162 bp for spn9802 and 227 bp for spn9828 were observed from all strains of S. pneumoniae (data not shown). Furthermore, Southern hybridization analysis with the digoxigenin-labeled ORF fragments including spn9802 and spn9828 as probes showed that the same 6.5-kb PstI fragments (spn9802) and 4.3-kb NcoI fragments (spn9828) hybridized from chromosomal DNA from WU2 and all S. pneumoniae clinical isolates (Fig. 1). Consequently, our results revealed that two primer sets, Spn9802 and Spn9828, were able to discriminate S. pneumoniae from all of the pneumococcus-like streptococci tested.
FIG. 1.
Southern hybridization with a digoxigenin-labeled probe to the ORF sequence containing spn9802 (A) and spn9828 (B) of S. pneumoniae WU2. Chromosomal DNAs from S. pneumoniae WU2 (lane 1), S. mitis 903 (lane 2), atypical clinical streptococci (lane 3 to 22), and clinical isolated pneumococci (lane 23 to 26) were digested by either PstI (A) or NcoI (B). The lack of clear results in lanes 3 to 20 is due to negative reactions to the digoxigenin-labeled probes.
DISCUSSION
Genomic subtractive hybridization allows DNA sequences that are unique to the tester organism relative to the driver organism to be easily isolated and analyzed. Using this technique, we identified 19 DNA fragments that are present in the genomic DNA of S. pneumoniae WU2 and absent from S. mitis 903. All 19 primer sets designed from the sequences of these subtracted DNA fragments were specific for S. pneumoniae WU2, as shown by PCR using chromosomal DNAs from typical laboratory strains of oral bacteria as templates. However, when PCR was conducted using DNA from atypical clinical isolates, only two primer sets, Spn9802 and Spn9828, discriminated S. pneumoniae from pneumococcus-like strains. The ORFs containing clones spn9802 and spn9828 encoded proteins of unknown function and had no significant homologies with any organisms in the database other than S. pneumoniae. We are currently undertaking experiments to characterize these genes, which may play important roles in S. pneumoniae.
The clinical isolates of S. mitis and S. oralis showed various patterns by PCR amplification with the 19 primer sets tested (Table 3). Strains 1 to 9 were isolated from one individual and the other 11 strains of S. mitis and S. oralis were isolated from different individuals. Strains 3, 4, 5, 7, 8, and 10, and strains 17 and 18 showed the same amplification patterns by PCR and were classified as S. mitis. Strains 1 and 11 also showed the same pattern by PCR but were classified as S. oralis and S. mitis, respectively, indicating that our primer sets could not detect the genetic differences between S. mitis and S. oralis clinical isolates.
The remaining isolates were found to display distinct patterns. The extensive diversity seen within the S. mitis and S. oralis groups is consistent with a number of previous reports (11, 14). In addition, interspecies recombination events between these organisms and S. pneumoniae have been previously reported, such as the transfer of genes encoding penicillin-binding proteins (9) and the lytA or ply genes (31).
Pneumococci can be divided into at least 90 serotypes according to the immunochemistry of their capsular polysaccharide (13) and approximately 90% of the invasive diseases worldwide are caused by 16 different serotypes (12). Therefore, more investigations using our primer sets in PCR assays with chromosomal DNA from many strains of S. pneumoniae and from various pneumococcus-like isolates are required. However, two primer sets designed in this study, Spn9802 and Spn9828, may contribute to the detection of S. pneumoniae by PCR as a means of providing definitive diagnoses, especially in combination with the classical primer sets, such as those for lytA and ply.
The viridans group streptococci, including S. mitis and S. oralis, constitute a major population of oral soft tissue, such as the ventral tongue, the floor of the mouth, buccal surfaces, and attached gingiva (18). These species are commensal organisms of healthy individuals but are also a frequent cause of bacterial endocarditis in patients with prosthetic valves (8) and bacteremia in neutropenic cancer patients (4). Several cell surface proteins of viridans group streptococci have been reported to contribute to platelet binding and endothelial cell invasion (5, 28). However, the mechanisms by which viridans group streptococci cause infections are not well understood.
The pathogenicity of clinical isolates that are most closely related to S. mitis and S. oralis but which harbor pneumococcal genes is unclear. Genomic subtractive hybridization has the potential to pinpoint regions of the chromosome that are likely to be involved in the differential virulence of bacterial pathogens (25). Our study indicates that this technique is also useful for designing species-specific primers for PCR detection, especially for organisms for which the whole genome sequence is not yet available.
Recently, to quantify early colonizer microorganisms in dental biofilms, we designed species-specific primers and probes for real-time TaqMan PCR using this technique in combination with Southern blots (29). The subtractive technique may be applicable to the rational design of new vaccines and to the study of microbial ecosystems consisting of related species in the oral cavity and the development of new diagnostic tests to distinguish pathogenic bacteria from closely related and atypical strains of lesser pathogenicity.
Acknowledgments
This investigation was supported primarily by the Sato Fund, Nihon University School of Dentistry (M.S.), by a Nihon University Research Grant for Assistants and Young Researchers (M.S.), by a Grant-in-Aid for Scientific Research (C) (no. 16592097) (M.S.), by a grant to promote multidisciplinary research projects from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by research fellowships from the Japan Society for the Promotion of Science for Young Scientists (N.S.), and by a research grant from the Fukushima Society for the Promotion of Medicine (N.S.).
REFERENCES
- 1.Akopyants, N. S., A. Fradkov, L. Diatchenko, J. E. Hill, P. D. Siebert, S. A. Lukyanov, E. D. Sverdlov, and D. E. Berg. 1998. PCR-based subtractive hybridization and differences in gene content among strains of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antelmann, H., C. Scharf, and M. Hecker. 2000. Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J. Bacteriol. 182:4478-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beighton, D., A. D. Carr, and B. A. Oppenheim. 1994. Identification of viridans streptococci associated with bacteraemia in neutropenic cancer patients. J. Med. Microbiol. 40:202-204. [DOI] [PubMed] [Google Scholar]
- 5.Bensing, B. A., I. R. Siboo, and P. M. Sullam. 2001. Proteins PblA and PblB of Streptococcus mitis, which promote binding to human platelets, are encoded within a lysogenic bacteriophage. Infect. Immun. 69:6186-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. D., E. I. Vivas, C. T. Walsh, and R. Kolter. 1995. MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J. Bacteriol. 177:4194-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, A. J. Fox, and E. B. Kaczmarski. 2001. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas, C. W., J. Heath, K. K. Hampton, and F. E. Preston. 1993. Identity of viridans streptococci isolated from cases of infective endocarditis. J. Med. Microbiol. 39:179-182. [DOI] [PubMed] [Google Scholar]
- 9.Dowson, C. G., V. Barcus, S. King, P. Pickerill, A. Whatmore, and M. Yeo. 1997. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. Soc. Appl. Bacteriol. Symp. Ser. 26:42S-51S. [PubMed] [Google Scholar]
- 10.Fittipaldi, N., A. Broes, J. Harel, M. Kobisch, and M. Gottschalk. 2003. Evaluation and field validation of PCR tests for detection of Actinobacillus pleuropneumoniae in subclinically infected pigs. J. Clin. Microbiol. 41:5085-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzsimmons, S., M. Evans, C. Pearce, M. J. Sheridan, R. Wientzen, G. Bowden, and M. F. Cole. 1996. Clonal diversity of Streptococcus mitis biovar 1 isolates from the oral cavity of human neonates. Clin. Diagn. Lab. Immunol. 3:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakenbeck, R., N. Balmelle, B. Weber, C. Gardès, W. Keck, and A. de Saizieu. 2001. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of Streptococcus pneumoniae. Infect. Immun. 69:2477-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohwy, J., and M. Kilian. 1995. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol. Immunol. 10:19-25. [DOI] [PubMed] [Google Scholar]
- 15.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura, Y., R. A. Whiley, S.-E. Shu, T. Ezaki, and J. M. Hardie. 1999. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 145:2605-2613. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura, Y., X.-G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406-408. [DOI] [PubMed] [Google Scholar]
- 18.Mager, D. L., L. A. Ximenez-Fyvie, A. D. Haffajee, and S. S. Socransky. 2003. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 30:644-654. [DOI] [PubMed] [Google Scholar]
- 19.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAvin, J. C., P. A. Reilly, R. M. Roudabush, W. J. Barnes, A. Salmen, G. W. Jackson, K. K. Beninga, A. Astorga, F. K. McCleskey, W. B. Huff, D. Niemeyer, and K. L. Lohman. 2001. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. J. Clin. Microbiol. 39:3446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai, K., Y. Shibasaki, K. Hasegawa, T. A. Davies, M. R. Jacobs, K. Ubukata, and P. C. Appelbaum. 2001. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and beta-lactam resistance, and to detect common macrolide resistance determinants. J. Antimicrob. Chemother. 48:915-918. [DOI] [PubMed] [Google Scholar]
- 23.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill, A. M., S. H. Gillespie, and G. C. Whiting. 1999. Detection of penicillin susceptibility in Streptococcus pneumoniae by pbp2b PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 37:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin, A., X. Nassif, and C. Tinsley. 1999. Identification of regions of the chromosome of Neisseria meningitidis and Neisseria gonorrhoeae which are specific to the pathogenic Neisseria species. Infect. Immun. 67:6119-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salo, P., A. Ortqvist, and M. Leinonen. 1995. Diagnosis of bacteremic pneumococcal pneumonia by amplification of pneumolysin gene fragment in serum. J. Infect. Dis. 171:479-482. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Stinson, M. W., S. Alder, and S. Kumar. 2003. Invasion and killing of human endothelial cells by viridans group streptococci. Infect. Immun. 71:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki, N., Y. Nakano, A. Yoshida, Y. Yamashita, and Y. Kiyoura. 2004. Real-time TaqMan PCR for quantifying oral bacteria during biofilm formation. J. Clin. Microbiol. 42:3827-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley, C. R., and X. Nassif. 1996. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc. Natl. Acad. Sci. USA 93:11109-11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiley, R. A., and D. Beighton. 1998. Current classification of the oral streptococci. Oral Microbiol. Immunol. 13:195-216. [DOI] [PubMed] [Google Scholar]