Abstract
An outbreak of Staphylococcus aureus intramammary infections on an organic dairy farm was monitored for 10 months. Environmental and milk samples were collected from a total of 26 cows and a group of 21 purchased heifers about to be introduced into the milking herd. There was variation in the rate of isolation of S. aureus (9.5 to 43.8%) from individual mammary quarters, although no S. aureus isolates were detected in the milk samples collected from the heifers. One hundred ninety-one S. aureus isolates were detected from cow milk samples (n = 182), milking machine clusters (n = 4), farm personnel (n = 4), and the environment (n = 1). Multilocus sequence typing (MLST) had a typeability of 100% when it was applied to the 191 isolates. Among the 191 isolates there was limited strain diversity, with seven sequence types (STs) dominated by two strains with closely related STs that differed at a single locus. Within individual mammary quarters there were naturally occurring dual infections, although this was identified in only 0.4% of milk samples. Different strains were associated with variable persistence within quarters. MLST is clearly a very suitable tool for the differentiation and analysis of S. aureus populations detected on dairy cattle farms.
Mastitis is the most frequently occurring health problem in organic dairy cattle herds, with Staphylococcus aureus and Streptococcus uberis being the most frequently isolated pathogens (41, 43). Control of contagious mastitis is more difficult on organic dairy farms than on conventional dairy farms because the routine use of antibiotic dry cow therapy (DCT) at the end of lactation is not permitted (12) and DCT is currently the most effective method of reducing contagious pathogen infections (32).
The lactating mammary gland is the primary reservoir of S. aureus (5, 24), although environmental S. aureus isolates have been detected on heifer body sites and in the dairy farm environment (20, 29). While nonmilk isolates may not form a large reservoir of infection (29), their presence may improve the ability of S. aureus to circumvent control mechanisms and persist within a herd, and their importance can be investigated through strain typing.
Molecular epidemiological investigations of S. aureus isolated from intramammary infections (IMIs) on individual dairy farms have identified clonal populations characterized by dominant strains and low strain diversity (16, 49). Strain dominance is likely to be associated with virulence and persistence within the mammary gland (31). Although some studies have reported the genetic diversity of S. aureus on individual farms (15, 34), these contrasting results may be due to variations in sampling and typing protocols or to differences in herd sizes and management techniques.
A range of different typing techniques have been employed to understand the epidemiology of S. aureus infections. Random amplified polymorphic DNA analysis, ribotyping, biotyping, pulsed-field gel electrophoresis, and binary typing are the major techniques previously used to identify persistent chronic infection of mammary quarters with the same strain (23, 27) and simultaneous infections within udders of more than one strain (27, 46). However, the presence of different strains within a quarter has been demonstrated only experimentally (45).
Multilocus sequence typing (MLST) is a technique suitable for global (40) and local (26) epidemiology of S. aureus and other bacterial pathogens. It offers advantages of unambiguous identification and high levels of discrimination based on the nucleotide sequences of 450- to 500-bp internal fragments of seven housekeeping genes (19, 35). In addition, the data obtained by MLST, unlike the data obtained by previous genomic analysis techniques, permit the investigation of the population structure and the development and testing of evolutionary hypotheses (8, 40).
This paper describes the isolation of S. aureus from the milk, humans, and environment of an organic dairy farm and the use of MLST to characterize these isolates. The relationships between the strains detected, the observations of strain variation, and the persistence within mammary quarters are reported.
MATERIALS AND METHODS
Herd selection.
An organic dairy herd which consisted of 100 (increasing to 150) Channel Island breed dairy cattle and which had completed conversion to full organic status in the summer of 1999 was selected for study based on the likelihood of a high level of S. aureus and the farmer's interest and compliance. The herd was milked twice a day through a 5/10 herringbone parlor, with each animal giving an annual milk yield of 5,000 to 6,000 kg/head. Calving was carried out all year round; the herd was housed in cubicles over the winter and fed a silage-based ration, supplemented with concentrate provided at in-parlor feeders. Over summer the herd was at pasture. Homeopathy and other nonantibiotic therapy were the treatments for mastitis.
Cow selection.
Fifteen cows were initially selected, 10 with somatic cell counts (SCCs) in the range 350,000 to 1,150,000 cells ml−1 and 5 with SCCs of <250,000 cells ml−1 (range, 120,000 to 240,000 cells ml−1); cows were also recruited into the study when their SCCs rose above 400,000 cells ml−1 for 2 consecutive months. A group of 21 purchased in-calf heifers, due to join the milking herd, was also studied. SCCs were recorded monthly from the farmer's own monitoring program.
Sampling strategy.
The farm was visited once per month from December 2001 to September 2002. Individual quarter milk samples were collected from the lactating cows and heifers enrolled in the study. Samples from the environment and personnel (outlined below) were collected, and heifer body sites were sampled before and for up to 3 months after parturition.
Sampling procedures.
Disposable latex examination gloves sterilized with 70% ethanol were worn for the collection of all samples.
(i) Milk samples.
At the initial visit, the teats were scrubbed with 70% ethanol and allowed to dry; the foremilk was discarded, up to 15 ml milk was collected, and the teats were dipped in the farm's postmilking teat disinfectant. At subsequent visits, the teats were initially dipped in a premilking teat dip [Total Control Dip, EmpraSan (Chemicals) Ltd., Birkenhead, United Kingdom], allowed a contact time of at least 30 s, and dried with individual paper towels. The teats were then scrubbed with a cotton wool ball moistened with 70% ethanol and allowed to dry before the sample was collected.
(ii) Body site samples.
Dirt was removed from heavily soiled teats before they were sampled with a transport swab (Appleton Woods Ltd., Selly Oak, United Kingdom) moistened in 0.5 ml sterile tryptone soy broth. Muzzle and vaginal samples were collected with unmoistened transport swabs (29).
(iii) Bedding and feedstuff samples.
Bedding and feedstuff samples representative of damp, dry, deep, and shallow areas were collected with a gloved hand and placed into large (300 mm by 539 mm) freezer bags (Safeway Stores plc, Hayes, United Kingdom) (29).
(iv) Environmental site samples.
Environmental site samples were collected with moistened transport swabs from cattle houses, animal handling and feeding equipment, parlor gates, and milkers' hands. Unmoistened swabs were used to collect samples from the parlor floor, milking machine clusters, nonbovine animals, and the nares of parlor workers. Air samples were collected from the milking parlor (when it was in use and between milkings), all cattle housing areas, and the calving area by exposing the surface of a modified Baird Parker (MBP) plate for 1 h (29). Water samples were collected from all drinking and wash water sources and placed into sterile 20-ml containers (Greiner Bio-One Ltd.).
All samples were stored on ice and taken to the laboratory within 24 h, where milk, feed, and bedding samples were frozen and where all swabs and air samples were processed immediately.
Bacteriological procedures. (i) Milk samples.
Milk samples were processed according to recommended protocols (11). Briefly, 100 μl of milk was inoculated onto sheep's blood agar (SBA; Oxoid, Basingstoke, United Kingdom) and incubated at 37°C for 24 to 48 h. Coagulase-positive staphylococci (CPS) were identified based on colony morphology, including hemolytic variation (α, β, and γ); where variation was detected, an example of each hemolytic variant was analyzed further. In addition, tests for reaction to KOH (1) and catalase and tube coagulase tests (Becton Dickinson, Oxford, United Kingdom) (11) were performed.
(ii) Bedding and feedstuff samples.
Bacteriological analyses were performed as described by Roberson et al. (29), with modifications. Briefly, the samples were thawed and chopped into approximately 50-mm lengths. After mixing of the samples, 10 g of each sample was then added to 100 ml sterile tryptone soy broth (Oxoid) and agitated at 120 rpm for 30 min (Gallenkamp orbital incubator; SANYO Gallenkamp Plc, Loughborough, United Kingdom) at room temperature. Serial 10-fold dilutions (final dilution, 1:1,000) of the resulting broth were made. One hundred microliters (0.1 ml) of the 1:10, 1:100, and 1:1,000 dilutions were inoculated onto MBP agar (Oxoid) and incubated at 37°C for 24 to 48 h. Colonies suspected of being CPS were streaked across one quadrant of an SBA plate for identification, as described above for the milk samples.
(iii) Environmental site samples.
Milking machine cluster samples were streaked across half of a SBA plate, incubated at 37°C, and processed as described above for the milk samples. Other samples collected on swabs were streaked across one quadrant of an MBP plate, and 100 μl of each water sample was inoculated onto MBP agar. All plates, including those with air samples, were then processed as described above for the bedding and feedstuff samples.
All CPS were stored frozen until species identification and MLST was performed.
Species identification.
The primers developed by Štepán et al. (36), based upon a species-specific 826-bp SmaI restriction fragment of genomic DNA, were used to confirm that the CPS isolates were S. aureus prior to MLST.
Multilocus sequence typing.
MLST was performed with the primers developed by Enright et al. (6), and the products were purified with a MinElute 96 UF PCR purification kit (QIAGEN), as recommended by the manufacturer. Each product was sequenced by using a BigDye Terminator (version 3.1) ready reaction cycle sequencing kit (Applied Biosystems, Warrington, United Kingdom) on an Prism Genetic Analyser 3100 DNA sequencer (Applied Biosystems), as recommended by the manufacturer. For the gmk locus, 1 μl dimethyl sulfoxide (Fisher Scientific United Kingdom, Loughborough, United Kingdom) was included in the reaction mixture.
Sequence data were compared to the data in the S. aureus MLST database (http://saureus.mlst.net) for allele number and sequence type (ST) assignment. Forward and reverse trace files of putative novel alleles and the allelic profiles of novel STs were sent to the database curator for allele or ST number assignment and entry into the database.
Data analysis.
Statistical analyses were carried out by using chi-square tables (EpiInfo, version 6.04d; Centers for Disease Control and Prevention, Atlanta, GA). The results of MLST were evaluated by determining typeability, discriminatory power, and reproducibility (37, 38). Isolate diversity was calculated by using the equation for Simpson's index of diversity (D) (13) with 95% confidence intervals (CIs) (9). Allele sequences were concatenated in the order arcC, aroE, glpF, gmk, pta, tpi, and yqiL to produce a 3,198-bp sequence for each ST; phylogenetic and molecular evolutionary analyses were conducted by using MEGA (Molecular Evolutionary Genetics Analysis), version 2.1 (17). Crude estimates of infection persistence were calculated by using SPSS (SPSS 10.0; SPSS Inc., Chicago, IL), having assumed that any infection present at visit 1 began at that visit.
Nucleotide sequence accession numbers.
The DNA sequences of the novel alleles detected in this study have been deposited in the EMBL database under accession numbers AJ849355 and AJ849356.
RESULTS
The average sampling interval was 29 days (range, 28 to 36 days), and 1,643 samples were collected for bacteriological analysis, ranging from 61 to 267 samples per visit. A total of 921 (56.1%) quarter milk samples were collected for bacteriology; the remaining 722 (43.9%) samples were from the environment and heifer body sites. Twenty-six cows were sampled, the mean lactation number was 4 (range, 1 to 8) at the start of the study; heifers were sampled an average of five times (range, two to six times).
Of the 921 quarter milk samples, 499 were from cows and 422 were from heifers; 11 (1.2%) samples (all from cows) were contaminated (more than three colony types were detected) (11). Of the 488 uncontaminated quarter milk samples, 142 (29.1%) yielded a detectable level of CPS; no CPS growth was detected in the heifer milk samples. The detectable level of CPS in each mammary quarter (left fore [LF], left hind [LH], right fore [RF], and right hind [RH] quarters) ranged from 9.5% to 43.8%. Significantly fewer CPS (P < 0.01) were detected in the LF quarter than in the other quarters, and significantly more (P < 0.01) were detected in hind quarters than in the fore quarters.
A total of 12 CPS were detected from sites other than milk, 5 (10.0%) were detected from milking machine clusters, 4 (8.7%) were detected from farm personnel, 2 (10.0%) were detected from nonbovine animals, and 1 (0.7%) was detected from a cubicle partition.
The 142 CPS-positive milk samples yielded 183 hemolytic isolates, of which 182 (99.5%) were S. aureus and 1 (0.5%) was a CPS other than S. aureus. Of the 12 nonmilk-associated CPS, 9 (75.0%) were S. aureus, giving a total of 191 S. aureus isolates for MLST.
MLST.
The rate of typeability of the S. aureus isolates was 100%, and five samples analyzed in duplicate produced the same result both times, giving a reproducibility of 100%. Two new alleles at the tpi locus (tpi-59 and tpi-60), and five new STs (ST116, ST117, ST118, ST119, and ST127) were identified and added to the S. aureus MLST database (http://saureus.mlst.net).
Isolate diversity (D) was 0.590 (95% CI = 0.547 to 0.633), indicating that if two isolates were selected at random from the population of 191, on 59% of occasions they would be of different STs.
Sequence types detected.
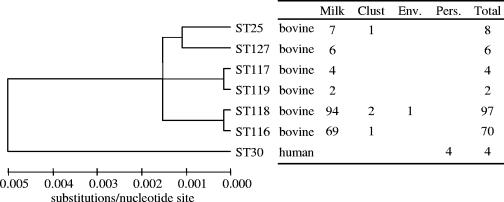
A total of six closely related STs (ST25, ST116, ST117, ST118, ST119, and ST127) were detected in milk (Fig. 1). ST116 and ST118 are single-locus variants of each other, as are ST117 and ST119. These two pairs of STs varied at only one nucleotide site, which was the result of a synonymous point mutation. Furthermore, STs ST116 and ST117 and STs ST118 and ST119 are double-locus variants. ST25 and ST127 are double-locus variants of each other and differ at three loci compared to the sequences of the other four STs detected in milk. At the nucleotide level, the STs detected in milk were very similar, with a maximum variation of 0.1% for each residue, with the exception of cytidine, which had a constant frequency of 15.0%.
FIG. 1.
Dendrogram displaying the number and distribution of sequence types detected on the farm. Clust, milking machine clusters; Env., environment; Pers., personnel.
The STs detected in individual mammary quarters over time are presented in Table 1. One hundred sixty-three (89.5%) of the isolates were ST116 (37.9%) or ST118 (51.6%); ST25, ST127, ST117, and ST119 formed 3.9, 3.3, 2.2, and 1.1% of the milk isolates, respectively. Two cows (cows 13 and 37) were persistently infected in different quarters with ST116 and ST118. These two sequence types were also detected in the same quarter of cow 6 at visit six, although neither ST was detected in this cow at subsequent samplings. In contrast, ST119, which was detected twice, was detected in the same quarter as ST118 on both occasions, although ST118 was the dominant strain in terms of the number of CFU and was present at at least a 10-fold higher concentration (data not shown). ST117 was detected once in the same quarter as ST116 and was detected twice in quarters which yielded ST116 at another sample point. ST25 and ST127 were detected in separate quarters (RH and RF) of the same cow (34), but they were never detected in the same quarter at the same time. While these data may have been biased by the analysis of only one or possibly two colonies from each sample, it is unlikely that the same ST would be repeatedly isolated by chance from the same quarter of a cow over time; and a more in-depth analysis of milk samples was outside the scope of this investigation.
TABLE 1.
Sequence types detected in cows and quarters over time
| Cow no. | Quarter | ST recovered at visit no.a:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | LF | 116 | 117 | Cull | |||||||
| 1 | LH | 118 | 118 | Cull | |||||||
| 1 | RH | 118 | 118 | Cull | |||||||
| 2 | LH | 117 | 116 | Cull | |||||||
| 2 | RH | 118 | * | Cull | |||||||
| 4 | LF | * | 116 | Cull | |||||||
| 4 | LH | 116 | 116 | Cull | |||||||
| 4 | RF | 116 | 116 | Cull | |||||||
| 4 | RH | 116 | 116 | Cull | |||||||
| 5 | LH | 116 | 116 | 116 | 116 | 116 | 116 | 116 | 116 | 116 | Dry |
| 5 | RF | 116 | 116 | 116 | 116 | 116 | 116 | 116/117 | 116 | 116 | Dry |
| 5 | RH | * | * | * | * | * | 116 | * | 116 | 116 | Dry |
| 6 | RH | * | * | Dry | Dry | Dry | 116/118 | * | * | * | * |
| 8 | RH | — | — | — | — | — | — | 118 | — | — | — |
| 11 | RF | * | Dry | Dry | * | * | * | * | * | * | 118 |
| 13 | LH | — | — | — | — | 118 | 118 | 118 | 118/119 | 118 | 118 |
| 13 | RF | — | — | — | — | 116 | 116 | 116 | 116 | 116 | 116 |
| 13 | RH | — | — | — | — | 118 | 118 | * | * | * | * |
| 16 | LF | — | — | — | — | — | — | 118 | 118 | 118 | 118 |
| 16 | LH | — | — | — | — | — | — | 118 | 118 | 118 | * |
| 16 | RH | — | — | — | — | — | — | 118 | 118 | 118 | 118 |
| 20 | LF | — | — | — | — | — | — | — | — | 118 | 118 |
| 20 | LH | — | — | — | — | — | — | — | — | 118 | 118 |
| 20 | RF | — | — | — | — | — | — | — | — | 118 | 118 |
| 20 | RH | — | — | — | — | — | — | — | — | 118 | 118 |
| 22 | LF | * | 118 | * | * | * | * | * | * | * | * |
| 22 | LH | * | 118 | * | * | 118 | 118 | 118 | 118 | 118 | 118 |
| 22 | RF | 118/119 | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 |
| 22 | RH | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 |
| 32 | LH | 118 | Dry | Dry | Dry | Dry | Dry | Dry | Dry | Dry | Cull |
| 32 | RH | * | Dry | Dry | Dry | * | 118 | * | * | * | Cull |
| 34 | RF | 127 | * | Dry | Dry | Dry | 127 | 127 | 25 | 25 | 25 |
| 34 | RH | * | 25 | Dry | Dry | Dry | * | * | * | * | * |
| 37 | LH | 116 | Dry | Dry | Dry | 116 | 116 | 116 | 116 | 116 | 116 |
| 37 | RH | * | Dry | Dry | Dry | * | * | 118 | 118 | 118 | 118 |
| 44 | LH | 116 | Dry | Dry | 116 | 116 | 116 | 116 | 116 | 116 | 116 |
| 44 | RH | 118 | Dry | Dry | 118 | 118 | * | * | * | * | * |
| 46 | LH | 116 | Dry | Dry | * | * | * | * | * | * | * |
| 64 | LF | — | — | — | — | — | — | 118 | — | — | — |
| 65 | LH | 116 | Dry | Dry | Dry | * | * | 116 | 118 | * | * |
| 71 | RF | — | — | — | — | — | — | 118 | 118 | 118 | 118 |
Visits were made monthly. Abbreviations and symbols: Cull, cow culled; Dry, dry period; *, no S. aureus detected; —, no sample collected; /, dual infection with both sequence types.
ST25 and ST116 were isolated once from milking machine clusters, and ST118 was isolated twice from milking machines and once from the farm environment (Fig. 1).
Infection persistence.
Crude estimates of the persistence of ST116 and ST118 within mammary quarters were >183 days (95% CI = 128 to 238 days) and >158 days (95% CI = 107 to 210 days), respectively. The true duration of infection is likely to have been greater than this, as infections present at the initial and final sampling points were included in the analysis.
Sequence types detected in personnel.
The four CPS isolated from milking personnel were all ST30. These were isolated from the nares of the same farm worker over time, and this strain was distinct from the other strains detected (Fig. 1).
DISCUSSION
The study described here is the first occasion that MLST has been used to analyze a large number of S. aureus isolates isolated from bovine mammary secretions and the first investigation into the number and distribution of S. aureus strains on an organic dairy farm.
MLST had high degrees of typeability and reproducibility when it was applied to these bovine isolates of S. aureus. This is the same level of typeability as pulsed-field gel electrophoresis, and this level of typeability compares favorably to those of phage and binary typing (47). Previous work has also demonstrated that large numbers of human strains are typeable by MLST (4, 6). The high discriminatory power of MLST (33, 35) revealed a low level of diversity of the S. aureus isolates on the farm, probably due to the clonal population structure and contagious transmission of the pathogen (8, 49).
It was surprising that CPS were not isolated from either the milk or the body sites of the heifers studied, especially since early lactation has a statistically significantly increased risk of S. aureus infection, particularly for cows in their first or second lactation (48). Previous reports have also indicated that approximately 8% of heifers are infected with CPS at parturition (21, 28) and that body sites can represent a source of IMI (30). While no CPS were detected and the majority (58.5%) of heifer milk samples were bacteriologically negative, there remained a substantial number (34.6%) of quarter milk samples with a detectable level of coagulase-negative staphylococci. The presence of coagulase-negative staphylococci in some samples may have helped to prevent infection by CPS (25). However, the number of bacteriologically negative samples demonstrates that maintenance of herd and milking hygiene can prevent the spread of contagious mastitis pathogens to some of the most susceptible animals, even when a substantial number of cows are infected.
The identification of novel alleles may be evidence of selection pressures that create specific localized variants of S. aureus (15, 39). It is not unusual for animal isolates to possess novel alleles compared to the sequences of those strains of the same species isolated from humans (4). However, this may also be merely due to the investigation of a new population, as most analyses have focused on human clinical isolates; greater sampling and additional analyses of other S. aureus populations would likely yield novel alleles. Furthermore, the limited crossover of bovine and human infecting strains may also explain the presence of novel alleles, with the restricted populations independently diversifying. The presence, at the time of writing, of no strains from other locations in the S. aureus MLST database (http://saureus.mlst.net) with alleles tpi-59 and tpi-60 supports the theory of localized diversification. The detection of previously undescribed alleles may help to explain the seemingly improved ability of some STs of S. aureus compared with those of other more human-associated strains to persist and cause disease following invasion of the mammary gland. Indeed, a bovine mastitis-associated S. aureus clone (RF122) has recently been demonstrated to have elevated rates of nonsynonymous substitutions compared with the sequences of human-associated clones Mu50 and N315, which may relate to host specificity (10).
The majority of the population detected were ST116 and ST118. While their difference in persistence was numerically, if not significantly, different, the possibility that ST118 had a shorter persistence is biologically plausible. As more cows were recruited (based upon high SCCs), higher levels of ST118 were detected, suggesting that this strain was associated with higher SCCs, although SCCs for individual quarters were not determined to confirm this. The higher SCCs putatively induced by infection with ST118 may have lead to the conclusion that these cows were “more infected” than cows with ST116. These cows may then have been identified as having an increased requirement for therapy and possibly suitable for culling, which would reduce the estimate of persistence. In practice, however, these effects would be blurred if a cow was infected with both strains. The potential of a variable SCC response to different strains is supported by the detection of ST25 and ST127 in a cow whose SCCs never rose above 200,000 cells ml−1, although this requires further study, as these strains were isolated from only a single cow. However, it is possible that ST116 and ST118 were equally virulent with respect to causing an increase in SCCs and that ST118 had a greater propensity than ST116 to spread throughout the herd. This scenario would also have caused the increased level of detection of ST118 compared to that of ST116. Further analysis of entire herd milk samples coupled with associated quarter SCCs would provide greater insight into the characteristics of individual strains.
Both transient and persistent S. aureus infections were detected, and the potential for strains to persist throughout the dry period was demonstrated, although the possibility of reinfection with the same strain is another interpretation of the data. As monthly sampling was used, it is possible that the infections detected were cleared and the quarter was reinfected between samplings, although reinfection with the same strain on multiple occasions is unlikely and infection persistence is the most probable explanation. It is also possible that the heifers suffered minor transient S. aureus infections which were missed because of the monthly sampling protocol. Persistent infections with both the same and various strains over a number of months have been demonstrated previously (27), although not at the quarter level. The apparent variation in infecting strains detected over time (27) may have been due to the serial selection of different strains from a dual infection rather than a change in the infecting strain. In addition, S. aureus was not detected in some cattle throughout the sampling period (data not shown). The variation in infection status over time detected compares well with the patterns of S. aureus carriage distinguished in humans: persistent, intermittent, and noncarriage. Persistent carriage is characterized by the uniform and consistent isolation of S. aureus, whereas with intermittent carriage, isolation is less frequent. Persistent carriage also tends to result in the detection of fewer genotypes than the numbers present in intermittent carriers (42); noncarriage classifies humans from whom S. aureus is never isolated.
The detection of dual infections suggests that some STs are clearly able to coexist for some period of time, although persistent dual infections for periods of greater than 1 month in the same quarter were not detected. However, this may have been due to a lack of sensitivity in the sampling protocol and would require more frequent analysis of multiple colonies to provide a better understanding of S. aureus IMI epidemiology within quarters (45). The isolation of more than one strain of S. aureus in individual mammary quarters has been demonstrated experimentally (45) but not previously in a natural infection until it was demonstrated here. Multiple strains isolated from a single quarter, although not a single sample, have, however, been reported previously for cases of S. uberis mastitis (14). It is likely that bacterial population dynamics within quarters promote the dominance of one strain, although further work is required to determine whether this simply relates to bacterial fitness or different interactions of strains with the host immune system. A better understanding of these factors and adaptation to the host environment would help to explain previous difficulties in establishing nonindigenous S. aureus in a naturally infected quarter (45). The observation of different S. aureus strains dominating within mammary quarters was not reflected within animals, with the two dominant strains able to infect different quarters of the same cow. This agrees with the findings presented in a previous report for S. aureus (46) and has also been demonstrated for cases of Escherichia coli (3) and S. uberis (14, 44) mastitis.
The low level of strains detected in the environment suggests that it is not a large reservoir, but it may be important with respect to elimination of disease. The detection of the two most prevalent intramammary strains in milking machine clusters demonstrates the importance of these as a vector of infection. The full extent of this could be assessed in future studies by more regular sampling of the cluster, if not individual liners, throughout milking, particularly after cattle which are known to be infected. ST118 was also detected on a cubicle partition, indicating that this strain was ubiquitous on the farm studied. The environmental survivability of ST118 is likely to have added to the ability of this strain to spread and persist within the herd.
The repeated detection of S. aureus from the nares of a farm worker was probably evidence of persistent infection. The strain detected (ST30) has been identified as the major methicillin-susceptible S. aureus clone associated with invasive disease in the United Kingdom (6). The fact that this strain differed from those detected in cattle and the fact that it was typical of those present in the human reservoir in the country of detection agree with the findings presented in previous reports (18, 31).
Variation in the rates of bacterial isolation from individual quarters is not unusual (2, 41) and may be explained by the lying behavior of cattle (7). It has also been suggested that increased levels of infection in rear quarters may be due to higher levels of milk production or because the teats are closer to the ground, putatively exposing them to an increased risk of injury (22). More detailed investigations of IMI in conjunction with behavioral observations may provide more data on this aspect of study.
Acknowledgments
E.M.S. was supported by the Biotechnology and Biological Sciences Research Council.
We express our gratitude to the management and staff of the farm involved for their cooperation.
REFERENCES
- 1.Arthi, K., B. Appalaraju, and S. Parvathi. 2003. Vancomycin sensitivity and KOH string test as an alternative to Gram staining of bacteria. Indian J. Med. Microbiol. 21:121-123. [PubMed] [Google Scholar]
- 2.Barkema, H. W., Y. H. Schukken, T. J. G. M. Lam, D. T. Galligan, M. L. Beiboer, and A. Brand. 1997. Estimation of interdependence among quarters of the bovine udder with subclinical mastitis and implications for analysis. J. Dairy Sci. 80:1592-1599. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, A. J., and M. J. Green. 2001. Adaptation of Escherichia coli to the bovine mammary gland. J. Clin. Microbiol. 39:1845-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, P. E., K. Begbie, and F. M. Thomson-Carter. 2003. Coagulase gene variants associated with distinct populations of Staphylococcus aureus. Epidemiol. Infect. 130:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson, I. 1961. Observations on the pathogenic staphylococci in a dairy herd during a period of six years. Res. Vet. Sci. 2:22-40. [Google Scholar]
- 6.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewbank, R. 1966. A possible correlation, in one herd, between certain aspects of the lying behaviour of tied-up dairy cows and the distribution of subclinical mastitis among the quarters of their udders. Vet. Rec. 78:299-303. [DOI] [PubMed] [Google Scholar]
- 8.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herron, L. L., R. Chakravarty, C. Dwan, J. R. Fitzgerald, J. M. Musser, E. Retzel, and V. Kapur. 2002. Genome sequence survey identifies unique sequences and key virulence genes with unusual rates of amino acid substitution in bovine Staphylococcus aureus. Infect. Immun. 70:3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan, J. S., R. N. Gonzalez, R. J. Harmon, S. C. Nickerson, S. P. Oliver, J. W. Pankey, and K. L. Smith. 1999. Laboratory handbook on bovine mastitis, revised edition. National Mastitis Council Inc., Madison, Wis.
- 12.Hovi, M., and S. Roderick. 1998. Mastitis therapy in organic dairy herds, p. 29-35. British Mastitis Conference, National Agricultural Centre, Stoneleigh, United Kingdom.
- 13.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayarao, B. M., S. P. Oliver, J. R. Tagg, and K. R. Matthews. 1991. Genotypic and phenotypic analysis of Streptococcus uberis isolated from bovine mammary secretions. Epidemiol. Infect. 107:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo, Y. S., L. K. Fox, W. C. Davis, G. A. Bohach, and Y. H. Park. 2001. Staphylococcus aureus associated with mammary glands of cows: genotyping to distinguish different strains among herds. Vet. Microbiol. 80:131-138. [DOI] [PubMed] [Google Scholar]
- 16.Kapur, V., W. M. Sischo, R. S. Greer, T. S. Whittam, and J. M. Musser. 1995. Molecular population genetic analysis of Staphylococcus aureus recovered from cows. J. Clin. Microbiol. 33:376-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 18.Larsen, H. D., A. Huda, N. H. R. Eriksen, and N. E. Jensen. 2000. Differences between Danish bovine and human Staphylococcus aureus isolates in possession of superantigens. Vet. Microbiol. 76:153-162. [DOI] [PubMed] [Google Scholar]
- 19.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matos, J. S., D. G. White, R. J. Harmon, and B. E. Langlois. 1991. Isolation of Staphylococcus aureus from sites other than the lactating mammary gland. J. Dairy Sci. 74:1544-1549. [DOI] [PubMed] [Google Scholar]
- 21.Matthews, K. R., R. J. Harmon, and B. E. Langlois. 1992. Prevalence of Staphylococcus species during the periparturient period in primiparous and multiparous cows. J. Dairy Sci. 75:1835-1839. [DOI] [PubMed] [Google Scholar]
- 22.Miller, R. H., M. J. Paape, and L. A. Fulton. 1991. Variation in milk somatic cells of heifers at first calving. J. Dairy Sci. 74:3782-3790. [DOI] [PubMed] [Google Scholar]
- 23.Myllys, V., J. Ridell, J. Björkroth, I. Biese, and S. Pyörälä. 1997. Persistence in bovine mastitis of Staphylococcus aureus clones as assessed by random amplified polymorphic DNA analysis, ribotyping and biotyping. Vet. Microbiol. 57:245-251. [DOI] [PubMed] [Google Scholar]
- 24.Newbould, F. H. S. 1968. Epizootiology of mastitis due to Staphylococcus aureus. J. Am. Vet. Med. Assoc. 153:1683-1687. [PubMed] [Google Scholar]
- 25.Nickerson, S. C., and R. L. Boddie. 1994. Effect of naturally occurring coagulase negative staphylococcal infections on experimental challenge with major mastitis pathogens. J. Dairy Sci. 77:2526-2536. [DOI] [PubMed] [Google Scholar]
- 26.Peacock, S. J., G. D. de Silva, A. Justice, A. Cowland, C. E. Moore, C. G. Winearls, and N. P. Day. 2002. Comparison of multilocus sequence typing and pulsed-field gel electrophoresis as tools for typing Staphylococcus aureus isolates in a microepidemiological setting. J. Clin. Microbiol. 40:3764-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raimundo, O., M. Deighton, J. Capstick, and N. Gerraty. 1999. Molecular typing of Staphylococcus aureus of bovine origin by polymorphisms of the coagulase gene. Vet. Microbiol. 66:275-284. [DOI] [PubMed] [Google Scholar]
- 28.Roberson, J. R., L. K. Fox, D. D. Hancock, C. C. Gay, and T. E. Besser. 1994. Coagulase-positive Staphylococcus intramammary infections in primiparous dairy cows. J. Dairy Sci. 77:958-969. [DOI] [PubMed] [Google Scholar]
- 29.Roberson, J. R., L. K. Fox, D. D. Hancock, J. M. Gay, and T. E. Besser. 1994. Ecology of Staphylococcus aureus isolated from various sites on dairy farms. J. Dairy Sci. 77:3354-3364. [DOI] [PubMed] [Google Scholar]
- 30.Roberson, J. R., L. K. Fox, D. D. Hancock, J. M. Gay, and T. E. Besser. 1998. Sources of intramammary infections from Staphylococcus aureus in dairy heifers at first parturition. J. Dairy Sci. 81:687-693. [DOI] [PubMed] [Google Scholar]
- 31.Schlegelová, J., M. Dendis, J. Benedík, V. Babák, and D. Ryšánek. 2003. Staphylococcus aureus isolates from dairy cows and humans on a farm differ in coagulase genotype. Vet. Microbiol. 92:327-334. [DOI] [PubMed] [Google Scholar]
- 32.Sears, P. M., and K. K. McCarthy. 2003. Management and treatment of staphylococcal mastitis. Vet. Clin. N. Am. Food Anim. Pract. 19:171-185. [DOI] [PubMed] [Google Scholar]
- 33.Smith, E. M., L. E. Green, G. F. Medley, H. E. Bird, L. K. Fox, Y. H. Schukken, J. V. Kruze, A. J. Bradley, R. N. Zadoks, and C. G. Dowson. 2005. Multilocus sequence typing of intercontinental bovine Staphylococcus aureus isolates. J. Clin. Microbiol. 43:4737-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommerhäuser, J., B. Kloppert, W. Wolter, M. Zschöck, A. Sobiraj, and K. Failing. 2003. The epidemiology of Staphylococcus aureus infections from subclinical mastitis in dairy cows during a control programme. Vet. Microbiol. 96:91-102. [DOI] [PubMed] [Google Scholar]
- 35.Spratt, B. G. 1999. Multilocus sequence typing: molecular typing of bacterial pathogens in an era of rapid DNA sequencing and the internet. Curr. Opin. Microbiol. 2:312-316. [DOI] [PubMed] [Google Scholar]
- 36.Štepán, J., R. Pantucek, V. Ruzicková, S. Rosypal, V. Hájek, and J. Doškar. 2001. Identification of Staphylococcus aureus based on PCR amplification of species specific genomic 826 bp sequence derived from a common 44-kb SmaI restriction fragment. Mol. Cell. Probes 15:249-257. [DOI] [PubMed] [Google Scholar]
- 37.Struelens, M. J., A. Bauernfeind, A. van Belkum, D. Blanc, B. D. Cookson, L. Dijkshoorn, N. El Solh, J. Etienne, J. Garaizar, P. Gerner-Smidt, N. Legakis, H. de Lencastre, M. H. Nicolas, T. L. Pitt, U. Römling, V. Rosdahl, and W. Witte. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2:2-11. [DOI] [PubMed] [Google Scholar]
- 38.Struelens, M. J., A. Bauernfeind, A. van Belkum, D. Blanc, B. D. Cookson, L. Dijkshoorn, N. El Solh, J. Etienne, J. Garaizar, P. Gerner-Smidt, N. Legakis, H. de Lencastre, M. H. Nicolas, T. L. Pitt, U. Römling, V. Rosdahl, and W. Witte. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2:5. (Erratum, 2: 236.) [DOI] [PubMed] [Google Scholar]
- 39.Su, C., C. Herbelin, N. Frieze, O. Skardova, and L. M. Sordillo. 1999. Coagulase gene polymorphism of Staphylococcus aureus isolates from dairy cattle in different geographical areas. Epidemiol. Infect. 122:329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urwin, R., and M. C. Maiden. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479-487. [DOI] [PubMed] [Google Scholar]
- 41.Vaarst, M., and C. Enevoldsen. 1997. Patterns of clinical mastitis manifestations in Danish organic dairy herds. J. Dairy Res. 64:23-37. [DOI] [PubMed] [Google Scholar]
- 42.VandenBergh, M. F. Q., E. P. F. Yzerman, A. van Belkum, H. A. M. Boelens, M. Sijmons, and H. A. Verbrugh. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weller, R. F., and P. J. Bowling. 2000. Health status of dairy herds in organic farming. Vet. Rec. 146:80-81. [DOI] [PubMed] [Google Scholar]
- 44.Wieliczko, R. J., J. H. Williamson, R. T. Cursons, S. J. Lacy-Hulbert, and M. W. Woolford. 2002. Molecular typing of Streptococcus uberis strains isolated from cases of bovine mastitis. J. Dairy Sci. 85:2149-2154. [DOI] [PubMed] [Google Scholar]
- 45.Young, B., D. Platt, D. Logue, H. Ternent, and J. Fitzpatrick. 2001. Bovine Staphylococcus aureus mastitis: strain recognition and dynamics of infection. J. Dairy Res. 68:377-388. [DOI] [PubMed] [Google Scholar]
- 46.Zadoks, R., W. van Leeuwen, H. Barkema, O. Sampimon, H. Verbrugh, Y. H. Schukken, and A. van Belkum. 2000. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J. Clin. Microbiol. 38:1931-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zadoks, R. N., W. B. van Leeuwen, D. Kreft, L. K. Fox, H. W. Barkema, Y. H. Schukken, and A. van Belkum. 2002. Comparison of Staphylococcus aureus isolates from bovine and human skin, milking equipment, and bovine milk by phage typing, pulsed-field gel electrophoresis, and binary typing. J. Clin. Microbiol. 40:3894-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zecconi, A., and R. Piccinini. 2002. Intramammary infections: epidemiology and diagnosis. XXII World Buiatrics Congress: Recent Developments and Perspectives in Bovine Medicine, Keynote Lectures, Hannover, Germany.
- 49.Zschöck, M., J. Sommerhäuser, and H. Castaneda. 2000. Relatedness of Staphylococcus aureus isolates from bovine mammary gland suffering from mastitis in a single herd. J. Dairy Res. 67:429-435. [DOI] [PubMed] [Google Scholar]