Abstract
Oat fermentation is often hampered by reduced enzymatic activity caused by kilning. We here investigate whether different enzymatic activities of oats at acidic-fermentation-relevant pH can enhance their nutritional and functional properties. Enzymatic activity (protease, xylanase, β-glucanase, amylase, phytase, and lipase) was assessed in kilned and non-kilned oat suspensions at pH 3.5–7.0. Then, changes in dietary fibre and protein extractability and starch and phytate hydrolysis were evaluated after a 24-h incubation (with added antimicrobials to ensure no fermentation occurred) at pH 6.4, pH 4.0, and under gradual acidification. Non-kilned oats exhibited higher enzymatic activities overall. Arabinoxylan and β-glucan extractability of non-kilned oats (23–26 % and up to 73 %, respectively) were higher than of kilned oats (11–12 % and 40–46 %, respectively). Incubated non-kilned oat demonstrated higher starch (4–5 %) and phytate hydrolysis (43–49 %). These findings highlight the potential of fermenting non-kilned oats to improve oat-based foods like sourdough bread and fermented dairy or meat alternatives.
Keywords: Oats, Enzymes, Protein, Dietary fibre, Extractability, Kilning
Highlights
-
•
Non-kilned oats show much higher enzymatic activities than kilned oats.
-
•
Oat lipases are inactive below pH 5.5.
-
•
Acidic non-kilned oat incubation boosts fibre, starch and phytate hydrolysis.
-
•
Oat protein extractability after incubation remains relatively low.
-
•
Non-kilned oats offer promising opportunities for developing liquid oat products.
1. Introduction
The growing interest in plant-based foods is mainly driven by environmental awareness, perceived health benefits and animal welfare considerations (Ramsing et al., 2023). Oats have emerged as a highly nutritious and versatile raw material for producing various plant-based foods (Yang et al., 2023). Oat groats contain, on a dry matter (dm) basis, around 10–15 % proteins with a well-balanced amino acid composition (Boukid, 2021; Holopainen-Mantila et al., 2024) and around 11–17 % of dietary fibre, with β-glucan (βG) (1–6 % dm) and arabinoxylan (AX) (2–4 % dm) being the most prominent (Maina et al., 2021; Prasadi & Joye, 2020; Sikora et al., 2013). The European Food Safety Authority has recognised several health benefits associated with the consumption of oat dietary fibre, such as βG. Furthermore, oat groats contain approximately 43–64 % of starch and are rich in vitamins, antioxidants and other bioactive compounds. Most minerals are located in the aleurone cells, where they are bound to phytate, which reduces their bioavailability (Butt et al., 2008). Finally, oat groats contain 5–9 % lipids (Klose & Arendt, 2012). To prevent oxidative rancidity caused by endogenous lipases and lipoxygenases, oat kernels in the food industry today are generally heat-treated or ‘kilned’, which generally involves treatment with steam at temperatures of 100–120 °C for an extended time (Norlander et al., 2024).
Oat-based milk and yoghurt alternatives, contain significantly less proteins and minerals than their dairy counterparts (Walther et al., 2022). In addition, despite oats being rich in βG and AX, such alternatives contain negligible amounts of these oat dietary fibres. Protein extraction from oats is challenging due to the low water solubility of oat globulins (Jiang et al., 2015), physical encapsulation by cell walls (Janssen et al., 2023), possible association with phytic acid (R. Wang & Guo, 2021) and structural changes as the result of kilning (Pynket et al., 2024; Runyon et al., 2015). AX and βG serve structural roles in (sub)aleurone and endosperm cell walls, making them entangled molecules that are inherently difficult to extract (He et al., 2021; Lazaridou & Biliaderis, 2007). Modifying the structural properties of oat proteins and dietary fibres to enhance their extractability in aqueous media would be beneficial both from nutritional and technological points of view for a range of foods.
The largely untapped potential of oat constituents in foods urges exploring alternative oat processing strategies. Food fermentation, defined as the “desired microbial growth and enzymatic conversions of food components” (Marco et al., 2021), holds significant promise as a natural approach to increase oat protein and dietary fibre extractability and mineral bio-accessibility (Suryamiharja et al., 2024). For wheat, the effectiveness of acidic food fermentation with regard to constituent transformations is primarily determined by the pH decrease-driven increase in the activity of wheat-associated enzymes (Arte et al., 2015; Coda et al., 2014; Maina et al., 2021). However, in kilned oats, enzymatic activity is significantly reduced if not fully eliminated, limiting the potential of food fermentation to increase the extractability of oat constituents. For instance, lactic acid fermentation had no effect on protein extractability in oat bran, likely due to the use of kilned oats with low enzymatic activity (Loponen et al., 2007). In addition, the absence of enzymatic activity in kilned oats limits the availability of fermentable sugars required for microbial growth (Poutanen et al., 2009). Since most studies in the literature have focused on kilned oats, the potential role of oat-associated enzymes in altering the extractability of its constituents remains largely unexplored.
The objective of this study was to investigate how oat-associated enzymes in kilned and non-kilned oats can alter the extractability of its constituents under pH conditions relevant to acidic food fermentation. The study was performed in conditions where microbial growth was inhibited so that only the effect of the oat-associated enzymes could be studied. The findings of this study could enable the application of non-kilned oats in food fermentation, enhancing their potential as raw materials for developing oat-based liquid and semi-solid foods with improved functionality and nutritional value.
2. Materials and methods
2.1. Materials
Hexane was purchased from Chem-lab Analytical (Zedelgem, Belgium), while sodium dodecyl sulfate, glacial acetic acid, ethanol (99 % v/v, denatured) and sodium dihydrogen phosphate dihydrate were obtained from VWR International (Leuven, Belgium). Sodium tetraborate decahydrate was purchased from Fisher Scientific (Brussel, Belgium) and glucose oxidase/peroxidase reagent buffer, glucose oxidase/peroxidase reagent enzymes, Xylazyme tablets, β-glucazyme tablets, amyloglucosidase, lichenase, β-glucosidase and d-Glucose Standard from Megazyme (Wicklow, Ireland). All other chemicals were obtained from Merck (Bornem, Belgium).
Dehulled kilned and non-kilned oat groats, consisting of a mixture of cultivars from the same harvest year and location, were kindly provided by Maselis (Roeselare, Belgium) and stored at −18 °C. Process specifications for kilning were not disclosed to the authors. However, it is known that the industrial target for the heat treatment is to achieve complete inactivation of the most heat-stable enzyme in oats, i.e. peroxidase activity. The groats were milled using a Cyclotec Sample mill 1093 (Foss, Höganäs, Sweden) with a 0.5 mm sieve. To minimise lipid oxidation and rancidity during storage, the whole meal was immediately defatted using a two-step hexane extraction (in a 1:5 w/v ratio) with centrifugation at 2000 xg for 10 min to separate the hexane phase and the pellet, the latter of which was air-dried overnight. The obtained defatted oat whole meal (DOW) samples of both kilned and non-kilned oats were stored at 4 °C before further use. It is expected that hexane defatting does not drastically affect the enzyme activities of the samples. Indeed, preliminary experiments confirmed that hexane defatting did not affect the peroxidase activity of the whole meal (data not shown).
2.2. Analysis of the enzymatic activity of kilned and non-kilned oats
The enzymatic activity in kilned and non-kilned oat suspensions was measured across a range of pH values using colourimetric assays (Fig. 1A). These assays, typically applied to extracts of plant materials, were optimised for DOW suspensions to obtain activities representative of those found in a food fermentation medium. To cover the pH spectrum 3.5–7.0, the following buffers were used: sodium acetate buffer (0.10 M; pH 3.5, 4.0, 4.5, 5.0, 5.5), sodium succinate buffer (0.10 M; pH 6.0) and sodium phosphate buffer (0.10 M; pH 6.5, 7.0). Table 1 provides detailed specifications for each enzyme assay.
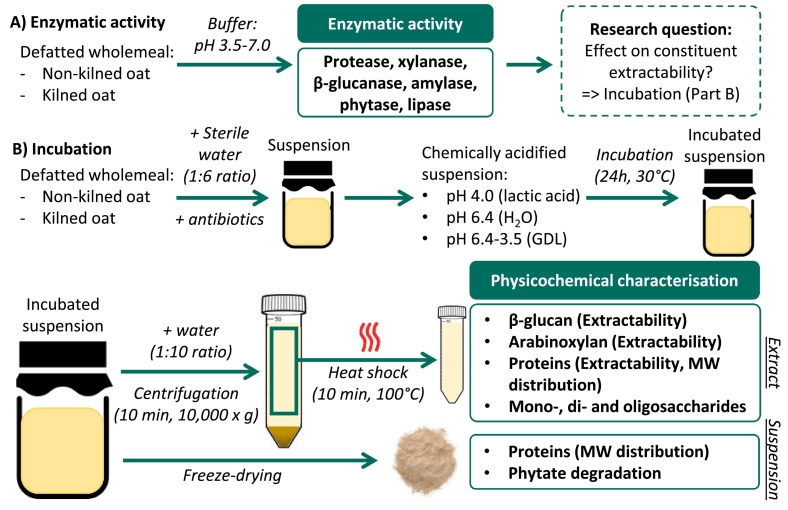
Fig. 1.
Graphical overview of the experimental set-up of this study – (A) Determination of enzymatic activity in defatted whole meal suspensions from kilned and non-kilned oats. (B) Outline of incubation conditions and subsequent sample treatments, including analyses conducted on resulting extracts or suspensions. GDL = Glucono-δ-lactone, MW = molecular weight.
Table 1.
Specifications for enzymatic activity assays in defatted whole meal suspensions from kilned and non-kilned oats.
| Enzyme activity | Flour:water ratio (w/v) | Enzyme substrate | Incubation time | Incubation temperature |
|---|---|---|---|---|
| Protease | 1:10 | Oat proteins | 20 min | 21 °C |
| Endo-1,4-β-xylanases | 1:10 | Azurine-crosslinked wheat arabinoxylan | 6 h | 40 °C |
| Endo-1,4-β-glucanases | 1:10 | Azurine-crosslinked barley β-Glucan | 6 h | 30 °C |
| α-amylase | 1:10 | Non-reducing-end blocked p-nitrophenyl maltoheptaoside | 20 min | 40 °C |
| Phytase | 1:5 | Sodium salt of phytic acid hydrate | 5 h | 37 °C |
| Lipase | 1:5 | p-nitrophenyl butyrate | 20 min | 21 °C |
2.2.1. Protease activity
The proteolytic activity in DOW from kilned and non-kilned oats was measured by quantifying the amount of free amino groups with the ortho-phthalaldehyde method of Spellman et al. (2003), with some modifications. Suspensions (in a 1:10 w/v ratio, with a total volume of 10 mL) of DOW from kilned and non-kilned oats prepared in buffer (pH 4.0–7.0) were incubated for 24 h at 30 °C in duplicate in the presence of antibiotics [chloramphenicol (0.05 % w/v) and cycloheximide (0.02 % w/v)] to prevent spontaneous fermentation. Non-incubated DOW suspensions were also made to measure the initial amount of free amino groups. Both incubated and non-incubated suspensions were diluted 1:8 v/v in sodium phosphate buffer (0.050 M, pH 6.8) containing 2.0 % w/v sodium dodecyl sulfate and 1.0 % w/v 1,4-dithiothreitol, and shaken (150 movements/min) for 15 min to extract all proteins. After centrifugation (10 min at 10,000 xg), 400 μL of supernatant was mixed (in triplicate, resulting in six replicates per oat type and pH) with 3.0 mL of freshly-made Borax solution (0.10 M) containing 0.08 % w/v ortho-phthalaldehyde [dissolved in 7.5 % v/v ethanol] and 0.088 % w/v 1,4-dithiothreitol. After incubation (Table 1), the absorbance at 340 nm was measured with an Ultraspec 2000 UV/VIS spectrophotometer (GE Healthcare, Uppsala, Sweden). This absorbance was converted into the amount of free amino groups (in mmol/mL) using a linear calibration curve based on L-Leucine. The degree of protein hydrolysis was then calculated as follows (Spellman et al., 2003):
with n the calculated hydrolysis-equivalents (mmol/g protein), N the total theoretical number of peptide bonds present in the sample (i.e. 7.8 mmol/g oat protein, based on Wang et al. (2015)), AbsI the absorbance at 340 nm of incubated DOW suspensions (Table 1), AbsNI the absorbance at 340 nm of non-incubated DOW suspensions, interceptcc and slopeCC respectively the intercept and slope of the calibration curve based on L-leucine, and c the protein concentration (mg/mL) in the DOW suspension (B. Wang et al., 2015).
2.2.2. Xylanase, β-glucanase and α-amylase activity
The activities of oat endo-1,4-β-xylanases (referred to as xylanase), endo-1,4-β-glucanases (referred to as β-glucanase) and α-amylases (referred to as amylase) were quantified using colourimetric Megazyme (Wicklow, Ireland) assays, with the substrates and incubation conditions listed in Table 1. Suspensions (in a 1:10 w/v ratio, with a total volume of 5 mL) of DOW from kilned and non-kilned oats were prepared in duplicate in buffer (pH 3.5–7.0) and shaken for 15 min at 150 movements/min. For the xylanase and β-glucanase assays, 1.0 mL of the suspension (in duplicate, resulting in four replicates per oat type and pH) was mixed with the respective substrate tablet (Table 1). For the amylase assay, 0.2 mL of liquid reagent [containing the substrate (Table 1) and thermostable α-glucosidase] was added to 0.2 mL of DOW suspension (in duplicate, resulting in four replicates per oat type and pH). Following incubation, 5.0 mL (for the xylanase and β-glucanase assays) or 3.0 mL (for the amylase assay) of 2.0 % w/v tris(hydroxymethyl)aminomethane buffer (pH 10.4) was added to stop the enzymatic reaction. The suspensions were vortexed and centrifuged (10 min at 4000 xg). The supernatant was filtered over filter paper (MN 615, Macherey-Nagel, Düren, Germany), followed by filtration using a Whatman Millex-HP membrane filter [0.45 μm, polyethersulfone; Millipore, Carrigtwohill, Ireland]. Absorbance measurements were performed at 590 nm for the xylanase and β-glucanase assays and 400 nm for the amylase assay using an Ultraspec 2000 UV/VIS spectrophotometer (GE Healthcare). Blank samples were made by adding the tris(hydroxymethyl)aminomethane buffer to the DOW suspension prior to the addition of the substrate. Enzyme activities were calculated as follows.
where Abs is the absorbance of the incubated sample, Abs0 the absorbance of the blank sample, V the volume of the DOW suspension (mL), mDOW the initial sample mass on dm basis (gdm) and t the incubation time (h). Xylanase and β-glucanase activities were expressed in units (U) per g of dm DOW, with one unit defined as the enzyme activity needed to increase the absorbance by one unit per hour under the assay-specific conditions (Table 1). Amylase activity was expressed in ceralpha units, with one ceralpha unit defined as the amount of enzyme activity required to release one μmol of p-nitrophenol from the substrate (Table 1) per minute.
2.2.3. Phytase activity
The phytase activity of DOW from kilned and non-kilned oats was determined by measuring the total amount of free phosphate released from phytic acid sodium salt hydrate (Heinonen & Lahti, 1981). Suspensions (in a 1:5 w/v ratio, with a total volume of 5 mL) of DOW from kilned and non-kilned oats were prepared in buffer (pH 4.0–7.0) in duplicate by shaking for 20 min at 150 movements/min. At pH 7.0, 0.10 M tris(hydroxymethyl)aminomethane buffer was used instead of 0.10 M sodium phosphate buffer to exclude a secondary phosphor source. An aliquot (0.2 mL) of the suspension was added (in duplicate, resulting in four replicates per oat type and pH) to 1.0 mL of 100 mM phytic acid solution (prepared in the same respective buffer (pH 4.0–7.0) and adjusted to pH 4.0–7.0 with 6.0 M HCl) and incubated (Table 1). The enzymatic reaction was stopped by adding 4.0 mL of a stopping solution containing 50 % v/v acetone, 25 % v/v aqueous ammonium molybdate solution (5 % w/v) and 25 % v/v sulfuric acid (5.0 N). Following mixing and centrifugation (10 min at 4000 xg), the supernatant was collected and filtered over paper and subsequently through a 0.45 μm membrane filter, both as in section 2.2.2. Absorbance values at 400 nm (Ultraspec 2000 UV/VIS spectrophotometer) were corrected for blank samples that consisted of 0.2 mL buffer, 1.0 mL phytic acid solution and 4.0 mL stopping solution. A calibration curve was prepared by adding 1.0 mL of the phytic acid solution to 0.1 mL of a potassium phosphate stock solution (50 mM in milli Q water) and 4.0 mL of stopping solution. The initial free phosphate content was measured by first heating suspensions of DOW in pure ethanol (1:10 w/v ratio) at 100 °C to inactivate phytases. After evaporation of the ethanol phase, the residue was suspended (in a 1:5 w/v ratio) in 0.10 M sodium acetate buffer at pH 4.0 and shaken for 20 min at 150 movements/min. An aliquot (0.2 mL) of this suspension was diluted in 1.0 mL 0.10 M sodium acetate buffer. Immediately afterwards, 4.0 mL of stopping solution was added. The samples were centrifuged and the supernatants were collected and filtered as above.
The total amount of phosphate released was calculated as follows (Heinonen & Lahti, 1981):
where measured is the total amount of phosphate measured after incubation (μmol/mL), DF the dilution factor of the suspension, V the extraction volume (mL), t the incubation time (h), mDOW the mass of DOW on dm basis (gdm), and free the free phosphate content already present in DOW (μmol/h*gdm).
2.2.4. Lipase activity
The lipase activity was determined colourimetrically based on the method of Yang et al. (2017) with some modifications. A substrate stock solution consisting of 3.5 % v/v p-nitrophenyl butyrate in pure dimethyl sulfoxide was freshly made before use. As substrate working solution and dimethyl sulfoxide blank solution, the substrate stock solution and dimethyl sulfoxide, respectively, were diluted in extraction buffer (pH 4.0–7.0) to obtain 2.0 % (v/v). Suspensions (in a 1:5 w/v ratio, with a total volume of 5 mL) of DOW from kilned and non-kilned oats were prepared in extraction buffer (pH 4.0–7.0) containing 0.10 % w/v Triton-X-100 in duplicate and the lipases extracted therefrom by shaking for 10 min at 150 movements/min and centrifuging (10 min at 10,000 xg). The obtained supernatant was filtered through a 0.45 μm membrane filter (as in section 2.2.2) and an aliquot (0.25 mL) was mixed (in duplicate, resulting in four replicates per condition) with 0.50 mL of substrate working solution and 2.0 mL of extraction buffer (pH 4.0–7.0). Following incubation (Table 1), the absorbance at 405 nm was measured using an Ultraspec 2000 UV/VIS spectrophotometer. This absorbance was corrected for that of (i) a sample blank (consisting of 0.25 mL filtered supernatant, 0.50 mL dimethyl sulfoxide blank solution and 2.0 mL extraction buffer [pH 4.0–7.0]), (ii) a substrate blank (consisting of 0.50 mL of substrate working solution and 2.25 mL of extraction buffer [pH 4.0–7.0]) and (iii) an extraction buffer (pH 4.0–7.0) blank. The lipase activity was calculated with the following equation:
where Abs is the absorbance at 405 nm of the sample, Abs0,E the absorbance of the sample blank, Abs0,S the absorbance of the substrate blank, Abs0,B the absorbance of the extraction buffer blank, V the suspension volume (mL), mDOW the mass of DOW (g), dm the sample dry matter content (%) and t the incubation time (h).
2.3. Analysis of the constituent extractability of kilned and non-kilned oats after incubation at food fermentation-relevant pH
2.3.1. Incubation of kilned and non-kilned oat whole meal suspensions
To investigate the effect of oat-associated enzymes on the extractability of proteins and dietary fibres and on the degradation of phytic acid and starch, whole meal of kilned and non-kilned oats was incubated at pH levels relevant to acidic food fermentation. The results were then compared with those of the non-incubated raw materials. DOW from kilned and non-kilned oats was suspended in a 1:6 w/v ratio in sterile milli-Q water containing antibiotics [0.05 % w/v chloramphenicol and 0.02 % w/v cycloheximide] to prevent spontaneous fermentation at a total suspension volume of 50 mL (Fig. 1B). These suspensions were incubated at pH 6.4 (the initial pH of the suspension) or pH 4.0 (using 5.0 % v/v lactic acid) for 24 h in closed 50 mL Schott bottles placed in a water bath at 30 °C, under continuous magnetic stirring at 300 rpm. To mimic the gradual pH decrease during an acidic fermentation, DOW suspensions were also gradually acidified from pH 6.4 to pH 3.5 by the stepwise addition of glucono-δ-lactone (GDL) powder (0.10 % w/v after 2 h, 0.10 % w/v after 3.5 h, and 0.60 % w/v after 5 h). The corresponding acidification profile is shown in supplementary information (Fig. A.1). All incubations were carried out in duplicate on each of two separate days (n = 4). Following incubation, an aliquot of the suspensions was freeze-dried and ground. The other part of the suspension was further diluted to 1:10 w/v with Milli-Q water and centrifuged (10 min at 10,000 xg). The supernatant (the ‘extract’) was immediately heat-treated (10 min, 100 °C) to stop any residual enzymatic activity. Heat-treated extracts and freeze-dried suspensions were stored at −18 °C until further analysis. Non-incubated suspensions and extracts derived from ethanol-treated DOW (as described in Section 2.2.3) were included as reference materials. The following sample codes will be used throughout the manuscript: , where X refers to kilned (K) or non-kilned (NK) oat groats, Y denotes either the non-incubated DOW suspensions (NI) or the DOW suspensions incubated for 24 h (I), and Z indicates the pH (pH 4.0, pH 6.4 or GDL).
2.3.2. Protein extractability and molecular weight distribution
The protein extractability was determined by analysing the protein content (N x 5.83) of the heat-treated extracts (see section 2.3.1) with a total combustion method (Dumas Elemental Analyzer model EA1108, Carlo Erba, Hindley Green, UK). Protein extractabilities (%) were defined as the mass of proteins recovered in the extracts expressed relative to the mass of proteins in the amount of DOW used for the incubations. The protein apparent molecular weight (MW) distribution was determined using size-exclusion high-performance liquid chromatography. Freeze-dried DOW suspensions (see section 2.3.1) were dispersed (in duplicate) at 1.0 mg protein per mL in sodium phosphate buffer (0.050 M, pH 6.8) containing 2.0 % w/v sodium dodecyl sulfate and 1.0 % w/v 1,4-dithiothreitol to dissolve all present proteins, shaken (60 min, 150 movements/min) and centrifuged (10 min at 10,000 xg). Heat-treated extracts (see section 2.3.1) were diluted tenfold in the same buffer. All samples were filtered through a 0.45 μm membrane filter (as in section 2.2.2) and an aliquot (10 μL for the DOW suspensions, 50 μL for the heat-treated extracts) was injected into a Biosep SEC-S2000 column (with a separation range of 0.2 to 75 kDa (in a 0.1 % w/v sodium dodecyl sulfate buffer), Phenomenex, Torrance, CA, USA) mounted in a Shimadzu (Kyoto, Japan) Prominence modular size exclusion high-performance liquid chromatography system. This system was equipped with an LC-20AT pump, SIL-20AC HT autosampler, CTO-20 AC oven and an SPD-20A UV–Vis detector (set at 214 nm). A sodium phosphate buffer (0.050 M, pH 6.8) containing 0.10 % w/v sodium dodecyl sulfate was used as the elution solvent at 0.5 mL/min and 30 °C. MW marker proteins [bovine serum albumin (66.5 kDa), carbonic anhydrase from bovine erythrocytes (30 kDa), α-lactalbumin (14 kDa), aprotinin (6.5 kDa) and (Ala)5 (373 Da)] were analysed under the same conditions as the samples. To enable comparison between chromatograms of heat-treated extracts and DOW suspensions, the extract profile areas were corrected for the injection volume and the quantity of starting material used in the extract preparation.
2.3.3. Arabinoxylan extractability
The total AX content in DOW and the heat-treated extracts (see section 2.3.1) was determined following the procedure of Courtin et al. (2000). In short, carbohydrates were hydrolysed using 4.0 N trichloroacetic acid to obtain monosaccharides which were reduced with NaBH4 and derivatised to alditol acetates using acetic acid anhydride. The alditol acetates were quantified using gas chromatography coupled with flame ionisation detection (GC-FID 6890 N, Agilent Technologies, Santa Clara, CA, USA) with the aid of a Supelco SP-2380 separation polar column (30 m × 0.32 mm, 0.2 μm film thickness, Bellefonte, PA, USA). The total AX content was determined by summing up the contents of arabinose and xylose and multiplying by 0.88 to correct for the release of water during hydrolysis. AX extractability (%) was defined as the mass of AX recovered in the extracts expressed relative to the mass of AX in the amount of DOW used for the incubations.
2.3.4. β-Glucan extractability
The βG content in DOW and the heat-treated extracts (see section 2.3.1) was determined with the mixed-linkage βG kit (Megazyme, Wicklow, Ireland; AACC Method 32–23.01) with minor adaptations. To precipitate extracted βG, an aliquot of extract (Section 2.3.1) was diluted to 90 % v/v ethanol. Following centrifugation (10 min at 1800 xg), the pellet was resuspended in sodium phosphate buffer (0.020 M, pH 6.5). To measure the βG content of DOW samples before incubation, these were ethanol-treated as in Section 2.2.3 to inactivate β-glucanases and subsequently suspended in the same sodium phosphate buffer. Next, lichenase and β-glucosidase hydrolysis steps were performed on all samples, followed by the use of glucose oxidase/peroxidase reagent to spectrophotometrically (Ultraspec 2000 UV/VIS) quantify the glucose level. A blank per sample (consisting of a sample treated with lichenase but not β-glucosidase and subsequently incubated with a glucose oxidase/peroxidase reagent), along with a glucose standard solution (1.5 mg/mL), was also measured to account for the amount of free glucose in the samples and to correlate the absorbance with glucose concentration, respectively. βG extractability (%) was defined as the mass of βG recovered in the extracts expressed relative to the mass of βG in the amount of DOW used for the incubations.
2.3.5. Starch hydrolysis
The degree of starch hydrolysis was assessed based on the total amount of starch hydrolysis products (i.e. glucose, maltose and dextrins) present in the incubated DOW extracts (Section 2.3.1). Heat-treated extracts were diluted 100 times in Milli-Q water, filtered through a Millex-GP 0.22 μm polyethersulfone syringe filter (MilliporeSigma, Burlington, MA, USA) and transferred into vials. Glucose, fructose, sucrose and maltose contents were quantified by injecting an aliquot (12.5 μL) of the diluted extracts into a Dionex ICS-5000 High-Performance Anion Exchange Chromatography system with a CarboPac PA100 separation column (Sunnyvale, CA, USA) and Integrated Pulsed Amperometric detection (HPAEC-IPAD, Sunnyvale, CA, USA). For elution, a 100 mM NaOH solvent was used at a flow rate of 1.0 mL/min for the first 5 min, followed by a gradual increase in sodium acetate concentration at 3.6 mM/min over the next 25 min. To determine the total dextrin content, 1.0 mL of the heat-treated extract was diluted in 1.0 mL of sodium acetate buffer (100 mM, pH 4.0, containing 5.0 mM CaCl2) and 10 μL of amyloglucosidase (Megazyme cat. No E-AMGDF, 3000 U/mL). Following incubation for 30 min at 50 °C, samples were diluted 20 or 200 times for kilned and non-kilned oat extracts, respectively, filtered over a 0.22 μm membrane filter and transferred into vials to quantify the total glucose content. The dextrin content was defined as the total glucose released following the amyloglucosidase treatment, with corrections made for the initial free glucose and maltose contents. The total initial starch content of kilned and non-kilned DOW was determined with the Rapid Total Starch Assay from Megazyme (K-TSTA-100 A). The degree of starch hydrolysis was calculated as follows:
The fructose content was subtracted from the glucose content to account for the glucose derived from sucrose hydrolysis by invertase activity. It should be noted that the analytical method used did not allow differentiating between glucose originating from starch and that from β-glucan. This may result in a slight overestimation of starch hydrolysis.
2.3.6. Phytate hydrolysis
The degradation of phytate was quantified by measuring the ratio of free to total phosphate content with the phytic acid assay (Megazyme, Wicklow, Ireland). In short, Freeze-dried DOW suspensions (see section 2.3.1) were hydrolysed in 0.66 M HCl for at least 16 h, centrifuged (10 min at 15,700 x g), and the resulting supernatant was neutralised by 1:1 v/v dilution in 0.75 M NaOH. The total phosphate content was determined by enzymatically hydrolysing phytic acid into myo-inositol phosphate and phosphate with phytase and subsequently to myo-inositol and phosphate with alkaline phosphatase. The amount of released phosphate was measured colourimetrically at 655 nm using an Ultraspec 2000 UV/VIS spectrophotometer after a reaction with ammonium molybdate colour reagent, which included ascorbic acid and sulfuric acid. The amount of free phosphate initially present in the DOW suspensions was determined by substituting the enzyme solutions with milli-Q water. Absorbance values were converted into phosphate concentrations using a phosphate calibration curve.
2.4. Statistical analysis
Significant differences were assessed using one-way analysis of variance in JMP Pro 17 statistical software (SAS Institute, Cary, NC, USA), followed by Tukey's multiple comparison test at a significance level of P < 0.05. Error bars or values represent standard deviations from the mean, calculated with Microsoft Excel.
3. Results and discussion
3.1. Enzymatic activity of kilned and non-kilned oats at food fermentation-relevant pH values
Fig. 2 shows the different enzymatic activities in DOW suspensions of kilned and non-kilned oats over a pH range. The assessed range (3.5–7.0) reflects the typical acidification profile observed in lactic acid bacteria fermentation, where an initial neutral to slightly acidic pH progressively decreases and stabilises below pH 4.5. Overall, kilned oats exhibit limited or even no enzymatic activities, consistent with the purpose of the kilning process, i.e. to completely inactivate peroxidases, which are known to be the most heat-resistant enzymes (Norlander et al., 2024). However, kilned oats still showed trace amounts of xylanase and phytase activity, though significantly lower than in non-kilned oats.
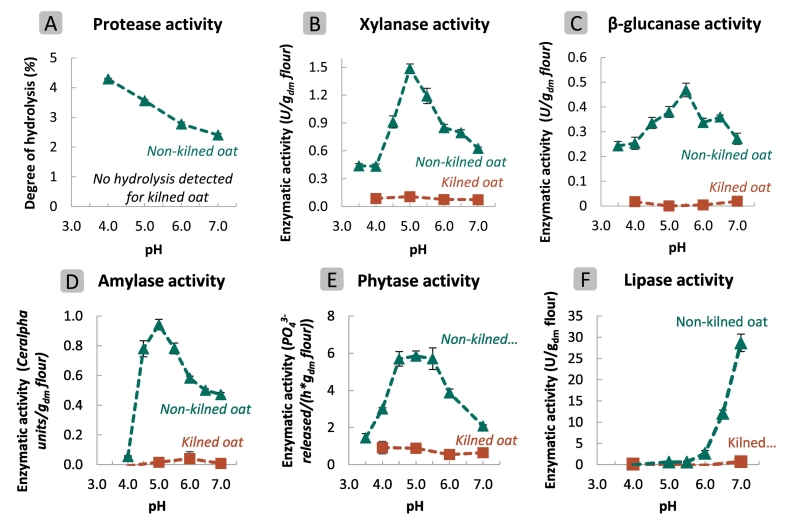
Fig. 2.
The pH-dependent activity of oat endogenous enzymes - The pH-dependent activity of protease (A), xylanase (B), β-glucanase (C), α-amylase (D), phytase (E), and lipase (F) in defatted whole meal (DOW) suspensions from kilned and non-kilned oats. Abbreviations: U = enzymatic units, dm = dry matter, = phosphate, h = hour.
Fig. 2A shows that in non-kilned DOW suspensions, the degree of protein hydrolysis decreased in an approximately linear way with pH, with a value of 4.3 % at pH 4.0 and 2.4 % at pH 7.0. These results are consistent with earlier findings by Mikola & Jones (2000), who reported that oat proteases exhibit optimal activity at acidic pH (around pH 3.8) during germination of hulled oats. Elsewhere, Arte et al. (2015) demonstrated that wheat bran proteolytic activity was highest under acidic conditions (pH 4.5). Non-kilned oats also demonstrated significantly (P < 0.05) higher xylanase (Fig. 2B) and β-glucanase (Fig. 2C) activities than kilned oats, with optimal pH values of 5.0 and 5.5, respectively. Even under more acidic (pH 4.0) and neutral (≥ pH 6.5) conditions, dietary fibre-degrading enzymes in non-kilned oat suspensions remained active. The amylase activity in non-kilned oats was highest at pH 5.0, with a marked decline to almost zero at pH values below 4.5. At pH values >5.0, intermediate amylase activity was observed (Fig. 2D). The highest phytase activities were measured in non-kilned oats between pH 4.5 and 5.5 (Fig. 2E). More acidic and neutral pH conditions resulted in a significant reduction in phytase activity. Notably, the highest phytase activity measured in non-kilned DOW suspensions (5.9 μmol released/h.gdm at pH 5.0) was much lower than what has been observed in wheat, barley and rye (Mayer et al., 2023; Reale et al., 2007). For instance, wheat phytase activity has been reported to reach 96 μmol released/h.gdm at pH 5.0, using a similar assay (Lemmens et al., 2018). It is important to note that a single batch of oat kernels comprising a mixture of cultivars was here used for enzymatic screening. It is likely that in different batches, different absolute values for the enzymatic activities here reported would be observed. Still, the potential enzymatic activities of non-kilned oats, as compared to kilned oats, at pH values relevant during acidic food fermentation were clearly demonstrated. In what follows, we will relate these enzymatic activities to changes in constituent properties.
For the sake of completeness, and since non-kilned oats were used, is was deemed relevant to also assess the pH dependence of the lipase activity. Notably, oat lipase activity was completely inhibited at pH values <5.5 (Fig. 2F), which are typically reached early (< 2–5 h) in most acidic food fermentation processes. This observation aligns with findings by Ekstrand et al. (1992), who reported higher lipase activity in non-kilned oats at pH 7.0 than at pH 4.6. As we here worked with defatted oats to prevent lipid oxidation from interfering with the different analyses performed, we do not further discuss the effect of lipase action in what follows. Still, our finding indicates that it might be possible to use non-kilned oats in low pH systems, and it would be interesting to investigate lipid oxidation in fermentation-type processing of non-kilned oats further.
3.2. Extractability of kilned and non-kilned oat constituents at food fermentation-relevant pH values
To assess the impact of enzymes in kilned and non-kilned oats on the extractability of protein and dietary fibre and the degradation of starch and phytate, DOW suspensions were incubated for 24 h at pH 6.4, pH 4.0 and under gradual acidification (GDL), representing key stages of lactic acid bacteria fermentation. Typically, such a process starts at a neutral to slightly acidic pH and gradually decreases the pH to a value below pH 4.0. Table 2 shows the changes in the extractability or hydrolysis of oat constituents following incubation under these conditions. The soluble protein, βG and AX contents, expressed on dm basis, in both incubated and non-incubated oat whole meal suspensions, are listed in Table A.1.
Table 2.
Overview of protein, arabinoxylan and β-glucan extractabilities as well as the extent of phytate and starch hydrolysis in defatted whole meal suspensions from kilned and non-kilned oat - Extractabilities are defined as the percentage of constituent recovered in the extracts relative to the constituent content in an equivalent amount of defatted oat whole meal (DOW). Measurements were conducted for non-incubated samples at pH 4.0 and pH 6.4, and after 24 h incubation at 30 °C at pH 4.0, pH 6.4 or under gradual acidification with glucono-δ-lactone (GDL). Samples are referred to as ‘’, where X indicates kilned (K) or non-kilned (NK) oat groats, Y denotes either the non-incubated starting material (NI) or the starting material incubated for 24 h (I), and Z indicates the pH. Significant differences (P < 0.05) for a given constituent and oat whole meal type are indicated by different lowercase letters, while differences between kilned and non-kilned oats for a given constituent and pH condition are shown using different capital letters. The abbreviation ‘na’ indicates ‘not analysed’.
| Kilned defatted oat whole meal | ||||||
| Protein | Extractability (%) | 4.0 ± 0.3 (c,B) | 3.9 ± 0.4 (c,B) | 6.1 ± 0.1 (b,B) | 8.2 ± 0.3 (a, B) | 6.4 ± 0.6 (b,B) |
| Arabinoxylan | Extractability (%) | 8.5 ± 0.3 (b,A) | 8.3 ± 0.6 (b,A) | 11.2 ± 0.2 (a,B) | 11.9 ± 0.5 (a,B) | 11.5 ± 0.6 (a,B) |
| β-glucan | Extractability (%) | 15.7 ± 0.5 (c,B) | 15.8 ± 1.9 (c,B) | 40.5 ± 1.5 (b,A) | 45.7 ± 4.0 (a,A) | 42.2 ± 0.7 (ab,B) |
| Starch | Hydrolysis (%) | na | na | 0.4 ± 0.1 (a,B) | 0.5 ± 0.1 (a,B) | 0.6 ± 0.1 (a,B) |
| Phytate | Free/total phosphate (%) | na | 16.9 ± 0.4 (a,A) | 15.2 ± 0.5 (b,B) | 16.4 ± 1.1 (ab,B) | 15.5 ± 0.5 (ab,B) |
| Non-kilned defatted oat whole meal | ||||||
| Protein | Extractability (%) | 10.9 ± 0.2 (c,A) | 17.3 ± 0.3 (b,A) | 26.4 ± 4.6 (a,A) | 23.2 ± 1.6 (a,A) | 27.9 ± 0.8 (a,A) |
| Arabinoxylan | Extractability (%) | 8.9 ± 2.5 (b,A) | 9.3 ± 0.2 (b,A) | 23.5 ± 1.3 (a,A) | 26.4 ± 4.0 (a,A) | 24.1 ± 1.5 (a,A) |
| β-glucan | Extractability (%) | 28.9 ± 1.2 (c,A) | 33.9 ± 6.0 (c,A) | 41.0 ± 2.6 (b,A) | 7.8 ± 0.5 (d,B) | 73.0 ± 2.6 (a,A) |
| Starch | Hydrolysis (%) | na | na | 4.0 ± 0.8 (a,A) | 5.3 ± 0.8 (a,A) | 4.9 ± 1.1 (a,A) |
| Phytate | Free/total phosphate (%) | na | 14.6 ± 0.3 (c,A) | 47.9 ± 3.8 (ab,A) | 48.8 ± 1.5 (a,A) | 43.3 ± 2.1 (b,A) |
3.2.1. Protein extractability and molecular weight distribution
As expected, the protein extractability of non-incubated kilned oats was low, at 4.0 % at pH 4.0 () and 3.9 % at pH 6.4 () (Table 2), corresponding to a soluble protein content of around 0.53 g/100 g dm (Table A.1). In comparison, non-incubated non-kilned oats at pH 4.0 () and pH 6.4 () samples exhibited slightly higher protein extractability (10.9 and 17.3 %, respectively) (Table 2). This observation aligns with previous studies by Runyon et al. (2015), Norlander et al. (2024) and Pynket et al. (2024), who reported a significantly lower protein extractability for kilned than for non-kilned oats.
The protein extractability of kilned oats, incubated for 24 h at pH 4.0, pH 6.4 or with GDL (, and respectively) was comparable to that of the sample, indicating minimal changes during incubation of kilned oats (Table 2). The lack of protease activity in DOW from kilned oats (Fig. 2A) likely explains this. In contrast, non-kilned oats, incubated for 24 h at pH 4.0, pH 6.4 or with GDL (, and respectively) showed a significant (P < 0.05) increase in protein extractability (23.2–27.9 %) compared to (10.9–17.3 %) (Table 2), consistent with the higher proteolytic activity observed for non-kilned oats (Fig. 2A). However, despite the combined effects of proteolytic activity (degree of protein hydrolysis between 2.4 and 4.3 %, Fig. 2A) and the lack of prior kilning, this increase in protein extractability was fairly limited. Moreover, the incubation pH did not significantly (P < 0.05) influence the protein extractability of non-kilned oats, despite protease activity being higher at 4.0 than at pH 6.4 (Fig. 2A). This may be attributed to the isoelectric point of oat 12S globulins at around pH 5.0 (Wouters & Nicolai, 2024), likely leading to protein aggregation and reduced extractability at pH 4.0 (Klose & Arendt, 2012). At pH 6.4, although proteolytic activity was lower than at pH 4.0 (Fig. 2A), the solubility of 12S globulins may have been higher due to greater electrostatic repulsion. The minor differences in soluble protein content between the three pH conditions likely reflect a balance between protease activity and protein aggregation.
To further assess proteolytic activity during incubation, the apparent MW distribution of the proteins present in DOW suspensions after incubation for 24 h at 30 °C was analysed with size-exclusion high-performance liquid chromatography. In addition, the supernatants obtained from the incubated suspensions (the extracts, see section 2.3.1) were also analysed to evaluate the protein composition of the extractable proteins. All analyses were performed under denaturing and reducing conditions to ensure complete protein solubilisation and the disruption of potentially present protein aggregates within the target samples. Chromatograms for suspensions and extracts of and samples are shown in Fig. 3, while the chromatograms of the other samples are presented in supplementary data (Fig. A.2 and Fig. A.3). Elution times for oat 12S globulin α- and β- subunits (12–13 min and 13–14 min, respectively) are indicated, along with annotations for 12S globulin monomers and protein aggregates (10–12 min) and low MW peptides (15–23 min). Notably, the peak corresponding to proteins near the 14 kDa marker could not be clearly identified, but it most likely represents water-soluble albumins. The chromatograms of the molecular weight markers are provided in the supplementary data (Fig. A.4). A key point to highlight here is that during liquid chromatography analysis quantification relies on UV absorption by mostly peptide bonds. This means that as protein hydrolysis progresses during incubation, the intensity associated with the elution of peptides is lower than that of an equivalent mass of intact protein.
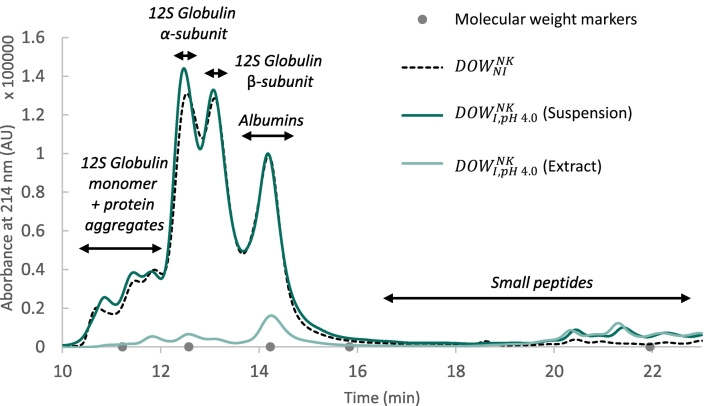
Fig. 3.
Apparent protein molecular weight distributions of non-incubated and incubated (at pH 4.0) oat whole meal suspensions - Size-exclusion high-performance liquid chromatography profiles of proteins from defatted non-kilned oat whole meal, analysed under denaturing and reducing conditions at 1.0 mg protein/mL. The samples include (i) proteins from defatted non-kilned oat whole meal powder, (ii) proteins from a non-kilned oat whole meal suspension incubated for 24 h at pH 4.0, and (iii) proteins from the aqueous extract of the incubated non-kilned oat whole meal suspension. To avoid overlap with the elution peak of 1,4 dithiothreitol (occurring at 24–30 min), chromatograms were truncated at 23 min. Molecular weight markers (from left to right: 66.5 kDa, 30.0 kDa, 14.0 kDa, 6.5 kDa, 373 Da) are indicated at their corresponding elution times, along with anticipated annotations for the different peaks. Samples are referred to as , where NK refers to non-kilned oat groats, Y denotes either the non-incubated starting material (NI) or the 24 h-incubated material (I), and Z corresponds to the (incubation) pH condition (pH 4.0). AU = absorbance units.
The main protein fraction in and samples, i.e. 12S globulins, remained largely intact after 24 h of incubation. Indeed, only a minor shift to lower MW fractions (elution times of 19–23 min) was observed (Fig. 3 and Fig. A.2), indicating some protein hydrolysis. The much lower area under the chromatogram of the extract from is in line with its relatively low protein extractability (Table 2). Notably, extracts from (Fig. 3) contained a higher content of peptides, while those from contained a greater proportion of 12S globulin monomers and subunits (Fig. A.2), in line with the higher degree of protein hydrolysis at pH 4.0 than at pH 6.4 (Fig. 2A). In samples, the formation of peptides was limited during incubation, which is in line with the lower proteolytic activity in these samples (Fig. A.3).
3.2.2. Arabinoxylan extractability
The arabinoxylan extractability of and samples was comparable (P > 0.05) and independent of pH (Table 2). In kilned DOW samples, AX extractability was slightly higher forincubated (11.3–11.9 %) than for the non-incubated samples (8.3–8.5 %), with no statistical differences (P > 0.05) observed between the different incubation conditions (Table 2). This small increase likely results from the combined effects of low xylanase activity in kilned DOW (Fig. 2B) and the hydration of cell walls and associated AX molecule solubilisation during prolonged incubation in excess water. In contrast, significantly higher AX extractabilities were observed in non-kilned samples (, and ), ranging from 23.5 % to 26.4 % (Table 2), corresponding to a 2.5-fold increase compared to . This increase can be ascribed to the higher xylanase activity in DOW suspensions from non-kilned than from kilned oats (Fig. 2B). Still, despite the higher xylanase activity at pH 6.5 than at pH 4.0, no significant (P > 0.05) differences in non-kilned oat AX extractability were observed among the different incubation conditions (Table 2). This suggests that the xylanase activity at pH 4.0 was already sufficient to solubilise the fraction of AX that was also solubilised at pH 6.4, or that hydration effects may have also contributed to this process.
3.2.3. β-Glucan extractability
Preliminary findings indicated that low β-glucanase activities during extraction can significantly influence the βG extractability, which was not observed for AX extractability. Therefore, the βG extractability of and samples at pH 4.0 and pH 6.4 was assessed for ethanol-refluxed flour to exclude these enzymatic effects during the extraction process itself, thereby providing insights into the impact of kilning on the inherent βG extractability (Fig. 4). For ethanol-refluxed , the βG extractability was around 16 %, with no significant (P > 0.05) differences between the extraction pH values. In contrast, ethanol-refluxed showed βG extractability of 28.9 % and 33.9 % at pH 4.0 and pH 6.4, respectively (Fig. 4). The observed differences in βG extractability between kilned and non-kilned oats might originate from kilning-induced changes in the cell wall structure, which could have rendered βG less extractable in kilned oats.
Fig. 4.
β-glucan extractabilities of (non-)incubated (non-)ethanol-refluxed defatted oat whole meal suspensions - Comparison between β-glucan extractability of ethanol-refluxed and non-ethanol-refluxed defatted oat whole meal suspensions from kilned and non-kilned oats, non-incubated at pH 4.0 and pH 6.4 and incubated for 24 h at 30 °C at pH 4.0 and 6.4. Samples are referred to as , where X refers to the kilned (K) or non-kilned (NK) oat groats, Y denotes either the non-incubated starting material (NI) or the 24 h-incubated material (I), and Z corresponds to the (incubation) pH condition (pH 4.0, pH 6.4). Statistical significant differences (P-value < 0.05) in β-glucan extractability between incubation conditions are indicated with different capital letters, while differences between ethanol-refluxed and non-ethanol-refluxed samples for a given condition are indicated with different lowercase letters.
Interestingly, after 24 h of incubation of ethanol-refluxed kilned DOW, and thus in the absence of enzymatic activity, βG extractability increased from 16 % to around 30 % (Fig. 4). This suggests that hydration of the cell walls, wherein water molecules gradually penetrate the βG chains, plays a key role in improving βG solubility and extractability as suggested by other authors as well (Lazaridou & Biliaderis, 2007; Vasanthan & Temelli, 2008). Similarly, for ethanol-refluxed and , βG extractability increased to 56.2 % and 46.9 %, respectively, due to similar hydration effects (Fig. 4). The higher βG extractability observed in the former compared to the latter sample may be attributed to pH-dependent charges on the phosphate groups of the βG chains, which may affect their hydration behaviour at different pH values (Ghotra et al., 2007). A more detailed investigation is needed to fully understand the impact of kilning on the microstructural organisation and hydration behaviour of oat cellular tissues.
Focusing on the non-ethanol-refluxed samples, as shown in Table 2, samples exhibited a significant (P < 0.05) two-fold increase in βG extractability compared to samples, irrespective of the pH. This increase is consistent with observations for ethanol-refluxed samples, and is primarily attributed to the previously discussed hydration effects. In contrast, the impact of pH on βG extractability in samples differed significantly (P < 0.05) from that observed in samples (Table 2). For samples, βG extractability increased to approximately 41 %, which was lower than that of ethanol-refluxed . This suggests some hydrolysis of soluble βG during incubation or extraction, resulting in the loss of low MW β-gluco-oligosaccharides or βG-derived glucose, as part of the supernatant, during the 90 % (v/v) ethanol precipitation step of the βG assay (section 2.3.4). For samples, βG extractability was much lower (7.8 %) than that of (Table 2). This reduced βG extractability is likely due to the complete degradation of βG into highly soluble β-gluco-oligosaccharides or glucose monomers, facilitated by β-glucanase activity (Fig. 2C). The highest βG extractability (around 73 %) was observed for samples (Table 2). This is likely due to a combination of hydration effects and the release of βG from cell walls due to β-glucanase activity. As the pH decreases during acidification, β-glucanase activity decreases (Fig. 2C), resulting in solubilised βG that is not further degraded or only partially degraded, thus remaining insoluble in the 90 % (v/v) ethanol precipitation step of the βG assay (section 2.3.4).
3.2.4. Starch hydrolysis
Starch plays a crucial role in cereal-based food fermentations by providing fermentable sugars (i.e. glucose, maltose and dextrins) through enzymatic degradation, which sustain microbial growth and activity. As shown in Table 2, starch hydrolysis in samples was limited (0.4–0.6 %), which can be explained by the lack of α-amylase activity (Fig. 2D). This is in line with mono-, di-, and oligosaccharide contents remaining largely unchanged and minimal dextrin production during the incubation of kilned oats (Fig. A.5). In contrast, although no amylase activity was detected in non-kilned oats at pH 4.0, a starch hydrolysis level of 4.0 % was observed in (Table 2). This discrepancy may stem from limitations in the assay used for α-amylase activity measurement (see Section 2.2.2), including the short assay duration (i.e. 20 min) and reduced α-glucosidase activity at pH 4.0, which could have hindered the formation of coloured reaction products (Y. H. Wang et al., 2009). Slightly higher starch hydrolysis values were observed in (5.3 %) and (4.9 %) (Table 2), consistent with the higher activity of oat amylase at neutral pH (Fig. 2D). Indeed, during incubation of non-kilned oats, glucose, maltose and dextrins were produced (Fig. A.6). After incubation, sucrose was no longer detectable, which is attributed to its conversion into glucose and fructose by oat invertase activity (Pressey & Avants, 1980). The remaining glucose was probably produced by the combined action of α-amylase, β-amylase and glucoamylase (Gänzle, 2014). These findings clearly demonstrate that significantly more fermentable sugars are produced during the incubation of non-kilned oats compared to kilned oats. This difference in sugar availability is expected to have a substantial impact on the fermentation behaviour (i.e. acidification, microbial growth etc.) of kilned and non-kilned oats.
3.2.5. Phytate hydrolysis
The ratio of free to total phosphate serves as an indicator of phytate hydrolysis. It should be mentioned that this measure does not show whether the observed degradation reflects complete hydrolysis to inositol monophosphate, partial hydrolysis to inositol di-, tri-, tetra- or pentaphosphates, or a combination of both. samples had a significantly (P < 0.05) higher free-to-total phosphate ratio than samples (Table 2). This difference might be caused by structural rearrangements in the oat kernel during kilning (Parmar, 2016). As expected based on the absence of phytase activity in kilned oat suspensions (Fig. 2E), no phytate hydrolysis was observed in samples under any of the incubation conditions (Table 2). In contrast, the relative free phosphate content in samples increased significantly (P < 0.05) to ±50 %, representing a 3.3-fold increase compared to samples. The relative free phosphate content of (47.3 %) was similar to that of (48.8 %), consistent with the similar phytase activities measured at these pH values (Fig. 2E). A slightly lower relative free phosphate content was observed for (43.3 %) samples, which could be related to the rapid pH decrease from 6.4 to 3.5 (Fig. A.1). At this lower pH, oat phytase activity was found to be reduced significantly (Fig. 2E). The phytate hydrolysis observed in this study is lower than that reported for other cereals such as wheat and rye (Reale et al., 2007). For example, Leenhardt et al. (2005) showed that acidification of wheat dough with organic acids decreased the phytate content by 70 %.
4. Conclusions
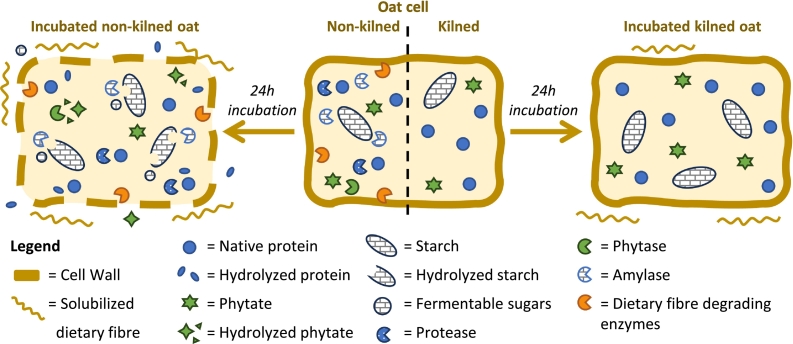
This study compared the enzymatic activities of kilned and non-kilned oats and investigated their effects on protein and dietary fibre extractability and starch and phytate hydrolysis at pH values relevant to acidic food fermentations. A schematic overview of the main findings is presented in Fig. 5. As expected, kilned oats showed minimal enzymatic activities, with, as a result, negligible changes in constituent degradation or extractability during incubation (Fig. 5). This limits the suitability of kilned oats as a raw material for food fermentation processes. The only notable change observed was an increase in β-glucan extractability to approximately 40 % after 24 h of incubation, which was here ascribed to hydration effects. In contrast, non-kilned oats had significantly higher enzymatic activities, which facilitated substantial changes in the oat matrix during incubation (Fig. 5). These included increased dietary fibre extractability, phytate breakdown, and starch hydrolysis. AX and βG extractabilities increased from 9 % to 24 % and 29 % to 73 %, respectively, under gradual acidification. In addition, starch hydrolysis reached ∼5 % at all pH conditions, providing a valuable source of fermentable sugars. Phytate hydrolysis was also notable, with a 40 % reduction for all tested pH values. Although protease activity was detected in non-kilned oats, protein extractability after incubation remained low (23–28 %).
Fig. 5.
Schematic overview of the main findings of this study – Graphical representation of kilned (right) and non-kilned (left) oat cells (simplified depiction of aleurone and starchy endosperm cells), illustrating the effect of 24 h incubation on key oat constituents, including protein, dietary fibre, starch and phytate. Enzymes such as proteases, phytases, amylases and dietary fibre-degrading enzymes are indicated, highlighting the differences in enzymatic activity between kilned and non-kilned oats.
The enhanced enzymatic activity in non-kilned oats enables more effective dietary fibre extraction and phytate hydrolysis. This presents a promising opportunity for developing liquid and semi-solid food products with enhanced technological and nutritional qualities, such as dairy alternatives. It should be considered that the use of non-kilned oats may well cause issues regarding rancidity development. In this regard, it is interesting that it was here observed that oat lipases are inactive at low pH values, which might facilitate the use of non-kilned oats in acidic food fermentation type processes. This should be investigated further. Moreover, the protein content in incubated oat extracts remains low, even in the case of non-kilned oats. Future research should aim at directing food fermentation processes to increase protein extractability. Additionally, understanding the kinetics of fermentable sugar release and consumption during food fermentation is crucial for process refinement and warrants further investigation. Finally, to further enhance phytate hydrolysis, the use of microbial strains capable of producing extracellular phytases could be a valuable strategy.
CRediT authorship contribution statement
Ewoud Blontrock: Writing – original draft, Visualization, Validation, Methodology, Formal analysis, Data curation. Eline Lambrechts: Writing – original draft, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Frederik Janssen: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Yamina De Bondt: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Sarah Vanhove: Methodology, Investigation, Formal analysis. Jolien Lemoine: Methodology, Investigation, Formal analysis. Christophe M. Courtin: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Arno G.B. Wouters: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
ChatGPT (OpenAI GPT-4-turbo, CA, USA) has been used to enhance the language quality of this manuscript. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors acknowledge financial support from the project HealthFerm, which is co-funded by the European Union under the Horizon Europe grant agreement No. 101060247 and the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract No. 22.00210. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Research Executive Agency (REA). Neither the European Union nor REA can be held responsible for them.
Frederik Janssen and Yamina De Bondt gratefully acknowledge the Research Foundation – Flanders (FWO Vlaanderen, Brussels, Belgium) for a position as postdoctoral researcher (grant number 1224123N and 12B3723N respectively). Eline Lambrechts would also like to thank The Research Foundation – Flanders (FWO Vlaanderen, Brussels, Belgium) for providing her with a doctoral grant (grant number 1S43725N). The Research Foundation – Flanders (FWO Vlaanderen, Brussels, Belgium) is also acknowledged for funding the research project “Establishing process-structure-function relationships for oat protein and dietary fibre from kernel to model foods” (grant G049824N).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2025.102834.
Contributor Information
Ewoud Blontrock, Email: ewoud.blontrock@kuleuven.be.
Eline Lambrechts, Email: eline.lambrechts@kuleuven.be.
Frederik Janssen, Email: frederik.janssen@kuleuven.be.
Yamina De Bondt, Email: yamina.debondt@kuleuven.be.
Christophe M. Courtin, Email: christophe.courtin@kuleuven.be.
Arno G.B. Wouters, Email: arno.wouters@kuleuven.be.
Appendix A. Supplementary data
Supplementary material
Data availability
All data is publicly available and accessible via https://doi.org/10.5281/zenodo.14639733.
References
- Arte E., Rizzello C.G., Verni M., Nordlund E., Katina K., Coda R. Impact of enzymatic and microbial bioprocessing on protein modification and nutritional properties of wheat bran. Journal of Agricultural and Food Chemistry. 2015;63(39):8685–8693. doi: 10.1021/ACS.JAFC.5B03495. [DOI] [PubMed] [Google Scholar]
- Boukid F. Oat proteins as emerging ingredients for food formulation: Where we stand? European Food Research and Technology. 2021;247(3):535–544. doi: 10.1007/s00217-020-03661-2. [DOI] [Google Scholar]
- Butt M.S., Tahir-Nadeem M., Khan M.K.I., Shabir R., Butt M.S. Oat: Unique among the cereals. European Journal of Nutrition. 2008;47(2):68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- Coda R., Rizzello C.G., Curiel J.A., Poutanen K., Katina K. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innovative Food Science & Emerging Technologies. 2014;25:19–27. doi: 10.1016/j.ifset.2013.11.012. [DOI] [Google Scholar]
- Courtin C.M., Van Den Broeck H., Delcour J.A. Determination of reducing end sugar residues in oligo- and polysaccharides by gas-liquid chromatography. Journal of Chromatography A. 2000;866(1):97–104. doi: 10.1016/S0021-9673(99)01064-X. [DOI] [PubMed] [Google Scholar]
- Gänzle M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiology. 2014;37:2–10. doi: 10.1016/J.FM.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ghotra B.S., Vasanthan T., Wettasinghe M., Temelli F. 31P-nuclear magnetic resonance spectroscopic analysis of phosphorus in oat and barley β-glucans. Food Hydrocolloids. 2007;21(7):1056–1061. doi: 10.1016/J.FOODHYD.2006.07.014. [DOI] [Google Scholar]
- He H.-J., Qiao J., Liu Y., Guo Q., Ou X., Wang X. Isolation, structural, functional, and bioactive properties of cereal Arabinoxylan - a critical review. Journal of Agricultural and Food Chemistry. 2021;69(51):15437–15457. doi: 10.1021/acs.jafc.1c04506. [DOI] [PubMed] [Google Scholar]
- Heinonen J.K., Lahti R.J. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Analytical Biochemistry. 1981;113(2):313–317. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Holopainen-Mantila U., Vanhatalo S., Lehtinen P., Sozer N. Oats as a source of nutritious alternative protein. Journal of Cereal Science. 2024;116 doi: 10.1016/j.jcs.2024.103862. [DOI] [Google Scholar]
- Janssen F., Lambrechts E., Pynket I., Wouters A.G.B. Ball milling alters the extractability and colloidal state of oat proteins. Journal of Cereal Science. 2023;112 doi: 10.1016/j.jcs.2023.103725. [DOI] [Google Scholar]
- Jiang, Z. qing, Sontag-Strohm, T., Salovaara, H., Sibakov, J., Kanerva, P., & Loponen, J. (2015). Oat protein solubility and emulsion properties improved by enzymatic deamidation. Journal of Cereal Science, 64, 126–132. doi: 10.1016/J.JCS.2015.04.010. [DOI]
- Klose C., Arendt E.K. Proteins in oats; their synthesis and changes during germination: A review. Critical Reviews in Food Science and Nutrition. 2012;52(7):626–639. doi: 10.1080/10408398.2010.504902. [DOI] [PubMed] [Google Scholar]
- Lazaridou A., Biliaderis C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. Journal of Cereal Science. 2007;46(2):101–118. doi: 10.1016/J.JCS.2007.05.003. [DOI] [Google Scholar]
- Leenhardt F., Levrat-Verny M.-A., Chanliaud E., Rémésy C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce Phytate content of whole wheat flour through endogenous Phytase activity. Journal of Agricultural and Food Chemistry. 2005;53(1):98–102. doi: 10.1021/jf049193q. [DOI] [PubMed] [Google Scholar]
- Lemmens E., De Brier N., Spiers K.M., Ryan C., Garrevoet J., Falkenberg G.…Delcour J.A. The impact of steeping, germination and hydrothermal processing of wheat (Triticum aestivum L.) grains on phytate hydrolysis and the distribution, speciation and bio-accessibility of iron and zinc elements. Food Chemistry. 2018;264:367–376. doi: 10.1016/j.foodchem.2018.04.125. [DOI] [PubMed] [Google Scholar]
- Loponen J., Laine P., Sontag-Strohm T., Salovaara H. Behaviour of oat globulins in lactic acid fermentation of oat bran. European Food Research and Technology. 2007;225:105–110. doi: 10.1007/s00217-006-0387-9. [DOI] [Google Scholar]
- Maina N.H., Rieder A., De Bondt Y., Mäkelä-Salmi N., Sahlstrøm S., Mattila O.…Poutanen K. Process-induced changes in the quantity and characteristics of grain dietary Fiber. Foods. 2021;10(11):2566. doi: 10.3390/foods10112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco M.L., Sanders M.E., Gänzle M., Arrieta M.C., Cotter P.D., De Vuyst L.…Hutkins R. The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews Gastroenterology & Hepatology. 2021;18(3):196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer N., Widderich N., Scherzinger M., Bubenheim P., Kaltschmitt M. Comparison of phosphorus and Phytase activity distribution in wheat, Rye, barley and oats and their impact on a potential Phytate separation. Food and Bioprocess Technology. 2023;16(5):1076–1088. doi: 10.1007/S11947-022-02981-3. [DOI] [Google Scholar]
- Norlander S., Dahlgren L., Sardari R.R.R., Marmon S., Tullberg C., Nordberg Karlsson E., Grey C. Effect of kilning on the macronutrient composition profile of three Swedish oat varieties. Cereal Chemistry. 2024;101(2):382–396. doi: 10.1002/cche.10757. [DOI] [Google Scholar]
- Parmar N. Changes in Phytate content of newly released wheat varieties during different processing methods. International Journal of Science and Research. 2016;391(10):1572–1576. [Google Scholar]
- Poutanen K., Flander L., Katina K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiology. 2009;26(7):693–699. doi: 10.1016/J.FM.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Prasadi N., Joye I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients. 2020;12(10) doi: 10.3390/nu12103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R., Avants J.K. Invertases in oat seedlings: Separation, properties, and changes in activities in seedling segments. Plant Physiology. 1980;65(1):136–140. doi: 10.1104/pp.65.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynket I., Janssen F., Van Gils J., Courtin C.M., Wouters A.G.B. Directing oat groat heat treatment conditions towards increased protein extractability. Current Research in Food Science. 2024;9 doi: 10.1016/J.CRFS.2024.100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsing R., Santo R., Kim B.F., Altema-Johnson D., Wooden A., Chang K.B.…Love D.C. Dairy and plant-based milks: Implications for nutrition and planetary health. Current Environmental Health Reports. 2023;10:291–302. doi: 10.1007/s40572-023-00400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale A., Konietzny U., Coppola R., Sorrentino E., Greiner R. The importance of lactic acid Bacteria for Phytate degradation during cereal dough fermentation. Journal of Agricultural and Food Chemistry. 2007;55(8):2993–2997. doi: 10.1021/JF063507N. [DOI] [PubMed] [Google Scholar]
- Runyon J.R., Sunilkumar B.A., Nilsson L., Rascon A., Bergenståhl B. The effect of heat treatment on the soluble protein content of oats. Journal of Cereal Science. 2015;65:119–124. doi: 10.1016/J.JCS.2015.06.008. [DOI] [Google Scholar]
- Sikora P., Tosh S.M., Brummer Y., Olsson O. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chemistry. 2013;137(1–4):83–91. doi: 10.1016/j.foodchem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Spellman D., McEvoy E., O’Cuinn G., FitzGerald R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. International Dairy Journal. 2003;13(6):447–453. doi: 10.1016/S0958-6946(03)00053-0. [DOI] [Google Scholar]
- Suryamiharja A., Gong X., Zhou H. Towards more sustainable, nutritious, and affordable plant-based milk alternatives: A critical review. Sustainable Food Proteins. 2024 doi: 10.1002/SFP2.1040. [DOI] [Google Scholar]
- Vasanthan T., Temelli F. Grain fractionation technologies for cereal beta-glucan concentration. Food Research International. 2008;41(9):876–881. doi: 10.1016/j.foodres.2008.07.022. [DOI] [Google Scholar]
- Walther B., Guggisberg D., Badertscher R., Egger L., Portmann R., Dubois S.…Rezzi S. Comparison of nutritional composition between plant-based drinks and cow’s milk. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.988707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Griffiths, &, Atungulu, G., Khir, R., Geng, J., Ma, H., Li, Y., & Wu, & B. (2015). Ultrasonic treatment effect on Enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophysics, 10, 244–252. doi: 10.1007/s11483-014-9375-y. [DOI]
- Wang R., Guo S. Phytic acid and its interactions: Contributions to protein functionality, food processing, and safety. Comprehensive Reviews in Food Science and Food Safety. 2021;20(2):2081–2105. doi: 10.1111/1541-4337.12714. [DOI] [PubMed] [Google Scholar]
- Wang Y.H., Jiang Y., Duan Z.Y., Shao W.L., Li H.Z. Expression and characterization of an α-glucosidase from Thermoanaerobacter ethanolicus JW200 with potential for industrial application. Biologia. 2009;64(6):1053–1057. doi: 10.2478/S11756-009-0197-1. [DOI] [Google Scholar]
- Wouters A.G.B., Nicolai T. Self-assembly of oat proteins into various colloidal states as function of the NaCl concentration and pH. Food Hydrocolloids. 2024;149 doi: 10.1016/J.FOODHYD.2023.109603. [DOI] [Google Scholar]
- Yang Z., Piironen V., Lampi A.M. Lipid-modifying enzymes in oat and faba bean. Food Research International. 2017;100:335–343. doi: 10.1016/J.FOODRES.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xie C., Bao Y., Liu F., Wang H., Wang Y. Oat: Current state and challenges in plant-based food applications. Trends in Food Science and Technology. 2023;134:56–71. doi: 10.1016/J.TIFS.2023.02.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data is publicly available and accessible via https://doi.org/10.5281/zenodo.14639733.