Abstract
Carbapenem-resistant Acinetobacter baumannii isolates were obtained from 24 patients between March and May 2004 at the Centre Hospitalier de Polynésie Française, Tahiti, French Polynesia. The isolates were multidrug resistant, produced the carbapenemase OXA-23, and belonged to a single clone presenting several subtypes, suggesting an endemic situation. This study further illustrates the global spread of this kind of β-lactamase-mediated resistance.
Imipenem and meropenem are among the drugs of choice used to treat nosocomial infections due to multidrug-resistant Acinetobacter baumannii isolates. However, their efficacy is being increasingly compromised by the emergence of carbapenem-hydrolyzing β-lactamases of molecular Ambler class B (metalloenzymes) and D enzymes (oxacillinases) (1, 2, 3, 12, 17). Whereas the metalloenzymes are of IMP and VIM types, the carbapenem-hydrolyzing oxacillinases are members of three subgroups of enzymes: the OXA-23, OXA-24, and OXA-58 enzymes (2, 5, 9, 15, 16, 17). Outbreaks of OXA-type carbapenemase-producing A. baumannii strains have been reported worldwide: OXA-24 in Madrid, Spain (4, 5); OXA-23 in Spain and in Curitiba, Brazil (2, 8); OXA-58 in Toulouse, France (11); and OXA-40 in Bilbao, Spain (13). The aim of this study was to analyze the molecular mechanism of carbapenem resistance in A. baumannii strains isolated at the Centre Hospitalier de Polynésie Française (CHPF), the main hospital of Papeete, Tahiti, French Polynesia, that is located in the middle of the Pacific Ocean.
From March to May 2004, 24 carbapenem-resistant A. baumannii isolates were isolated from 24 patients hospitalized at the CHPF, a 353-bed tertiary care hospital. The patients' ages ranged from 8 to 80 years (mean age, 54 years). Nineteen patients (80%) were colonized and 5 were infected (20%) according to the French national recommendations, which derived from those of the Centers for Disease Control and Prevention (7, 10). The sites of infection were urinary tract (2 patients), intravenous catheters (2 patients), and the respiratory tract (1 patient). The patients were scattered throughout the hospital, being mostly in the intensive care unit (41.7%), and the motives for hospital admission were diverse (Table 1). Seventy-seven percent of the patients had a bacterial infection prior to the isolation of A. baumannii isolates and received an appropriate antibiotic treatment. Thirty-three percent of the patients died after A. baumannii isolation, but the death rate was 26.3% with colonization and 60% when the patient was infected. Infection with multidrug-resistant A. baumannii was considered by the clinicians to have contributed to the death of patient 3, despite amikacin and colistin treatment. The four other infected patients were cured from their infection without treatment. Two of them died from their underlying disease (patients 2 and 10; Table 1).
TABLE 1.
Case history of patients with OXA-23-producing A. baumanniie
| Isolate no. | Date of hospitalization (mo/day/yr) | Age (yr) | Sex | Ward | Date of isolation (mo/day/yr) | Underlying condition | Site of isolation | Diagnosisa | Treatmentb | Outcomec | PFGE pulsotypef |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 03/03/04 | 44 | F | Gastroenterology | 03/22/04 | Heart failure | Rectal swab | C | A1 | ||
| 2 | 03/05/04 | 58 | M | Cardiology ICU | 03/22/04 | Heart failure | Central catheter | Infection | A6 | ||
| 3 | 02/15/04 | 45 | F | ICU | 04/10/04 | Asthma | Tracheal aspiration | Infection | AM, CS | Deceased | A1 |
| 4 | 03/28/04 | 50 | M | ICU | 04/13/04 | Respiratory failure | Rectal swab | C | A6 | ||
| 5 | 02/09/04 | 62 | M | Internal medicine | 04/14/04 | Paraplegia | Rectal swab | C | A6 | ||
| 6 | 04/10/04 | 22 | M | ICU | 04/20/04 | Polytraumatism | Rectal swab | C | A6 | ||
| 7 | 03/31/04 | 39 | M | Surgery | 04/19/04 | Arthritis | Rectal swab | C | A6 | ||
| 8 | 04/08/04 | 79 | M | Internal medicine | 04/20/04 | Cerebral vascular | Urinary | Infection | A1 | ||
| 9 | 04/06/04 | 35 | F | Endocrinology | 04/22/04 | Diabetes | Urinary | Infection | A5 | ||
| 10 | 03/23/04 | 68 | F | ICU | 04/22/04 | Heart failure | Catheter | Infection | A4 | ||
| 11 | 03/28/04 | 61 | M | ICU | 04/23/04 | Tetraplegia | Catheter | C | A4 | ||
| 12 | 08/30/03 | 58 | M | Internal medicine | 04/23/04 | Tetraplegia | Rectal swab | C | A3 | ||
| 13 | 04/03/04 | 76 | M | Internal medicine | 04/26/04 | Erysipelas | Rectal swab | C | A3 | ||
| 14 | 04/20/04 | 30 | M | ICU | 04/27/04 | Respiratory failure | Rectal swab | C | A4 | ||
| 15 | 03/16/04 | 20 | M | Gastroenterology | 05/02/04 | Leptospirosis | Rectal swab | C | A1 | ||
| 16 | 04/16/04 | 50 | F | ICU | 05/04/04 | Meningitis | Rectal swab | C | A5 | ||
| 17 | 04/28/04 | 43 | F | Emergency | 05/04/04 | Erysipela | Rectal swab | C | A1 | ||
| 18 | 05/02/04 | 8 | F | Surgery | 05/10/04 | Polytraumatism | Rectal swab | C | A5 | ||
| 19 | 05/05/04 | 80 | M | ICU | 05/11/04 | Cellulitis | Rectal swab | C | A2 | ||
| 20 | 03/14/04 | 77 | M | Internal medicine | 05/12/04 | Cellulitis | Rectal swab | C | A1 | ||
| 21 | 04/13/04 | 67 | M | Pneumology | 05/14/04 | Leukemia | BAFd | C | A3 | ||
| 22 | 05/01/04 | 66 | M | Surgery | 05/18/04 | Digestive bleeding | Rectal swab | C | A1 | ||
| 23 | 04/21/04 | 76 | M | ICU | 05/19/04 | Respiratory failure | Central catheter | C | A3 | ||
| 24 | 05/09/04 | 59 | M | ICU | 06/01/04 | Epilepsy | Urinary catheter | C | A3 |
Infection or colonization (C) status was established based on clinical data.
Only patients that received antibiotic treatment for their A. baumannii infections are shown. The treatment consisted of amikacin (AM) and colymicine (CS).
Unless-specified, the outcome was favorable with respect to the A. baumannii infection.
BAF, bronchoalveolar fluid.
All isolates were imipenem resistant and positive for blaOXA-23 by PCR. ICU, intensive care unit.
Pulsotypes are according to Tenover et al. (20).
Isolates were identified using the API 32GN system (BioMérieux, Marcy l'Etoile, France). Pulsed-field gel electrophoresis (PFGE) was performed with ApaI-restricted whole-cell DNAs embedded in 1% agarose plugs and separated in a 1% pulsed field-certified agarose gel using a contour-clamped homogeneous electric field DRII system (Bio-Rad, Marnes-La-Coquette, France), as previously described (11). Routine antibiograms were determined by the disk diffusion method on Mueller-Hinton agar (Bio-Rad) and interpreted as recommended by the Clinical and Laboratory Standards Institute (6). MICs of carbapenems were determined with E-tests (AB BIODISK, Solna, Sweden) performed on Mueller-Hinton agar plates (Oxoid, Basingstoke, United Kingdom) with incubation at 37°C for 24 h. DNA extractions (genomic and plasmid) and analysis, isoelectric focusing, and conjugation assays with rifampin-resistant A. baumannii strain CIP 7020 were performed as described previously (11).
Genes coding for Ambler class B and D carbapenemases were sought by PCR using primers specific for the blaIMP (19), blaVIM (19), blaOXA-23-like (9), blaOXA-26-like (2), and blaOXA-58 genes (16). Similarly, the class A β-lactamase blaTEM gene and the chromosomal class C β-lactamase blaAMPC gene were sought by PCR (11, 18). The presence of ISAbaI inserted upstream of a blaAMPC-β-lactamase gene was sought by PCR as previously described (14, 18). PCR products were purified using a QIAquick PCR purification kit (QIAGEN, Courtaboeuf, France) and sequenced on both strands with an automated sequencer (ABI 3100; Applied Biosystems, Foster City, Calif.). The nucleotide and deduced amino acid sequences were analyzed with software available over the Internet (http://www.ncbi.nlm.nih.gov/).
The 24 A. baumannii isolates were resistant to all β-lactams, including carbapenems (with imipenem and meropenem MICs of >32 μg/ml), ciprofloxacin, fosfomycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole. The isolates remained susceptible to colistin (MIC, 0.5 μg/ml), rifampin (MIC, 1 μg/ml), and to aminoglycosides, except isolates 3, 4, 6, and 7, which were resistant to gentamicin and netilmicin.
The A. baumannii isolates were positive for blaOXA-23-like, blaTEM-like, and the natural and chromosomally located blaAMPC genes. Sequencing of the amplified fragments confirmed the presence of blaOXA-23, blaTEM-1, and blaAMPC genes. As previously described, the blaOXA-23 gene was not embedded in a class 1 integron (9, 17, and data not shown). The genetic environment was similar to that of the prototype blaOXA-23 gene, with insertion sequence ISabaI inserted upstream of this gene, as revealed by PCR analysis (9, 14, 18).
Isoelectric focusing confirmed that in addition to OXA-23 (pI 6.9), the chromosomal class C β-lactamase (pI >9.0) and TEM-1 (pI 5.4) were also expressed (data not shown).
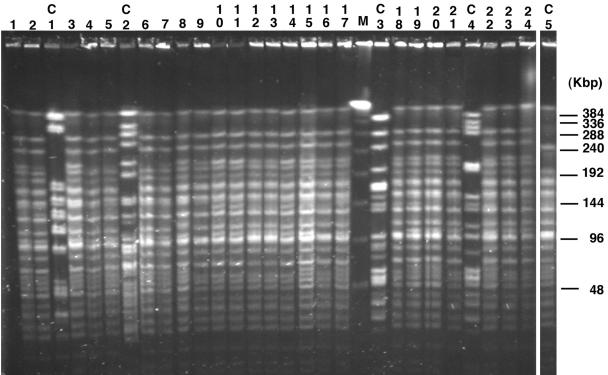
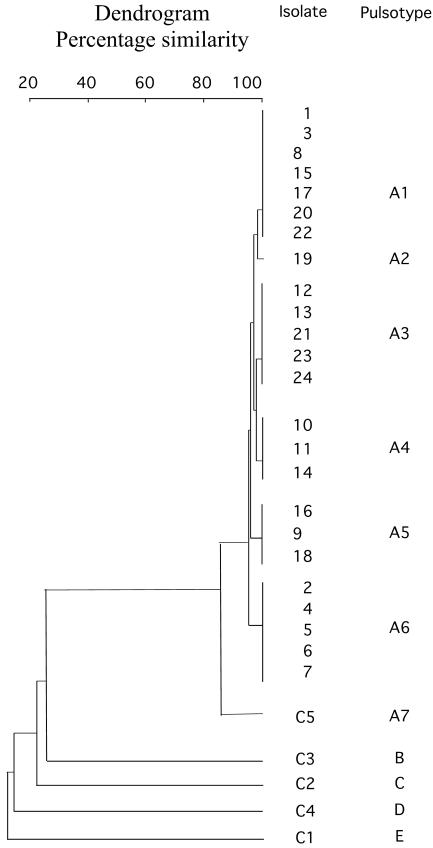
Plasmid analysis revealed a 60-kb plasmid in all strains that was mobilizable to rifampin-resistant A. baumannii CIP 7020 (frequency of transfer, 10−6). This plasmid expressed only β-lactamase OXA-23, and no additional resistance marker was identified according to results of routine antibiograms performed with the transconjugants and testing the same antibiotics as for the parental strains. The isolates from the different wards of the hospital gave similar PFGE patterns (Fig. 1), differing by a maximum of three bands. Six different subtypes were observed, suggesting clonal dissemination of the isolates (Fig. 2). The four carbapenem-susceptible control isolates from the same hospital differed from the carbapenem-resistant strain according to their PFGE patterns (Fig. 1 and 2).
FIG. 1.
PFGE patterns of A. baumannii isolates. The assigned numbers of A. baumannii isolates are shown over the lanes of the gel and correspond to those of Table 1. The positions of molecular size markers in kilobases (M) are shown on the right side of the gel. Lanes C1, C2, C3, and C4 correspond to carbapenem-susceptible strains isolated during the study period. Lane C5 corresponds to A. baumanii strain C5, which was isolated in Montpellier in 1999 from a patient directly transferred from Papeete.
FIG. 2.
Dendrogram obtained after digitization of the PFGE gel. ApaI macrorestriction patterns were digitized and analyzed using Taxotron software (Institut Pasteur, Paris, France) to calculate Dice coefficients of correlation and to generate a dendrogram by the unweighted pair group method using arithmetic averages clustering. The scale indicates the level of pattern similarity. Capital letters on the right side indicate macrorestriction types based on visual interpretation of PFGE results according to the criteria of Tenover et al. (20).
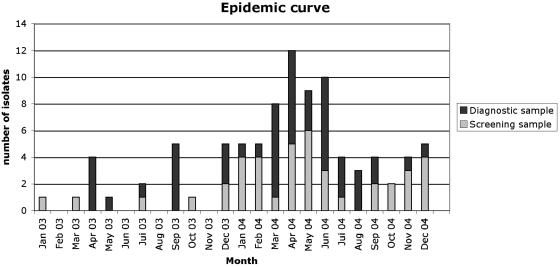
Carbapenem-resistant A. baumannii strains have been isolated in Europe, Asia, and North and South America (1, 2, 4, 5, 8, 13, 16). Outbreaks of OXA-23-producing A. baumannii isolates have also been reported in Spain and in Brazil (2, 4, 8). Our study identified OXA-23-positive A. baumannii in Tahiti, a remotely located island in the Pacific Ocean, further illustrating the global spread of this broad-spectrum β-lactamase. The genetic variability observed among the OXA-23-positive A. baumannii isolates (six pulsotypes) suggested that these isolates may have been present earlier in the hospital and thus reflect an endemic situation. Indeed, retrospective analysis of a bacteriology database based on antibiotic resistance susceptibility patterns revealed that the index case had been isolated in January 2003, with several sporadic cases until December 2003, when the outbreak started (Fig. 3). Interestingly, in 1999, A. baumannii strain C5 was identified at the university hospital of Montpellier (in southern France) from a French tourist previously hospitalized in a private hospital in Papeete. This strain had an identical antibiotic susceptibility profile and a similar PFGE pattern (Fig. 1 and 2) and was OXA-23 positive. These data suggested that this strain could have been present for a much longer period of time on the island.
FIG. 3.
Epidemic curve of the outbreak.
After May 2004, the epidemic strain was still isolated in screening samples and in clinical specimens (Fig. 3), but implementation of infection control measures (isolation precautions, chlorhexidine hand washing, and barrier protections) and thorough biodecontamination of the rooms of colonized patients led to control of the outbreak but did not eradicate the epidemic strain (Fig. 3). The persistence of this strain is probably a consequence of frequent rehospitalization of colonized patients in the different wards. Indeed, the CHPF is the main hospital of the French Polynesian islands, dealing with acute care patients but also with long-term-care patients. The closest larger hospital is in Auckland, New Zealand, which is 2,600 miles away from Tahiti, thus limiting patient exchange. Overcoming the combination of clonal spread, multidrug resistance, and long-term-care patients that remain carriers is a challenge for the effective control of nosocomial A. baumannii infections.
Acknowledgments
We are grateful to Claire Héritier and Hélène Jean-Pierre for helpful discussions.
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, and by the European Community (6th PCRD, LSHMCT-2003-503-335).
REFERENCES
- 1.Afzal-Shah, M., H. E. Villar, and D. M. Livermore. 1999. Biochemical characteristics of a carbapenemase from an Acinetobacter baumannii isolate collected in Buenos Aires, Argentina. J. Antimicrob. Chemother. 43:127-131. [DOI] [PubMed] [Google Scholar]
- 2.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bou, G., G. Cervero, M. A. Dominguez, C. Quereda, and J. Martinez-Beltran. 2000. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of beta-lactamases. J. Clin. Microbiol. 38:3299-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou, G., A. Oliver, and J. Martinez-Beltran. 2000. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 7.Comité Technique National des Infections Nosocomiales. 1999. 100 recommandations pour la surveillance et la Prévention des Infections Nosocomiales, 2nd ed. Ministére de l'Emploi et de la Solidarité, Secrétariat d'Etat à la Santé et l'Action Sociale, Paris, France.
- 8.Dalla-Costa, L. M., J. M. Coelho, H. A. Souza, M. E. Castro, C. J. Stier, K. L. Bragagnolo, A. Rea-Neto, S. R. Penteado-Filho, D. M. Livermore, and N. Woodford. 2003. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. J. Clin. Microbiol. 41:3403-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donald, H. M., W. Scaife, S. G. Amyes, and H. K. Young. 2000. Sequence analysis of ARI-1, a novel OXA beta-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 11.Héritier, C., A. Dubouix, L. Poirel, N. Marty, and P. Nordmann. 2005. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J. Antimicrob. Chemother. 55:115-118. [DOI] [PubMed] [Google Scholar]
- 12.Livermore, D. M. 2002. The impact of carbapenemases on antimicrobial development and therapy. Curr. Opin. Investig. Drugs 3:218-224. [PubMed] [Google Scholar]
- 13.Lopez-Otsoa, F., L. Gallego, K. J. Towner, L. Tysall, N. Woodford, and D. M. Livermore. 2002. Endemic carbapenem resistance associated with OXA-40 carbapenemase among Acinetobacter baumannii isolates from a hospital in northern Spain. Antimicrob. Agents Chemother. 40:4741-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammeri H., L. Poirel, N. Mangeney, and P. Nordmann. 2003. Chromosomal integration of a cephalosporinase gene from Acinetobacter baumannii into Oligella urethralis as a source of acquired resistance to beta-lactams. Antimicrob. Agents Chemother. 47:1536-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton, R. H., R. S. Miles, J. Hood, and S. G. Amyes. 1993. ARI-1:β-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2:81-88. [DOI] [PubMed] [Google Scholar]
- 16.Poirel, L., S. Marque, C. Héritier, C. Segonds, G. Chabanon, and P. Nordmann. 2005. OXA-58, a novel class D beta-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirel, L., and P. Nordmann. 2002. Acquired carbapenem-hydrolyzing beta-lactamases and their genetic support. Curr. Pharm. Biotechnol. 3:117-127. [DOI] [PubMed] [Google Scholar]
- 18.Segal, H., E. C. Nelson, and B. G. Elisha. 2004. Genetic environment and transcription of ampC in an Acinetobacter baumannii clinical isolate. Antimicrob. Agents Chemother. 48:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senda, K., Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato, and M. Ohta. 1996. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenover, F. C., R. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]