Abstract
The indirect hemagglutination assay routinely used to detect antibodies to Burkholderia pseudomallei was modified to detect cross-reactivity of antibodies to B. pseudomallei, B. mallei, and B. thailandensis antigens. We demonstrate a lack of cross-reactivity between B. pseudomallei and B. thailandensis but marked cross-reactivity between B. pseudomallei and B. mallei.
Burkholderia pseudomallei is the causative agent of melioidosis and a recognized biothreat agent. The organism is present in mud and pooled surface water in Southeast Asia and northern Australia, where natural infection is acquired following inoculation or inhalation (11). Culture of B. pseudomallei is the gold standard for the diagnosis of melioidosis. However, serological testing is commonly used, particularly in resource-poor regions that lack microbiological culture facilities. Serological testing may also be used as an epidemiological marker of exposure in regions where melioidosis is not known to be endemic and in patients (such as returned travelers or military personnel) from areas where melioidosis is not endemic to investigate suspected exposure to B. pseudomallei or Burkholderia mallei (the causative agent of glanders).
In areas where melioidosis is endemic, high rates of background seropositivity have been noted (4, 8); this limits the specificity of the most commonly used indirect hemagglutination assay (IHA). Seropositivity may be due to prior exposure to B. pseudomallei or exposure to the antigenically similar but rarely pathogenic B. thailandensis which is present in the environment in Thailand. This may also explain the higher rate of seropositivity seen in Thailand than that seen in Australia, where B. thailandensis is not present (4). The aim of this study was to define whether patients with culture-proven melioidosis have antibodies with cross-reactivity to B. pseudomallei, B. thailandensis, or B. mallei antigens presented in a modified IHA.
Patients with melioidosis were prospectively recruited over a 5-month period between June and October 2004 by a study team based at Sappasithiprasong Hospital, Ubon Ratchathani, northeast Thailand. Patients presenting with febrile illnesses were identified by active surveillance of the medical and intensive care wards. Culture of B. pseudomallei from any sample was considered diagnostic for melioidosis (5). Patients were excluded if they were <14 years of age or declined to participate. Ethical approval was obtained from the Ministry of Public Health, Royal Government of Thailand.
Pooled antigens were prepared from the following isolates: (i) B. pseudomallei clinical isolates 199a and 207a, obtained from patients with melioidosis in northeast Thailand; (ii) B. thailandensis isolates E27, E32, and E256, obtained from soil in northeast Thailand; and (iii) B. mallei isolates EY2233 (kindly provided by Sumalee Tungpredabkul, Faculty of Science, Mahidol University) and ATCC 23344. A loopful of each isolate was inoculated into 5 ml Trypticase soy broth and incubated for 18 h with shaking at 37°C in air, after which 200 μl was spread onto 30 plates of either Columbia agar (for B. pseudomallei and B. thailandensis) or Columbia agar plus 4% glycerol (for B. mallei). Bacteria were harvested after incubation for 72 h at 37°C in air, suspended in 15 ml phosphate-buffered saline, vortexed vigorously, and then autoclaved at 121°C for 15 min. Preparations were centrifuged at 4,000 rpm for 30 min, the supernatants were filtered through a Millipore 0.22-μm filter, phenol was added (0.5%), and the preparation was stored at 4°C until use. An IHA was then performed as previously described (1). Growth of isolates prior to harvest and preparation of antigen represent a departure from the previously reported method in which bacteria are incubated in Rice medium at 37°C in air for 14 days prior to autoclaving, centrifugation, and filtration, as described above. In view of this difference, the B. pseudomallei IHA titers were initially compared between the two methods. Overall IHA titers were not significantly different between the two groups. Seven of 117 sera (5.9%) gave IHA titers that were ≥2 dilutions different between the methods (five samples were 2 dilutions different, and two samples had a 3-dilution difference); of these titers, three were higher using the conventional method, and four were higher using the modified method. We propose that growth of bacteria on agar plates prior to antigen preparation for IHA is a reasonable alterative to prolonged growth in broth culture.
Sera from 117 patients with culture-confirmed melioidosis were evaluated. Patient ages ranged from 15 to 82 years (median, 48.5 years; interquartile range [IQR], 38 to 57); 56 patients (48%) were female. The majority of patients were rice farmers (79%), and 70% had diabetes mellitus. Infection was associated with septic shock in 22 patients (19%), and the overall in-hospital mortality was 28%.
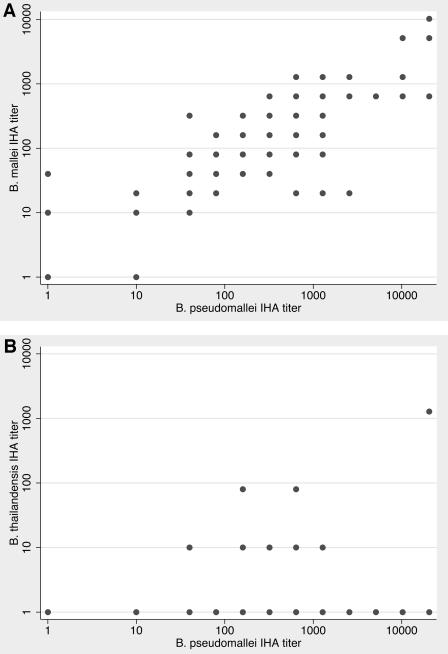
The B. pseudomallei titers ranged from 0 to 1:20,480 (median, 1:320; IQR, 1:80 to 1:1,280), and those for B. mallei ranged from 0 to 1:10,240 (median, 1:160; IQR, 1:40 to 1:640). There was significant cross-reactivity between the two assays (Spearman's ρ = 0.78, P < 0.0001 [Fig. 1 ]). The IHA titers were identical between the IHAs using B. pseudomallei or B. mallei antigens in 46 (39%) of the 117 cases. The titer was greater with the B. pseudomallei IHA than with the B. mallei IHA in 57 cases and less in 14 cases. Antibodies recognizing B. thailandensis were not detected in 98 (84%) samples. Of the remainder, 14 samples had a titer of 1:10, one had a titer of 1:20, three had a titer of 1:80, and one had a titer of 1:1,280 (the B. pseudomallei titer in this case was 1:20,480). There was no significant cross-reactivity between the B. thailandensis assay and those based on B. pseudomallei (Spearman's ρ = 0.17, P = 0.06) and B. mallei antigens (Spearman's ρ = 0.09, P = 0.35); the borderline P value in the case of B. pseudomallei was entirely due to the single high B. thailandensis case mentioned above.
FIG. 1.
IHA titers of (A) B. mallei and (B) B. thailandensis versus B. pseudomallei in patients with melioidosis. Each dot represents one or more patients.
The finding of cross-reactivity of B. mallei antigens by antibodies raised against B. pseudomallei is consistent with previous studies demonstrating antigenic relatedness between the two species (2). In addition, there is increasing evidence of a close phylogenetic relationship based on similarities in the genome sequence and multilocus sequence typing (6, 7, 9). It is unlikely that our patient group had been exposed to B. mallei previously, as human glanders is not recognized to occur in Thailand.
However, our patient population is likely to be repeatedly exposed to both B. thailandensis and B. pseudomallei, which are both present in soil in northeast Thailand (10). We found that there was little detectable cross-reactivity between B. thailandensis and B. pseudomallei antigens by the IHA. The generally absent or very low titers to B. thailandensis in our patient population indicate that environmental exposure to this organism is not very immunogenic or that these antibodies are not recognized by the IHA; in this way, B. thailandensis differs from B. pseudomallei, for which background seropositivity is a major problem in areas of endemicity. This study suggests that exposure of individuals to B. thailandensis is unlikely to interfere with the results from the B. pseudomallei IHA.
Some antigens, such as lipopolysaccharide, are known to be conserved between these Burkholderia species (3), while others are immunologically unique (12). A limitation of the IHA is that the antigens generated are poorly characterized. It is generally assumed that the use of multiple strains would result in a broad representation of antigens, but cell-free supernatant is likely to be depleted of many of the major cell surface-associated antigens.
The explanation for the different rates of seropositivity between Australia and Thailand remains unclear. It is unlikely that the intensity of exposure, maintaining seropositivity in Southeast Asian patients, is responsible, as the rates of seropositivity were higher in immigrants to Australia from Southeast Asia (4). However, it is possible that intense exposure earlier in life may result in a prolonged seropositivity. Differences in immunological responses to B. pseudomallei in Asian and Australian patients have not been described, but variations in Th1/Th2 responses may be important. Finally, the IHA is a poorly standardized test with different isolates (typically local clinical strains) used in Australia and Thailand; the effect of these differences has not been defined.
Future publication of the B. thailandensis genome sequence will shed new light on the similarities and degree of homology between these saprophytic Burkholderia species. We conclude that the B. pseudomallei IHA is unlikely to be confounded by antibodies to B. thailandensis, but that this assay is unable to distinguish between infection by B. pseudomallei and infection by B. mallei.
Acknowledgments
We are grateful for the support of the staff at Sappasithiprasong Hospital and the Wellcome Trust-Mahidol University-Oxford University Tropical Medicine Research Program.
S.J.P. is supported by a Wellcome Trust Career Development Award in Clinical Tropical Medicine. This study was funded by the Wellcome Trust.
REFERENCES
- 1.Alexander, A. D., D. L. Huxsoll, A. R. Warner, Jr., V. Shepler, and A. Dorsey. 1970. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl. Microbiol. 20:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anuntagool, N., and S. Sirisinha. 2002. Antigenic relatedness between Burkholderia pseudomallei and Burkholderia mallei. Microbiol. Immunol. 46:143-150. [DOI] [PubMed] [Google Scholar]
- 3.Anuntagool, N., P. Intachote, V. Wuthiekanun, N. J. White, and S. Sirisinha. 1998. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara− clinical isolates. Clin. Diagn. Lab Immunol. 5:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashdown, L. R., and R. W. Guard. 1984. The prevalence of human melioidosis in Northern Queensland. Am. J. Trop. Med. Hyg. 33:474-478. [DOI] [PubMed] [Google Scholar]
- 5.Dance, D. A., V. Wuthiekanun, P. Naigowit, and N. J. White. 1989. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API 20NE. J. Clin. Pathol. 42:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godoy, D., G. Randle, A. J. Simpson, D. M. Aanensen, T. L. Pitt, R. Kinoshita, and B. G. Spratt. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden, M. T., R. W. Titball, S. J. Peacock, A. M. Cerdeno-Tarraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 101:14240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaphun, P., N. Thirawattanasuk, Y. Suputtamongkol, P. Naigowit, D. A. Dance, M. D. Smith, and N. J. White. 1993. Serology and carriage of Pseudomonas pseudomallei: a prospective study in 1,000 hospitalized children in northeast Thailand. J Infect. Dis. 167:230-233. [DOI] [PubMed] [Google Scholar]
- 9.Nierman, W. C., D. DeShazer, H. S. Kim, H. Tettelin, K. E. Nelson, T. Feldblyum, R. L. Ulrich, C. M. Ronning, L. M. Brinkac, S. C. Daugherty, T. D. Davidsen, R. T. Deboy, G. Dimitrov, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, H. Khouri, J. F. Kolonay, R. Madupu, Y. Mohammoud, W. C. Nelson, D. Radune, C. M. Romero, S. Sarria, J. Selengut, C. Shamblin, S. A. Sullivan, O. White, Y. Yu, N. Zafar, L. Zhou, and C. M. Fraser. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 101:14246-14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trakulsomboon, S., V. Vuddhakul, P. Tharavichitkul, N. Na-Gnam, Y. Suputtamongkol, and V. Thamlikitkul. 1999. Epidemiology of arabinose assimilation in Burkholderia pseudomallei isolated from patients and soil in Thailand. Southeast Asian J. Trop. Med. Public Health 30:756-759. [PubMed] [Google Scholar]
- 11.White, N. J. 2003. Melioidosis. Lancet 361:1715-1722. [DOI] [PubMed] [Google Scholar]
- 12.Wuthiekanun, V., N. Anuntagool, N. J. White, and S. Sirisinha. 2002. Short report: a rapid method for the differentiation of Burkholderia pseudomallei and Burkholderia thailandensis. Am. J. Trop. Med. Hyg. 66:759-761. [DOI] [PubMed] [Google Scholar]