Abstract
The 51 serotypes of human adenoviruses (HAdVs) of the genus Mastadenovirus are classified into the six species HAdV-A to HAdV-F. For the detection of genus- and species-specific antibodies in human sera an immunoblot assay was developed. The recombinant long fiber of HAdV-41[F] (Ad41Fi) and the native hexon of HAdV-5[C] were used as genus-specific antigens. The recombinant capsid protein IX (pIX) of HAdV-2 (Ad2pIX[C]) and HAdV-41 (Ad41pIX[F]), the C-terminal pIX part of HAdV-3 (Ad3pIXC[B]), and the fiber knob of HAdV-8 (Ad8FiKn[D]) were evaluated as representative species-specific antigens. Hence, the pIX amino acid sequences of numerous serotypes of all HAdV species were compared, and the cross-reactivities of pIX antigens with rabbit hyperimmune sera among HAdV-A to -F were analyzed. In an epidemiological study, 667 human patient sera, not selected for viral infection, were screened for adenovirus seroprevalence. The genus-specific antibody prevalences directed against the Ad41Fi and HAdV-5 hexon were 82.8 and 98.8%, respectively. The species-specific antibody prevalence of 44.7% against Ad2pIX[C], 36.6% against Ad41pIX[F], 26.4% against Ad8FiKn[D], and 18% against Ad3pIXC[B] showed an age-dependent distribution and correlated well with the frequency of isolated serotypes of the respective species in earlier studies (except HAdV-D). In conclusion, the immunoblot assay using pIX, fiber, and hexon antigens represents a valuable and new serological tool for refined adenovirus diagnosis as shown in an epidemiological study.
Human adenoviruses (HAdVs) cause epidemic, endemic, and sporadic infections worldwide and can infect and replicate in the respiratory tract, as well as in the gastrointestinal tract, eye, urinary bladder, and liver. In immunocompetent individuals subclinical adenovirus infections often result only in antibody production. Immunosuppressed patients are more susceptible to adenovirus infections and carry a significantly higher risk of mortality. In these patients fatal infections with serotypes of the different species have been described (24). After allogeneic stem cell transplantation, it was possible to demonstrate that a positive adenovirus antibody test in the donor is a risk factor for adenovirus infection of the recipient (29). Over the last few years, adenovirus vectors have become one of the most important systems for gene transfer, which was previously hampered by preexisting adenovirus antibodies (19, 37). Diagnosis of adenovirus infections is currently based on virus isolation in cell culture and genus-specific antibody and antigen detection by enzyme immunoassay (38), as well as adenovirus DNA detection by PCR (19, 30). Until now serotype- and species-specific antibodies have been detected by neutralization or hemagglutination inhibition assays. These expensive methods of adenovirus typing have mainly been used in epidemiological studies. For a refined diagnosis of adenovirus infections, it would be advantageous to know the individual patients' history of adenovirus infections (10). Therefore, the medical interest for simple typing has increased due to the advancements made in understanding differences in virulence among several serotypes (4).
The human adenoviruses of the genus Mastadenovirus comprise 51 distinct serotypes that are grouped into the six species HAdV-A to -F (previously named subgroups or subgenera) based on the various immunological, biological, and biochemical characteristics (7, 32). The genus-specific epitopes on the major antigen hexon are often used for immunological routine diagnosis of adenoviruses. The components of the outer virus capsid hexon and penton are further possessed of the epitopes of neutralizing antibodies. The fiber, which contains a shaft with mainly genus-specific epitopes and a knob with mainly species-specific epitopes, should be suitable for the genus-specific but also for the species-specific immunological diagnosis as already used for serotyping of adenoviruses by hemagglutination inhibition assay based on fiber determinants. The structural species-dependent differences were demonstrated by an amino acid sequence alignment, which showed the location of the linear epitopes in the fiber knobs of different serotypes of species HAdV-B, -C, and -D (22). Species-dependent clustering of serotypes based on the amino acid sequences of the fiber knob was illustrated in a phylogenetic tree generated by parsimony analysis (16).
An important candidate antigen for species-specific adenovirus diagnosis is protein IX (pIX) based on its species-dependent sequence and its virion surface localization. The small hexon-associated pIX of HAdV-5[C] is a 14.3-kDa minor structural component that stabilizes hexon-hexon interactions (15). There are 240 pIX molecules per virion and 12 per group of nine hexons inserted as trimers (33). The adenoviral pIX is characterized by three structural elements: the structure-forming more conserved N terminus, the alanine-rich middle region, and the variable C-terminal region, which contains a leucine zipper motif (1, 28). The C-terminal part of Ad3pIX[B] is located on the surface of the virus capsid shown by immunoelectron microscopy (3). This finding was confirmed by the fact that, after modification of the variable pIX C terminus by adding a coreceptor-binding motif, HAdV-5 vectors can change their cell tropism (13, 36). Antibodies to the pIX are detectable in human sera (8). The pIX seems to have species-specific epitopes, shown for the serotypes HAdV-3[B] and HAdV-2[C] (2). The detection of species-specific antibodies to pIX in human sera and their diagnostic use in an immunoblot assay has not been reported thus far.
The aim of the present study was to establish an immunoblot assay for genus- and species-specific detection of adenovirus antibodies directed against pIX, fiber/fiber knob, and hexon antigens in human sera and to evaluate this test with rabbit hyperimmune sera, as well as with human sera.
MATERIALS AND METHODS
Viruses and cells.
HAdV-1, -6, -8, -11, -15, -17, -34, -35, -37, and -41 were originally obtained from the American Type Culture Collection and HAdV-2, -3, -4, -5, -7, -9, -10, -12, -14, -21, -31, and -40 were from T. Adrian and A. Heim, German National Reference Laboratory for Adenoviruses, Hannover Medical School, Hannover, Germany. To produce rabbit hyperimmune sera, the viruses were propagated on HeLa cells, concentrated by precipitation with 8% polyethylene glycol, and purified by two cesium chloride density gradient centrifugations as previously described (3, 21).
Recombinant adenovirus antigens.
The adenovirus proteins were expressed as fusion proteins with His tag and with (Ad3pIXC[B], Ad4pIX[E], and Ad41pIX[F]) or without (Ad2pIX[C], Ad3pIX[B], Ad12pIX[A], Ad15pIX[D], Ad37pIX[D], Ad8FiKn[D], and Ad41Fi) dihydrofolate reductase (DHFR) by using the bacterial expression vectors pQE40 and pQE30 (QIAGEN, Hilden, Germany), respectively. The recombinant DNA constructs were prepared by using previously described primers (3, 6, 27). For Ad41pIX[F], the following primers containing restriction enzyme cleavage sites (underlined) at the 5′ end were used (forward and reverse, as indicated): 5′-TGG ATC CAG CGG CTC CAT GGA AGG-3′ (forward) and 5′-CAA GCT TTT AAG AAG CAT TAG CGG-3′ (reverse). For cross-reactivity investigations in parts before but mostly after finishing the seroepidemiological study, we prepared recombinant pIX DNA with the help of the following primers: 5′-AGA TCT AGC GGA AGC GGC TCC TTT-3′ (forward) and 5′-AAG CTT TTA TTT GGA TTT CAC CGT-3′ (reverse) for Ad4pIX[E], 5′-GAG ATC TAA CGG AAC TAC TCA GAA-3′ (forward) and 5′-CCC CGG GTT ATT GGG TTA CAG GTT-3′ (reverse) for Ad12pIX[A], and 5′-CGG ATC CAA CGG GAC GGG CGG GGC CT-3′ (forward) and 5′-TAA GCT TTC ATT TAT TTT GCT GCT GTT-3′ (reverse) for Ad15pIX[D] and Ad37pIX[D]. The Escherichia coli strain M15[pREP4] transformed with the plasmid constructs was grown at 37°C until it reached an optical density at 600 nm of 0.7, and then a total of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce protein expression. The proteins were purified by affinity chromatography on Ni-NTA (QIAGEN) and analyzed by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) as published previously (3).
Hyperimmune sera.
After stepwise dialysis against 10 mM Tris (pH 7.5)-100 mM NaCl containing 4, 2, 1, or 0.5 M urea, the expressed proteins were used for immunization of rabbits to produce polyclonal sera. For anti-protein sera, a total of 100 μg of recombinant protein of Ad12pIX[A], Ad3pIX[B], Ad3pIXC[B], Ad2pIX[C], Ad8FiKn[D], Ad15pIX[D], Ad37pIX[D], Ad4pIX[E], and Ad41pIX[F] and for anti-virus sera a total of 10 μg of purified adenovirus (HAdV-12[A], HAdV-3[B], HAdV-7[B], HAdV-21[B], HAdV-35[B], HAdV-2[C], HAdV-5[C], HAdV-8[D], HAdV-9[D], HAdV-15[D], HAdV-4[E], Ad41Fi, and HAdV-41[F]) per injection emulsified with an equal volume of Freund complete adjuvant was used for initial immunization. For the following two booster immunizations after 4 weeks and then after 15 days with Freund incomplete adjuvant, the same antigen amounts were used (2). Rabbit sera were collected before and at different times after immunization and analyzed by enzyme-linked immunosorbent assay and immunoblot. Most sera were also tested for their specificity by indirect immunofluorescence and by radioimmunoprecipitation of adenovirus-infected HeLa cells (data not shown).
Patient sera.
Patient sera, not selected for virus infections, were from the Greifswald surrounding area. To have a representative collection of study material, sera of hospitalized and nonhospitalized patients of all age groups were included. Patient sera were obtained as unpaired sera from hospitals of the University of Greifswald in the years 1993 to 1999. We divided the patients into eight age groups and chose approximately the same number of sera per age group. The project protocol was reviewed and approved by the local institutional review board, and informed consent was obtained from all patients.
Immunoblot and line assay.
The reactivity of adenovirus antigens was analyzed after immobilization onto nitrocellulose (NC) membrane by different methods.
(i) Protein application by electrophoresis.
For the epidemiological study, mixed purified Ad3pIXC[B], Ad2pIX[C], Ad8FiKn[D], Ad41pIX[F], and Ad41Fi antigens (ca. 10 to 50 ng per protein and per strip) were separated by SDS-12% PAGE and blotted onto NC membrane (150 by 250 mm; Schleicher & Schuell, Dassel, Germany). Two individual thin control lines were additionally sprayed as a thin line (0.1 μl/cm) onto the NC membrane after blotting: a native hexon preparation of HAdV-5[C] (6) (20 ng per strip; Micromun, Greifswald, Germany) as genus-specific protein and anti-human immunoglobulin G (IgG; 0.5 ng per strip) as a control for correct test performance. Spraying was done by a Western blot-producing company (Mikrogen, Munich-Martinsried, Germany) using an Isoflow Reagent Dispenser (Imagene Technology, Hanover, N.H.). After that the NC membrane was saturated with 5% skim milk powder solution and cut into strips. Strips were incubated with human sera (diluted with phosphate-buffered saline buffer 1:102) for ca. 14 h at room temperature. After the strips were washed, they were incubated with anti-human IgG (POD conjugated, diluted 1:2 × 103) for 45 min at room temperature. The positive enzyme reactions were visualized by the addition of POD substrate (3,3′,5,5′-tetramethylbenzidine) for 7 min.
(ii) Protein application by line assay.
For the detection of pIX cross-reactivity with rabbit hyperimmune sera diluted adenovirus antigens (10.3 to 199.9 μg/ml, diluted in Tris-HCl buffer) and two dilutions of anti-human IgG (0.05 and 0.5 ng per strip) for showing a weak reaction as cutoff and a strong reaction as a control for correct test performance (reaction control), respectively, were sprayed onto NC membranes as individual thin lines. The recombinant antigens were used after further chromatographic purification procedures (depending on the characteristics of the antigens such as pI or hydrophobicity; Mikrogen). POD-conjugated anti-rabbit IgG (diluted 1:2 × 103) was used.
Sequencing and phylogeny.
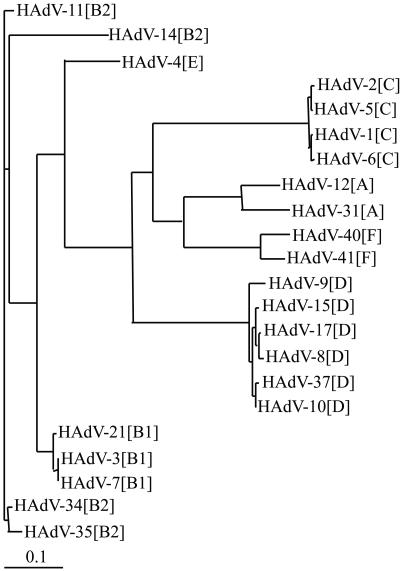
The following pIX amino acid sequences of the GenBank were used: HAdV-1[C] (AAQ10541.1), HAdV-2[C] (P03282), HAdV-3[B] (P03283), HAdV-5[C] (SXAD95), HAdV-7[B] (SXAD97), HAdV-9[D] (AAD16306), HAdV-11[B] (DAA01647.1), HAdV-12[A] (SXAD12), HAdV-17[D] (AF108105), HAdV-35[B] (AAN17474.1), HAdV-40[F] (AAC13959), and HADV-41[F] (SXADF1). To carry out phylogenic investigations for adenovirus serotypes with the help of pIX amino acid sequence, additional sequencing was needed. For PCR amplification and sequencing of the pIX region of HAdV-4[E], -6[C], -8[D], -10[D], -14[B], -15[D], -21[B], -31[A], -34[B], and -37[D], the primer sequences 5′-GTG ACC GAG GAG CTG-3′ and 5′-GGT CCA GGG CGT ACC-3′ were used. Amplified pIX DNA was sequenced using the Prism ready-reaction cycle sequencing protocol on an ABI 310 automated DNA sequencer (Applied Biosystems GmbH, Darmstadt, Germany). The nucleotide sequences were deposited in the GenBank under the following accession numbers: Ad4pIX[E] under AY178667, Ad6pIX[C] under AY675302, Ad8pIX[D] under AY675301, Ad10pIX[D] under AY178666, Ad14pIX[B] under AY675300, Ad15pIX[D] under AF480441, Ad21pIX[B] under AY675298, Ad31pIX[A] under AY178668, Ad34pIX[B] under AY675299, and Ad37pIX[D] under AF481737. The amino acid alignment was accomplished with CLUSTAL X (9, 34), and the phylogenetic tree was constructed by using the program TreeView (26). The length of the branches indicates the phylogenetic distance between the different serotypes and the species, respectively.
RESULTS
Selection of adenovirus antigens used for immunoblot assay.
As genus-specific antigens, the native HAdV-5 hexon and the recombinant long-fiber protein of HAdV-41[F] (Ad41Fi) were tested because of their cross-reacting properties. For the detection of species-specific adenovirus antibodies in human sera, the antigens should be highly immunogenic and display low cross-reactivity from HAdV-A to -F. In contrast to hexon and fiber, the small protein IX seems to have such properties. Therefore, the bacterially expressed His-tagged pIX fusion proteins Ad3pIXC[B], Ad2pIX[C], and Ad41pIX[F] were used as representative species-specific antigens for all serotypes of HAdV-B, -C, and -F in immunoblotting. The Ad8FiKn[D] antigen was evaluated as representative antigen for serotypes of HAdV-D. A representative pIX antigen of HAdV-A, -D, and -E were left in the immunoblot system because no pIX sequences of these species were known at the time point of starting the seroepidemiological study. The use of His-tagged fusion proteins with or without DHFR facilitated a size differentiation by electrophoresis for the immunoblot.
Based on the pIX amino acid sequences we found that within HAdV-A (HAdV-12 and -31) the identity is 84.7%, within HAdV-B (HAdV-3, -7, -11, -14, -21, -34, and -35) the identity is 83.3 to 99.3%, within HAdV-C (HAdV-1, -2, -5, and -6) the identity is 99.5 to 100%, within HAdV-D (HAdV-8, -9, -10, -15, -17, and -37) the identity is 96.2 to 99.3%, and within HAdV-F (HAdV-40 and -41) the identity is 90.9%, whereas between the serotypes of different adenovirus species identities of 47.7 to 70.5% were obtained (except for Ad4pIX[E] and pIX of the HAdV-B serotypes with identities between 75.5 and 82.6%) (9). After comparison of C- and N-terminal parts of pIX, we found for all species that the identity of the amino acid sequences of the variable C-terminal parts (amino acid position 71 till the end) were only 29.8 to 73.9% in contrast to higher identities of 61.9 to 96.9% in the conserved N-terminal parts (amino acid positions 1 to 70). This comparison of amino acid sequences indicated that the cross-reactivity should be localized on the N terminus of pIX and that especially antibodies directed against epitopes of the C-terminal pIX parts should be valuable for the species-specific differentiation of adenovirus infections by immunoblot assay. Furthermore, a phylogram (Fig. 1) based on the pIX amino acid sequences of 22 serotypes showed a good correlation with the classification of these serotypes into the six species from HAdV-A to -F. A phylogeny based on pIX nucleotide sequences of all HAdV serotypes (except 10 HAdV-D serotypes) also confirms this classification (not shown).
FIG. 1.
Phylogenetic tree based on amino acid sequences of pIX genes of different human adenovirus serotypes of HAdV-A to -F.
After that, only for HAdV-C (HAdV-2[C]) and -F (HAdV-41[F]) was the full-length pIX used as the representative antigen. To eliminate cross-reacting pIX domains between HAdV-3[B] and HAdV-2[C], we evaluated the C-terminal part of HAdV-3[B] (Ad3pIXC[B]) as representative antigen of HAdV-B. For the 32 serotypes of HAdV-D, the fiber knob of HAdV-8[D] (Ad8FiKn[D]) was chosen as the representative species-specific antigen instead of pIX because at the time of the seroepidemiological study no sequence data of pIX of any serotype of this adenovirus species were available. However, a recently generated phylogenetic tree of the fiber knob amino acid derived from serotypes of all adenovirus species, including 30 serotypes of HAdV-D, showed considerable differences between serotypes from HAdV-A to -F but minor sequence differences between serotypes of HAdV-D (16). Thereafter, the amino acid sequence of Ad8FiKn[D] represents a typical fiber knob sequence of HAdV-D.
Reaction pattern of adenovirus antigens obtained with rabbit hyperimmune sera.
With virus- and pIX-specific rabbit hyperimmune sera we investigated whether the antigens HAdV-5[C] hexon and HAdV-41[F] long fiber were enabled for determination of genus-specific immune response and, on the other hand, whether the antigens Ad2pIX[C], Ad3pIXC[B], Ad8FiKn[D], and Ad41pIX[F] are useful for determination of species-specific immune response (Fig. 2 and Table 1).
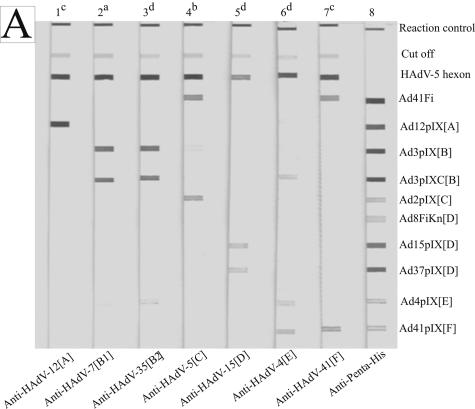
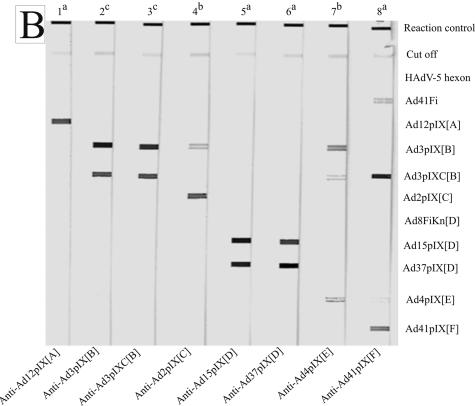
FIG. 2.
Reaction patterns of sprayed adenovirus antigens, analyzed with homologous and heterologous rabbit antibodies obtained by line assay. (A) The membrane strips were incubated with hyperimmune sera against whole virus (lanes 1 to 7) at serum dilutions of 1:5 × 102 (a), 1:103 (b), 1:104 (c), and 1:105 (d). Anti-Penta-His (lane 8; QIAGEN, Hilden, Germany; 1:103 diluted) marks all recombinant His-tagged antigens. (B) The membrane strips were incubated with hyperimmune sera against pIX antigens at serum dilutions of 1:104 (a), 1:105 (b), and 1:106 (c). The antigens are indicated on the right side.
TABLE 1.
Reaction patterns of adenovirus antigens detected by line assay with rabbit hyperimmune sera
| Evaluated antigen | Reaction pattern of sera directed against recombinant antigens or against purified virions (in parentheses)a
|
|||||
|---|---|---|---|---|---|---|
| Ad12pIX[A] (HAdV-12[A]) | Ad3pIX[B]/Ad3pIXC[B] (HAdV-3[B] and -35[B]) | Ad2pIX[C] (HAdV-5[C]) | Ad15pIX[D] (HAdV-15[D]) | Ad4pIX[E] (HAdV-4[E]) | Ad41pIX[F] (HAdV-41[F]) | |
| Hexon | − (++++) | −/− (++++) | − (++++) | − (++++) | − (++++) | − (++++) |
| Ad41Fi | + (+++) | −/− (+) | − (++++) | + (+++) | − (+) | + (+++) |
| Ad12pIX[A] | ++++ (++++) | ++/− (+) | − (−) | − (−) | − (+) | + (−) |
| Ad3pIX[B] | ++ (−) | ++++/++++ (+++) | ++ (−) | + (+) | ++ (−) | + (−) |
| Ad3pIXC[B] | + (−) | ++++/++++ (+++) | − (−) | + (−) | ++ (++) | ++ (−) |
| Ad2pIX[C] | − (−) | +/− (+) | ++++ (++++) | − (+) | − (+) | + (−) |
| Ad8FiKn[D] | − (−) | −/− (+) | − (−) | − (+++) | − (−) | − (−) |
| Ad15pIX[D] | + (−) | ++/− (−) | − (−) | ++++ (++++) | − (−) | + (−) |
| Ad37pIX[D] | + (−) | ++/− (+) | − (−) | ++++ (++) | − (−) | + (−) |
| Ad4pIX[E] | − (−) | +/++ (−) | − (−) | − (−) | ++ (++) | + (−) |
| Ad41pIX[F] | + (−) | +/+ (−) | − (+) | − (+) | − (++) | ++ (+++) |
Abbreviations for reaction intensity: −, no reaction; +, very weak reaction; ++, weak reaction; +++, strong reaction; ++++, very strong reaction. Recombinant antigens were tested at dilutions of 1:104 to 1:106. Purified virions were tested at dilutions of 1:103 to 1:105.
(i) Hyperimmune sera directed against whole virus.
HAdV-5 hexon always reacted very strongly with rabbit hyperimmune sera directed against whole virus of HAdV-12[A], -3[B], -7[B], -35[B], -5[C], -15[D], -4[E], and -41[F]. The other genus-specific antigen Ad41Fi reacted weakly with the sera of HAdV-B and -E serotypes, strongly with the sera of HAdV-A, -D, and -F serotypes, and very strongly with the sera of HAdV-C serotypes (Table 1). All sera showed a species-specific reaction with representative pIX antigens. For example, the representative Ad2pIX[C] antigen reacted with the serum against HAdV-5[C] virus but did not react with the sera against HAdV-12[A], -7[B], -35[B], -15[D], and -41[F]. In the same way, the representative antigens Ad3pIXC[B], Ad41pIX[F], and Ad8FiKn[D] only had a strong reaction to homologous hyperimmune sera and sera against serotypes of the same species. The rabbit hyperimmune sera against HAdV-3, -7 (both HAdV-B1), and -35 (HAdV-B2) showed a strong reaction to the complete Ad3pIX[B], as well as to the C-terminal part (Ad3pIXC[B]). Only anti-HAdV-4[E] cross-reacted with Ad3pIXC[B] and Ad41pIX[F] (Fig. 2A). Table 1 summarizes the reaction patterns of rabbit anti-virion sera (values in round asterisks) tested at serum dilutions of 1:103 to 1:105. Generally, at suitable serum dilutions rabbit sera against the purified virus reacted strongly with the pIX and fiber knob, respectively, of the same species and showed no to weak cross-reactivities with the representative antigens of other species.
(ii) PIX-specific hyperimmune sera.
For the detection of pIX cross-reactivity furthermore rabbit hyperimmune sera directed against representative pIX antigens were used. The reaction patterns of sprayed pIX antigens generally showed a strong species-specific signal by line assay (Fig. 2B and Table 1). The hyperimmune sera against Ad12pIX[A], Ad15pIX[D], and Ad37pIX[D] indicated no or very weak cross-reactivity with the pIX antigens of other species. Therefore, no to little interfering cross-reactivities were expected between representative pIX antigens of HAdV-B, -C, and -F with pIX antibodies against serotypes of HAdV-A and -D within the seroepidemiological study. The serum against the C-terminal part of Ad3pIX[B] reacted as strongly as the serum against the complete Ad3pIX[B] with Ad3pIXC[B] antigen, and the representative antigens Ad3pIXC[B] and Ad2pIX[C] did not show cross-reactivity. The Ad4pIX[E] antigen cross-reacted with Ad3pIX[B] and Ad41pIX[F] (investigated after completion of the seroepidemiological study). In contrast to antibodies to the purified virions of HAdV-41[F], the antibodies to the recombinant protein Ad41pIX[F] cross-reacted with Ad3pIXC[B]. Such weak cross-reactivities or nonspecific reactions were eliminated at higher serum dilutions. The rabbit hyperimmune sera were tested for disturbing antibodies, e.g., against DHFR or bacterial antigens.
Reaction pattern of adenovirus antigens within a seroepidemiological study.
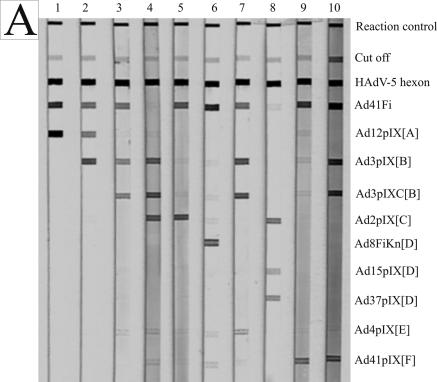
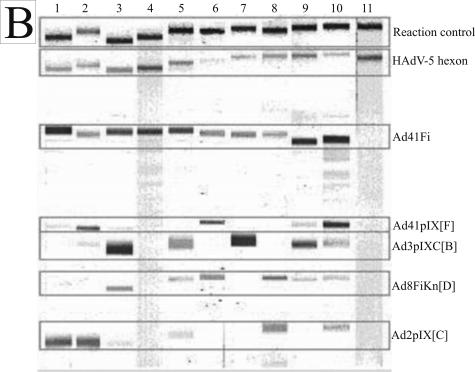
All human sera demonstrated in Fig. 3 showed a genus-specific reaction with HAdV-5 hexon and mostly with Ad41Fi antigen. The sera reacted with at least one but often with more than one of the representative antigens. In Fig. 3A examples of typical reaction patterns of human sera with sprayed adenovirus antigens in the line assay are shown; Fig. 3B shows examples for immunoblot assay. The immunoblot results of weak and strong antibody reactions of patient sera with adenovirus proteins separated by electrophoresis and blotted onto the NC membrane are demonstrated in Tables 2 and 3.
FIG. 3.
Reaction patterns of adenovirus antigens with human sera (1:102 diluted) on NC membrane. (A) Line assay of sprayed antigens. (B) Immunoblot assay of recombinant adenovirus fiber and pIX antigens separated by electrophoresis, as well as sprayed HAdV-5 hexon and reaction control. The minimal differences in the position of each protein band from one NC strip to another based on different polyacrylamide gels are bordered. The adenovirus antigens and controls are indicated on the right side.
TABLE 2.
Age-dependent IgG seroprevalence against representative species- and genus-specific adenovirus antigens in 667 patients by immunoblot assay
| Age group (no. of sera) | No. (%) of sera positive with species-representing antigens
|
No. (%) of sera positive with genus- specific antigens
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad3pIXC[B] | Ad2pIX[C] | Ad8FiKn[D] | Ad41pIX[F] | Single speciesa | All pIXb | All antigensc | Ad41Fi | Ad41Fid alone | Hexon | |
| 0-3 mo (48) | 8 (16.7) | 17 (35.4) | 9 (18.8) | 19 (39.6) | 14 (29.2) | 27 (56.2) | 29 (60.4) | 37 (77.1) | 12 (25.0) | 46 (95.8) |
| 4-11 mo (65) | 3 (4.6) | 9 (13.8) | 7 (10.8) | 11 (16.9) | 11 (16.9) | 17 (26.2) | 20 (30.8) | 29 (44.6) | 14 (21.5) | 59 (90.8) |
| 1-4 yr (104) | 14 (13.5) | 58 (55.8) | 20 (19.2) | 38 (36.5) | 33 (31.7) | 65 (62.5) | 70 (67.3) | 80 (76.9) | 16 (15.4) | 104 (100) |
| 5-14 yr (126) | 19 (15.1) | 75 (59.5) | 37 (29.4) | 44 (34.9) | 43 (34.1) | 85 (67.5) | 95 (75.4) | 112 (88.9) | 26 (20.6) | 125 (99.2) |
| 15-29 yr (100) | 21 (21.0) | 45 (45.0) | 23 (23.0) | 32 (32.0) | 31 (31.0) | 62 (62.0) | 67 (67.0) | 89 (89.0) | 27 (27.0) | 100 (100) |
| 30-50 yr (100) | 17 (17.0) | 47 (47.0) | 30 (30.0) | 44 (44.0) | 40 (40.0) | 73 (73.0) | 82 (82.0) | 95 (95.0) | 14 (14.0) | 100 (100) |
| 51-65 yr (86) | 32 (37.2) | 35 (40.7) | 35 (40.7) | 44 (51.2) | 22 (25.6) | 63 (73.3) | 70 (81.4) | 78 (90.7) | 12 (14.0) | 86 (100) |
| >65 yr (38) | 6 (15.8) | 12 (31.6) | 15 (39.5) | 12 (31.6) | 14 (36.8) | 18 (47.4) | 25 (65.8) | 32 (84.2) | 7 (18.4) | 38 (100) |
| Total (667) | 120 (18.0) | 298 (44.7) | 176 (26.4) | 244 (36.6) | 208 (31.2) | 410 (61.5) | 458 (68.7) | 552 (82.8) | 128 (19.2) | 658 (98.8) |
Single positive reaction per serum with any species-representing antigen.
Single or multiple positive reactions per serum with the species-representing pIX antigens.
Single or multiple positive reactions per serum with the species-representing pIX and fiber knob antigens.
No reaction with species-representing antigens.
TABLE 3.
Single and multiple species-specific versus genus-specific reaction patterns of 667 human sera
| No. of species-specific reactions per serum | No. of sera | No. of genus-specific reactions
|
|
|---|---|---|---|
| Ad41Fi | HAdV-5 hexon | ||
| 0 | 209 | 130 | 200 |
| 1 | 208 | 182 | 208 |
| 2 | 148 | 138 | 148 |
| 3 | 74 | 74 | 74 |
| 4 | 28 | 28 | 28 |
A total of 667 human sera were screened for adenovirus antibody prevalence (Table 2). There were 658 (98.8%) sera positive for genus-specific hexon antibodies. A total of 72 sera had no additional reaction beside the anti-hexon reactivity. With the second genus-specific antigen Ad41Fi 552 (82.9%) human sera did react. Thirty-four sera (5.1%) were positive with representative antigens but negative for Ad41Fi. In 458 (68.7%) of 667 sera antibodies to at least one of the four representative antigens were detected. In 586 of the 658 anti-hexon positive sera antibodies to at least one of the recombinant adenovirus pIX, fiber knob or fiber antigens were found. Antibodies against at least one of the three pIX antigens were found in 410 (61.5%) sera. In 384 of the 410 anti-pIX positive sera antibodies to Ad41Fi were detected.
An immune response to only one of the representative species-specific antigens showed 208 (31.2%) of the 667 sera, followed by 148 sera (22.2%) with antibodies to two species-specific antigens and by 74 sera (11.1%) against three species-representing antigens (6). A total of 28 (4.2%) of the 667 human sera possessed antibodies to all four species-specific antigens (Table 3). Two hundred (30%) of the 667 sera reacted with HAdV-5 hexon but did not react with the used species-specific antigens. Of these 200 sera, 130 (65%) contained antibodies to Ad41Fi. A weaker anti-fiber immune response was also found in 182 of the 208 sera (87.5%), with only one species-specific antigen reaction. If there were two or more species-specific reactions for one serum, the genus-specific Ad41Fi antigen reacted in every case (100%). Thereafter, the frequency of the detection of antibodies directed against the receptor-binding fiber seems to correlate with the frequency of detection of the representative antigens in one serum and the frequency of adenovirus infections in individual patients' history, respectively. These frequencies increased age dependently till the 50th year of life (Tables 2 and 3).
Furthermore, in 667 sera, 298 sera (44.7%) reacted with Ad2pIX[C], 244 (36.6%) reacted with Ad41pIX[F], 176 (26.4%) reacted with Ad8FiKn[D], and 120 (18%) reacted with Ad3pIXC[B]. To analyze the age-dependent antibody distribution, we divided the patients into eight age groups 0 to 3 months (n = 48), 4 to 11 months (n = 65), 1 to 4 years (n = 104), 5 to 14 years (n = 126), 15 to 29 years (n = 100), 30 to 50 years (n = 100), 51 to 65 years (n = 86), and >65 years (n = 38). The age-dependent adenovirus antibody distribution within the 667 patients' sera is summarized in Table 2. In the first age group of 0 to 3 months the antibody prevalence against adenovirus proteins was significantly higher than in the age group 4 to 11 months, the age group that contained six of the nine anti-hexon negative sera. It can be assumed that the majority of these antibodies in the age group of 0 to 3 months have a maternal origin and are no longer available after a while. Most of the antibody reactions were found against HAdV-F and -C antigens. A comparable seroprevalence for HAdV-F and slightly more HAdV-C antibodies were detected in the two age groups of potential mothers (15 to 29 years and 30 to 50 years). The age group of 1 to 4 years was the first age group with 100% anti-hexon positive sera, and 80 (76.9%) of them already possessed anti-fiber (Ad41Fi) antibodies. In all following age groups the amount of anti-hexon positive sera stayed at ∼100%. In the age group from 30 to 50 years the highest number of Ad41Fi-positive sera (95%) was detected. In the age group of >65 years antibodies against species- and genus-specific (Ad41Fi) antigens were found in a reduced number of sera (65.8 and 84.2%, respectively).
Altogether, the following characteristic species-specific distribution of the antibodies through the age groups was obtained (Table 2). (i) For HAdV-B, in the age group of 4 to 11 months fewer than 5% of the children had antibodies to the representative antigen Ad3pIXC[B]. In the age group of 1 to 4 years the HAdV-B-specific seroprevalence increased to 13.5%, and then in the age group of 51 to 65 years it increased to 37.2%. After the age of 65 years there was a significant drop to 15.8% of HAdV-B-positive sera. (ii) For HAdV-C, nearly 14% of the sera in the age group of 4 to 11 months reacted with the antigen Ad2pIX[C] and 55.8% of the sera were already positive in the age group of 1 to 4 years. The highest percentage of HAdV-C-positive sera (59.5%) was found in the age group of 5 to 14 years. In the subsequent age groups the antibody prevalence against Ad2pIX[C] decreased to 31.6%. (iii) For HAdV-D, in the age group of 4 to 11 months ca. 10% of the children possessed antibodies that reacted with Ad8FiKn[D]. The number of positive sera increased in the age group of 51 to 65 years to ca. 40%. The representative antigen of HAdV-D was the only species-specific antigen with nearly no decline of antibody prevalence after the 65th year of life. This result indicates also that one part of the Ad8FiKn[D] reactions was only induced after repeated adenovirus infections. This was supported by the fact that some Ad8FiKn[D] positive sera did not show a reaction with Ad15pIX[D] and Ad37pIX[D]. (iv) For HAdV-F, in the age group of 4 to 11 months antibodies against the representative antigen Ad41pIX[F] were detected in 16.9% of the sera. In the age group of 1 to 4 years there was a peak with 36.5% positive sera. In the age group of 51 to 65 years the second peak was found with 51.2% seroprevalence. After the 65th year of life the HAdV-F-specific seroprevalence decreased to 31.6%.
DISCUSSION
Evaluation of the immunoblot assay.
For adenovirus diagnosis the detection of HAdV antibodies is performed by enzyme-linked immunosorbent assay or complement fixation test based on the genus-specific hexon antigen (19). The epitopes on hexon and penton are important for serotyping of adenoviruses by neutralization and hemagglutination inhibition tests. However, these methods are not suitable for routine diagnosis (30). Besides antibodies directed against the capsid components hexon and penton the sera against whole virus contain also non-neutralizing antibodies against the outer capsid protein IX (1, 2, 8). For the extensive formation of pIX-specific antibodies and its use in serodiagnosis it seems to be advantageous that hexon-associated pIX is synthesized in far greater quantity than needed for encapsidation; furthermore, the hexon can provide an adjuvant effect for other antigens (25).
The detection of adenovirus antibodies with an immunoblot assay using recombinant pIX and fiber proteins had not been described until now. To investigate the ability of pIX protein to detect adenovirus antibodies in a species-specific manner, we analyzed at first the variability of the amino acid sequences of pIX of 22 HAdV serotypes of HAdV-A to -F. Identities of 47.7 to 70.5% as we have found between serotypes of two HAdV species are low for an adenovirus structural protein (except for Ad4pIX[E] and the pIX of different HAdV-B serotypes with higher identities of 75.5 to 82.6%) (9). For the hexon protein of different HAdV species the amino acid sequence identities are 65 to 81% (5). The average identity of pIX has a value between that of structural proteins and that of nonstructural E3 proteins (31 to 57%; unpublished results). In phylogenetic studies a clustering of serotypes into the species HAdV-A to -F as found for the pIX genes was also obtained for other regions of the adenovirus genome, such as the genes of the E1B-55K protein, the protease, the E3 proteins, and the fiber knob protein (5, 7, 16). However, these proteins are probably fewer suitable for species-specific differentiation of adenovirus infections due to the lack of appropriate antibodies in human sera. An immunoblot assay like that described here, which uses the strong immune response to species-specific determinants of pIX, constitutes a new tool in the serodiagnosis of adenovirus infections. The used genus-specific marker antigens HAdV-5 hexon and Ad41Fi cross-reacted as strongly, as expected with the tested rabbit anti-virion sera of serotypes of the HAdV-A to -F (except serotypes of HAdV-B and -E with weaker reactivity to Ad41Fi). The representative antigens Ad3pIXC[B], Ad2pIX[C], Ad8FiKn[D], and Ad41pIX[F] showed species-specific reaction patterns and no disturbing cross-reacting effects with rabbit antibodies to Ad12pIX[A], Ad15pIX[D], and Ad37pIX[D]. Therefore, although only anti-virion sera of one to four serotypes per species were tested, it can be presumed that the representative species-specific antigens are also suitable for the detection of antibodies against other nontested serotypes of HAdV-B, -C, -D, and -F in human sera. A significant cross-reactivity could only be found between antibodies against Ad4pIX[E] and pIX of HAdV-B and -F serotypes. However, serotyping determined with reference horse antisera by neutralization test (17), amino acid alignment for pIX sequences, and the phylogenetic tree based on hexon genes (not shown) are also an indication for antigenic and structural relationships between HAdV-4[E] and HAdV-B serotypes (in particular HAdV-16). After these results with rabbit sera it should be of interest to compare the prevalence of species-specific antibodies in human sera detected by immunoblot assay with the isolation rate of different serotypes of HAdV-B, -C, -D, and -F in earlier studies.
Antibody prevalence against pIX, fiber, and hexon in human sera.
Our epidemiological study with 667 human sera of the Greifswald area showed a species-specific IgG prevalence in an age-dependent manner. Often a seroprevalence was found against more than one representative species-specific antigen, indicating earlier infections with serotypes of different adenovirus species in the patients' history. These findings correlate with the prevalence of neutralizing antibodies against more than one adenovirus serotype in patients' sera of different age groups (12, 37). The age-specific attack rates based on the prevalence of neutralizing adenovirus antibodies showed that in the age group of 6 to 12 months more than 30% of the children were infected with at least one serotype, and in the age group of 3 to 6 years more than 50% of the children already had three or four different adenovirus infections (12).
For a World Health Organization (WHO) report of the years 1967 to 1976, 24,184 specimens of symptomatic patients worldwide were analyzed to find out by virus isolation the frequency of different adenovirus serotypes (31). In a European study from 1982 to 1996 in the Greater Manchester area, United Kingdom, adenoviruses of 3,098 patient specimens with symptoms severe enough to warrant laboratory diagnosis were isolated and typed (11). These studies should be suitable to compare the frequency of isolated adenovirus serotypes of HAdV-B, C-, -D, and -F with the species-specific immune responses in our study, if the differences between the virus isolation of different serotypes in symptomatic individuals and the detection of circulating antibodies in mainly asymptomatic patients are considered. The most frequently occurring adenoviruses in children and adults were the serotypes HAdV-1, -2, and -5 of HAdV-C. Schmitz et al. (31) found HAdV-C viruses in 59.2% and Cooper et al. (11) in 39.8% of all isolations. We detected 59.5% HAdV-C-positive sera in the childrens' age group of 5 to 14 years and 44.7% in all patients' sera. After childhood, the number of virus isolations of the HAdV-C in the greater Manchester area was decreasing, in agreement with our results. Adenoviruses of HAdV-F were first described after the study of Schmitz et al. (31). Cooper et al. (11) found HAdV-F in 42.1% of isolates. In our study with asymptomatic patients we found with 36.6% positive sera slightly lower results. HAdV-B viruses were detected in 33.7% (31) and 23.3% (11) of all isolations. We found antibodies to Ad3pIXC[B] in 18% of human sera. This lower number of positive sera in comparison to the Manchester study is understandable because we analyzed patient sera at random and not only those with suspected viral infection. Patients with HAdV-B infections showed more frequent clinical symptoms in comparison to that seen with serotypes of other adenovirus species (20). The C-terminal part of pIX of the serotypes of HAdV-B does not induce antibodies as often as the full-length pIX. We found few sera that reacted with the complete pIX but did not react with the C-terminal pIX part (Fig. 3A).
Serotypes of HAdV-D were typed in 4.1% (11) and 6.4% (31) of the isolates, respectively, but our study showed 26.4% Ad8FiKn[D]-positive sera. There are several possible explanations for this discrepancy. Apart from a different epidemiological situation, the higher seroprevalence against HAdV-D viruses in our study might be caused by the fact that HAdV-D infections are often asymptomatic and thus not included in the WHO and greater Manchester studies but leading to an antibody formation detectable by immunoblot assay. The species HAdV-D is the one with the most serotypes. Some HAdV-D viruses are difficult to cultivate, which might play a role in the lower HAdV-D level in the virus isolation studies. Unlike our immunoblot study, which detected former as well as fresh, symptomatic as well as asymptomatic infections, the isolation studies included only fresh symptomatic infections. Recent experiments with Ad15pIX[D] and Ad37pIX[D] antigens showed that not all human sera that reacted with Ad8FiKn[D] also reacted with these pIX antigens of HAdV-D. Further, an exhibited immune response similar to that against Ad41Fi, which seems to also be dependent on the frequency of adenovirus infections in an individual, can also be presumed for the Ad8FiKn[D] antigen. Therefore, for diagnostic purposes pIX seems to be better suited than the fiber knob antigen of HAdV-8[D] as a representative for HAdV-D.
Adenoviruses of HAdV-A represented only 0.5 and 1.4% of the typed virus isolates, respectively (11, 31). HAdV-E contains only the serotype HAdV-4, which was isolated in 8.9% of typed isolates reported in the Manchester study (11) and in 2.4% of the WHO study (31). At the time of our epidemiological study no pIX sequences for pIX antigens of HAdV-A, -D, and -E serotypes were available for the immunoblot assay. However, a part of human sera which were positive for genus-specific antigens and negative for the species-specific antigens might probably represent infections with serotypes of HAdV-A and -E.
The inability to clear an infection is primarily a failure of T-cell response, whereas reinfections are prevented by the host's antibody response (19, 23). Neutralizing antibody responses to adenoviruses are directed against components of the virion surface, primarily against fiber, penton base, and hexon (35). Neutralizing antibodies against fiber are qualified to block binding of the fiber knob to its cellular attachment receptor and to aggregate virions by cross-linking fibers on separate virus particles (14). Neutralizing antibodies against penton bases are mainly directed against the integrin-binding RGD motif and prevent virus internalization (18). Viral attachment, cell entry, and intracellular transport to the nuclear pore still occur in presence of neutralizing levels of monoclonal hexon antibodies. Thereafter, for neutralization of adenovirus by anti-hexon the hexon capsid is cross-linked by antibodies. This prevents virus uncoating and nuclear entry of viral DNA (35). With pIX hyperimmune sera we only found insignificant neutralizing titers of <10 (not shown). However, it is conceivable that the anti-pIX level could intensify the anti-hexon effect during virus neutralization. Apart from the virus neutralization the species-specific detection of pIX antibodies seems to be an important indication for the individual patients' history of adenovirus infections. The described immunoblot assay might be a valuable contribution to a refined adenovirus diagnosis and for getting a profound knowledge of adenovirus pathogenesis. The immunoblot assay will be completed with suitable representative pIX antigens of HAdV-A, -D, and -E. A further proofing on clinical specimens seems to be necessary and worthwhile.
Acknowledgments
This research was supported by a grant from Mikrogen GmbH, Munich-Martinsried, Germany, and by the German Federal Ministry for Education and Research (NBL3 program, reference 01220103).
We thank Leopold Doehner (Micromun GmbH, Greifswald, Germany) for the HAdV-5 hexon; Egbert Mundt (Friedrich-Loeffler-Instituts, Insel Riems/Greifswald, Germany) for rabbit immunization against pIX of HAdV-4, -15, and -37; and Patricia Pring-Åkerblom (Medical School, Hannover, Germany) for the Ad41Fi and Ad8FiKn plasmids. We are also thankful to Millie Heitmann, Anke Meinzer, and Katrin Schooff for technical assistance.
REFERENCES
- 1.Akalu, A. 1997. Antigenic characterization and posttranslational analysis of the protein IX of human adenovirus serotypes 2 and 3. Ph.D. thesis. University of Greifswald, Greifswald, Germany. (In German.)
- 2.Akalu, A., W. Seidel, H. Liebermann, U. Bauer, and L. Doehner. 1998. Rapid identification of subgenera of human adenovirus by serological and PCR assays. J. Virol. Methods 71:187-196. [DOI] [PubMed] [Google Scholar]
- 3.Akalu, A., H. Liebermann, U. Bauer, H. Granzow, and W. Seidel. 1999. The subgenus-specific C-terminal region of protein IX is located on the surface of the adenovirus capsid. J. Virol. 73:6182-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard, A., B. Albinson, and G. Wadell. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey, A., and V. Mautner. 1994. Phylogenetic relationships among adenovirus serotypes. Virology 205:438-452. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, U. 2000. Subgenus-specific detection of adenovirus antibodies in human sera by immunoblot. Ph.D. thesis. University of Greifswald, Greifswald, Germany. (In German.)
- 7.Benkö, M., B. Harrach, and W. C. Russell. 2000. Family Adenoviridae, p. 227-238. In M. H. V. van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy: classification and nomenclature of viruses. Academic Press, Inc., San Diego, Calif.
- 8.Boulanger, P., P. Lemay, G. E. Blair, and W. C. Russell. 1979. Characterization of adenovirus protein IX. J. Gen. Virol. 44:783-800. [DOI] [PubMed] [Google Scholar]
- 9.Bruss, K. 2003. Identification of adenoviruses by using the pIX region. Ph.D. thesis. University of Greifswald, Greifswald, Germany. (In German.)
- 10.Carrigan, D. R. 1997. Adenovirus infections in immunocompromised patients. Am. J. Med. 102:71-74. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, R. J., R. Hallett, A. B. Tullo, and P. E. Klapper. 2000. The epidemiology of adenovirus infections in Greater Manchester, UK 1982-96. Epidemiol. Infect. 125:333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dmitriev, I. P., E. A. Kashentseva, and D. T. Curiel. 2002. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J. Virol. 76:6893-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fender, P., A. H. Kidd, R. Brebant, M. Oberg, E. Drouet, and J. Chroboczek. 1995. Antigenic sites on the receptor-binding domain of human adenovirus type 2 fiber. Virology 214:110-117. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh-Choudbury, G., Y. Haj-Ahmad, and F. L. Graham. 1987. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full-length genomes. EMBO J. 6:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havenga, M. J. E., A. A. C. Lemckert, O. J. A. E. Ophorst, M. van Meijer, W. T. V. Germeraad, J. Grimbergen, M. A. van den Doel, R. Vogels, J. van Deutekom, A. A. M. Janson, J. D. de Bruijn, F. Uytdehaag, P. H. A. Quax, T. Logtenberg, M. Mehtali, and A. Bout. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hierholzer, J. C., Y. O. Stone, and J. R. Broderson. 1991. Antigenic relationships among the 47 human adenoviruses determined in reference horse antisera. Arch. Virol. 121:179-197. [DOI] [PubMed] [Google Scholar]
- 18.Hong, S. S., N. A. Habib, L. Franqueville, S. Jensen, and P. A. Boulanger. 2003. Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors. J. Virol. 77:10366-10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz, M. S. 2001. Adenoviruses, p. 2301-2326. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 20.Larranaga, C., A. Kajon, E. Villagra, and L. F. Avendano. 2000. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988-1996). J. Med. Virol. 60:342-346. [PubMed] [Google Scholar]
- 21.Liebermann, H., R. Mentel, U. Bauer, P. Pring-Åkerblom, R. Dölling, S. Modrow, and W. Seidel. 1998. Receptor-binding sites and antigenic epitopes on the fiber knob of human adenovirus serotype 3. J. Virol. 72:9121-9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebermann, H., K. Lotz, and W. Seidel. 2002. Mapping of epitopes on the fiber knobs of human adenovirus serotypes 8 and 15. Intervirology 45:59-66. [DOI] [PubMed] [Google Scholar]
- 23.Lord, A., A. S. Bailey, P. E. Klapper, N. Snowden, and S. H. Khoo. 2000. Impaired humoral responses to subgenus D adenovirus infections in HIV-positive patients. J. Med. Virol. 62:405-409. [PubMed] [Google Scholar]
- 24.Lukashok, S. A., and M. S. Horwitz. 1997. Adenovirus persistence, p. 147-164. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons, Chichester, United Kingdom.
- 25.Molinier-Frenkel, V., R. Lengagne, F. Gaden, S. Hong, J. Choppin, H. Gahery-Ségard, P. Boulanger, and J. Guillet. 2002. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 76:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 27.Pring-Åkerblom, P., and T. Adrian. 1995. Characterization of adenovirus subgenus D fiber genes. Virology 206:564-571. [DOI] [PubMed] [Google Scholar]
- 28.Rosa-Calatrava, M., L. Grave, F. Puvion-Dutilleul, B. Chatton, and C. Kedinger. 2001. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol. 75:7131-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Runde, V., S. Ross, R. Trenschel, E. Lagemann, O. Basu, K. Renzing-Köhler, U. W. Schaefer, M. Roggendorf, and E. Holler. 2001. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant. 28:51-57. [DOI] [PubMed] [Google Scholar]
- 30.Ruuskanen, O., O. Meurman, and G. Akusjärvi. 1997. Adenoviruses, p. 525-548. In D. Douglas and M. D. Richman (ed.), Clinical virology. Churchill Livingstone, New York, N.Y.
- 31.Schmitz, H., R. Wigand, and W. Heinrich. 1983. Worldwide epidemiology of human adenovirus infections. Am. J. Epidemiol. 117:455-466. [DOI] [PubMed] [Google Scholar]
- 32.Shenk, T. 2001. Adenoviridae: the viruses and their replication, p. 2265-2300. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 33.Steward, P. L., S. D. Fuller, and R. M. Burnett. 1993. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 12:2589-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmongin, and D. G. Higgins. 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varghese, R., Y. Mikyas, P. L. Stewart, and R. Ralston. 2004. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J. Virol. 78:12320-12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vellinga, J., M. J. W. E. Rabelink, S. J. Cramer, D. J. M. van den Wollenberg, H. van der Meulen, K. N. Leppard, F. J. Fallaux, and R. C. Hoeben. 2004. Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. J. Virol. 78:3470-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. de Béthune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg, A., M. C. D. S. Fink, S. Takimoto, M. A. Ishida, and M. C. O. Souza. 1989. Enzyme linked immunosorbent assay: determination of anti-adenovirus antibodies in an infant population. Rev. Inst. Med. Trop. Sao Paulo 31:336-340. [DOI] [PubMed] [Google Scholar]