Abstract
Serologic tests play an important role in diagnosis of typhoid fever. In an effort to develop a more defined reagent for these tests, purified Salmonella enterica serovar Typhi (ST) O:1,9,12 polysaccharide was conjugated to human serum albumin (HSA), and the conjugate was purified chromatographically to yield a reagent with 2 moles ST O polysaccharide per mole HSA. In 40 patients with bacteriologically confirmed typhoid fever, significant dot immunobinding titers (≥20,000) were present in 28 (70%) tested with 100 ng of ST O antigen-HSA (ST O-HSA) conjugate, in 38 (95%) tested with 100 ng of ST lipopolysaccharide, and in 16 (40%) tested with purified unconjugated ST O chains. In sera from 22 patients with other nontyphoid fevers, 2 (9.1%) had such reactivities with 100 ng of ST O-HSA, 1 (4.5%) had such reactivity with 100 ng of ST lipopolysaccharide (4.5%), and none reacted with 100 ng of unconjugated ST O chains. None of the 17 healthy-control sera reacted significantly with any of the ST reagents. None of the patient or control sera reacted with unconjugated HSA. The sensitivity of dot immunobinding for typhoid fever was 70% with 100 ng of ST O-HSA, somewhat lower than that with 100 ng of ST lipopolysaccharide (95%) but similar to that of the Widal H agglutination test with a ≥1/160 cutoff (74%). Specificities of these tests were 91%, 95%, and 86%, respectively. These preliminary results suggest that ST O polysaccharide-protein conjugates could provide a nontoxic, easily quality-controlled synthetic reagent for analysis of human immune responses to ST as well as for the development of new diagnostics and vaccines for typhoid fever.
Typhoid fever is an enteric fever of humans caused by infection with Salmonella enterica serovar Typhi (ST). It is transmitted by the ingestion of water or food contaminated with infected feces (20) and is an important public health problem, especially in the developing world, where sanitary measures are lacking and/or do not keep up with the pace of rapid urban growth (28). The estimated worldwide annual incidence of this disease is about 16 million cases (7 million cases in the areas of typhoid fever endemicity in Southeast Asia alone), with approximately 600,000 deaths (19). Sporadic cases of typhoid fever occurring in developed countries are concentrated in immigrant populations and in tourists who have visited zones of high typhoid fever endemicity (6).
Diagnosis of typhoid fever can be difficult because its nonspecific symptoms and signs can be easily confused with those of other acute and subacute infectious and noninfectious febrile diseases (20). Culturing of the causative organism provides definitive diagnosis. While up to 95% of bone marrow cultures can be positive, only 60 to 80% of the more commonly obtained blood cultures are positive, and serological tests for the presence of anti-ST lipopolysaccharide (LPS) O antigens and flagellar H antigens in patients' sera provide an important adjunct to diagnosis (20). Unfortunately, the Widal agglutination assay, introduced over 100 years ago but still in common use, is unreliable, especially in areas of typhoid fever endemicity (18, 21, 22, 28). Furthermore, its interpretation is often problematic (6, 11, 22). More-recent assays to detect anti-ST O and H antibodies with sensitivities and specificities greater than those of the Widal tests have employed enzyme-linked immunosorbent assay (ELISA), immunoblotting, dot immunobinding, and dipstick methodologies (1, 5, 7, 11-13, 18, 24), but none has been widely adopted as yet (23).
At least some of the lack of specificity and sensitivity in these serodiagnostic assays for typhoid might be a result of the use of poorly characterized and/or standardized antigens (15). Conjugates of purified ST O polysaccharide to well-defined proteins provide a ready means of obtaining chemically defined antigens free from contamination from other LPS components for use in serodiagnosis. Such polysaccharide-protein conjugates have been previously shown to be immunogenic in mice and to generate high levels of protective anti-ST immunity (26). They also exhibited high specificities and sensitivities for detection of anti-ST O in commercially available rabbit and patient sera (1). We have now prepared ST O chain conjugated to human serum albumin (HSA) and have characterized and used it in a dot immunobinding assay to detect antibodies in patients with culture-positive typhoid fever.
MATERIALS AND METHODS
Study population.
A convenience sample of sera from 79 hospitalized patients and healthy controls obtained in the course of diagnosis and treatment was tested. Patients and controls of 12 to 63 years old were seen at the Lucio Córdova Infectious Diseases, Sotero del Rio, and Catholic University hospitals in Santiago, Chile. All patients were Hispanic, 60% were male, and 70% were under the age of 30. Diagnosis of typhoid fever was made in 40 patients on the basis of one or more positive blood cultures of ST. Etiologic diagnosis of 22 patients with other acute febrile diseases due to systemic infections with gram-negative bacteria, gram-positive bacteria, or fungi was made on the basis of positive blood cultures; diagnoses of these patients included urinary tract infection, renal sepsis, pyelonephritis, pneumonia, bronchopneumonia, acute pancreatitis, thrombophlebitis, catheter infection, wound infection, and coma. Blood donors (17 subjects) at the hospital with no illnesses were used as healthy controls. The median duration of fever in typhoid patients at the time of diagnosis was 12 days (range, 3 to 60 days); seven patients had had fever for 21 to 60 days. Of the patients with typhoid, four had stool cultures positive for ST and one had a stool culture positive for Salmonella enteritidis B. The following complications occurred in 13 patients with typhoid: gastrointestinal hemorrhage (7 patients), hepatitis (1 patient), hepatitis with cholecystitis (1 patient), septic shock (1 patient), and relapse (3 patients). Results of agglutination titers for Widal O and H were available for 35 patients with typhoid and for 22 patients with other acute febrile diseases. Patients with typhoid were treated with chloramphenicol for an average of 5 days before blood samples were withdrawn. Sera from patients and healthy controls were obtained with informed consent and stored frozen at −80°C until analyzed. All serum samples were coded, and the code was not broken until all assay measurements had been completed.
Preparation of ST O-HSA conjugate.
A conjugate of ST O chains and low-endotoxin-level-containing HSA (Sigma Chemical Co., St. Louis, Mo.) was prepared using essentially the same procedures previously described for the preparation of ST O chains conjugated to bovine serum albumin (1). Briefly, ST LPS (Sigma) was purified by enzymatic treatment and ultracentrifugation and hydrolyzed in 1% acetic acid for 1.5 h at 100°C, and the precipitated lipid A was removed by centrifugation. The high-molecular-mass glycose-positive fractions excluded from gel filtration chromatography (Sephadex G-50; Amersham Biosciences, Piscataway, N.J.) were pooled, lyophilized, dissolved in water, and oxidized with 100 mM sodium metaperiodate at 4°C for 1 h, a procedure followed by a Smith hydrolysis in 1% acetic acid for 1 h to yield oxidized ST O chain polysaccharide. Oxidized ST O chain polysaccharide was conjugated to HSA by reductive amination in the presence of sodium cyanoborohydride for 7 days at 25°C, and the conjugate was purified by gel filtration chromatography (Bio-Gel A 0.5m; Bio-Rad Laboratories, Hercules, Calif.). Conjugated material was dialyzed against distilled water and lyophilized. Protein in the conjugate was determined using HSA as a standard (4). Carbohydrate in the conjugate was determined using purified ST O polysaccharide as a standard; galactose was used in assay standardization and validation (8).
Endotoxin measurement.
The endotoxin content of HSA was measured by the end-point method using the Limulus amebocyte lysate test (Associates of Cape Cod, East Falmouth, Mass.) according to the manufacturer's instructions.
Immunoblotting.
Purified ST O chains, ST O-HSA conjugate, ST LPS, and HSA were separated electrophoretically on a 7.5% sodium dodecyl sulfate-polyacrylamide gel, transferred electrolytically to a nitrocellulose membrane (14), and developed using optimal dilutions of rabbit anti-Salmonella O:1,9,12 (group D) (DIFCO, Detroit, Mi.) and alkaline phosphatase-conjugated anti-rabbit immunoglobulin G secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.) and enhanced chemiluminescence (ECL) technology (Amersham, Arlington Heights, Ill.) (14). Developed blots were analyzed using a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Dot immunobinding.
Anti-ST O titers were determined in duplicate by dot immunobinding using 100 ng of HSA, 100 ng and 10 ng of ST O-HSA, 100 ng and 10 ng of ST LPS, and 100 ng of ST O chain adsorbed to nitrocellulose in a filtration manifold (Bio-Dot; Bio-Rad) and serial twofold dilutions of human sera (initial dilution, 1/1,000) (14). All blots contained positive and negative control dots. Blots were blocked and developed with alkaline phosphatase-conjugated secondary antibodies and enhanced chemiluminescence technology (14). Developed blots were read using a Storm 860 PhosphorImager. The densities of dots in scans were evaluated visually by comparison with control dots containing no protein and scored as follows: 0, none; +, minimal; ++, moderate; +++, high; ++++, very high. Titer was defined as the reciprocal of the last serum dilution to give a score for moderate density (++ response). Previous studies have indicated that visual and densitometric quantitations yield equivalent results (3, 27, and our own unpublished observations). Titers are reported as geometric means (± standard errors); for purposes of computation, sera showing no immunodot reactivity at 1/1,000 dilutions (the lowest dilution tested) were deemed to be reactive at 1/500 dilutions. A positive dot immunobinding response was defined as a titer of ≥20,000 in this population with heavy exposure to bacterial enteric infections (9).
Statistical analysis.
Significance of differences between mean titers measured with the various reagents was determined by a Kruskal-Wallis test (nonparametric analysis of variance) with a Dunn multiple-comparison posttest. A significance level of P < 0.01 was used. Sensitivity (the percentage of patients with typhoid whose sera had dot immunobinding titers of ≥20,000) and specificity (the percentage of control subjects without typhoid whose sera had dot immunobinding titers of <20,000) of the dot immunobinding assay were analyzed by Fisher's exact test. A significance level of P < 0.05 was used for these data.
RESULTS
Characterization of ST O-HSA conjugate.
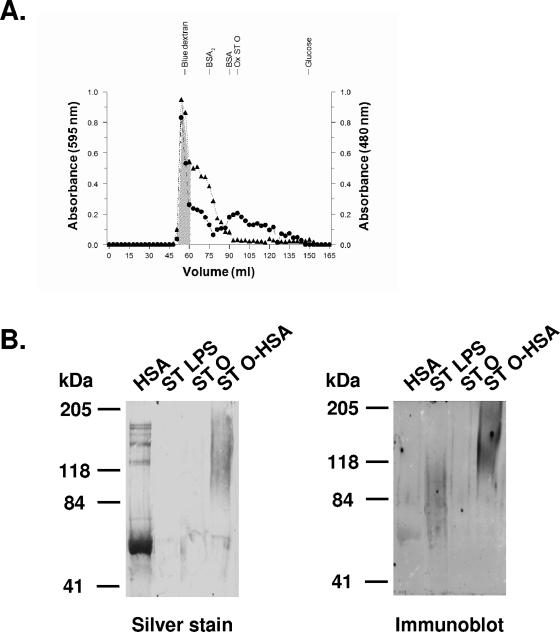
Analysis of individual chromatographic fractions revealed that almost all of the HSA (98%) in the conjugation reaction mixture of HSA and purified ST O chains was recovered as conjugate and was well separated from fractions containing unbound oxidized ST O chains (Fig. 1A). The salt-free 12 mg of conjugate with the highest molecular mass (shaded region in Fig. 1A) contained 29% O polysaccharide and 60% protein. If it is assumed that carbohydrate and protein are the only macromolecular components of the conjugates (lipid components of ST LPS are removed in the course of ST O chain purification) and that purified O chains have average molecular masses of 15 to 20 kDa, the conjugate was calculated to contain two O chains per HSA molecule (1). Although this conjugate was aggregated in solution (it was present in the excluded volume of the gel filtration column [Fig. 1A]), it was disaggregated under the denaturing conditions of sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting (Fig. 1B), where it had a molecular mass of about 120 kDa (Fig. 1B), consistent with the calculated number of O chain polysaccharide units per mole HSA. The diffuse staining of ST O-HSA in the silver-stained blot and immunoblot results from the heterogeneity of the conjugated ST O chains. Similarly, the diffuse staining of ST LPS in the immunoblot is due to polysaccharide heterogeneity; this low dose was not detected by silver staining (Fig. 1B). The reactivity of HSA with anti-ST O antiserum (Fig. 1B) is consistent with its low endotoxin content (0.15 endotoxin unit/mg). The ST O chains ran out of the gel under the conditions of electrophoresis.
FIG. 1.
Characterization of ST O antigen-protein conjugate. (A) Gel filtration chromatography (Bio-Gel A 0.5m) of reaction mixture containing 10 mg of HSA and 10 mg of purified ST O chains eluted with phosphate-buffered saline, pH 7.2. See Materials and Methods for details of the conjugation reaction. Contents of protein at 595 nm (▴) and carbohydrate at 480 nm (•) were determined in each fraction. The shaded area indicates fractions pooled for further analysis. Note the clear separation of conjugated material from unconjugated oxidized ST O chains. BSA, bovine serum albumin; BSA2, dimer of BSA; Ox ST O, purified oxidized ST O chains. (B) Immunoblot of 7.5% polyacrylamide gel of 2 μg of HSA, ST LPS, purified ST O chains (ST O), and ST O-HSA conjugate developed with commercial rabbit anti-ST O:1,9,12. Rabbit anti-ST O reacts with endotoxin-contaminated (Limulus amebocyte lysate assay-positive) HSA, ST LPS, and ST O-HSA conjugate. ST O did not stain because it ran out of the gel under these conditions.
Dot immunobinding titers to ST antigen-containing reagents in patients with typhoid fever and controls.
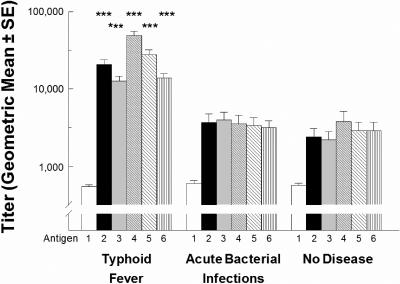
Dot immunobinding titers to HSA were low and similar in typhoid patients and controls (Fig. 2). In contrast, dot immunobinding titers to ST O-HSA, ST LPS, and unconjugated ST O chain polysaccharide were significantly greater in typhoid patients than in patient or healthy controls (P < 0.001; Kruskal-Wallis test with Dunn multiple-comparison posttest). Dot immunobinding of sera from 40 patients with bacteriologically confirmed typhoid fever revealed significant titers (titers of ≥20,000) in 28 (70%) and 12 (30%) tested with 100 ng and 10 ng of ST O-HSA, respectively; in 38 (95%) and 34 (85%) tested with 100 ng and 10 ng of ST LPS, respectively; and in 16 (40%) tested with purified unconjugated ST O chains (Table 1). In sera from 22 patients with other nontyphoid fevers, similar reactivities to 100 ng and 10 ng of ST O-HSA were observed only in two sera (9.1%) and one (4.5%) serum sample, respectively, and to 100 ng and 10 ng of ST LPS in a single serum sample to both doses (4.5%) (Table 1). None of these patient controls showed any such reactivity with unconjugated ST O chains, nor did any of the 17 healthy-control sera show significant reactivities with any of the ST reagents. The numbers of typhoid patients showing significant responses to ST O-HSA, ST LPS, or unconjugated ST O chains were significantly greater than the numbers of patient or healthy controls showing such responses to these reagents (P = 0.0226 to <0.0001) (Table 1).
FIG. 2.
Dot immunobinding titers (geometric mean ± standard errors [SE]) of sera from 40 patients with bacteriologically confirmed typhoid fever, 22 patients with other acute microbial infections, and 17 healthy controls measured with 100 ng of HSA (antigen 1), 100 ng of ST O-HSA (antigen 2), 10 ng of ST O-HSA (antigen 3), 100 ng of ST LPS (antigen 4), 10 ng of ST LPS (antigen 5), and 100 ng of purified ST O polysaccharide (antigen 6). Titers of individual sera were determined in duplicate. See Materials and Methods for details. ***, P < 0.001 by a Kruskal-Wallis test with a Dunn multiple-comparison posttest.
TABLE 1.
Number of patients with significantly elevated dot immunobinding or Widal agglutination titers
| Group | No. of sera with positive result when tested with indicated antigen in dot immunobinding (titer ≥ 20,000)
|
No. of sera with positive result in indicated Widal test
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ST O-HSA
|
ST LPS
|
ST O (100 ng) | n | Widal H
|
Widal O
|
|||||
| 100 ng | 10 ng | 100 ng | 10 ng | ≥1/80 | ≥1/160 | ≥1/80 | ≥1/160 | ||||
| Typhoid fever | 40 | 28 | 12 | 38 | 34 | 16 | 35 | 27 | 26 | 25 | 22 |
| Nontyphoid fevers | 22 | 2a | 1b | 1a | 1a | 0d | 22 | 7f | 3a | 13 | 6g |
| Healthy controls | 17 | 0a | 0c | 0a | 0a | 0g | NDh | ND | ND | ND | ND |
Significantly different from patients with typhoid fever (P < 0.0001; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.0226; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.0114; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.0005; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.0012; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.001; Fisher's exact test).
Significantly different from patients with typhoid fever (P = 0.014; Fisher's exact test).
ND, not determined.
The sensitivities of dot immunobinding for typhoid fever patients compared with those of nontyphoid fever patient controls were 70% and 30% with 100 ng and 10 ng of ST O-HSA, respectively; 95% and 85% with 100 ng and 10 ng of ST LPS, respectively; and 40% with 100 ng of unconjugated ST O chains (Table 2). Differences between the sensitivities with ST LPS and unconjugated ST O chains were significant (P < 0.05; Fisher's exact test), but the differences in sensitivities between ST O-HSA and ST LPS were not. The specificities of dot immunobinding with any of these reagents were similar (91 to 100%) (Table 2). Sensitivity and specificity of dot immunobinding for typhoid fever were essentially the same when the control group included healthy controls as well as nontyphoid fever patient controls (Table 2).
TABLE 2.
Sensitivity and specificity of dot immunobinding and Widal assays for diagnosis of typhoid fever
| Comparison | Dot immunobinding (titer ≥ 20,000)
|
Widal test
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ST O-HSA
|
ST LPS
|
ST O (100 ng) | Widal H
|
Widal O
|
|||||
| 100 ng | 10 ng | 100 ng | 10 ng | ≥1/80 | ≥1/160 | ≥1/80 | ≥1/160 | ||
| Typhoid fever versus patient controls | |||||||||
| Sensitivity (%) | 70 | 30 | 95a | 85a | 40 | 77 | 74 | 71 | 63 |
| Specificity (%) | 91 | 97 | 95 | 95 | 100 | 68 | 86 | 41 | 73 |
| Typhoid fever versus all controls | |||||||||
| Sensitivity (%) | 70 | 30 | 95a | 85a | 40 | NDb | ND | ND | ND |
| Specificity (%) | 95 | 97 | 97 | 97 | 100 | ND | ND | ND | ND |
Sensitivity is significantly different from that with ST O (P < 0.05; Fisher's exact test).
ND, not determined.
Widal H and O agglutination titers in patients with typhoid fever and controls.
Widal agglutination titers were available for 35 of the 40 patients with typhoid fever and for all 22 of the patient controls with other fevers (Table 1). Of the typhoid fever patients, 27 had Widal H titers of ≥1/80, while 26 had titers of ≥1/160, significantly higher than those of the 7 and 3 nontyphoid fever patient controls with responses at these levels (P = 0.001 and P < 0.0001, respectively) (Table 1). The diagnostic sensitivities and specificities of Widal H titers were 77% and 68%, respectively, for titers of ≥1/80 and 74% and 86%, respectively, for titers of ≥160. With respect to Widal O titers, the difference in numbers of typhoid fever patients and controls was significant only with titers of ≥160 (P = 0.014); the difference in numbers of typhoid patients and controls was not significant for Widal O titers of ≥80 (Table 1). The diagnostic sensitivity and specificity of Widal O titers were 63% and 73%, respectively, for titers of ≥160. Only the Widal H assay using a cutoff of ≥160 was comparable in diagnostic sensitivity and specificity to the dot immunobinding assay with 100 ng of ST O-HSA conjugate or 100 ng of ST LPS.
DISCUSSION
We have used an ST O-HSA conjugate containing 2 moles of ST O chain polysaccharide per mole of low-endotoxin-level HSA to detect anti-ST O antibodies in typhoid patients in a dot immunobinding assay. At the 100-ng dose, its diagnostic sensitivity (70%) and specificity (91%) were similar to those of the Widal H agglutination assay with a titer cutoff of ≥1/160 (74% and 86%, respectively) and somewhat better than those of the Widal O assay with a titer cutoff of ≥1/160 (63% and 73%, respectively). A comparison of results from the Widal O assay and dot immunobinding using ST O-HSA conjugate suggests that the antigenicity of the ST O polysaccharide was not decreased by the chemical modifications of oxidation and reductive amination involved in producing the conjugate (1). It should be noted that the sensitivities of the Widal assays in the present study at the 1/160 cutoff were similar or somewhat higher than the usual quoted values (15) but not appreciably different from those in several recent studies (18, 22).
The diagnostic sensitivity of the ST O-HSA conjugate assay was lower than that of a comparable dot immunobinding assay using 100 ng of ST LPS (95%) and that reported for an anti-ST LPS ELISA (94%) (24); the specificity of dot immunobinding with ST O-HSA was similar to those of both dot immunoassays using ST LPS (92 to 95%) (24). Sensitivity and specificity of ST O-HSA in dot immunobinding were, however, comparable to recently reported sensitivities and specificities found for two rapid diagnostic tests, TyphiDot and TUBEX (18). The similarity of sensitivities obtained with ST LPS in dot immunobinding and ELISA suggests that the difference between the sensitivities of ST O-HSA and ST LPS in dot immunobinding represents a real difference in the diagnostic sensitivities of these two reagents. If the decreased sensitivity of ST O-HSA compared to that of purified ST LPS were a result of the limited number of ST O antigen epitopes present in the conjugate, modified conjugation procedures could be devised to increase the amount of ST O antigen conjugated to each molecule of protein (1). The decreased sensitivity of the conjugate might also be a result of the fact that it contains none of the protein epitopes closely associated with ST LPS which are not removed during purification and which have been shown to be responsible for some of the antigenicity of the purified material (16, 17, 23; L. Aron-Hott and F. C. Cabello, data not shown).
The hospitalized typhoid fever patients in this study may possibly have been sicker than those in some recently reported groups (10, 28), although they appear to be comparable to those in another recent study using hospitalized patients (18). One or more blood cultures were positive in all patients, as opposed to the expected 60 to 80% (10, 20, 28). Our patients were thus similar to those studied by Olsen and coworkers (18). The median length of fever before diagnosis was almost 2 weeks, again not markedly different from that in the group studied by Olsen and coworkers (18). Not surprisingly for a group of patients who had been ill for this length of time with typhoid, the rate of complications was 33% rather than the expected rate of 10 to 15% (20). On the other hand, the 7.5% rate of relapse was similar to the usual quoted rate of 5 to 10% (20).
The advantage of the ST O-HSA conjugate is that it represents a nontoxic, chemically well-defined material that can be produced in large lots under carefully controlled and documented conditions. Its use offers the possibility of employing well-defined reagents for analyzing the immune response in human beings following infection or vaccination with ST. For example, the ST O-HSA conjugate described here had only two O chains per molecule of HSA. As suggested above, increased numbers of O chains per mole of conjugate could increase both sensitivity and specificity. A similar increase might also be achieved by conjugating the O chain to specific highly immunogenic ST proteins (2). However, even with improved methodology and more-specific antigens, the serodiagnosis of typhoid can be problematic. First, ST O antigens are shared with other group D Salmonella serotypes (the O:9,12 antigen of ST is also found in S. enterica serovar Dublin, S. enterica serovar Panama, and S. enterica serovar Gallinarum [20]), and the ST LPS core and lipid A contain epitopes that are cross-reactive with other Enterobacteriaceae LPS (25). Second, patients with typhoid fever may develop no detectable antibody response or have no increase in titer to these antigens (15, 20). In one recent study, for example, the Widal test failed to detect 28% of typhoid cases seen in the first week of illness and was useful only in the second week of illness or subsequently (22). Furthermore, infections with other bacterial pathogens may generate an anamnestic response against ST antigens in regions of typhoid endemicity (2), a phenomenon somewhat evident in sera from patients with other bacterial fevers (Fig. 2). One approach to these problems has been to develop local cutoff points (21, 28). Another has been to apply new methodologies or to standardize the reagents and controls used in the assay (6, 8-11, 22). Unfortunately, the variable serum immune responses in ST infections may ultimately make long-term success impossible even if the practical problems associated with the use of these assays under conditions of limited resources are solved.
In sum, we have found that an ST O-HSA conjugate is as active as purified ST LPS in detecting anti-ST immune responses in patients with typhoid fever, suggesting that the chemical modifications involved in generating this conjugate did not decrease the antigenicity of the ST O polysaccharide. Improved conjugates may prove useful in the future for analyzing human immune responses to ST and for developing new typhoid fever diagnostics and vaccines.
Acknowledgments
We thank J. Di Fabio for his encouragement and assistance and Harriett Harrison for her help in manuscript preparation.
This study was supported by NIH grants AI43782 (to L.A.-H.) and AI37014 (to F.C.C.) and by the New York Medical College Research Endowment Fund (Intramural Research Support Program) (to H.P.G.). J.Z. was a recipient of a fellowship from the Andes Foundation (Santiago, Chile).
REFERENCES
- 1.Aron, L., J. Di Fabio, and F. C. Cabello. 1993. Salmonella typhi O:9,12 polysaccharide-protein conjugates: characterization and immunoreactivity with pooled and individual normal human sera, sera from patients with paratyphoid A and B and typhoid fever, and animal sera. J. Clin. Microbiol. 31:975-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron, L., G. Faundez, C. Gonzalez, E. Roessler, and F. Cabello. 1993. Lipopolysaccharide-independent radioimmunoprecipitation and identification of structural and in vivo induced immunogenic surface proteins of Salmonella typhi in typhoid fever. Vaccine 11:10-17. [DOI] [PubMed] [Google Scholar]
- 3.Bentley-Hibbert, S. I., X. Quan, T. Newman, K. Huygen, and H. P. Godfrey. 1999. Pathophysiology of antigen 85 in patients with active tuberculosis: antigen 85 circulates as complexes with fibronectin and immunoglobulin G. Infect. Immun. 67:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cardona-Castro, N., E. Gotuzzo, M. Rodriguez, and H. Guerra. 2000. Clinical application of a dot blot test for diagnosis of enteric fever due to Salmonella enterica serovar Typhi in patients with typhoid fever from Colombia and Peru. Clin. Diagn. Lab. Immunol. 7:312-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chart, H., J. S. Cheesbrough, and D. J. Waghorn. 2000. The serodiagnosis of infection with Salmonella typhi. J. Clin. Pathol. 53:851-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chart, H., L. R. Ward, and B. Rowe. 1998. An immunoblotting procedure comprising O = 9,12 and H = d antigens as an alternative to the Widal agglutination assay. J. Clin. Pathol. 51:854-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 9.Fica, A. E., S. Prat-Miranda, A. Fernandez-Ricci, K. D'Ottone, and F. C. Cabello. 1996. Epidemic typhoid in Chile: analysis by molecular and conventional methods of Salmonella typhi strain diversity in epidemic (1977 and 1981) and nonepidemic (1990) years. J. Clin. Microbiol. 34:1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatta, M., M. G. Goris, E. Heerkens, J. Gooskens, and H. L. Smits. 2002. Simple dipstick assay for the detection of Salmonella typhi-specific IgM antibodies and the evolution of the immune response in patients with typhoid fever. Am. J. Trop. Med. Hyg. 66:416-421. [DOI] [PubMed] [Google Scholar]
- 11.House, D., J. Wain, V. A. Ho, T. S. Diep, N. T. Chinh, P. V. Bay, H. Vinh, M. Duc, C. M. Parry, G. Dougan, N. J. White, T. T. Hien, and J. J. Farrar. 2001. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J. Clin. Microbiol. 39:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail, T. F., H. Smits, M. O. Wasfy, J. L. Malone, M. A. Fadeel, and F. Mahoney. 2002. Evaluation of dipstick serologic tests for diagnosis of brucellosis and typhoid fever in Egypt. J. Clin. Microbiol. 40:3509-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan, E., I. Azam, S. Ahmed, and R. Hassan. 2002. Diagnosis of typhoid fever by dot enzyme immunoassay in an endemic region. J. Pak. Med. Assoc. 52:415-417. [PubMed] [Google Scholar]
- 14.Landowski, C. P., H. P. Godfrey, S. I. Bentley-Hibbert, X. Liu, Z. Huang, R. Sepulveda, K. Huygen, M. L. Gennaro, F. H. Moy, S. A. Lesley, and M. Haak-Frendscho. 2001. Combinatorial use of antibodies to secreted mycobacterial proteins in a host immune system-independent test for tuberculosis. J. Clin. Microbiol. 39:2418-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losonsky, G. A., and M. M. Levine. 2002. Immunologic methods for diagnosis of infections caused by diarrheagenic members of the families Enterobacteriaceae and Vibrionaceae, p. 447-461. In N. R. Rose, R. G. Hamilton, and B. Detrick (ed.), Manual of clinical laboratory immunology. ASM Press, Washington, D.C.
- 16.Luk, J. M., S. M. Lind, R. S. Tsang, and A. A. Lindberg. 1991. Epitope mapping of four monoclonal antibodies recognizing the hexose core domain of Salmonella lipopolysaccharide. J. Biol. Chem. 266:23215-23225. [PubMed] [Google Scholar]
- 17.Luk, J. M., and A. A. Lindberg. 1991. Anti-Salmonella lipopolysaccharide monoclonal antibodies: characterization of Salmonella BO-, CO-, DO-, and EO-specific clones and their diagnostic usefulness. J. Clin. Microbiol. 29:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen, S. J., J. Pruckler, W. Bibb, T. M. T. Nguyen, M. T. Tran, T. M. Nguyen, S. Sivapalasingam, A. Gupta, T. P. Phan, T. C. Nguyen, V. C. Nguyen, D. C. Phung, and E. D. Mintz. 2004. Evaluation of rapid diagnostic tests for typhoid fever. J. Clin. Microbiol. 42:1885-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang, T., M. M. Levine, B. Ivanoff, J. Wain, and B. B. Finlay. 1998. Typhoid fever—important issues still remain. Trends Microbiol. 6:131-133. [DOI] [PubMed] [Google Scholar]
- 20.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 21.Parry, C. M., N. T. Hoa, T. S. Diep, J. Wain, N. T. Chinh, H. Vinh, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J. Clin. Microbiol. 37:2882-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakash, P., O. P. Mishra, A. K. Singh, and A. K. Gulati. 2005. Evaluation of nested PCR in diagnosis of typhoid fever. J. Clin. Microbiol. 43:431-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri, A., S. K. Gupta, and G. P. Talwar. 1988. Monoclonal antibodies delineate multiple epitopes on the O antigens of Salmonella typhi lipopolysaccharide. J. Clin. Microbiol. 26:2292-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quiroga, T., M. Goycoolea, R. Tagle, F. Gonzalez, L. Rodriguez, and L. Villarroel. 1992. Diagnosis of typhoid fever by two serologic methods. Enzyme-linked immunosorbent assay of antilipopolysaccharide of Salmonella typhi antibodies and Widal test. Diagn. Microbiol. Infect. Dis. 15:651-656. [DOI] [PubMed] [Google Scholar]
- 25.Rahman, M. M., J. Guard-Petter, and R. W. Carlson. 1997. A virulent isolate of Salmonella enteritidis produces a Salmonella typhi-like lipopolysaccharide. J. Bacteriol. 179:2126-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena, M., and J. L. Di Fabio. 1994. Salmonella typhi O-polysaccharide-tetanus toxoid conjugated vaccine. Vaccine 12:879-884. [DOI] [PubMed] [Google Scholar]
- 27.Van Vooren, J. P., M. Turneer, J. C. Yernault, J. De Bruyn, E. Burton, F. Legros, and C. M. Farber. 1988. A multidot immunobinding assay for the serodiagnosis of tuberculosis. Comparison with an enzyme-linked immunosorbent assay. J. Immunol. Methods 113:45-49. [DOI] [PubMed] [Google Scholar]
- 28.Willke, A., O. Ergonul, and B. Bayar. 2002. Widal test in diagnosis of typhoid fever in Turkey. Clin. Diagn. Lab. Immunol. 9:938-941. [DOI] [PMC free article] [PubMed] [Google Scholar]