ABSTRACT
Connections between the multi‐ome and epigenetic age acceleration (EAA), and especially whether these are influenced by genetic or environmental factors, remain underexplored. We therefore quantified associations between the multi‐ome comprising four layers—the proteome, metabolome, external exposome (here, sociodemographic factors), and specific exposome (here, lifestyle)—with six different EAA estimates. Two twin cohorts were used in a discovery‐replication scheme, comprising, respectively, young (N = 642; mean age = 22.3) and older (N = 354; mean age = 62.3) twins. Within‐pair twin designs were used to assess genetic and environmental effects on associations. We identified 40 multi‐omic factors, of which 28 were proteins, associated with EAA in the young twins while adjusting for sex, smoking, and body mass index. Within‐pair analyses revealed that genetic confounding influenced these associations heterogeneously, with six multi‐omic factors —matrix metalloproteinase 9, complement component C6, histidine, glycoprotein acetyls, lactate, and neighborhood percentage of nonagenarians— remaining significantly associated with EAA, independent of genetic effects. Replication analyses showed that some associations assessed in young twins were consistent in older twins. Our study highlights the differential influence of genetic effects on the associations between the multi‐ome and EAA and shows that some, but not all, of the associations persist into adulthood.
Keywords: environment, epigenetic age acceleration, genetics, multi‐omics, twins
The connections between epigenetic age acceleration (EAA) and the multi‐ome, as well as the extent to which these connections are driven by genetic and environmental factors, remain underexplored. We identified blood proteins, metabolites, and exposures associated with EAA in young adults and, using twin study designs, show that these associations are heterogeneously influenced by genetic and environmental effects. Some associations persisted after controlling for all genetic influences, and replication in 60‐year‐old twins confirmed several of the identified associations.

1. Introduction
Globally, life expectancy has increased by one year every three years since the beginning of the 21st century according to the World Health Organization (2019). As a result, the number of older people is increasing and is expected to continue to increase in the coming years. Since age is a major risk factor for a variety of diseases (Kaeberlein 2017), such as COVID‐19 and Alzheimer's disease (Williamson et al. 2020; Hou et al. 2019), such a situation represents a pressing public health and societal issue. A better understanding of the biological mechanisms of aging, which progresses at different rates for different people, is needed. Thus, a better grasp of the role of genetics and the environment in the variation of aging is a key to addressing this challenge.
The advent of high‐throughput technologies has made it possible to generate large amounts of so‐called omics data, which ultimately makes it possible, for example, to assess the effect of genes on health using genotype data. Unlike the genotype, which remains essentially the same from birth to death, methylation of the DNA, a common epigenetic modification, has been shown to be dynamic across the life span. Both genotype and environmental factors (e.g., diet or early life experiences) interact to shape DNA methylation patterns during the life course, giving them a high potential to be used as biomarkers of environmental exposures, behavior, or disease. For example, DNA methylation patterns have been identified as biomarkers for smoking and perfluoroalkyl substances (Tsai et al. 2018; Liu et al. 2022). In addition, many studies have provided evidence for age‐related changes in DNA methylation levels at specific CpG sites (Christensen et al. 2009). This led to the development of DNA methylation‐based epigenetic clocks with the goal of estimating biological age, which is a more accurate reflection of health and a better biologically informed measure of age than chronological age (Salameh et al. 2020). Because the study of biological age can be insightful in comparison to chronological age, another measure used is epigenetic age acceleration (EAA), which is determined by regressing biological age on chronological age. EAA values are therefore an indication of how many years a person is older than their chronological age would suggest at the time the blood sample is taken.
Several algorithms have been developed to estimate biological aging from DNA methylation data, including six that we used in the current study: Horvath (Horvath 2013), Hannum (Hannum et al. 2013), GrimAge (Lu et al. 2019), GrimAge2 (Lu et al. 2022), PhenoAge (Levine et al. 2018) and DunedinPACE (Belsky et al. 2022). The EAA estimates derived from each of these clocks (except DunedinPACE, which is used as is, since it is a measure that already controls for chronological age) have been widely used in studies targeting phenotypes such as lifestyle (Drouard et al. 2024a), environmental exposures (de Prado‐Bert et al. 2021) and diseases (Lundgren et al. 2022; Yusupov et al. 2023; Joyce et al. 2021; Foster et al. 2023; Carbonneau et al. 2024), all of which have demonstrated adverse health outcomes in individuals with greater EAA. However, studies investigating associations between EAA and multiple omics data remain scarce. Studies of biological aging integrating both blood omics and the concept of exposome appear to be even more limited, partly due to the complexity of the exposome, which includes diverse exposures ranging from proximal factors (e.g., lifestyle) to distal factors (e.g., sociodemographics). Classification of the exposome has been proposed in the literature, and we considered lifestyle, representing proximal factors, as an independent part of the exposome (sometimes referred to as the “specific exposome”) (Vrijheid 2014). The identification of blood biomarkers and exposures related to DNA methylation‐based aging would provide a more holistic picture of the plausible biological and sociodemographic determinants of aging and a deeper understanding of the underlying processes that translate into acceleration of biological age. While associations between EAA and blood omics are likely bidirectional, certain external exposome factors are more likely to drive EAA than the reverse. Lifestyle factors, such as substance use, are highly influenced by changes in the exposome (e.g., relocating for university), which may, in turn, impact EAA rather than the other way around. This highlights the potential for public health interventions to mitigate accelerated aging at the population level, as multiple factors likely contribute to EAA.
Mavromatis and colleagues (Mavromatis et al. 2023) investigated associations of EAA with genomic, transcriptomic and metabolomic data and reported several genes and metabolites to be associated with EAA and longevity. However, mendelian randomization analysis did not indicate any causal relationships between metabolites and EAA. Whether metabolites and plasma biomolecules are causally associated with EAA is therefore largely undetermined. The use of twin study designs may be a first step in identifying putative causal mechanisms (sometimes called ‘quasi‐causal’ mechanisms), as they allow for control of genetic confounding in associations (McAdams et al. 2021; Gustavson et al. 2024). Biological age has also been shown to be heritable (McCartney et al. 2021; Marioni et al. 2015), but the influence of genetic and environmental factors on the associations between EAA and several layers of the multi‐ome remains underexplored. In addition, whether genetics and environment influence these associations similarly across the different omic layers, and whether these associations are consistent across different EAA estimates and age groups are to be investigated. The use of twin cohorts and related designs would provide a platform to assess genetic and environmental influences on associations that would be difficult to assess in cohorts of unrelated individuals.
We designed a multi‐omic study of EAA in a twin cohort (Figure 1). First, we sought to identify markers of EAA in young adults (mean age: 22.3 years old) in four domains: the plasma proteome, the plasma metabolome, the external exposome (including physical and social factors), and the specific exposome (here, lifestyle‐related traits). Together, these four domains are hereafter referred to as the multi‐ome. We quantified associations between the multi‐ome and six different estimates of EAA using linear mixed‐effects models. We then investigated the role of genetics and the environment in the observed associations using twin study designs. Finally, we used an independent cohort of older (mean age: 62.3 years old) twins with available EAA, proteomic, and metabolomic data with the aim to replicate the associations identified in the young twins. As the age difference between the two twin cohorts is substantial, this allowed us to discuss which markers of EAA were consistent throughout adulthood and whether environmental and genetic influences on the associations remained the same.
FIGURE 1.
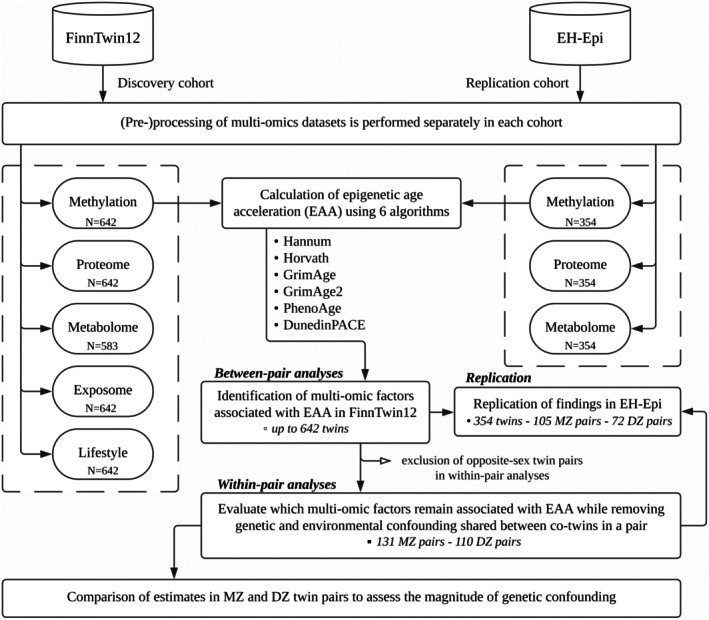
Study flow chart. The study was divided into three stages. The first was to quantify the associations between EAA and multi‐omic factors with all twin individuals from the FinnTwin12 cohort, referred to as between‐pair analyses. Next, within‐pair analyses were carried out on all complete same‐sex twin pairs. Finally, replication of the proteins and metabolites identified in FinnTwin12 was performed in the external EH‐Epi sample. EAA: Epigenetic Age Acceleration. MZ pairs: Monozygotic pairs. DZ pairs: Dizygotic pairs.
2. Methods
2.1. Cohorts and Participants
Main analyses were performed on young adult twins (mean age: 22.3; range: 21.0–24.7) from the FinnTwin12 cohort, including both between‐pair and within‐pair analyses (Figure 1). Significant associations between EAA and plasma biomolecules (i.e., proteins and metabolites) were replicated in an independent sample of older twins (mean age: 62.3; range: 56.0–70.0) from the Old Twin Cohort Essential Hypertension Epigenetics (EH‐Epi) study. Data processing is presented separately for each cohort, but the statistical analysis methodology applies to both cohorts.
2.1.1. FinnTwin12
FinnTwin12 is one of the core sub‐cohorts of the nationwide Finnish Twin Cohort (FTC), whose initial aim was to study adolescent behavior and mental health (Kaprio 2006; Rose et al. 2019). Participants were first identified from the Digital and Population Data Services Agency of Finland, and later asked to complete a series of questionnaires at the ages of 11/12, 14, 17, and as young adults (mean age: 22 years). In the latest assessment wave, a sample of these twins participated in a more detailed in‐person study with clinical measures, interviews, and questionnaires (Rose et al. 2019). In the morning of the assessment, a fasting venous blood sample was drawn, processed, and immediately stored at −80°C. Multiple omics were generated, including metabolomic, proteomic, and epigenetic data from plasma and DNA.
The present study is based on 642 twins from FinnTwin12 with all of the aforementioned complete omics data, except for 9% of them with no metabolomics data (Figure 1). All molecular omics were derived from the same blood sample. Other datasets depicting lifestyle and external exposome data were generated to be chronologically consistent with the time at which blood sampling was performed. The sample included 60% female participants. Age at blood sampling ranged from 21.0 to 24.7 years (mean: 22.3) in this sample (Table 1).
TABLE 1.
Description of the FinnTwin12 and EH‐Epi samples.
| FinnTwin12 (N = 642) | EH‐Epi (N = 354) | |
|---|---|---|
| Mean (sd)/Frequencies | Mean (sd)/Frequencies | |
| Age at blood sampling | 22.3 (0.6) | 62.3 (3.8) |
| Body mass index | 23.2 (3.9) | 27 (4.9) |
| Current smokers * | 249 | 56 |
| Never smokers * | 102 | 168 |
| Sex (females) * | 386 | 212 |
| Complete MZ pairs * | 131 | 105 |
| Complete DZ pairs * | 110 | 72 |
Note: Variable descriptions are given in terms of mean and standard deviation (sd) for continuous variables, and in terms of frequencies for binary variables marked with the symbol *.
2.1.2. Essential Hypertension Epigenetics (EH‐Epi) Study
The Old Cohort is another sub‐cohort of the FTC, which was initiated in the 70s and for which longitudinal data have been collected over time (Kaprio et al. 2019). Based on the fourth survey in 2011, a sample of this cohort, referred to as EH‐Epi, was constructed a few years later to study pairs of twins with a difference in blood pressure, as described elsewhere (Kaprio et al. 2019). These twins were invited to come in for a one‐day in‐person study, including measurement of their blood pressure (Huang et al. 2020). During their visit, the twins completed interviews and questionnaires, and their weight and height were measured. Fasting venous blood samples were also collected, from which multiple omics were generated (Drouard et al. 2022).
We used 354 twins (177 complete twin pairs) from EH‐Epi with complete DNA methylation, proteomic, and metabolomic data in the sample to replicate some of the findings from the main analyses (Figure 1). The twins in this sample were approximately 40 years older than those in FinnTwin12 (mean: 62.3; range: 56.0–70.0). Body mass index (BMI) ranged from 18.1 to 45.9 kg m−2 (mean: 27.0; SD: 4.9) (Table 1). The mean systolic blood pressure was 148.4 (SD: 16.0) and 139.7 (16.6) mmHg in men and women, respectively. The mean diastolic blood pressure was 85.8 (10.7) and 81.9 (9.4) mmHg in men and women, respectively.
2.2. Data Processing in FinnTwin12
2.2.1. DNA Methylation and EAA Calculation
DNA methylation levels were quantified using the Infinium Illumina HumanMethylation450K array and preprocessed using the R‐package meffil (Min et al. 2018), as described in detail elsewhere (Sehovic et al. 2023). EAA estimates, defined as the residuals of chronological age regressed on epigenetic age, were calculated using six different algorithms. Horvath (Horvath 2013), Hannum (Hannum et al. 2013), PhenoAge (Levine et al. 2018) and GrimAge (Lu et al. 2019) EAA estimates were calculated using their PC score version (Higgins‐Chen et al. 2022). Details about their calculations are described elsewhere (Kankaanpää et al. 2022). These aging estimates were used in their PC score versions to reduce the influence of technical variation on the age estimates. Original versions of these clocks, calculated without the PC‐based method, were highly correlated in all twins from the Finnish Twin Cohort with available DNA methylation data (450 K); the pairwise correlations were r = 0.93 for GrimAge, r = 0.97 for Horvath and PhenoAge, and r = 0.98 for Hannum. In addition, we calculated estimates of EAA with the DunedinPACE (Belsky et al. 2022) and GrimAge2 (Lu et al. 2022) clocks. Although the DunedinPACE clock does not strictly follow the definition of EAA, we refer to it as an EAA estimate in the current study for ease of reading. Higher values of the DunedinPACE estimates indicate a greater pace of aging, whereas higher values of the other aging clocks used in the current study indicate an accelerated age at the time of blood sampling. All 642 participants had a complete sextuplet of EAA values. All EAA estimates were positively associated with BMI in this sample (p < 0.05), as assessed using linear mixed‐effects models. Males exhibited higher accelerated aging across all clocks except for GrimAge2 (p = 0.20). Additionally, GrimAge, GrimAge2, and DunedinPACE estimates were higher in current smokers (p < 0.05). EAA estimates showed relatively moderate phenotypic correlations in all twin individuals (mean Pearson correlation: r = 0.53; Table S1).
2.2.2. Proteomics
Proteins from the plasma samples of 786 participants were precipitated and subjected to in‐solution digestion according to the standard protocol of the Turku Proteomics Facility (Turku Proteomics Facility, Turku, Finland). Details about protein depletion, precipitation, and digestion in this sample have been described elsewhere (Afonin et al. 2024). Samples were first analyzed by independent data acquisition LC–MS/MS using a Q Exactive HF mass spectrometer and further analyzed using Spectronaut software. Data were locally normalized (Callister et al. 2006), and the raw matrix was processed and quality controlled as described elsewhere (Drouard et al. 2023). Briefly, protein levels were log2‐transformed, and proteins with > 10% missing values were excluded. Missing values were imputed by the lowest observed value for each protein carrying missing values. Corrections for batch effects were performed with Combat (Leek et al. 2012) and the final proteomic dataset comprised 439 proteins, which were scaled such that one unit corresponded to one standard deviation (sd). Protein descriptions are available in the Supporting Information (Table S2).
2.2.3. Metabolomics
Metabolites were quantified from plasma samples using high‐throughput proton nuclear magnetic resonance spectroscopy (1H‐NMR) (Nightingale Health Ltd., Helsinki, Finland) (Soininen et al. 2015; Bogl et al. 2016; Rose et al. 2019). Detailed data description (Whipp et al. 2022) are available elsewhere. Data processing included (1) exclusion of pregnant women and individuals taking cholesterol‐lowering medications, (2) imputation of missing values using the sample minimum value for metabolites having less than 10% of missing values (NA) and metabolites with > 10% missing values were excluded, and (3) examination for the presence of outliers. Pregnant women were excluded only from metabolomic data processing, but not from other omics analyses. Outlier presence was assessed by verifying that no participant had any of its first three principal components with an absolute value greater than 5 SD from the mean. Metabolites were normalized using inverse normal rank transformation so that one unit corresponded to a change of one SD, with a mean of zero. The list of metabolites included in the analyses is available in the Supporting Information (Table S2).
2.2.4. External Exposome
The external exposome data comprised a total of 62 exposures derived from Statistics Finland and other sources available for all participants. These exposures represented the sociodemographic environment of the twins. We used twin's residential geocodes in 2005–2006 to merge the exposures, which were described elsewhere (Wang et al. 2023). Briefly, exposures were continuous and included: sizes of and access to green spaces, percentage of built‐up areas, population ages and headcounts, crime rates, and voting patterns at municipal elections. Missing values, representing 2.1% of the total data points, were imputed by the median, with the highest missing value rate per variable being 7.5%. A complete description of the exposome's variables is available in the Supporting Information (Table S2).
2.2.5. Lifestyle
A total of 13 binary variables were selected to describe the lifestyle of the twins, and in particular their education, leisure time activities, substance use, and social behavior. These were derived from questionnaires completed at home and at the study visit when blood samples were taken, as was described elsewhere (Drouard et al. 2024a). Eleven of these variables were initially categorical and represented frequencies: playing video games, watching videos, playing an instrument, reading, going out, dancing, taking part in a club, going to a fast food restaurant, going to a bar, drinking alcohol, and having alcohol‐induced blackouts. These variables were dichotomized into binary variables so that the modalities were defined by a frequency of at most once a month vs. at least once a week. Additionally, obtaining a vocational degree (modalities: yes/no) and age of first sexual intercourse (modalities: strictly before age 18/at or after age 18) were included. The list of lifestyle‐related variables is available in the Supporting Information (Table S2).
We created within‐pair, binary lifestyle variables for use in within‐pair analyses as well (Drouard et al. 2024a). Briefly, these were coded so that “1” indicated a discordant pair, while “0” indicated a concordant pair. That is, “1” denoted a twin pair in which one twin expressed a feature that the other did not (e.g., one twin often plays video games while the other twin does not), and “0” denoted two co‐twins expressing the same trait.
2.2.6. Covariates
Between‐pair analyses included four covariates: sex, body mass index (BMI), and lifetime and current smoking exposure. BMI was calculated from measured height and weight taken during in‐person visits at the time of blood sample and was available for all but 10 FinnTwin12 participants; imputation was performed using the median BMI for these 10 participants. BMI ranged from 16.4 to 51.2 kg m−2 (mean: 23.2; SD: 3.9) in the FinnTwin12 sample. Smoking covariates were binary and represented lifetime and current smoking exposure. Lifetime smoking exposure was coded in such a way that twins who had never smoked in their lifetime were coded as “1” and the others as “0”. Current smoking exposure was coded so that twins who never smoked, tried smoking but did not smoke, or quit smoking were coded as “1” and compared with twins who currently smoke, coded as “0”. Of the 642 individuals included in the analyses, 102 had never smoked and 249 were current smokers (Table 1).
The same covariates were included in within‐pair analyses, namely: sex of the pair, within‐pair BMI differences, and lifetime and current smoking discordance. These two latter binary covariates were constructed such that twins within a pair with similar smoking habits (i.e., (“0” “0”) (“1” “1”)) were considered to form a concordant pair, whereas a pair was discordant if two co‐twins did not have the same smoking habits (i.e., (“0” “1”) (“1” “0”)). Of the 241 pairs of twins included in the within‐pair analyses, 41 and 61 were discordant for lifetime and current smoking exposure, respectively.
2.3. Data Processing in External EH‐Epi Sample
2.3.1. DNA Methylation and EAA Calculation
DNA methylation levels were quantified using Infinium Illumina HumanMethylation450K. Data preprocessing is identical to that detailed for the FinnTwin12 sample. A total of 354 participants, including 105 MZ pairs and 72 same‐sex DZ pairs, had complete sextuplet EAA values. Correlations between EAA values are in the Supporting Information (Table S1).
2.3.2. Proteomics
Proteomic profiling was initially performed on 415 plasma samples from participants in the EH‐Epi sample. The samples were analyzed using an antibody‐based technology (Olink Proteomics AB, Uppsala, Sweden; Olink Explore 384) and processed as described elsewhere (Drouard et al. 2024b). Briefly, the data were quality controlled according to Olink's internal quality control criteria, which led to the rejection of a few outlier samples. Proteins for which the frequency of samples with NPX values below the plate‐specific limit of detection (LoD) was greater than 20% were excluded, and values of remaining proteins below the LoD, representing less than 1% of the total data points, were replaced by the LoD value. Final proteomic data were available for 401 EH‐Epi twins, of which 354 were included in the current study (Figure 1; Table 1). A total of 2321 proteins were quantified and their values were expressed as Normalized Protein eXpression (NPX) values, where NPX is Olink's unit for quantifying relative protein levels on a log2 scale. Proteins were then matched by gene name to those in the FinnTwin12 set if they overlapped. Protein descriptions of overlapping proteins used for replication analyses are available in the Supporting Information (Table S3).
2.3.3. Metabolomics
Metabolomic data were generated using high‐throughput proton nuclear magnetic resonance (1H‐NMR) spectroscopy using the same platform as for the FinnTwin12 sample (Nightingale Health Ltd., Helsinki, Finland). Metabolite values below the limit of quantification were reported as missing, as were metabolite values that deviated from the mean by more than 5 standard deviations (SD). Metabolites with more than 10% missing values were discarded. Missing values (rate of missing values in the dataset: 0.6%) were imputed using the sample minimum of each metabolite for which imputation was to be performed. Outlier presence was assessed by verifying that no participant had any of its first three principal components with an absolute value greater than 5 SD from the mean. Metabolite values were normalized using inverse normal rank transformation, with a mean of zero and variance one. In the current study, only metabolites that could be matched to the FinnTwin12 set were used (Table S3).
2.3.4. Covariates
The use and construction of covariates for between‐pair and within‐pair analyses was identical to that described for FinnTwin12. BMI was calculated from height and weight measurements taken during in‐person visits at the time of blood sample. Description of the EH‐Epi sample is shown in Table 1.
2.4. Statistical Analyses
Analyses were divided into two main parts. First, between‐pair analyses were performed to calculate the associations between EAA and multi‐omic factors for all twins. For these analyses, linear mixed‐effects models were used. Second, within‐pair analyses were performed for all twin pairs, for MZ twin pairs only, and for DZ twin pairs only (Figure 1).
2.4.1. Between‐Pair Analyses
Linear mixed‐effects models were used to quantify the associations between EAA and multi‐omic factors. For each EAA variable derived from one of the 6 different algorithms considered, these analyses were performed independently. EAA estimates were modeled as dependent variables to enable downstream comparisons of effect sizes across multi‐omic factors. This approach also supports interpreting estimates under the assumption that certain factors, such as lifestyle and exposome variables, are more likely to contribute to EAA than vice versa. However, our models do not establish causal direction in these associations. Fixed‐effect covariates included sex, BMI, and the two binary variables representing lifetime and current smoking habits (Table 1). To correct for data clustering due to familial relationships among participants, we used family identifiers as a random effect.
The strength of the association between EAA and a multi‐omic factor k was assessed by testing the nullity of its coefficient . Satterthwaite's approximation was used to calculate resulting p values. To counteract the occurrence of false positives, multiple testing corrections were applied. In FinnTwin12 analyses, we corrected the nominal p values using the number of principal components (PCs) that are needed to cover at least 95% of the initial variance of the omic to which they belong, as calculated by Principal Component Analysis (Gao et al. 2008; Li and Ji 2005). This multiple testing correction was used to account for the high correlations within the omics data and to avoid using too conservative multiple testing corrections as FinnTwin12 was used as the discovery cohort. A nominal p value was considered significant if it was below α/ Neff , with α = 0.05 and Neff the number of PCs needed to cover > 95% of the original variance. The proteins and metabolites to be replicated in the EH‐Epi sample were selected using this last criterion. As the EH‐Epi sample was used for replication, we adjusted the nominal p values using the Bonferroni method.
2.4.2. Within‐Pair Analyses
To assess the genetic and environmental effects underlying associations between multi‐omic layers and EAA, we used within‐pair twin designs. These designs exploit the fact that differences in values (e.g., EAA or protein levels) between MZ co‐twins arise solely from environmental differences, as MZ co‐twins share identical genomic sequences. In contrast, differences between DZ co‐twins may be larger, since DZ twins share only part of their genetic makeup with their co‐twin. By using within‐pair twin designs in MZ pairs only, one can control for all genetic confounding in associations, allowing for quasi‐causal inferences where causality is probable—albeit without providing full proof of causality or indicating the direction of the association (McAdams et al. 2021; Gustavson et al. 2024).
In within‐pair analyses, only complete same‐sex twin pairs were included (Figure 1). Analyses were performed on all complete twin pairs, on DZ twin pairs only, and on MZ twin pairs only. The stratification of within‐pair analyses by zygosity was motivated by the fact that while the use of all pairs corrects for environmental factors shared between co‐twins while maintaining a high level of statistical power, only within‐pair analyses using MZ pairs additionally ensure the removal of all genetic confounding in multi‐omic associations of EAA. Linear regression models were fitted to model differences in EAA values within a pair, modeled here as the dependent variable, by differences in the multi‐omic factors within the pair. Covariates added were sex of the pair, differences in BMI, and lifetime and current smoking binary variables indicating discordance in smoking habits (Table 1). Differences in values were all calculated in such a way that one of the two co‐twins was randomly annotated as twin1, from which the value of twin2 was subtracted. This was done in the same order across all datasets. Nullity testing of the coefficient of the multi‐omic factors was used to assess the strength of the associations. Correction for multiple testing was performed identically as for between‐pair analyses, that is, correction for effective tests (95% inertia covered by PCs at the omic level) in FinnTwin12 and Bonferroni correction for all associations tested in EH‐Epi.
Depending on the degree of genetic confounding affecting the multi‐omic associations of EAA, differences in the coefficients obtained in all pairs, DZ pairs only, and MZ pairs only are expected to differ accordingly, as the correction for genetic confounding is either complete in MZ pairs or partial in DZ pairs. Therefore, we compared the coefficients obtained in all pairs ((within all pairs)), DZ pairs only ((within DZ pairs)), and MZ pairs only ((within MZ pairs)). To do so, we notably calculated ratios of the coefficients, such as (within MZ pairs)/(within DZ pairs), for which values close to 1 indicate weak genetic effects in the studied association, as the coefficient in MZ twin pairs does not differ substantially to that obtained in DZ twin pairs. Conversely, the lower the (within MZ pairs)/(within DZ pairs) ratio, the more likely genes confound the studied association.
3. Results
The main analyses were divided into three parts (Figure 1) and involved two independent samples of twins, as described in Table 1. First, associations between EAA and multi‐omic factors were quantified in all twin individuals from FinnTwin12. These analyses are referred to as between‐pair analyses. A list of the multi‐omic factors included in these analyses is available in the Supporting Information (Table S2). Second, to investigate whether associations were robust to correction for shared environmental and genetic effects, within‐pair analyses were performed. In parallel, we investigated the contribution of genetics in the observed associations by comparing within‐pair estimates in monozygotic (MZ) twin pairs to those in dizygotic (DZ) twin pairs, as MZ twins in a pair are identical at the DNA sequence level, whereas DZ twins in a pair share on average only half of their segregating genes. Finally, significant associations observed between EAA and proteins and metabolites were examined in a replication set of older twins from the Older Cohort EH‐Epi sample.
3.1. Between‐Pair Analyses Reveal a Broad Range of Multi‐Omic Associations With EAA
Linear mixed‐effects models were fitted to quantify associations between six different EAA estimates (Figure 1) and the multi‐ome in the FinnTwin12 participants. Models were adjusted for sex, BMI, and smoking. We identified 40 unique multi‐omic factors associated with one or multiple EAA estimates after correcting p values for multiple testing (total number of associations: 77) (Figure 2, Table S4). All omics showed associations with at least one EAA measure, with the DunedinPACE estimate showing the greatest number (n = 19) of associations with multi‐omic factors (Table S4). As could be expected, the clocks that were developed to estimate chronological age (Hannum and Horvath) showed significantly fewer associations compared with the clocks that were developed to estimate mortality and phenotypic aging (GrimAge, GrimAge2 and PhenoAge) (Table S4). Similarities and differences between the EAA estimates in their associations with multi‐omic factors are shown in Figure 3. The direction of the observed multi‐omic factor associations shared across the EAA estimates was consistent (i.e., the sign of the coefficients was the same across all the EAA estimates, Table S4).
FIGURE 2.
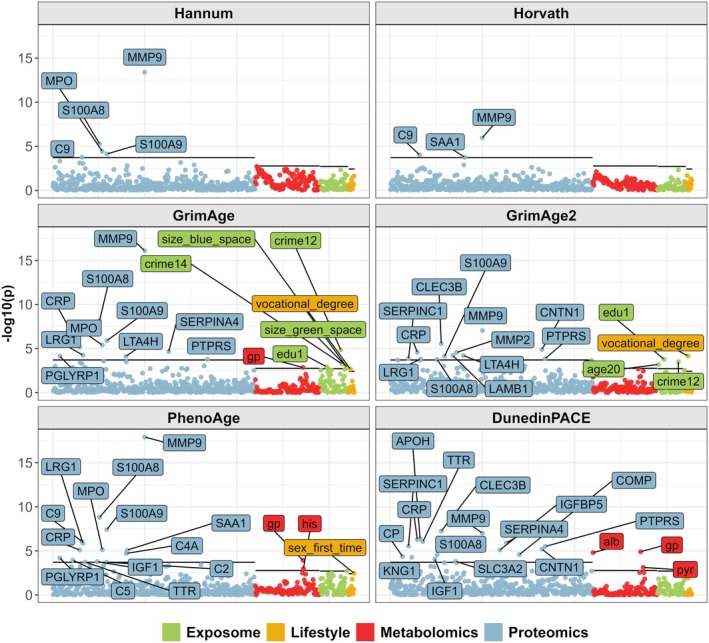
Between‐pair analyses reveal numerous associations between epigenetic age acceleration and multi‐omic factors. Linear mixed‐effect models were used to quantify associations between EAA and multi‐omics factors in between‐pair analyses. Sex, body mass index, and smoking were used as covariates. Correction for multiple testing was based on the number of principal components needed to cover 95% of the initial variance for each omic and is indicated by a solid line. A description of the multi‐omics factors is available in the Supporting Information (Table S2). EAA: Epigenetic age acceleration. p: p value resulting from testing the nullity of the multi‐omic factor coefficient.
FIGURE 3.
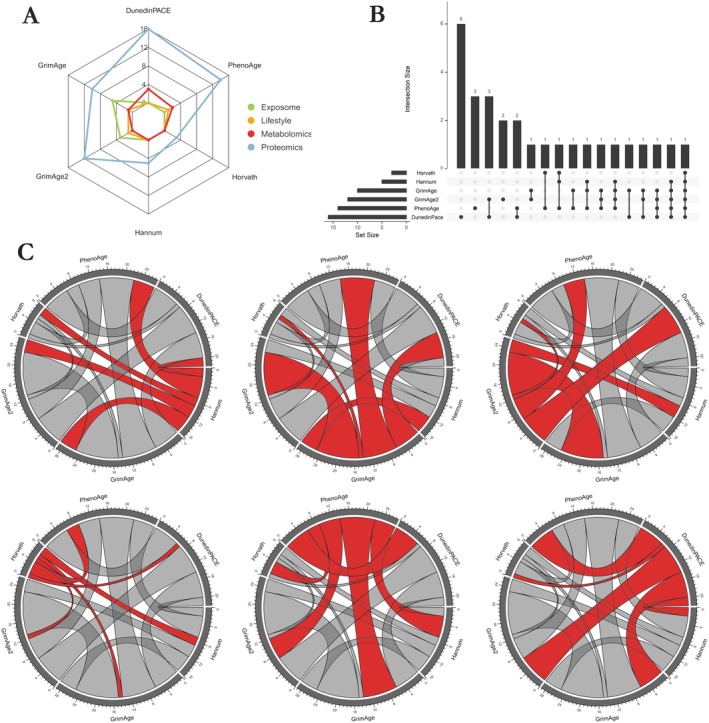
Common associations between multi‐omic factors and EAA across different EAA estimates, and omic specificities of different EAA estimates. (A) Radar plot showing the number of associations between EAA and proteins, metabolites, and lifestyle or exposome variables for each EAA estimate. Only the GrimAge and GrimAge2 estimates were associated with exposome variables, whereas the DunedinPACE estimate showed a greater number of associations with plasma omics. (B) Upset plot showing the overlap of identified proteins across the 6 EAA estimates. 62% of these proteins were associated with more than one EAA estimate. (C) Circos plots showing the pairwise number of shared associations between EAA estimates. The six circos plots are identical, with connections between an EAA estimate and others colored in red. The thicker the connection between two EAA estimates, the more they share common associations with multi‐omic factors. For example, the DunedinPACE EAA estimate is associated with several multi‐omic factors that are also associated with the GrimAge, GrimAge2, and PhenoAge estimates, but very few with Hannum and Horvath (see circos plot at the bottom right).
3.1.1. Associations Between Proteins and EAA Estimates
Altogether 60 associations were identified between EAA estimates and plasma proteins. These involved 28 unique proteins, of which 11 were associated with only one estimate of EAA. The rest were found to be associated with multiple EAA estimates (Figure 3B). Here, the protein nomenclature will follow the gene nomenclature for clarity. Matrix metallopeptidase 9 (coding gene: MMP9, with the respective protein referred to as MMP9) was the only protein associated with all six EAA estimates, with standardized coefficients ranging from 4.9 to 9.1. Proteins associated with three or more EAA estimates also included S100‐A8 (S100A8), S100‐A9 (S100A9), C‐reactive protein (CRP), complement component C9 (C9), leucine‐rich alpha‐2‐glycoprotein (LRG1), myeloperoxidase (MPO), and receptor‐type tyrosine‐protein phosphatase S (PTPRS). The full set of proteins associated with EAA estimates and detailed summary statistics is available in the Supporting Information (Table S4).
3.1.2. Associations Between Metabolites and EAA Estimates
Significantly fewer associations were observed between metabolites and EAA estimates compared with proteins and EAA estimates, and none of the associations were with EAA estimates developed for chronological age. Four metabolites, albumin (variable: alb), glycoprotein acetyls (gp), histidine (his) and pyruvate (pyr) were associated with EAA estimates (Figure 2; Table S4). Albumin and histidine were negatively associated with EAA estimates; the other two were positively associated. Glycoprotein acetyls was the only metabolite associated with multiple EAA estimates (standardized coefficient range: 3.2–4.4): PhenoAge, GrimAge, and DunedinPACE (Figure 3).
3.1.3. Associations Between Exposome and Lifestyle, and EAA
Several factors associated with EAA other than proteins and metabolites were also identified (Figure 2; Table S4). Associations between the external exposome and EAA were identified only with GrimAge and GrimAge2 estimates. Neighborhood crime rates per capita at the municipal level (crimes against life and health: crime12; crimes against authority and public order: crime14), proportion of individuals aged 20 to 29 (age20) and proportion of the population with unknown or no education after primary or lower secondary education in the population aged 16 and over (edu1) at the postal code level, and sizes of the nearest green (size_green_space) and blue (size_blue_space) spaces were associated with EAA. Associations with EAA were positive for edu1 and size_green_clc, otherwise negative. Finally, we identified two lifestyle‐related variables associated with EAA. Twins who had their first sexual intercourse before the age of 18 had a higher PhenoAge EAA than those who had their first sexual intercourse after the age of 18 (standardized coefficient: 2.9). Twins who only had a vocational degree were also found to have a higher EAA than twins who did not have a vocational degree (standardized coefficient with GrimAge: 3.1; with GrimAge2: 4.0).
3.1.4. Confounding Effects of BMI and Smoking
We also performed additional between‐pair analyses adjusting only for sex to assess the confounding effects of BMI and smoking on the association between EAA and the multi‐ome. These analyses resulted in the identification of 142 unique multi‐omic factors associated with EAA, including 89 proteins, 41 metabolites, 7 exposome variables, and 5 lifestyle‐related variables (Table S5). While the increase in the number of exposome and lifestyle variables was modest compared to the fully adjusted models, not adjusting for BMI and smoking dramatically increased the number of associations with plasma omics. These results suggest that many associations between plasma omics and EAA in models adjusted for sex only are likely to be confounded by BMI and smoking habits.
3.2. Within‐Pair Models Reveal Associations Robust to Correction for Shared Environmental Factors and Genetics
We used within‐pair analyses to investigate whether there would be significant associations between EAA and the multi‐ome while correcting for shared environment and genetics. We performed three sets of within‐pair analyses with different degrees of correction for shared environmental and genetic effects: all same‐sex twin pairs, same‐sex DZ twin pairs only, and MZ twin pairs only. While within‐pair analyses using all pairs correct for environmental factors and genetic polymorphisms that are shared between the co‐twins while maintaining a high level of statistical power, only within‐pair analyses using MZ pairs additionally ensure the removal of all genetic confounding in the associations.
A total of 53 associations were significant in the within‐pair analyses using all pairs of twins (n = 241 pairs), involving 31 multi‐omic factors (Table S6). About half of these associations (27/53) were also identified in between‐pair analyses (Figure S1). These findings suggest that environmental factors and genetic polymorphisms shared by the co‐twins either do not have an influence or cannot be the only influence on part of the associations between EAA and the multi‐ome. Significant associations found in both between‐pair and within‐pair analyses were mostly between proteins and EAA estimates, with the exception of the metabolite albumin, which was negatively associated with the DunedinPACE estimate of EAA. MMP9 was associated with all six EAA estimates, as observed in the between‐pair analyses.
In the within‐pair analyses of DZ pairs only (n = 110 pairs), 23 associations were significant (involving 14 multi‐omic factors), 16 of which were with proteins (Table S7). We observed associations between several crime rate variables from the exposome domain and EAA, as well as an association between the lifestyle‐related variable indicating participation in clubs and Horvath estimate of EAA, all of which were negative.
In MZ pairs only (n = 131 pairs), we observed 8 significant associations (involving six multi‐omic factors), three of which were with proteins, three with metabolites, and two with exposome variables (Table S8). MMP9 was positively associated with GrimAge (standardized coefficient: 5.5) and PhenoAge (standardized coefficient: 5.7) estimates of EAA. The complement component C6 (C6) protein was positively associated with GrimAge's estimate of EAA (standardized coefficient: 4.0). Regarding metabolites: histidine was negatively associated with PhenoAge's estimate of EAA (standardized coefficient: −3.3). Glycoprotein acetyls metabolite was negatively associated with DunedinPACE's estimate of EAA (standardized coefficient: −3.3), and lactate was positively associated with Phenoage's estimate of EAA (standardized coefficient: 3.3). These results suggest that either a scenario in which these molecules are causally related to EAA is plausible, or that environmental factors not shared by the co‐twins influence these associations. Finally, we identified that the proportion of individuals aged 90 and older in the area linked to the twins residential geocode (exposome variable age 90) was positively associated with both PhenoAge (standardized coefficient: 3.3) and GrimAge (standardized coefficient: 3.4) estimates of EAA within MZ pairs. This echoes the negative associations that were identified in between‐pair analyses between the proportion of young individuals aged 20 to 29 in the geocode (age20) and EAA.
3.3. Within‐Pair Analyses Provide Evidence for Heterogeneous Genetic Influences in the Observed Associations
While within‐pair analyses allow for the identification of associations between EAA and the multi‐ome that are independent of shared environmental and genetic factors, they also provide insight into how genetics underlie these associations: larger estimates in DZ pairs compared with MZ pairs reflect greater genetic influence in the associations. Therefore, we examined in detail the associations that were significant in all twin pairs and compared the estimates of these associations in MZ twin pairs only with those in DZ twin pairs only (Figure 4A, Figure 4B). Of the 53 significant associations identified in within‐pair analyses in all twin pairs, 49 showed higher estimates in DZ pairs compared with MZ pairs (Table S9). On average, the estimates in DZ pairs were 76% higher than in MZ pairs (interquartile range (%): 17.5–87.7).
FIGURE 4.
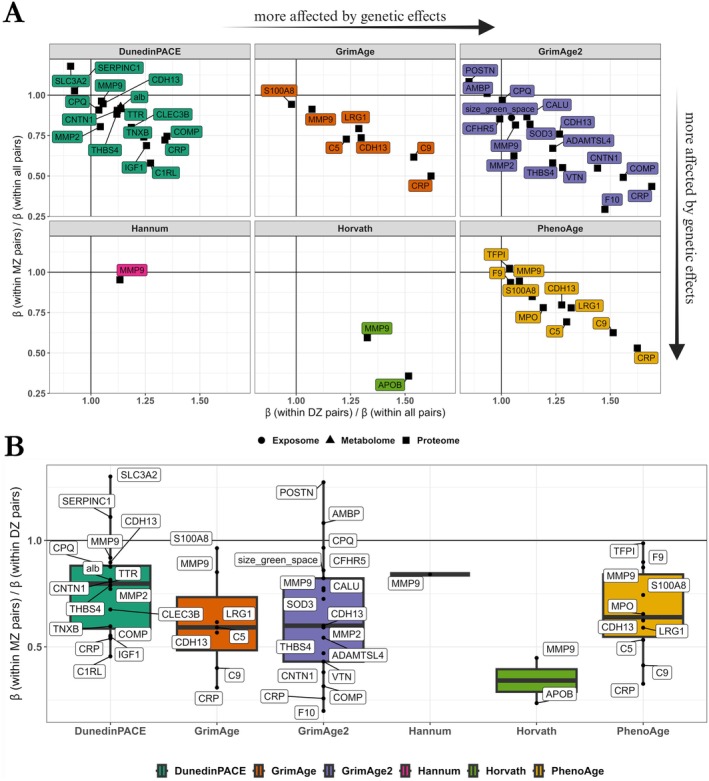
Comparisons between MZ and DZ pairs in within‐pair analyses provide evidence for genetic confounding in associations. (A) Comparison of estimates in MZ [(within MZ pairs)] and DZ [(within DZ pairs)] pairs for multi‐omics factors significantly associated with EAA in all pairs [(within all pairs)]. Multi‐omics factors located farthest down and to the right are those for which the genetic effects in the association are most intense, as indicated by the arrows on the side of the plot. Conversely, weak genetic effects in associations are indicated by multi‐omics factors with coefficient ratios close to 1. (B) For most multi‐omic factors associated with EAA in all pairs, estimates in DZ twins [(within DZ pairs)] are higher than those in MZ twins [(within MZ pairs)]. The lower the ratio of MZ to DZ pair coefficients, the more likely the genetic confounding in these associations with EAA. EAA: Epigenetic age acceleration. Variable descriptions are available in the Supporting Information (Table S2).
Thirteen associations, involving 10 unique proteins, had estimates in DZ pairs that were at least twice as large as in MZ pairs (Figure 4B, Table S9), indicating that genetic effects are likely the main and only driver for these associations. These proteins were CRP, coagulation factor X, cartilage oligomeric matrix protein (COMP), apolipoprotein B‐100 (APOB), C9, vitronectin, thrombospondin‐4, CNTN1, complement C1r subcomponent‐like protein, and MMP9. Interestingly, while the association between MMP9 and Horvath EAA showed a high degree of genetic confounding (ratio coefficient in MZ pairs vs. DZ pairs: 0.45; Figure 4B), the associations of MMP9 with other clocks showed a lower degree of genetic influence (ratio coefficient in MZ pairs vs. DZ pairs ranged from 0.77–0.92 for the remaining five clocks; Figure 4B). This suggests that there may be differences in genetic influences between MMP9 and EAA across clocks.
3.4. Replication of Associations With Plasma Proteins and Metabolites in an Independent Sample of Older Twins
We sought to replicate the findings from the FinnTwin12 study of young adult twins in an independent cohort of older twins (Figure 1; Table 1).
Of the 40 multi‐omic factors identified in the between‐pair analyses in FinnTwin12, 4 were metabolites and 28 were proteins. Of these, 13 proteins could not be investigated in the EH‐Epi sample due to proteomics platform differences between the cohorts. The standardized coefficients of 4 metabolites and 15 proteins estimated in this independent EH‐Epi twin sample correlated highly with those of FinnTwin12 (Pearson correlation estimate: r = 0.73; confidence interval: [0.55,0.85]). Altogether, 17 of the 39 associations replicated in the EH‐Epi sample with nominal p value below 0.05 (Figure 5A, Table S10). Nine associations were robust to Bonferroni correction, six of which were with proteins. MMP9 was positively associated with DunedinPACE (standardized coefficient: 3.8; Bonferroni p = 0.006) and GrimAge2 (standardized coefficient: 3.6; Bonferroni p = 0.013) estimates of EAA. CNTN1 was negatively associated with DunedinPACE (standardized coefficient: −3.6; Bonferroni p = 0.014) and GrimAge2 (standardized coefficient: −3.9; Bonferroni p = 0.004) estimates of EAA. PTPRS was associated with both GrimAge (standardized coefficient: −4.0; Bonferroni p = 0.003) and GrimAge2 (standardized coefficient: −3.7; Bonferroni p = 0.010) estimates of EAA. Among metabolites, the glycoprotein acetyls metabolite was associated with both GrimAge (standardized coefficient: 4.6; Bonferroni p = 2.2e‐4) and DunedinPACE (standardized coefficient: 4.5; Bonferroni p = 3.7e‐4) EAA. In addition, albumin was negatively associated with DunedinPACE's EAA (standardized coefficient:‐4.6; Bonferroni p = 2.7e‐4).
FIGURE 5.
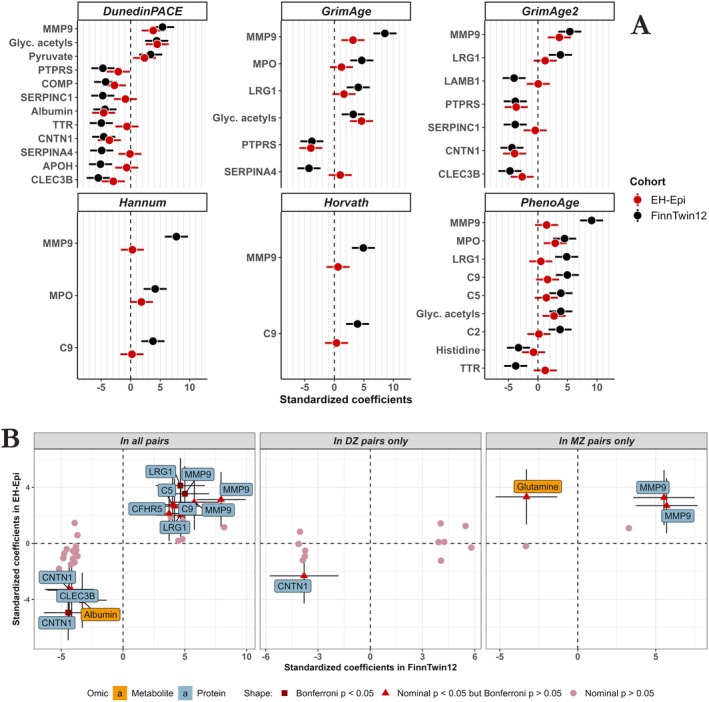
Replication of between‐pair and within‐pair findings from FinnTwin12 young adult twins in the EH‐Epi sample of older adults. (A) Standardized coefficients and their 95% confidence intervals in the EH‐Epi sample next to those found in FinnTwin12. Almost half of the replicated associations had a nominal p value testing the null of the coefficients below 0.05, 9 of which were robust to Bonferroni correction. (B) Comparison of standardized coefficients obtained in within‐pair analyses in the EH‐Epi sample with those found in FinnTwin12 in three configurations: In all pairs, in DZ pairs only, and in MZ pairs only. Associations with nominal p values below 0.05 are labeled and their 95% confidence intervals are shown. The shape of the dots indicates whether the associations were robust to the Bonferroni correction considering all associations tested. Metabolites are annotated with their complete name, with the exception of Glyc. acetyls, being glycoprotein acetyls. Proteins are annotated with their coding genes. C9: Complement component C9. MPO: Myeloperoxidase. MMP9: Matrix metalloproteinase‐9. LRG1: Leucine‐rich alpha‐2‐glycoprotein. CLEC3B: Tetranectin. SERPINA4: Kallistatin. PTPRS: Receptor‐type tyrosine‐protein phosphatase S. SERPINC1: Antithrombin‐III. APOH: Beta‐2‐glycoprotein 1. LAMB1: Laminin subunit beta‐1. CNTN1: Contactin‐1. C5: Complement component C5. TTR: Transthyretin. DPP4: Dipeptidyl peptidase 4. COMP: Cartilage oligomeric matrix protein. CFHR5: Complement factor H‐related protein 5.
In within‐pair analyses, we were able to test a total of 53 associations in the EH‐Epi sample out of the 84 identified in the FinnTwin12 including all pairs, DZ, and MZ pairs only (Figure 5B, Table S11). Standardized coefficients of metabolites and proteins in the EH‐Epi sample were moderately correlated with those obtained in FinnTwin12 (Pearson correlation estimate: r = 0.67; confidence interval: [0.49, 0.80]). A total of 16 associations were replicated with a nominal p value below 0.05 (Figure 5B) and four associations were robust to Bonferroni correction (i.e., nominal p value below 0.05/53 = 9.4e‐4) in within‐pair analyses using all twin pairs. CNTN1 was most strongly associated with EAA (clock: GrimAge2; standardized coefficient: −4.9; Bonferroni p = 1.0e‐4), followed by LRG1 (GrimAge; standardized coefficient: 4.1; Bonferroni p = 0.003), albumin (DunedinPACE; standardized coefficient: −4.1; Bonferroni p = 0.004) and MMP9 (DunedinPACE; standardized coefficient: 3.5; Bonferroni p = 0.028). In addition, associations within MZ pairs showed high evidence of significance despite not reaching the Bonferroni threshold, such as the metabolite glutamine (nominal p = 1.3e‐3; Bonferroni p = 0.067) and MMP9 (nominal p = 1.5e‐3; Bonferroni p = 0.079) with DunedinPACE and GrimAge EAA estimates, respectively. Interestingly, the association between glutamine and DunedinPACE EAA was negative in the FinnTwin12 sample of young adults, but positive in the older twins of the EH‐Epi sample.
4. Discussion
Our study provides a comprehensive, in‐depth epidemiological view of the connections between EAA and the multi‐ome. We identified 40 unique multi‐omic factors associated with accelerated epigenetic aging—including proteins, metabolites, external exposures, and lifestyle‐related traits—while adjusting for BMI and smoking. A larger number of associations was identified through sensitivity analyses not corrected for BMI and smoking, suggesting that most of such associations between plasma omics and EAA are likely confounded by body weight and smoking habits. Using twin study designs, we evaluated the influence of genetic and environmental factors on multi‐omic associations with EAA and demonstrated that these associations show varying degrees of evidence for genetic confounding. Of note, only a fraction of the associations in between‐pair analyses were significant in within‐pair analyses, suggesting an overall large role for genetic effects underlying multi‐omic associations of EAA, although the loss of statistical power in within‐pair analyses may partially explain this observation. In addition, we identified eight significant associations with EAA in within‐pair analyses of MZ twins, suggesting either plausible causality in these associations and/or unique environmental factors influencing them independently of genetic factors. Finally, by replicating our findings obtained in a sample of 20‐year‐old twins in an independent sample of 60‐year‐old twins, we demonstrated that a small proportion of multi‐omic factors reflect accelerated epigenetic aging across age groups.
We identified 28/439 proteins associated with EAA in between‐pair analyses. Most of these proteins were associated with more than one estimate of EAA, such as MMP9, which was strongly positively associated with all six estimates of EAA. This is consistent with the known role of metalloproteinases in aging (Cancemi et al. 2020; Freitas‐Rodríguez et al. 2017), but also echoes, for example, how the GrimAge and GrimAge2 clocks were developed (Lu et al. 2019, 2022), since they involved the use of tissue inhibitors of metalloproteinases, which are regulators of metalloproteinases. Matrix metalloproteinase‐9 (MMP‐9) is associated with several aging and age‐related pathologies. Elevated MMP‐9 levels are implicated in neurodegenerative disorders such as Alzheimer's disease (Bašić et al. 2024) as well as depression (Li et al. 2022). MMP9 activity in vascular tissues is associated with age‐related conditions such as atherosclerosis (Yabluchanskiy et al. 2016). Associations between MMP9 and EAA estimates were present in within‐pair models as well, two of which (with PhenoAge and GrimAge) remained significant within MZ pairs. This suggests a scenario in which associations of MMP9 with PhenoAge and Grimage estimates of EAA may be plausible, or that these associations are at least partially due to environmental effects not shared between siblings. Attempts to replicate these two associations in older MZ twin pairs of the EH‐Epi sample failed to reach Bonferroni significance despite nominal p values below 0.05 (GrimAge: nominal p = 1.5e‐3, Bonferroni p = 0.08; PhenoAge: nominal p = 8.4e‐3, Bonferroni p = 0.45) and effect sizes in the same direction (i.e., positive) as in FinnTwin12.
Among the proteins associated with multiple estimates of EAA were those involved in metabolic health regulation and inflammatory processes, highlighting biological links between systemic inflammation, metabolic dysfunction, and accelerated aging. PTPRS associated negatively with three EAA estimates (GrimAge, GrimAge2 and DunedinPACE) in the FinnTwin12 sample, and the finding was replicated in the EH‐Epi for GrimAge and GrimAge2. PTPRS plays a multifaceted role in aging through its impact in neural development and regeneration (Shen et al. 2009; Martin et al. 2011), metabolic regulation, and inflammation (Zheng et al. 2025). Its dysregulation can lead to inflammaging, changes in metabolism, and age‐related diseases. LRG1 was positively associated with three EAA estimates (GrimAge, GrimAge2 and PhenoAge) in FinnTwin12, and associations with both GrimAge and PhenoAge remained significant in the within‐pair analysis of all FinnTwin12 pairs and with GrimAge in all EH‐Epi pairs. LRG1 is an important factor of innate immunity, where it has been postulated to act as an acute phase protein, responding to infections and other inflammatory stimuli (Codina et al. 2010). It has been further shown to be upregulated in a plethora of human diseases and to contribute to the disease process in multiple organs (Camilli et al. 2022). CNTN1 showed a consistent negative association with DunedinPACE and GrimAge2 in both FinnTwin12 and EH‐Epi, and it was replicated in the within‐pair analyses. CNTN1 has been previously associated with both frailty (Liu et al. 2024) and frailty trajectories (Verghese et al. 2021) in proteomic studies in mid‐ and late life. These and other studies (Osawa et al. 2020) provide evidence for CNTN1 having a protective role in the development of frailty, which has been associated with EAA (Gale et al. 2018). These findings are in line with our observations on higher plasma CNTN1 levels associated with lower EAA both in early adulthood and later life.
A total of 4/140 metabolites were found to be associated with EAA in the between‐pair analyses, three of which are well known to be associated with biological aging. Glycoprotein acetyls were positively associated with EAA in both early and late adulthood in our study. Glycoprotein acetyls are markers of inflammation, and they may contribute to inflammaging, the low‐grade inflammation occurring during aging predisposing to many age‐related diseases (Franceschi and Campisi 2014). A recent study showed that glycoprotein acetyls are positively associated with the frailty index in older age (Bålsrud et al. 2024), and glycoprotein acetyls have been shown to be associated with all‐cause mortality and consequently used to create a metabolomic aging clock (Deelen et al. 2019). While our findings are supported by these aforementioned studies, it also adds to the literature by demonstrating a direct link between glycoprotein acetyls and EAA, which is present already in early adulthood. We observed both in the younger and older twins that the higher the albumin levels, the lower the EAA, which is consistent with the literature on epigenetic aging (Uchehara et al. 2023) and aging in general (Lau et al. 2024), where lower albumin levels are associated with higher chronological age. The same has been observed for mortality risk among adults at all ages (Fulks et al. 2010; Deelen et al. 2019). We verified the negative association between albumin and EAA in within‐pair analyses in all younger and older twin pairs, but no significant differences were observed within MZ twin pairs, and therefore, genetic confounding could not be completely ruled out in these associations. Finally, histidine was negatively associated with EAA in the current study. Histidine has been shown to predict chronological age and all‐cause mortality (Lau et al. 2024; Deelen et al. 2019). In the current study, histidine was significantly associated with the PhenoAge estimate of EAA in both between‐pair and within‐pair analyses of MZ twins, suggesting a direct effect between histidine level and EAA, independent of genetic effects.
Pyruvate was the only metabolite identified in between‐pair analyses that was associated with EAA, and to our knowledge, its relationship with biological aging has not yet been established in the literature. Further investigation is needed to disentangle the role of pyruvate in aging. Another metabolite we identified to differ within the MZ twin pairs was glutamine, which was negatively associated with the DunedinPACE estimate of EAA in FinnTwin12. In contrast, when we attempted to replicate this association in older twins of the EH‐Epi sample, we found a substantial (but not significant after Bonferroni correction) positive association. A positive association between glutamine and chronological age was also observed in a large metabolomic study of aging including adults ranging from 24 to 86 years old (Lau et al. 2024). This suggests that the role of glutamine in biological aging may change over decades, with higher levels indicating a lower rate of aging in young adulthood but a higher rate of aging in older adults. Further studies to disentangle the relationship between glutamine and biological aging are needed, particularly in younger populations.
Lifestyle variables were also associated with EAA. We observed a greater acceleration of epigenetic aging (PhenoAge) in individuals who had their first sexual intercourse before the age of 18 in between‐pair analyses. This echoes the results of a study based on women from the ALSPAC cohort, in which an earlier age at first sexual intercourse was associated with greater EAA during childhood (Schlomer 2024). Another study showed the same direction of association in women and demonstrated the role of BMI as a mediator in this association (Zhang et al. 2023). Thus, our findings consolidate the existing literature by showing that an earlier age at sexual intercourse is associated with greater EAA in a sample including both women and men, and adjusting for BMI but also for smoking. The other lifestyle variable that we observed to be significantly associated with EAA was the variable representing having a vocational degree, where individuals with a vocational degree (i.e., individuals with low educational attainment) had higher EAA values calculated with GrimAge and GrimAge2 than others. This is consistent with the known association between lower education and greater GrimAge EAA reported in the literature (Crimmins et al. 2021). Associations between EAA and lifestyle variables, such as education level and age at first sexual intercourse, may collectively reflect an underlying association between lower socioeconomic status and higher biological aging (Schmitz et al. 2022).
We identified multiple associations between the external exposome and the GrimAge and GrimAge2 estimates of EAA in the between‐pair analyses. Significant associations were observed with crime rates, the proportion of 20‐year‐olds, sizes of nearest blue and green spaces areas, and average educational attainment, all in the residential neighborhood indexed by geocode. For all these or related characteristics, similar associations have been reported (Kim et al. 2023; Dos Santos Oliveira et al. 2023; Jovanovic et al. 2017). For instance, we observed that the higher the proportion of individuals with no education after primary or secondary education among individuals over 16 years old at the geocode level, the higher the acceleration of epigenetic aging of individuals living in such an area. Our study therefore suggests that lower educational attainment both at the geocode level (Exposome domain) and at the individual level (Lifestyle domain) is associated with greater EAA. As with lifestyle‐related traits, associations between the external exposome and EAA may be strongly confounded by factors such as socioeconomic status, and causality remains to be investigated. For example, socioeconomic status may influence both an individual's place of residence and dietary patterns, with the latter being a more likely contributor to reduced biological aging. Thus, multiple complex factors may explain the observed associations between the external exposome and EAA. The external exposome, which in our study represents physical and social exposures, and lifestyle are strongly interrelated, which in studies of biological aging may result in one domain confounding EAA associations of the other.
Within‐pair analyses showed only a few associations between EAA and the external exposome, suggesting that a large proportion of the associations observed in the between‐pair analyses are likely due to shared environmental effects and genetic confounding. However, the proportion of individuals aged over 90 years at the geocode level was significantly associated with increased EAA in within‐pair analyses in MZ twin pairs, suggesting a strong connection between age structure in a neighborhood tagged by a geocode and EAA independent of all genetic and shared environmental factors. While the literature on EAA in relation to the prenatal, childhood, or adolescent exposome is growing, that is not the case for the adult exposome. Further studies between EAA and the adult external exposome are therefore warranted.
While some multi‐omic associations are shared between different EAA estimates, we also observed differences at the level of domains of the multi‐ome. As each EAA estimate is constructed using different populations and outcomes, they characterize different aspects and functions of biological aging, resulting in greater affinity to either deep (i.e., molecular) or shallow (e.g., external exposome) layers of the multi‐ome. Hannum and Horvath clocks that were trained to predict chronological age only captured age acceleration‐related alterations in proteins. All the observed associations attenuated in the within‐pair analyses of MZ twin pairs. This may reflect rather strong effects of underlying genetics, which affect methylation at age‐associated CpG sites as well as protein expression. Genetic effects may be more direct and less affected by the environment for clocks that estimate calendar age compared with the clocks that were trained to predict phenotypes that reflect the effect of aging on health and mortality risk. Also, the DunedinPACE estimate of EAA showed the strongest connections with metabolites and proteins. It should be noted that DunedinPACE is a measure for which higher values indicate an acceleration in the pace of aging rather than accelerated age, unlike the other estimates of EAA we used in the current study. Consequently, the strongest association with plasma omics was found with the only aging clock that represents the pace of aging. The GrimAge, GrimAge2, and PhenoAge estimates of EAA were the only clocks whose connections with the external exposome and lifestyle‐related traits were significant. Our study therefore provides an excellent platform for assessing which omics of the multi‐ome, shallow or deep, and which EAA clock is best suited to a given research question. Extending multi‐omic approaches to epigenetic aging to other omics than those investigated in the current study may lead to a better understanding of biological aging, and such attempts should be fostered.
Our study had limitations that may have affected its results. The first is sample size, as the number of twin pairs included in the analyses was modest with regard to the number of variables included in our analyses and therefore to the number of tests performed. However, twin cohorts with metabolomic, proteomic, external exposome, and lifestyle data in a similar or larger number of twins are rare, demonstrating the uniqueness of our study and its unprecedented contribution to the literature. In addition, since the FinnTwin12 twins are young adults, they have had only limited time to build their own experiences outside of their familial environment, which the twins in a pair share. This may have led to relatively modest within‐pair differences in the omic profiles and therefore a failure to detect significant associations. By design, differences in biological age estimates were modest, as the twins—particularly those from FinnTwin12—were relatively homogeneous in age. This resulted in relatively modest variability in biological aging and only moderate correlations between these estimates and chronological age, suggesting that our ability to identify associations was not optimal. In addition, because the environment of twins differs whether they are in their 20s or in their 60s, the small number of within‐pair associations that remained significant in the independent EH‐Epi sample could also be due to different environmental factors influencing associations between the multi‐ome and EAA throughout adulthood. The apparent age effects may also be due to cohort effects, as the younger twins were born in 1983–87 and the older twins in 1945–57. Another limitation of the EH‐Epi sample is that it consists of selected twin pairs invited based on blood pressure discordance. However, their blood pressure measurements were broadly similar to those of their age group in Finland (Borodulin et al. 2013), suggesting that the sample is not overly enriched for high blood pressure and likely represents its age group well in the population. Another limitation that prevented us from validating all the observed associations in the FinnTwin12 sample in the independent sample (EH‐Epi) is the fact that the same exposome and lifestyle data were not available for both samples, and the proteomic data were generated using different platforms. Studies have demonstrated varying levels of concordance between assays across platforms (Eldjarn et al. 2023; Bhardwaj et al. 2021; Raffield et al. 2020). While some assays exhibit strong correlations across platforms, others do not; this could also account for the lack of replication of protein‐related findings in EH‐Epi. Also, the statistical models used in the current study assumed linearity in associations between the multi‐ome and EAA. As a result, increases in identified proteins, metabolites, or exposures are assumed to translate equally to increases or decreases in EAA across their value ranges, which may be an oversimplified view of how the multi‐ome relates to EAA. In addition to potential non‐linearity patterns in associations, EAA itself may be nonlinearly distributed across the age range, and including age as a covariate in models predicting EAA may help account for this pattern. Further studies investigating non‐linear patterns in omic associations with biological aging are encouraged, as the assessment of non‐linearity presents its own statistical challenges.
In conclusion, our study provides an in‐depth view of the connections that link the multi‐ome and acceleration of epigenetic aging. We have shown that associations between multi‐omic factors and EAA are due to different contributions of genetic and environmental factors, which may ultimately allow the identification of biomarkers of interest, for example, when conducting intervention or genetic studies. However, whether the determinants of biological aging in young adults are similar to those in children or in old adults remains to be further explored. Although the replication of our findings in an independent sample of 60‐year‐old participants partially met this challenge, intergenerational studies are encouraged to harmonize findings across age groups.
Author Contributions
G.D., J.K., and M.O. conceived and designed the study. G.D. and S.S. analyzed the data and performed the statistical analyses. S.S. and A.H. preprocessed the methylation data and generated EAA estimates in both FinnTwin12 and EH‐Epi samples. Z.W. preprocessed the exposome data of the FinnTwin12 sample. G.D. preprocessed the proteomic, metabolomic, and covariate data in both FinnTwin12 and EH‐Epi samples, as well as the lifestyle data in the FinnTwin12 sample. M.O. and J.K. contributed to the data collection by providing funding and resources. G.D. wrote the original draft. All authors provided critical feedback on the original draft, participated in its revision, and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. Scatter plot of standardized coefficients in both between‐pair and within‐pair analyses in FinnTwin12.
Table S1. Phenotypic correlations between EAA estimates in FinnTwin12 and EH‐Epi twin individuals.
Table S2. Description of multi‐omic factors included in between‐ and within‐pair discovery analyses using the FinnTwin12 sample.
Table S3. Description of multi‐omic factors included in between‐ and within‐pair replication analyses using the EH‐Epi sample.
Table S4. Associations between multi‐omic factors and epigenetic age acceleration in between‐pair analyses using the FinnTwin12 sample.
Table S5. Repeated between‐pair analyses without model adjustment by smoking and body mass index using the FinnTwin12 sample.
Table S6. Within‐pair results in FinnTwin12 using all same‐sex twin pairs.
Table S7. Within‐pair results in FinnTwin12 using same‐sex dizygotic twin pairs only.
Table S8. Within‐pair results in FinnTwin12 using monozygotic twin pairs only.
Table S9. Within‐pair coefficients in MZ and DZ pairs separately for associations found in within‐pair models using all pairs.
Table S10. Associations between multi‐omic factors and epigenetic age acceleration in between‐pair analyses using the EH‐Epi replication sample.
Table S11. Replication of within‐pair results from FinnTwin12 in external EH‐Epi sample in all same‐sex twin pairs, same‐sex DZ pairs only and MZ pairs only.
Acknowledgements
We thank M. Foraster, A. Ambros, and B. Raimbault of ISGlobal for enriching the external exposome data for this study. The graphical abstract was created in BioRender (license: https://biorender.com/a54z511). Open access publishing facilitated by Helsingin yliopisto, as part of the Wiley ‐ FinELib agreement.
Funding: M.O. and J.K. acknowledge support from the Sigrid Juséliuksen Säätiö. G.D. and Z.W. have been supported by the doctoral programs of the University of Helsinki. Data collection in the twin cohort has been supported by the Academy of Finland (grants 100499, 205585, 118555, 141054, 264146, 308248 to JKaprio) and the Academy of Finland Center of Excellence in Complex Disease Genetics (grant # 352792 to Kaprio).
Contributor Information
Gabin Drouard, Email: gabin.drouard@helsinki.fi.
Jaakko Kaprio, Email: jaakko.kaprio@helsinki.fi.
Miina Ollikainen, Email: miina.ollikainen@helsinki.fi.
Data Availability Statement
The data analyzed in this study is not publicly available due to the restrictions of informed consent. Requests to access these datasets should be directed to the Institute for Molecular Medicine Finland (FIMM) Data Access Committee (DAC) (fimmdac@helsinki.fi) for authorized researchers who have IRB/ethics approval and an institutionally approved study plan. To ensure the protection of privacy and compliance with national data protection legislation, a data use/transfer agreement is needed, the content and specific clauses of which will depend on the nature of the requested data.
References
- Afonin, A. M. , Piironen A. K., de Sousa Maciel I., et al. 2024. “Proteomic Insights Into Mental Health Status: Plasma Markers in Young Adults.” Translational Psychiatry 14: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bålsrud, P. , Ulven S. M., Christensen J. J., Ottestad I., and Holven K. B.. 2024. “Inflammatory Markers and Frailty in Home‐Dwelling Elderly, a Cross‐Sectional Study.” BMC Geriatrics 24, no. 1: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bašić, J. , Milošević V., Djordjević B., et al. 2024. “Matrix Remodeling Enzymes as Potential Fluid Biomarkers of Neurodegeneration in Alzheimer's Disease.” International Journal of Molecular Sciences 25, no. 11: 5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, D. W. , Caspi A., Corcoran D. L., et al. 2022. “DunedinPACE, a DNA Methylation Biomarker of the Pace of Aging.” eLife 11: e73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj, M. , Terzer T., Schrotz‐King P., and Brenner H.. 2021. “Comparison of Proteomic Technologies for Blood‐Based Detection of Colorectal Cancer.” International Journal of Molecular Sciences 22, no. 3: 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogl, L. H. , Kaye S. M., Ramo J. T., et al. 2016. “Abdominal Obesity and Circulating Metabolites: A Twin Study Approach.” Metabolism 65: 111–121. [DOI] [PubMed] [Google Scholar]
- Borodulin, K. , Levälahti E., and Saarikoski L.. 2013. “Kansallinen FINRISKI 2012 – Terveystutkimus: Osa 2: Tutkimuksen Taulukkoliite” (THL Report No. 22/2013) Terveyden ja Hyvinvoinnin Laitos (THL).
- Callister, S. J. , Barry R. C., Adkins J. N., et al. 2006. “Normalization Approaches for Removing Systematic Biases Associated With Mass Spectrometry and Label‐Free Proteomics.” Journal of Proteome Research 5: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli, C. , Hoeh A. E., De Rossi G., Moss S. E., and Greenwood J.. 2022. “LRG1: An Emerging Player in Disease Pathogenesis.” Journal of Biomedical Science 29, no. 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancemi, P. , Aiello A., Accardi G., et al. 2020. “The Role of Matrix Metalloproteinases (MMP‐2 and MMP‐9) in Ageing and Longevity: Focus on Sicilian Long‐Living Individuals (LLIs).” Mediators of Inflammation 2020: 8635158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonneau, M. , Li Y., Prescott B., et al. 2024. “Epigenetic Age Mediates the Association of Life's Essential 8 With Cardiovascular Disease and Mortality.” Journal of the American Heart Association 13: e032743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, B. C. , Houseman E. A., Marsit C. J., et al. 2009. “Aging and Environmental Exposures Alter Tissue‐Specific DNA Methylation Dependent Upon CpG Island Context.” PLoS Genetics 5: e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina, R. , Vanasse A., Kelekar A., Vezys V., and Jemmerson R.. 2010. “Cytochrome c‐Induced Lymphocyte Death From the Outside in: Inhibition by Serum Leucine‐Rich Alpha‐2‐Glycoprotein‐1.” Apoptosis 15, no. 2: 139–152. [DOI] [PubMed] [Google Scholar]
- Crimmins, E. M. , Thyagarajan B., Levine M. E., Weir D. R., and Faul J.. 2021. “Associations of Age, Sex, Race/Ethnicity, and Education With 13 Epigenetic Clocks in a Nationally Representative U.S. Sample: The Health and Retirement Study.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 76: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prado‐Bert, P. , Ruiz‐Arenas C., Vives‐Usano M., et al. 2021. “The Early‐Life Exposome and Epigenetic Age Acceleration in Children.” Environment International 155: 106683. [DOI] [PubMed] [Google Scholar]
- Deelen, J. , Kettunen J., Fischer K., et al. 2019. “A Metabolic Profile of All‐Cause Mortality Risk Identified in an Observational Study of 44,168 Individuals.” Nature Communications 10: 3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Oliveira, N. C. , Katrinli S., de Assis S. G., Smith A. K., and Serpeloni F.. 2023. “Community and Domestic Violence Are Associated With DNA Methylation GrimAge Acceleration and Heart Rate Variability in Adolescents.” European Journal of Psychotraumatology 14: 2202054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouard, G. , Hagenbeek F. A., Ollikainen M., et al. 2024b. “Twin Study Provides Heritability Estimates for 2321 Plasma Proteins and Assesses Missing SNP Heritability.” https://www.medrxiv.org/content/10.1101/2024.04.24.24306270v1. [DOI] [PMC free article] [PubMed]
- Drouard, G. , Hagenbeek F. A., Whipp A. M., et al. 2023. “Longitudinal Multi‐Omics Study Reveals Common Etiology Underlying Association Between Plasma Proteome and BMI Trajectories in Adolescent and Young Adult Twins.” BMC Medicine 21: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouard, G. , Ollikainen M., Mykkänen J., et al. 2022. “Multi‐Omics Integration in a Twin Cohort and Predictive Modeling of Blood Pressure Values.” OMICS 26: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouard, G. , Wang Z., Heikkinen A., et al. 2024a. “Lifestyle Differences Between Co‐Twins Are Associated With Decreased Similarity in Their Internal and External Exposome Profiles.” Scientific Reports 14: 21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldjarn, G. H. , Ferkingstad E., Lund S. H., et al. 2023. “Large‐Scale Plasma Proteomics Comparisons Through Genetics and Disease Associations.” Nature 622: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, C. A. , Barker‐Kamps M., Goering M., Patki A., Tiwari H. K., and Mrug S.. 2023. “Epigenetic Age Acceleration Correlates With BMI in Young Adults.” Aging (Albany NY) 15: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi, C. , and Campisi J.. 2014. “Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age‐Associated Diseases.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 69, no. Suppl 1: S4–S9. [DOI] [PubMed] [Google Scholar]
- Freitas‐Rodríguez, S. , Folgueras A. R., and López‐Otín C.. 2017. “The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and Beyond.” Biochimica et Biophysica Acta, Molecular Cell Research 1864, no. 11 Pt A: 2015–2025. [DOI] [PubMed] [Google Scholar]
- Fulks, M. , Stout R. L., and Dolan V. F.. 2010. “Albumin and All‐Cause Mortality Risk in Insurance Applicants.” Journal of Insurance Medicine 42, no. 1: 11–17. [PubMed] [Google Scholar]
- Gale, C. R. , Marioni R. E., Harris S. E., Starr J. M., and Deary I. J.. 2018. “DNA Methylation and the Epigenetic Clock in Relation to Physical Frailty in Older People: The Lothian Birth Cohort 1936.” Clinical Epigenetics 10, no. 1: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Starmer J., and Martin E. R.. 2008. “A Multiple Testing Correction Method for Genetic Association Studies Using Correlated Single Nucleotide Polymorphisms.” Genetic Epidemiology 32, no. 4: 361–369. [DOI] [PubMed] [Google Scholar]
- Gustavson, K. , Torvik F. A., Davey Smith G., Røysamb E., and Eilertsen E. M.. 2024. “Familial Confounding or Measurement Error? How to Interpret Findings From Sibling and Co‐Twin Control Studies.” European Journal of Epidemiology 39, no. 6: 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum, G. , Guinney J., Zhao L., et al. 2013. “Genome‐Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates.” Molecular Cell 49: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins‐Chen, A. T. , Thrush K. L., Wang Y., et al. 2022. “A Computational Solution for Bolstering Reliability of Epigenetic Clocks: Implications for Clinical Trials and Longitudinal Tracking.” Nature Aging 2: 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S. 2013. “DNA Methylation Age of Human Tissues and Cell Types.” Genome Biology 14: R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Y. , Dan X., Babbar M., et al. 2019. “Ageing as a Risk Factor for Neurodegenerative Disease.” Nature Reviews. Neurology 15: 565–581. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Ollikainen M., Muniandy M., et al. 2020. “Identification, Heritability, and Relation With Gene Expression of Novel DNA Methylation Loci for Blood Pressure.” Hypertension 76, no. 1: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic, T. , Vance L. A., Cross D., et al. 2017. “Exposure to Violence Accelerates Epigenetic Aging in Children.” Scientific Reports 7: 8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, B. T. , Gao T., Zheng Y., et al. 2021. “Epigenetic Age Acceleration Reflects Long‐Term Cardiovascular Health.” Circulation Research 129: 770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein, M. 2017. “Translational Geroscience: A New Paradigm for 21st Century Medicine.” Translational Medicine of Aging 1: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpää, A. , Tolvanen A., Heikkinen A., Kaprio J., Ollikainen M., and Sillanpää E.. 2022. “The Role of Adolescent Lifestyle Habits in Biological Aging: A Prospective Twin Study.” eLife 11: e80729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio, J. 2006. “Twin Studies in Finland 2006.” Twin Research and Human Genetics 9: 772–777. [DOI] [PubMed] [Google Scholar]
- Kaprio, J. , Bollepalli S., Buchwald J., et al. 2019. “The Older Finnish Twin Cohort ‐ 45 Years of Follow‐Up.” Twin Research and Human Genetics 22: 240–254. [DOI] [PubMed] [Google Scholar]
- Kim, K. , Joyce B. T., Nannini D. R., et al. 2023. “Inequalities in Urban Greenness and Epigenetic Aging: Different Associations by Race and Neighborhood Socioeconomic Status.” Science Advances 9: eadf8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, C. E. , Manou M., Markozannes G., et al. 2024. “NMR Metabolomic Modeling of Age and Lifespan: A Multicohort Analysis.” Aging Cell 23, no. 7: e14164. 10.1111/acel.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek, J. T. , Johnson W. E., Parker H. S., Jaffe A. E., and Storey J. D.. 2012. “The Sva Package for Removing Batch Effects and Other Unwanted Variation in High‐Throughput Experiments.” Bioinformatics 28, no. 6: 882–883. 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. E. , Lu A. T., Quach A., et al. 2018. “An Epigenetic Biomarker of Aging for Lifespan and Healthspan.” Aging (Albany NY) 10: 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Sheng Z., Khan S., et al. 2022. “Matrix Metalloproteinase‐9 as an Important Contributor to the Pathophysiology of Depression.” Frontiers in Neurology 13: 861843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , and Ji L.. 2005. “Adjusting Multiple Testing in Multilocus Analyses Using the Eigenvalues of a Correlation Matrix.” Heredity 95: 221–227. [DOI] [PubMed] [Google Scholar]
- Liu, F. , Schrack J. A., Walston J., et al. 2024. “Mid‐Life Plasma Proteins Associated With Late‐Life Prefrailty and Frailty: A Proteomic Analysis.” Geroscience 46, no. 5: 5247–5265. 10.1007/s11357-024-01219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Eliot M. N., Papandonatos G. D., et al. 2022. “Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome‐Wide Association Study.” Environmental Health Perspectives 130: 37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A. T. , Binder A. M., Zhang J., et al. 2022. “DNA Methylation GrimAge Version 2.” Aging (Albany NY) 14: 9484–9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A. T. , Quach A., Wilson J. G., et al. 2019. “DNA Methylation GrimAge Strongly Predicts Lifespan and Healthspan.” Aging (Albany NY) 11: 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, S. , Kuitunen S., Pietiläinen K. H., et al. 2022. “BMI Is Positively Associated With Accelerated Epigenetic Aging in Twin Pairs Discordant for Body Mass Index.” Journal of Internal Medicine 292: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni, R. E. , Shah S., McRae A. F., et al. 2015. “DNA Methylation Age of Blood Predicts All‐Cause Mortality in Later Life.” Genome Biology 16: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, K. R. , Xu Y., Looyenga B. D., et al. 2011. “Identification of PTPsigma as an Autophagic Phosphatase.” Journal of Cell Science 124, no. Pt 5: 812–819. 10.1242/jcs.080341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromatis, L. A. , Rosoff D. B., Bell A. S., Jung J., Wagner J., and Lohoff F. W.. 2023. “Multi‐Omic Underpinnings of Epigenetic Aging and Human Longevity.” Nature Communications 14: 2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams, T. A. , Rijsdijk F. V., Zavos H. M. S., and Pingault J. B.. 2021. “Twins and Causal Inference: Leveraging Nature's Experiment.” Cold Spring Harbor Perspectives in Medicine 11, no. 6: a039552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney, D. L. , Min J. L., Richmond R. C., et al. 2021. “Genome‐Wide Association Studies Identify 137 Genetic Loci for DNA Methylation Biomarkers of Aging.” Genome Biology 22: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, J. L. , Hemani G., Davey Smith G., Relton C., and Suderman M.. 2018. “Meffil: Efficient Normalization and Analysis of Very Large DNA Methylation Datasets.” Bioinformatics 34: 3983–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa, Y. , Semba R. D., Fantoni G., et al. 2020. “Plasma Proteomic Signature of the Risk of Developing Mobility Disability: A 9‐Year Follow‐Up.” Aging Cell 19, no. 4: e13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffield, L. M. , Dang H., Pratte K. A., et al. 2020. “Comparison of Proteomic Assessment Methods in Multiple Cohort Studies.” Proteomics 20, no. 12: e1900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, R. J. , Salvatore J. E., Aaltonen S., et al. 2019. “FinnTwin12 Cohort: An Updated Review.” Twin Research and Human Genetics 22: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh, Y. , Bejaoui Y., and El Hajj N.. 2020. “DNA Methylation Biomarkers in Aging and Age‐Related Diseases.” Frontiers in Genetics 11: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomer, G. L. 2024. “Epigenetic Age Acceleration and Reproductive Outcomes in Women.” Evolution and Human Behavior 45: 91–98. [Google Scholar]
- Schmitz, L. L. , Zhao W., Ratliff S. M., et al. 2022. “The Socioeconomic Gradient in Epigenetic Ageing Clocks: Evidence From the Multi‐Ethnic Study of Atherosclerosis and the Health and Retirement Study.” Epigenetics 17, no. 6: 589–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehovic, E. , Zellers S. M., Youssef M. K., Heikkinen A., Kaprio J., and Ollikainen M.. 2023. “DNA Methylation Sites in Early Adulthood Characterised by Pubertal Timing and Development: A Twin Study.” Clinical Epigenetics 15: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Y. , Tenney A. P., Busch S. A., et al. 2009. “PTPsigma Is a Receptor for Chondroitin Sulfate Proteoglycan, an Inhibitor of Neural Regeneration.” Science 326, no. 5952: 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen, P. , Kangas A. J., Würtz P., Suna T., and Ala‐Korpela M.. 2015. “Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics.” Circulation. Cardiovascular Genetics 8: 192–206. [DOI] [PubMed] [Google Scholar]
- Tsai, P. C. , Glastonbury C. A., Eliot M. N., et al. 2018. “Smoking Induces Coordinated DNA Methylation and Gene Expression Changes in Adipose Tissue With Consequences for Metabolic Health.” Clinical Epigenetics 10: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchehara, B. , Coulter Kwee L., Regan J., et al. 2023. “Accelerated Epigenetic Aging Is Associated With Multiple Cardiometabolic, Hematologic, and Renal Abnormalities: A Project Baseline Health Substudy.” Circulation: Genomic and Precision Medicine 16: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese, J. , Ayers E., Sathyan S., et al. 2021. “Trajectories of Frailty in Aging: Prospective Cohort Study.” PLoS One 16, no. 7: e0253976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid, M. 2014. “The Exposome: A New Paradigm to Study the Impact of Environment on Health.” Thorax 69, no. 9: 876–878. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Zellers S., Whipp A. M., et al. 2023. “The Effect of Environment on Depressive Symptoms in Late Adolescence and Early Adulthood: An Exposome‐Wide Association Study and Twin Modeling.” Nature Mental Health 1: 751–760. [Google Scholar]
- Whipp, A. M. , Heinonen‐Guzejev M., Pietiläinen K. H., van Kamp I., and Kaprio J.. 2022. “Branched‐Chain Amino Acids Linked to Depression in Young Adults.” Frontiers in Neuroscience 16: 935858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, E. J. , Walker A. J., Bhaskaran K., et al. 2020. “Factors Associated With COVID‐19‐Related Death Using OpenSAFELY.” Nature 584: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2019. “Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability.” Accessed 18 January 2024. https://www.who.int/data/gho/data/themes/mortality‐and‐global‐health‐estimates.
- Yabluchanskiy, A. , Ma Y., DeLeon‐Pennell K. Y., et al. 2016. “Myocardial Infarction Superimposed on Aging: MMP‐9 Deletion Promotes M2 Macrophage Polarization.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71, no. 4: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov, N. , Dieckmann L., Erhart M., et al. 2023. “Transdiagnostic Evaluation of Epigenetic Age Acceleration and Burden of Psychiatric Disorders.” Neuropsychopharmacology 48: 1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Yuan Q., Luan Y., and Xia J.. 2023. “Effect of Women's Fertility and Sexual Development on Epigenetic Clock: Mendelian Randomization Study.” Clinical Epigenetics 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Zhou C., Hu Y., et al. 2025. “Mass Spectrometry‐Based Proteomics Analysis Unveils PTPRS Inhibits Proliferation and Inflammatory Response of Keratinocytes in Psoriasis.” Inflammation 48, no. 1: 89–103. 10.1007/s10753-024-02044-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatter plot of standardized coefficients in both between‐pair and within‐pair analyses in FinnTwin12.
Table S1. Phenotypic correlations between EAA estimates in FinnTwin12 and EH‐Epi twin individuals.
Table S2. Description of multi‐omic factors included in between‐ and within‐pair discovery analyses using the FinnTwin12 sample.
Table S3. Description of multi‐omic factors included in between‐ and within‐pair replication analyses using the EH‐Epi sample.
Table S4. Associations between multi‐omic factors and epigenetic age acceleration in between‐pair analyses using the FinnTwin12 sample.
Table S5. Repeated between‐pair analyses without model adjustment by smoking and body mass index using the FinnTwin12 sample.
Table S6. Within‐pair results in FinnTwin12 using all same‐sex twin pairs.
Table S7. Within‐pair results in FinnTwin12 using same‐sex dizygotic twin pairs only.
Table S8. Within‐pair results in FinnTwin12 using monozygotic twin pairs only.
Table S9. Within‐pair coefficients in MZ and DZ pairs separately for associations found in within‐pair models using all pairs.
Table S10. Associations between multi‐omic factors and epigenetic age acceleration in between‐pair analyses using the EH‐Epi replication sample.
Table S11. Replication of within‐pair results from FinnTwin12 in external EH‐Epi sample in all same‐sex twin pairs, same‐sex DZ pairs only and MZ pairs only.
Data Availability Statement
The data analyzed in this study is not publicly available due to the restrictions of informed consent. Requests to access these datasets should be directed to the Institute for Molecular Medicine Finland (FIMM) Data Access Committee (DAC) (fimmdac@helsinki.fi) for authorized researchers who have IRB/ethics approval and an institutionally approved study plan. To ensure the protection of privacy and compliance with national data protection legislation, a data use/transfer agreement is needed, the content and specific clauses of which will depend on the nature of the requested data.


